Microbial Degradation of Herbicide Residues in Australian Soil: An Overview of Mechanistic Insights and Recent Advancements
Abstract
1. Introduction
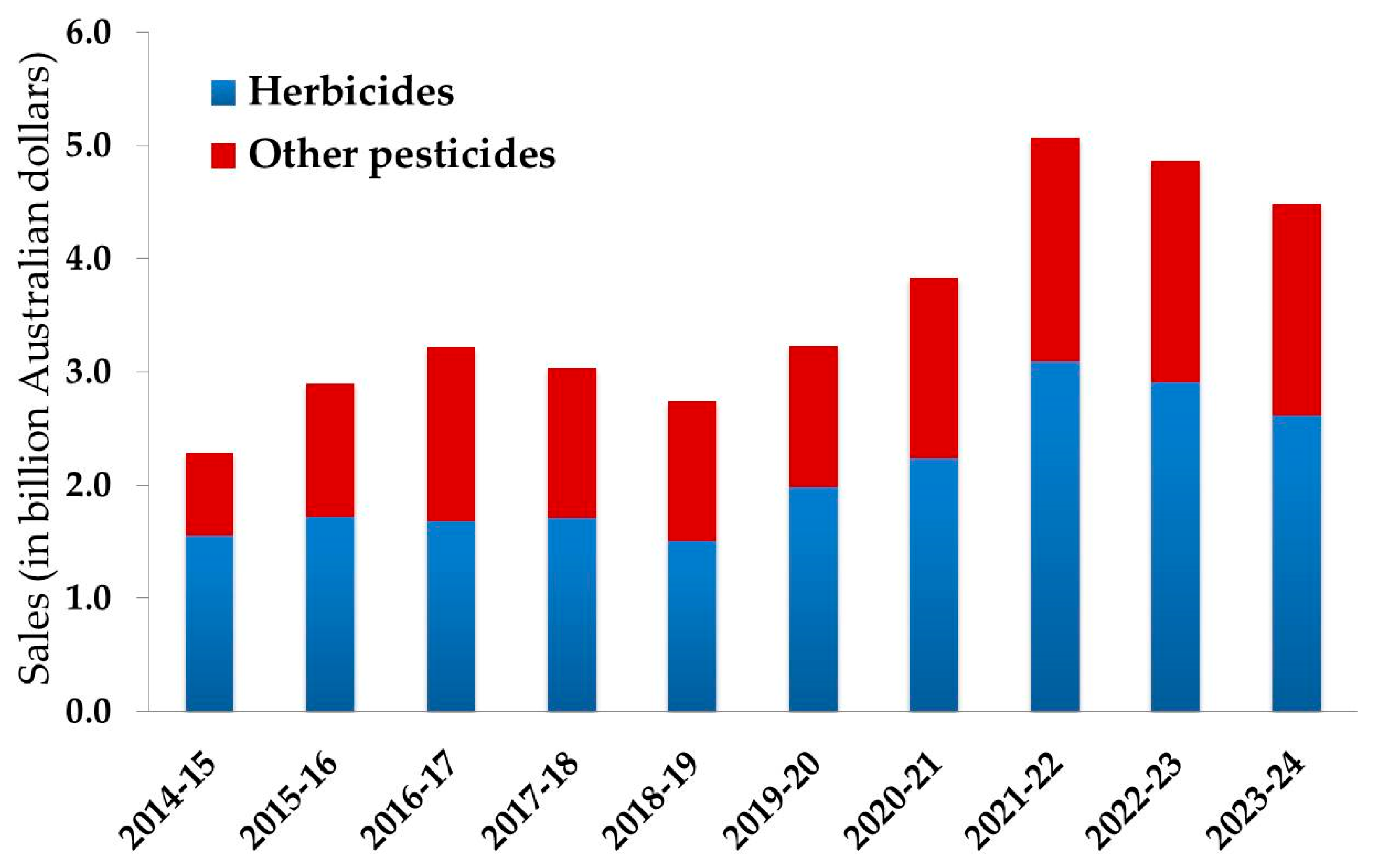
2. Herbicides in Australian Agriculture
3. Significance of Herbicide Residues in Australian Soil
4. Distribution and Functions of Microorganisms in Soil
5. Microorganisms Involved in Microbial Degradation
6. Mechanisms Involved in Microbial Degradation
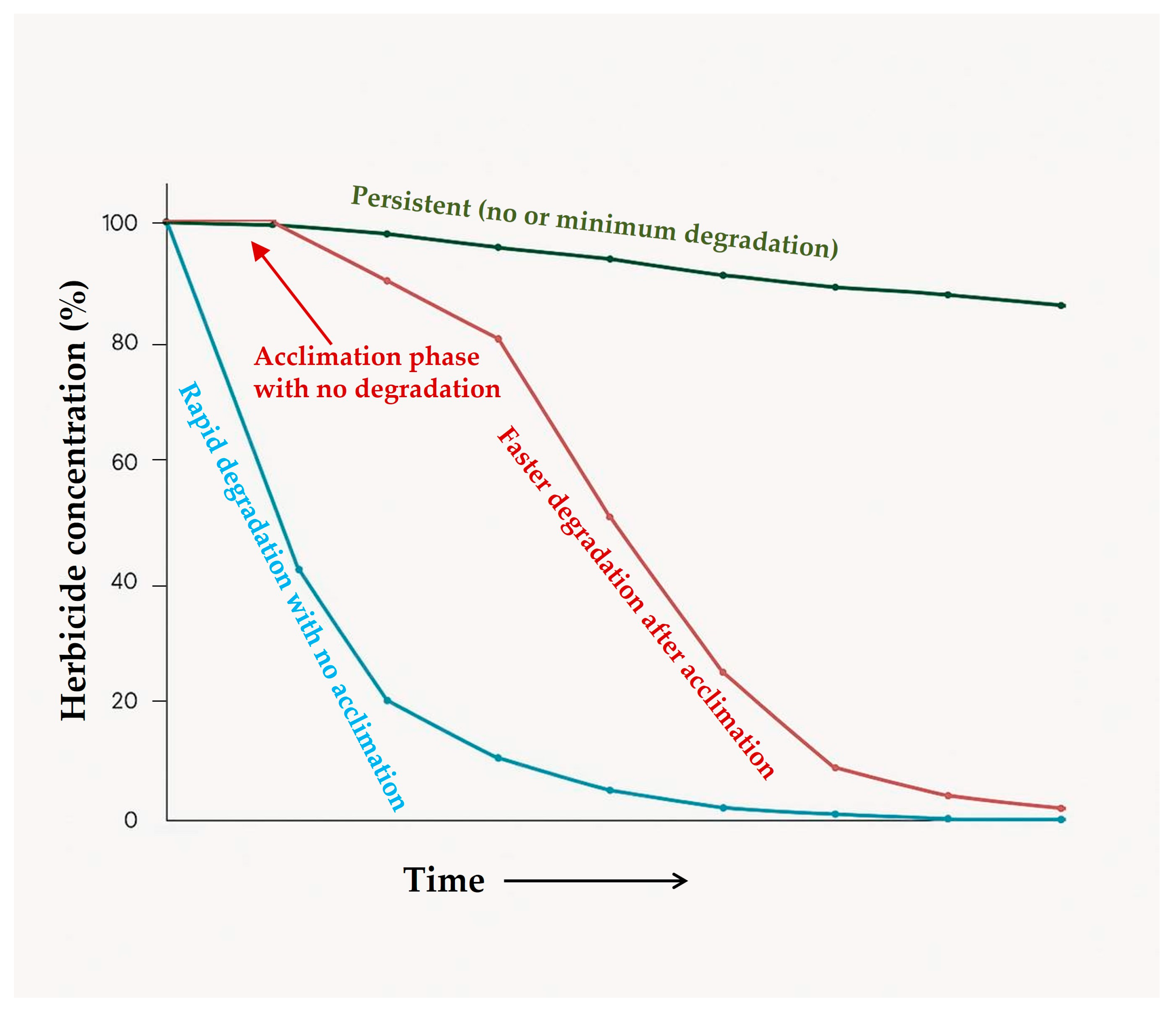
6.1. Adaptation
6.2. Co-Metabolism
7. Factors Affecting Microbial Degradation
7.1. Temperature
7.2. Soil Moisture
7.3. Soil pH
7.4. Soil Organic Matter
7.5. Herbicide Structural Properties and Concentration
7.6. Dissolved Organic Matter (DOM)
8. Approaches Used to Study Microbial Degradation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swinton, S.M.; Van Deynze, B. Hoes to herbicides: Economics of evolving weed management in the United States. Eur. J. Dev. Res. 2017, 29, 560–574. [Google Scholar] [CrossRef]
- Shah, M.H.; Garai, S.; Mandal, B.; Mukherjee, D.; Aktar, S.N.; Hossain, A.; Islam, M.M. Weed control in wheat: Assessment of herbicide efficacy, economic, and yield impacts. Phytoparasitica 2025, 53, 1–15. [Google Scholar] [CrossRef]
- Parish, S. A review of non-chemical weed control techniques. Biol. Agric. Hortic. 1990, 7, 117–137. [Google Scholar] [CrossRef]
