Copper-Based MOF-Derived Core–Shell Materials via N/P/S Ternary Doping for Peroxymonosulfate Activation: Efficient Degradation and Removal of Sulfamethazine
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Experimental Chemical Reagents
2.3. Material Preparation Methods
2.3.1. Synthesis of Cu-MOFs400
2.3.2. Synthesis of Cu-MOFs400@PSN
2.4. Degradation Experiment
3. Results and Discussion
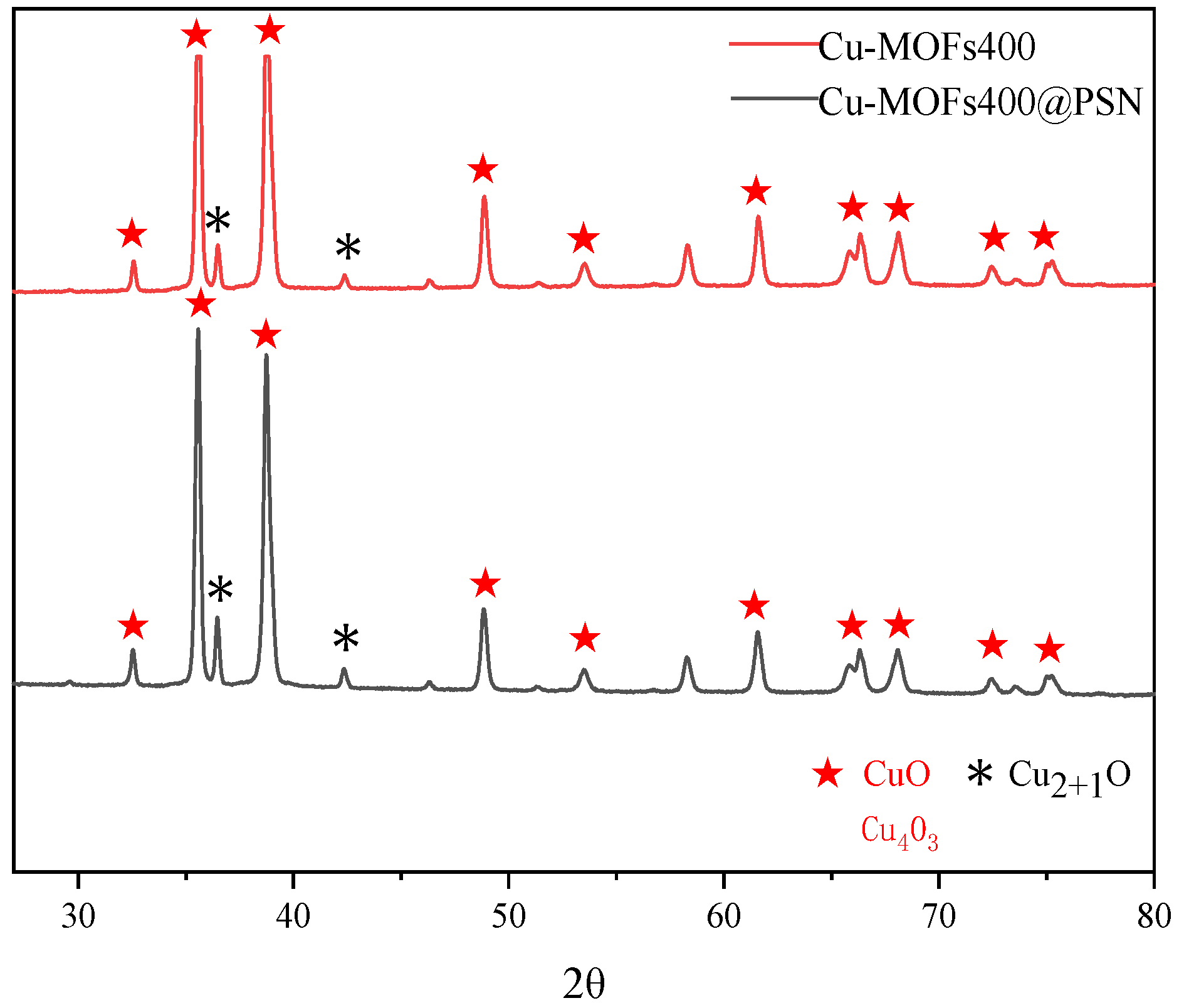
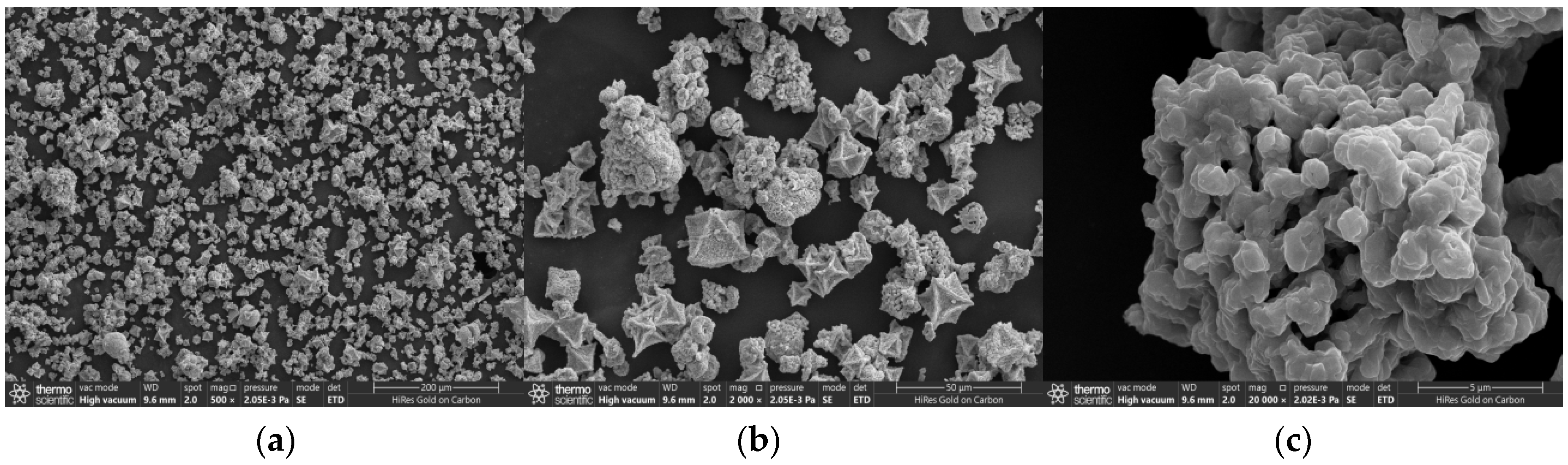
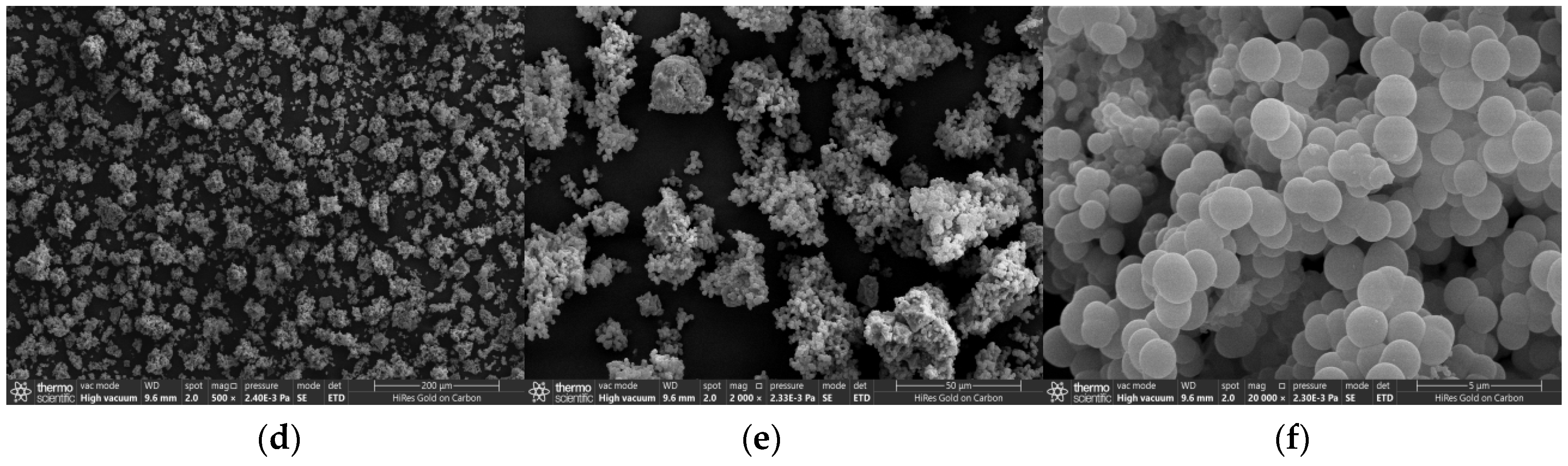
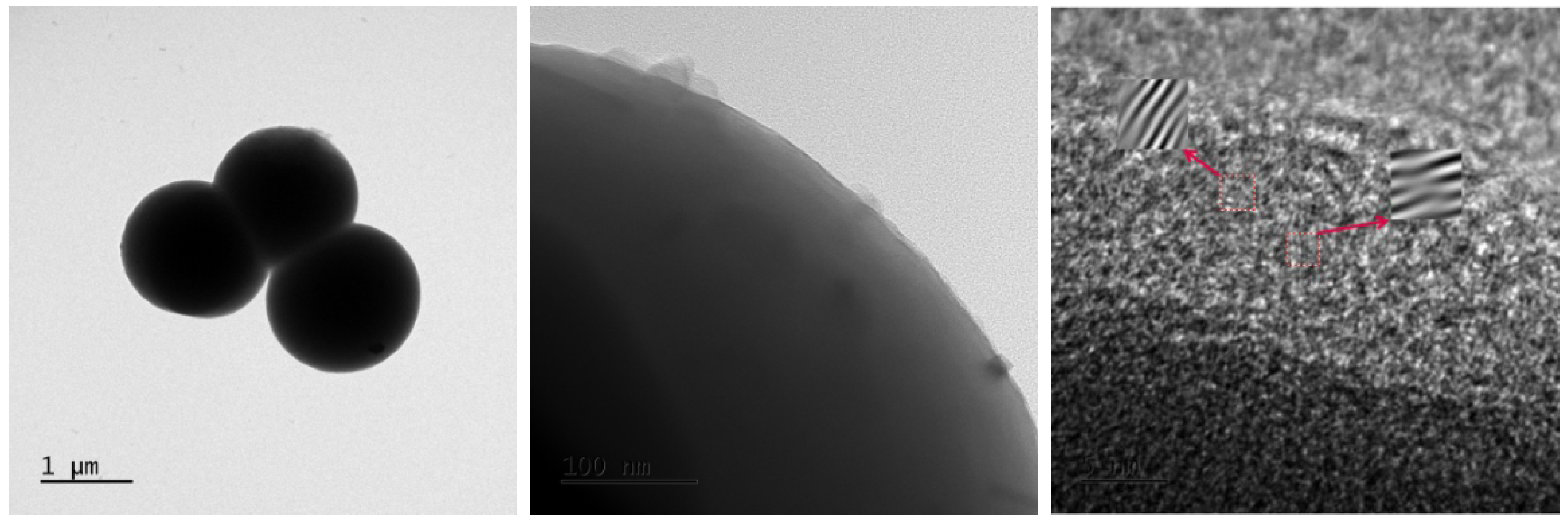
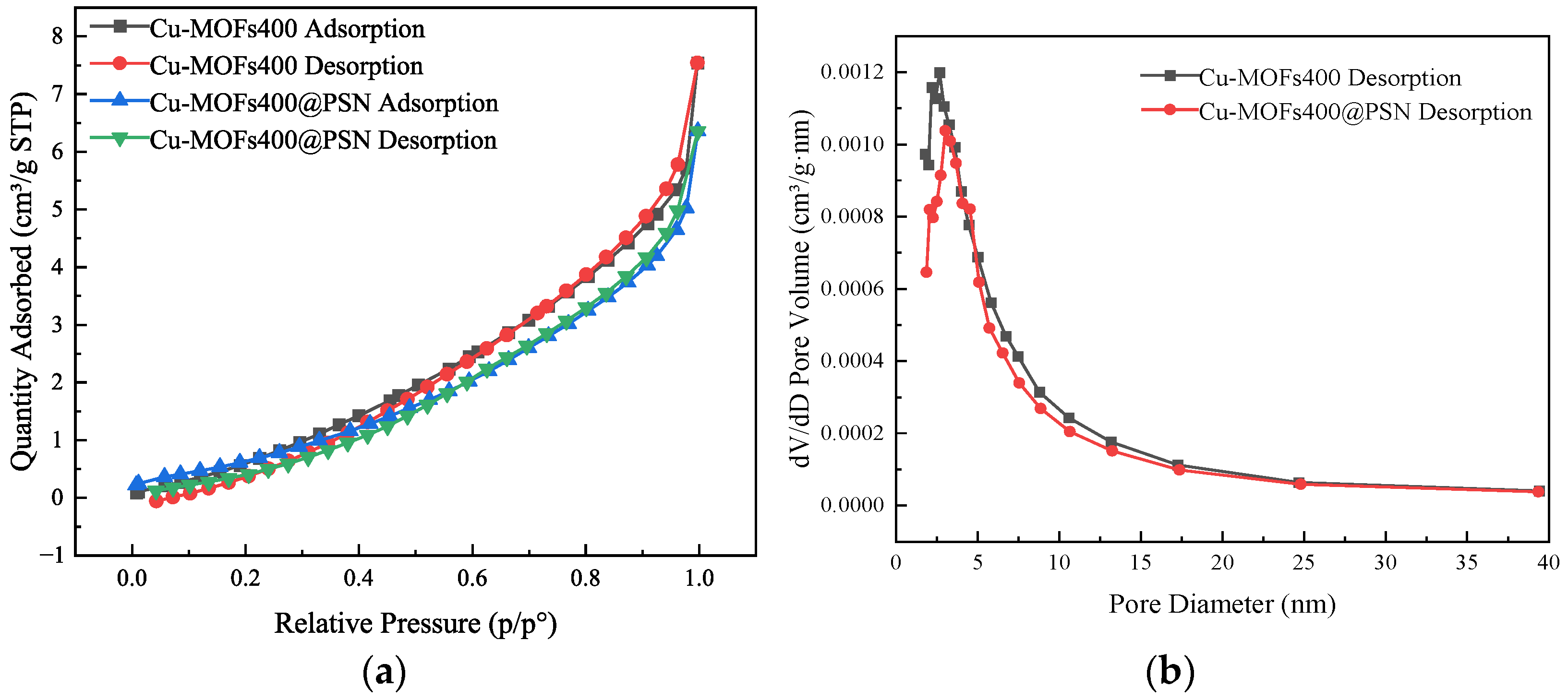
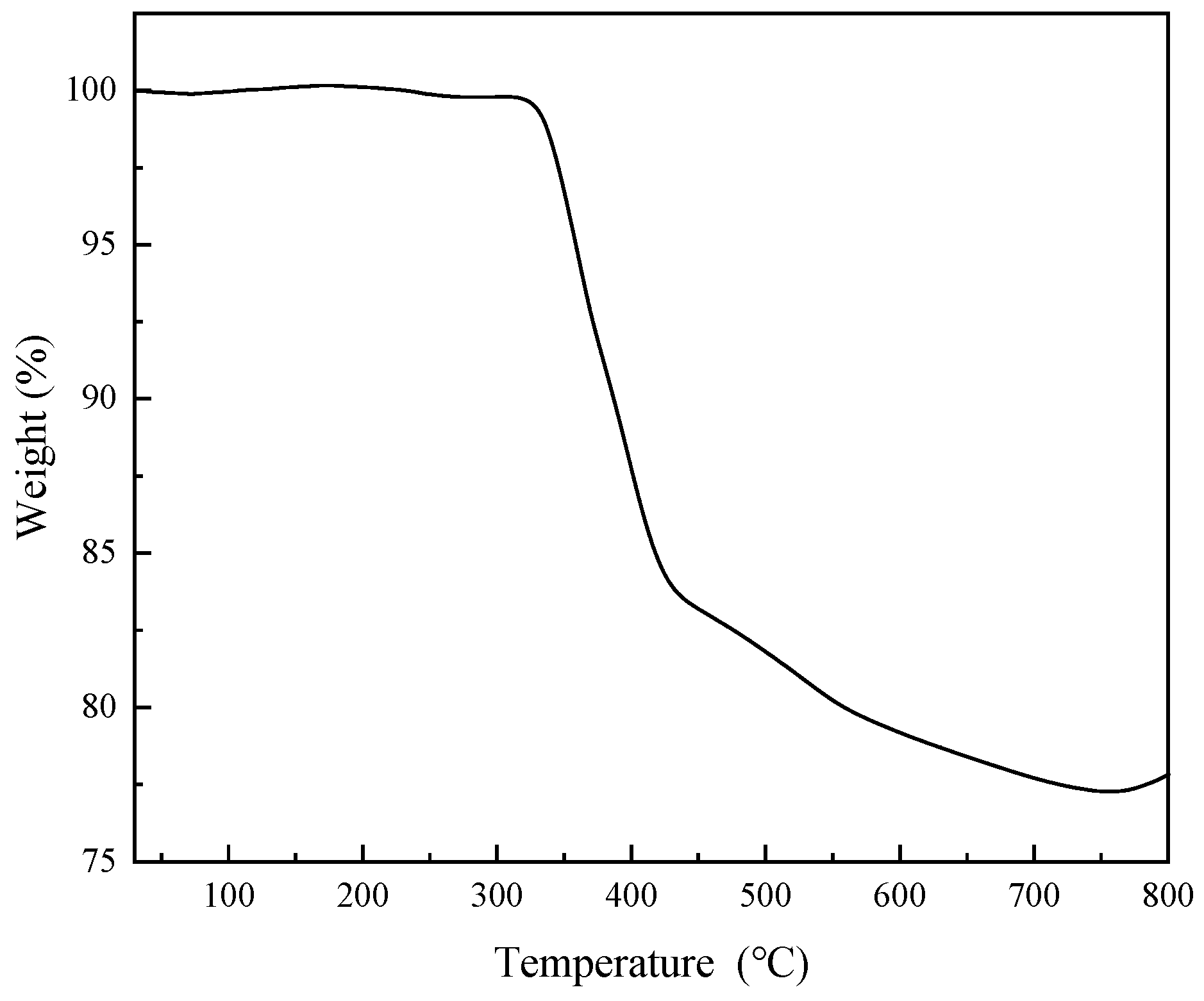
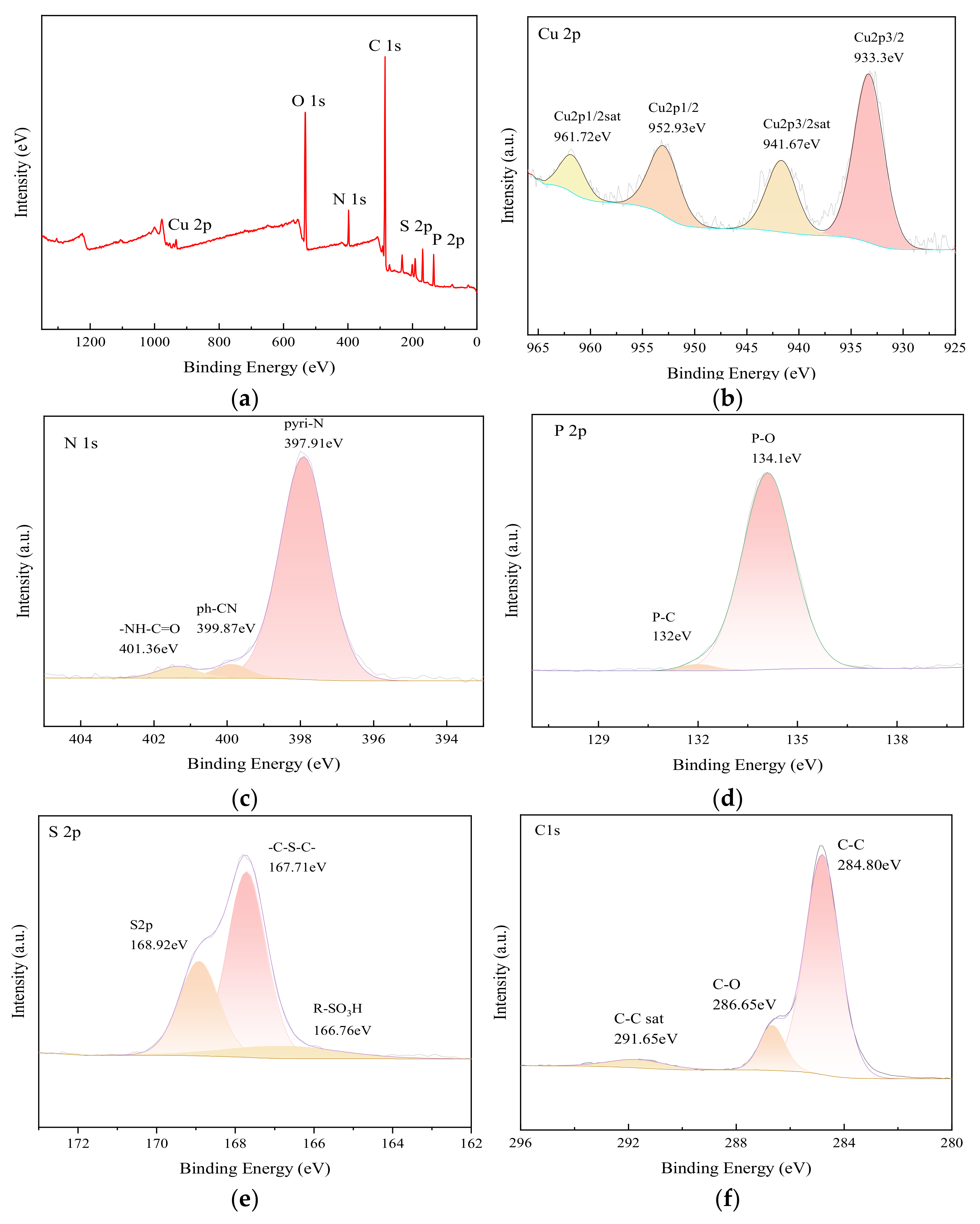
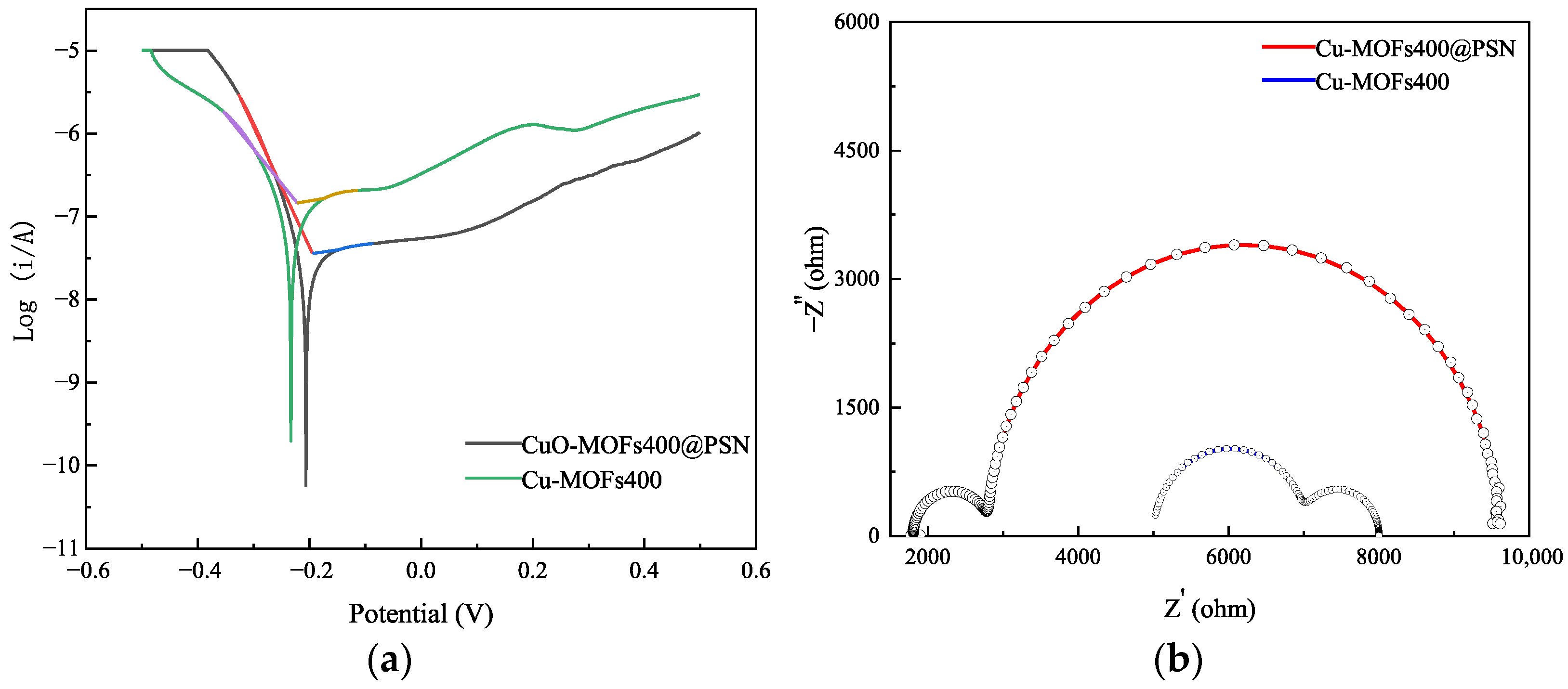
3.1. Material Characterization and Analysis
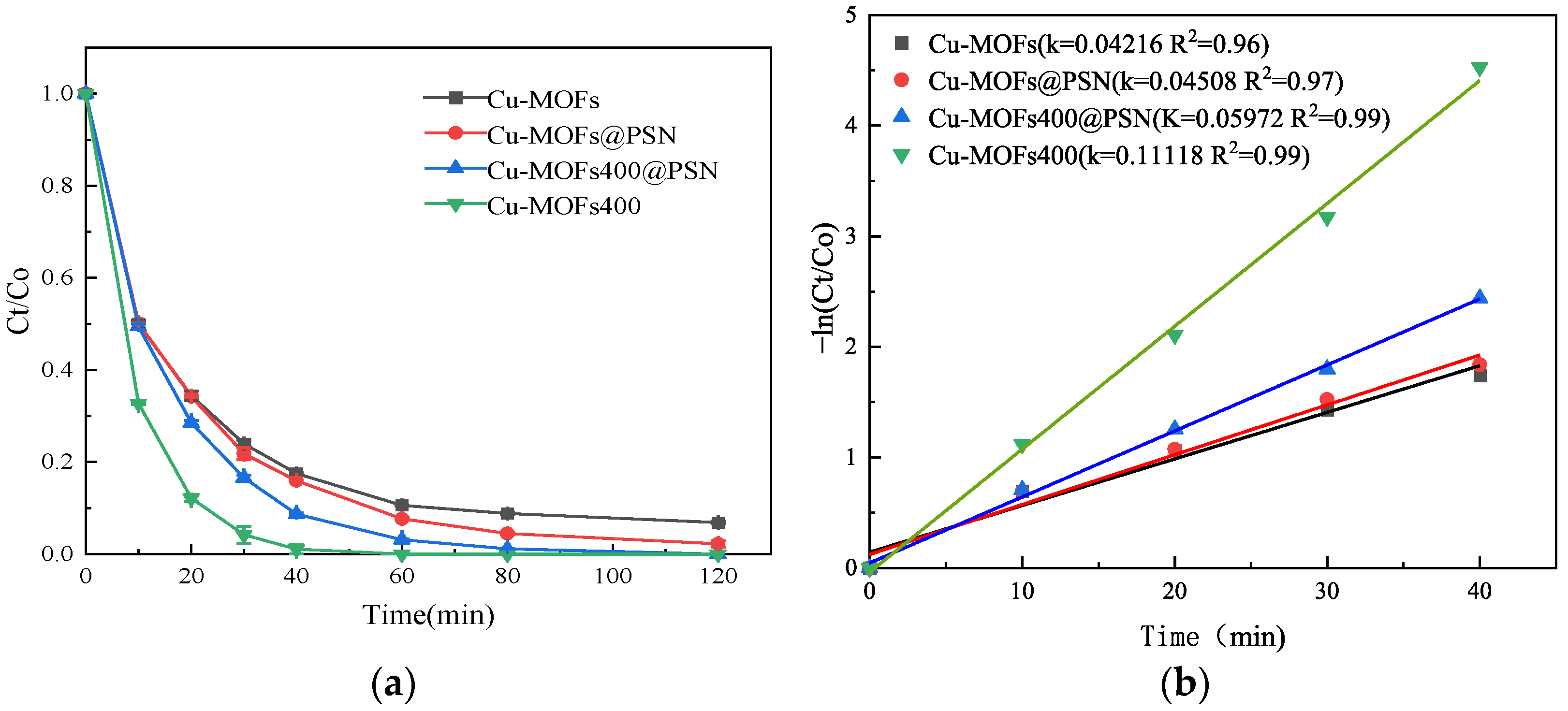
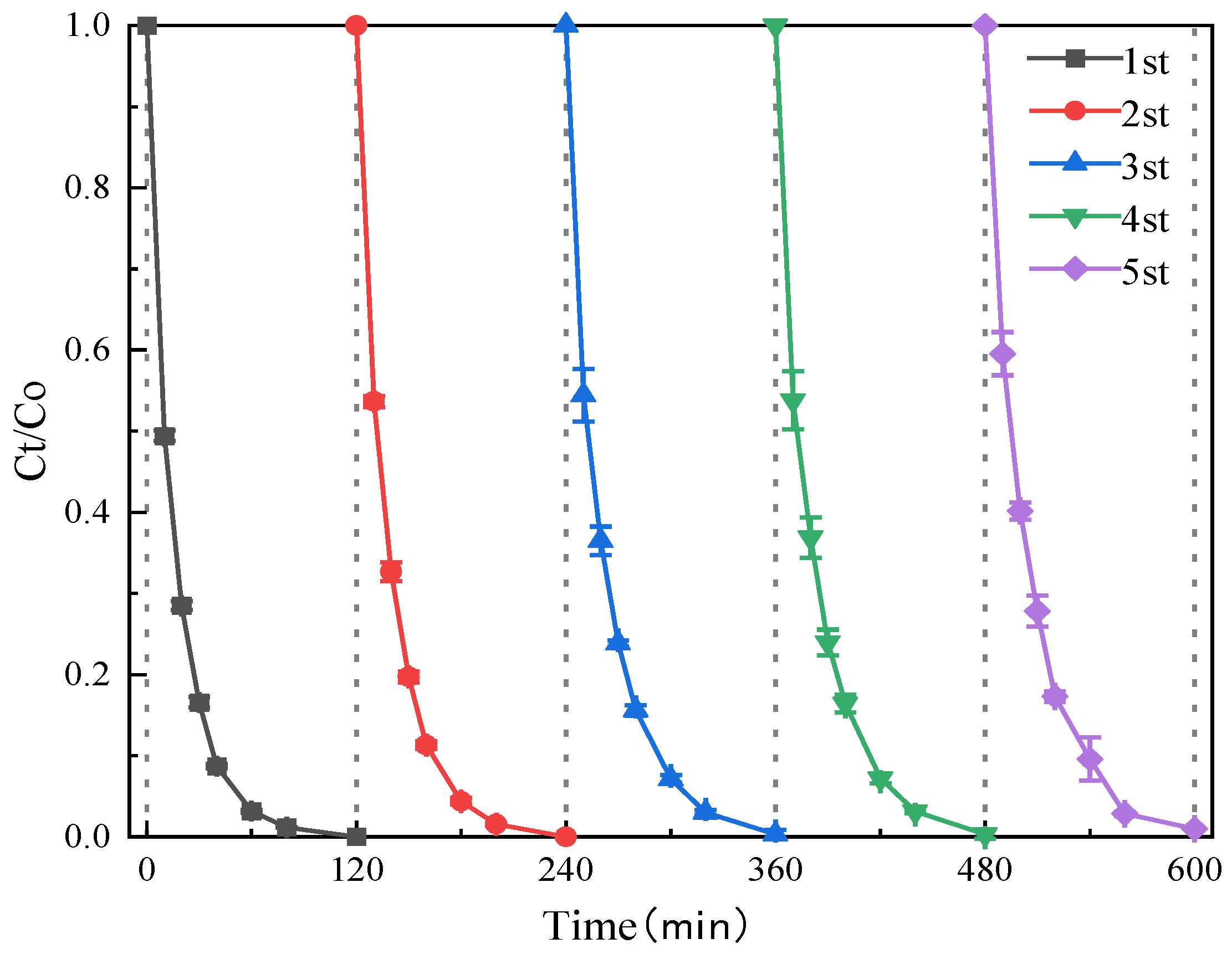
3.2. Effect of Reaction Systems on SMT Degradation Efficiency
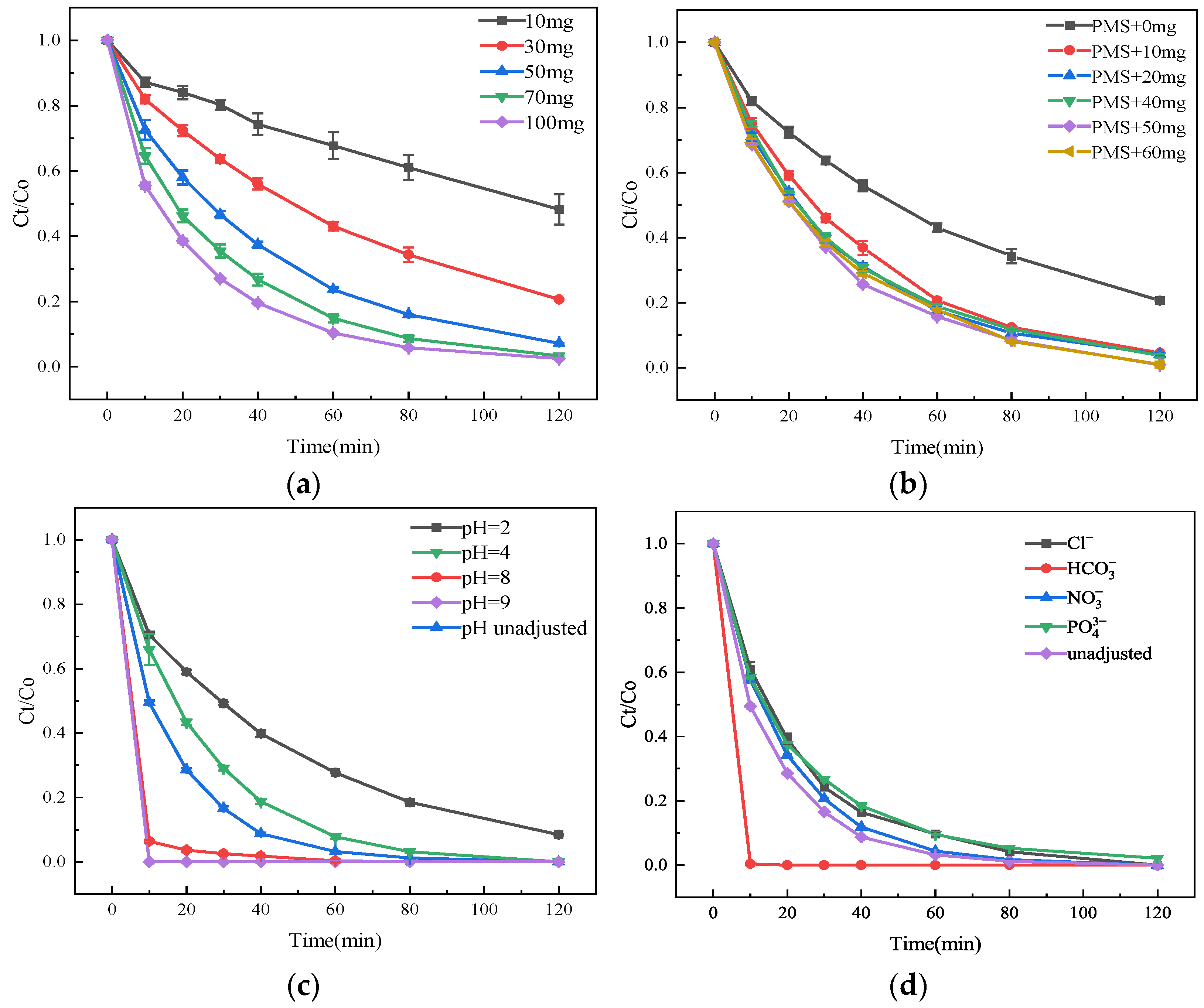
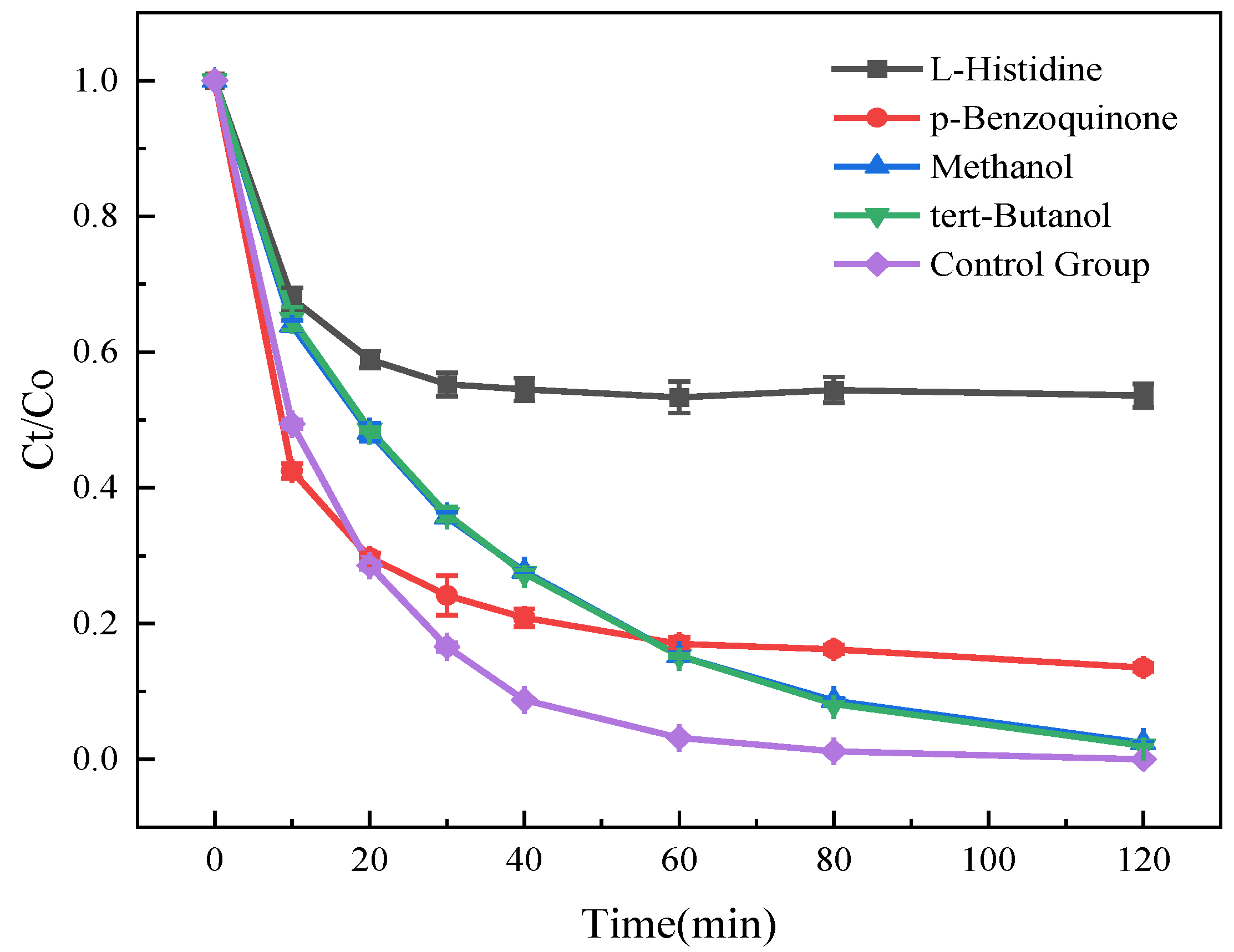
3.3. Influence of Reaction Conditions on the Degradation and Removal of SMT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMT | Sulfamethazine |
| PMS | peroxymonosulfate |
| Cu-MOFs400@PSN | core–shell structured activator |
| N/P/S | Nitrogen–phosphorus–sulfur |
| AR | Antibiotic resistance |
| ARB | antibiotic-resistant bacteria |
| ARGs | antibiotic resistance genes |
| AOPs | Advanced Oxidation Processes |
| MOFs | Metal–organic frameworks |
References
- Xiao, C.; Hu, Y.; Li, Q.; Liu, J.; Li, X.; Shi, Y.; Chen, Y.; Cheng, J. Carbon-doped defect MoS2 co-catalytic Fe3+/peroxymonosulfate process for efficient sulfadiazine degradation: Accelerating Fe3+/Fe2+ cycle and 1O2 dominated oxidation. Sci. Total Environ. 2023, 858, 159587. [Google Scholar] [CrossRef]
- Brillas, E.; Peralta-Hernández, J.M. Antibiotic removal from synthetic and real aqueous matrices by peroxymonosulfate-based advanced oxidation processes. A review of recent development. Chemosphere 2024, 351, 141153. [Google Scholar] [CrossRef]
- Zhu, G.; Ding, J.; Dou, A.; Xu, Q.; Yang, J.; Ji, Y.; Lu, G.; Zhu, M. Insights into sulfamethazine degradation by peroxymonosulfate activation using H2 reduced hematite in high-salinity wastewater: Performances and mechanisms. Chem. Eng. J. 2024, 500, 157259. [Google Scholar] [CrossRef]
- Xiao, C.; Li, S.; Yi, F.; Zhang, B.; Chen, D.; Zhang, Y.; Chen, H.; Huang, Y. Enhancement of photo-Fenton catalytic activity with the assistance of oxalic acid on the kaolin–FeOOH system for the degradation of organic dyes. RSC Adv. 2020, 32, 18704–18714. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chen, Y.; Guan, X.; Qin, Z.; Ke, X.; Li, Z.; Qiu, G.; Wu, H.; Wei, C. Total nitrogen self-purification driven by cyclic endogenous electron donors in coking wastewater treatment: Materials flow in A-OHHO process. Chem. Eng. J. 2025, 518, 164732. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, L.; Xie, Y.; Huang, W.; Liu, H.; Liu, X.; Chen, D. Trans-membrane piezoelectric activation of peroxymonosulfate for effective control of waterborne antibiotic resistance dissemination. npj Clean Water 2024, 7, 70. [Google Scholar] [CrossRef]
- Hazra, M.; Watts, J.E.M.; Williams, J.B.; Joshi, H. An evaluation of conventional and nature-based technologies for controlling antibiotic-resistant bacteria and antibiotic-resistant genes in wastewater treatment plants. Sci. Total Environ. 2024, 917, 170433. [Google Scholar] [CrossRef]
- Chen, A.; Li, H.; Wu, H.; Song, Z.; Chen, Y.; Zhang, H.; Pang, Z.; Qin, Z.; Wu, Y.; Guan, X.; et al. Anaerobic cyanides oxidation with bimetallic modulation of biological toxicity and activity for nitrite reduction. J. Hazard. Mater. 2024, 472, 134540. [Google Scholar] [CrossRef]
- Maniakova, G.; Salmerón, I.; Nahim-Granados, S.; Malato, S.; Oller, I.; Rizzo, L.; Polo-López, M.I. Sunlight advanced oxidation processes vs ozonation for wastewater disinfection and safe reclamation. Sci. Total Environ. 2021, 787, 147531. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Zhou, Y. Ultrasonic enhancement of persulfate oxidation system governs emerging pollutants decontamination. Green Energy Environ. 2024, 9, 1666–1678. [Google Scholar] [CrossRef]