- Beste, C.E. Herbicide Handbook of the Weed Science Society of America, 5th ed.; Weed Science Society of America (WSSA): Lawrence, KS, USA, 1983. [Google Scholar]
- Zimdahl, R.L. Introduction to chemical weed control. In Fundamentals of Weed Science, 5th ed.; Zimdahl, R.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 391–416. [Google Scholar]
- Smith, D.T.; Richard, E.P., Jr.; Santo, L.T. Weed control in sugarcane and the role of triazine herbicides. In The Triazine Herbicides; LeBaron, H.M., McFarland, J.E., Burnside, O.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 185–197. [Google Scholar]
- Heri, W.; Pfister, F.; Carroll, B.; Parshley, T. Production, development, and registration of triazine herbicides. In The Triazine Herbicides; LeBaron, H.M., McFarland, J.E., Burnside, O.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 31–43. [Google Scholar]
- Bamal, D.; Duhan, A.; Pal, A.; Beniwal, R.K.; Kumawat, P.; Dhanda, S.; Goyat, A.; Hooda, V.S.; Yadav, R. Herbicide risks to non-target species and the environment: A review. Environ. Chem. Lett. 2024, 22, 2977–3032. [Google Scholar] [CrossRef]
- Papadakis, E.N.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Makris, K.C.; Papadopoulou-Mourkidou, E. A pesticide monitoring survey in rivers and lakes of northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol. Environ. Saf. 2015, 116, 1–9. [Google Scholar] [CrossRef]