- Liu, J.; Yang, R.; Jiang, Q.; Chen, S.; Fang, M.; Wang, S.; Xu, Z. Sulfamethazine degradation via PMS activated by Co-Cu catalyst supported on modified geopolymer. J. Environ. Chem. Eng. 2025, 13, 118825. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Yuan, M.; Ni, B.-J.; Xia, S.; Zhao, J. Insights into the removal of sulfamethazine and sulfonamide-resistant bacteria from wastewater by Fe-Mn spinel oxide modified cow manure biochar activated peroxymonosulfate: A nonradical pathway regulated by enhanced adsorption and 3d orbital electron reconstruction. Appl. Catal. B Environ. Energy 2025, 361, 124652. [Google Scholar] [CrossRef]
- Li, J.; Jing, B.; Tian, Y.; Li, G.; Li, X. Electrochemical activation of peroxymonosulfate with commercial electrodes in the mixed reaction chamber: A cost-effective way for sulfamethazine degradation. Desalination Water Treat. 2025, 323, 101279. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Yu, X.; Cheng, J.; Zhu, Y.; Zhu, B.; Cao, Q.; Cai, H.; Yang, X. Engineering Co(OH)2/mesoporous g-C3N4 composites for synergistic radical/non-radical antibiotic degradation via electron-bridged peroxymonosulfate activation. J. Environ. Chem. Eng. 2025, 13, 118457. [Google Scholar] [CrossRef]
- Li, R.; Huang, D.; Tao, J.; Wei, Z.; Wang, G.; Zhou, W.; Xu, W.; Huang, H.; Li, S.; Tang, L. In-Depth Investigation of Role of -BCO2 in the Degradation of Sulfamethazine by Metal-Free Biochar/Persulfate: The Mechanism of Occurrence of Nonradical Process. ACS Appl. Mater. Interfaces 2024, 16, 44850–44862. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-Y.; Wu, J.; Yin, G.; Jiao, F.-Z.; Yu, Z.-Z.; Qu, J. Dynamic Regulation of Hydrogen Bonding Networks and Solvation Structures for Synergistic Solar-Thermal Desalination of Seawater and Catalytic Degradation of Organic Pollutants. Nano-Micro Lett. 2024, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Bouzayani, B.; Lomba-Fernández, B.; Fdez-Sanromán, A.; Elaoud, S.C.; Sanromán, M.Á. Advancements in Copper-Based Catalysts for Efficient Generation of Reactive Oxygen Species from Peroxymonosulfate. Appl. Sci. 2024, 14, 8075. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Zhang, Y.; Zhou, L.; Huang, S.; Zhou, S. MOFs-derived Mn/C composites activating peroxymonosulfate for efficient degradation of sulfamethazine: Kinetics, pathways, and mechanism. Surf. Interfaces 2023, 36, 102551. [Google Scholar] [CrossRef]
- Dong, Y.; Huang, W.; Liang, C.; Gao, Y.; Wei, Z.; Meng, L.; Zhong, F.; Zhang, J.; Zhou, L.; Xu, J. Sulfamethazine degradation and copper transformation in Cu(II)/PMS system: In-depth investigation of the interaction between intermediates and copper. J. Water Process Eng. 2024, 58, 104929. [Google Scholar] [CrossRef]
- Qiu, Y.; Huang, Y.; Wang, Y.; Liu, X.; Huang, D. Facile synthesis of Cu-doped manganese oxide octahedral molecular sieve for the efficient degradation of sulfamethoxazole via peroxymonosulfate activation. Int. J. Miner. Metall. Mater. 2024, 31, 2770–2780. [Google Scholar] [CrossRef]
- Maged, A.; Elgarahy, A.M.; Elwakeel, K.Z.; El-Gohary, F.A.; Abd El-Ghaffar, M.A.; El-Kousy, S.M.; El-Shorbagy, K.M. Sustainable functionalized smectitic clay-based nanoscale hydrated zirconium oxides for enhanced levofloxacin sorption from aqueous medium. J. Hazard. Mater. 2023, 452, 131325. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qi, W.; Xie, Y.; Huang, C.; Su, Q.; Xue, X.; Han, Y.; Wei, D. Environmental geopolymer microsphere composite catalysts as highly efficient activators of PMS for the degradation of Rhodamine B. J. Mater. Sci. 2024, 59, 20575–20592. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, H.; Xiao, C.; Ni, L.; Chen, S.; Qi, J.; Zhou, Y.; Zhu, Z.; Li, J. Improvement of peroxymonosulfate utilization efficiency for sulfamethazine degradation by photo-electron activating peroxymonosulfate: Performance and mechanism. J. Colloid Interface Sci. 2023, 633, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Yang, R.; Liu, M.; Feng, Y.; Li, Z.; Zhu, W. A highly-efficient peroxymonosulfate activator using a sewage sludge derived biochar supported cobalt oxide: Mechanism and characteristics. Process Saf. Environ. Prot. 2024, 192, 1319–1329. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Hao, R.; Shao, M.; Yang, X.; Li, X.; Li, Y.; Liu, J.; Zhang, S. Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal. Nanotechnol. Rev. 2023, 12, 20220542. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Zhou, J.; Huang, Y.; Li, W.; Li, Y.; Li, Y.; Bi, H.; Chang, F.; Zhang, H.; et al. Amorphous Co@TiO2 heterojunctions: A high-performance and stable catalyst for the efficient degradation of sulfamethazine via peroxymonosulfate activation. Chemosphere 2022, 307, 135681. [Google Scholar] [CrossRef]
- Shen, Q.; Song, X.; Fan, J.; Chen, C.; Li, Z. UV/Advanced Oxidation Process for Removing Humic Acid from Natural Water: Comparison of Different Methods and Effect of External Factors. Water 2024, 16, 1815. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, Y.; Feng, L.; Yang, S.; Tan, X.; Liu, Z. Magnetic Fe3O4-C@MoS2 composites coordinated with peroxymonosulfate catalysis for enhanced tetracycline degradation. J. Alloys Compd. 2024, 989, 174318. [Google Scholar] [CrossRef]
- Li, X.; Wen, X.; Lang, J.; Wei, Y.; Miao, J.; Zhang, X.; Zhou, B.; Long, M.; Alvarez, P.J.J.; Zhang, L. CoN1O2 Single-Atom Catalyst for Efficient Peroxymonosulfate Activation and Selective Cobalt(IV)=O Generation. Angew. Chem. Int. Ed. 2023, 62, e202303267. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, S.; Zhang, Z.; Liu, S.; Guo, H.; Qi, X.; Wu, Z.; Guo, J. Cobalt/Nitrogen-Doped carbon hollow spheres for highly efficient degradation of tinidazole with peroxymonosulfate. Mater. Res. Bull. 2024, 175, 112755. [Google Scholar] [CrossRef]