- Chowdhury, I.F.; Doran, G.S.; Stodart, B.J.; Chen, C.; Wu, H. Trifluralin and Atrazine sensitivity to selected cereal and legume crops. Agronomy 2020, 10, 587. [Google Scholar] [CrossRef]
- Cornish, P.S.; Tullberg, J.N.; Lemerle, D.; Flower, K. No-till farming systems in Australia. In No-Till Farming Systems for Sustainable Agriculture; Dang, Y., Dalal, R., Menzies, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 511–531. [Google Scholar]
- Adeux, G.; Guinet, M.; Courson, E.; Lecaulle, S.; Munier-Jolain, N.; Cordeau, S. Multicriteria assessment of conservation agriculture systems. Front. Agron. 2022, 4, 999960. [Google Scholar] [CrossRef]
- Llewellyn, R.S.; D’Emden, F.H.; Kuehne, G. Extensive use of no-tillage in grain growing regions of Australia. Field Crops Res. 2012, 132, 204–212. [Google Scholar] [CrossRef]
- Kushwah, S.S.; Tiwari, R.; Sharma, K.; Sharma, B. Role of herbicides and tillage on weeds and wheat (Triticum aestivum) yield in northern region of Madhya Pradesh. Indian J. Agric. Sci. 2025, 95, 383–388. [Google Scholar] [CrossRef]
- APVMA. Australian Pesticides and Veterinary Medicines Authority. Annual Product Sales Data. 2024. Available online: https://www.apvma.gov.au/about/accountability-and-reporting/annual-product-sales-data (accessed on 12 October 2025).