- Demïr, A.; Akçay, H.T.; Özçïfçï, Z.; Keleş, T. The effective polymeric macromolecule as peroxymonosulfate activator as an alternative to metal oxides in the treatment of organic dye pollutants. Mater. Today Commun. 2024, 39, 109144. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Erk, N.; Karaman, C.; Karaman, O. An electrochemical molecularly imprinted sensor based on CuBi2O4/rGO@MoS2 nanocomposite and its utilization for highly selective and sensitive for linagliptin assay. Chemosphere 2022, 291, 132807. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, S.; Hou, X.; Li, J.; Yang, L.; Liu, X.; Li, M. Efficient electrocatalytic nitrate reduction via boosting oxygen vacancies of TiO2 nanotube array by highly dispersed trace Cu doping. J. Hazard. Mater. 2022, 438, 129455. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, P.P.; Pan, U.N.; Paudel, D.R.; Kandel, M.R.; Kim, N.H.; Lee, J.H. Cobalt–manganese sulfide hybridized Fe-doped 1T-Vanadium disulfide 3D-Hierarchical core-shell nanorods for extreme low potential overall water-splitting. Mater. Today Nano 2022, 20, 100272. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, X.; Ji, M.; Jiang, F. Densely Stacked CoCu-MOFs Coated with CuAl/LDH Enhance Sulfamethoxazole Degradation in PMS-Activated Systems. Nanomaterials 2025, 15, 432. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Yan, Y.; Luo, J.; Liu, T.; Wu, B.; Qian, F. Effects of peroxide types on the removal performance and mechanism of sulfonamide antibiotics using graphene-based catalytic membranes. Process Saf. Environ. Prot. 2023, 179, 362–372. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Yang, Y.; Xie, X.; Wang, X.; Wu, Z. Adsorption-activation dual-site synergy in nitrogen-doped mesoporous carbon nanosheets for enhanced pollutant degradation via peroxymonosulfate activation. Appl. Catal. B Environ. 2026, 380, 125762. [Google Scholar] [CrossRef]
- Mendes, P.C.D.; Song, Y.; Ma, W.; Gani, T.Z.H.; Lim, K.H.; Kawi, S.; Kozlov, S.M. Opportunities in the design of metal@oxide core-shell nanoparticles. Adv. Phys. X 2023, 8, 2175623. [Google Scholar] [CrossRef]
- Wang, B.; Song, Z.; Liu, F.; Yang, W.; Wan, Q.; Du, Y.; Sun, L. Novel Fe3O4@TiO2 core–shell nanostructure catalyst for NO removal through heterogeneous Fenton processes. J. Clean. Prod. 2024, 434, 140100. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Zhang, Z.; Feng, K.; Liang, J.; Wang, D.; Zhou, L. Organic carbon modified Fe3O4/schwertmannite for heterogeneous Fenton reaction featuring synergistic in-situ H2O2; generation and activation. Sep. Purif. Technol. 2021, 276, 119344. [Google Scholar] [CrossRef]
- Dai, H.; Li, N.; Ye, J.; Zhao, J.; He, X.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Confinement boosted heterogeneous advanced oxidation processes. Chem. Eng. J. 2023, 472, 144861. [Google Scholar] [CrossRef]
- Li, H.; Qin, X.; Wang, K.; Ma, T.; Shang, Y. Insight into metal-based catalysts for heterogeneous peroxymonosulfate activation: A critical review. Sep. Purif. Technol. 2024, 333, 125900. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, W.; Cao, S.; Yin, X.; Zhu, H.; Gu, L.; Chen, H.; Zhang, X.; Han, W.; Wei, K. CoOh-O-MnOh edge-sharing engineering in Co-Mn inverse spinel oxides for PMS activation: Electronic reconfiguration-driven nonradical oxidation via synergistic CoIV=O and 1O2. Appl. Catal. B Environ. Energy 2026, 384, 126149. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Qin, L.; Ma, D.; Zhou, X.; Yan, H.; Xu, F.; Tang, L.; Yuan, X. Peroxydisulfate activation by copper metal-organic framework derivatives: Deciphering the role of copper species. Environ. Res. 2025, 283, 122142. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Gu, Y.; He, B.; Chen, Z.; Hu, H.; Liu, H.; Ding, W. Biochar-Supported FeCo-MOF Derivative Catalyzes PDS-Mediated Degradation of Tetracycline. Sep. Purif. Technol. 2024, 349, 127841. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Wang, H.; Zhang, S.; Wu, C.; Huang, H.; Pang, Y.; Isung, C.; Li, T. Uncovering Two Kinetic Factors in the Controlled Growth of Topologically Distinct Core–Shell Metal–Organic Frameworks. Chem. Sci. 2019, 10, 7755–7761. [Google Scholar] [CrossRef]
- Li, Y.; Teng, J.; Wu, J.; Zhang, S.; Li, L.; Zhao, Z. Mechanistic Insights into Carbonate Radical-Driven Reactions: Selectivity and the Hydrogen Atom Abstraction Route. SSRN Electron. J. 2024. [Google Scholar] [CrossRef]
- Dou, J.B.; Cheng, J.; Lu, Z.J.; Tian, Z.Q.; Xu, J.M.; He, Y. Biochar Co-Doped with Nitrogen and Boron Switching the Free Radical Based Peroxydisulfate Activation into the Electron-Transfer Dominated Nonradical Process. Appl. Catal. B Environ. 2022, 301, 120832. [Google Scholar] [CrossRef]
- Liu, X.Y.; Yan, X.Y.; Liu, W.Y.; Yan, Q.Y.; Xing, M.Y. Switching of Radical and Nonradical Pathways Through the Surface Defects of Fe3O4/MoOxSγ in a Fenton-Like Reaction. Sci. Bull. 2023, 68, 603–612. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhang, Z.; Zhang, S.; Wu, X.; Li, H.; Qiu, Y.; Nengzi, L. Copper-Based MOF-Derived Core–Shell Materials via N/P/S Ternary Doping for Peroxymonosulfate Activation: Efficient Degradation and Removal of Sulfamethazine. Toxics 2025, 13, 1023. https://doi.org/10.3390/toxics13121023
Zhou H, Zhang Z, Zhang S, Wu X, Li H, Qiu Y, Nengzi L. Copper-Based MOF-Derived Core–Shell Materials via N/P/S Ternary Doping for Peroxymonosulfate Activation: Efficient Degradation and Removal of Sulfamethazine. Toxics. 2025; 13(12):1023. https://doi.org/10.3390/toxics13121023
Chicago/Turabian StyleZhou, Haiyang, Zhijing Zhang, Shan Zhang, Xiaofeng Wu, Haitao Li, Yong Qiu, and Lichao Nengzi. 2025. "Copper-Based MOF-Derived Core–Shell Materials via N/P/S Ternary Doping for Peroxymonosulfate Activation: Efficient Degradation and Removal of Sulfamethazine" Toxics 13, no. 12: 1023. https://doi.org/10.3390/toxics13121023
APA StyleZhou, H., Zhang, Z., Zhang, S., Wu, X., Li, H., Qiu, Y., & Nengzi, L. (2025). Copper-Based MOF-Derived Core–Shell Materials via N/P/S Ternary Doping for Peroxymonosulfate Activation: Efficient Degradation and Removal of Sulfamethazine. Toxics, 13(12), 1023. https://doi.org/10.3390/toxics13121023