- Llewellyn, R.S.; Powles, S.B. High levels of herbicide resistance in rigid ryegrass (Lolium rigidum) in the wheat belt of Western Australia. Weed Technol. 2001, 15, 242–248. [Google Scholar] [CrossRef]
- D’Emden, F.H.; Llewellyn, R.S.; Burton, M.P. Adoption of conservation tillage in Australian cropping regions: An application of duration analysis. Technol. Forecast. Soc. Change 2006, 73, 630–647. [Google Scholar] [CrossRef]
- Wolejko, E.; Wydro, U.; Odziejewicz, J.I.; Koronkiewicz, A.; Jablonska-Trypuc, A. Biomonitoring of soil contaminated with herbicides. Water 2022, 14, 1534. [Google Scholar] [CrossRef]
- Beaumelle, L.; Tison, L.; Eisenhauer, N.; Hines, J.; Malladi, S.; Pelosi, C.; Thouvenot, L.; Phillips, H.R. Pesticide effects on soil fauna communities—A meta-analysis. J. Appl. Ecol. 2023, 60, 1239–1253. [Google Scholar] [CrossRef]
- Curran, W.S. Persistence of herbicides in soil. Crop Soils Mag. 2016, 49, 16–21. [Google Scholar] [CrossRef]
- Rose, M.T.; Zhang, P.; Rose, T.J.; Scanlan, C.A.; McGrath, G.; Van Zwieten, L. Herbicide residues in Australian grain cropping soils at sowing and their relevance to crop growth. Sci. Total Environ. 2022, 833, 155105. [Google Scholar] [CrossRef] [PubMed]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 1–15. [Google Scholar] [CrossRef]
- Harries, M.; Flower, K.C.; Scanlan, C.A.; Rose, M.T.; Renton, M. Interactions between crop sequences, weed populations and herbicide use in Western Australian broadacre farms: Findings of a six-year survey. Crop Pasture Sci. 2020, 71, 491–505. [Google Scholar] [CrossRef]
- Aparicio, V.C.; De Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Silva, V.; Montanarella, L.; Jones, A.; Fernández-Ugalde, O.; Mol, H.G.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Silva, T.L.; Andreani, T.; Silva, A.M. Glyphosate vs. glyphosate-based herbicides exposure: A review on their toxicity. J. Xenobiot. 2022, 12, 21–40. [Google Scholar] [CrossRef]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: A review. Pest Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Chowdhury, I.F.; Rohan, M.; Stodart, B.J.; Chen, C.; Wu, H.; Doran, G.S. Persistence of atrazine and trifluralin in a clay loam soil undergoing different temperature and moisture conditions. Environ. Pollut. 2021, 276, 116687. [Google Scholar] [CrossRef]
- Jolley, A.V.; Johnstone, P.K. Degradation of trifluralin in three Victorian soils under field and laboratory conditions. Aust. J. Exp. Agric. 1994, 34, 57–65. [Google Scholar] [CrossRef]
- Johnstone, P.K.; Jolley, A.V.; Code, G.R.; Moerkerk, M.R.; Corbett, A. Degradation of trifluralin in three Victorian soils—Long-term field trials. Aust. J. Exp. Agric. 1998, 38, 363–374. [Google Scholar] [CrossRef]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Hofman, J. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613, 361–370. [Google Scholar] [CrossRef]
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nélieu, S.; Fritsch, C. Residues of currently used pesticides in soils and earthworms: A silent threat? Agric. Ecosyst. Environ. 2021, 305, 107167. [Google Scholar] [CrossRef]
- Conte, E.; Morali, G.; Galli, M.; Imbroglini, G.; Leake, C.R. Long-term degradation and potential plant uptake of diflufenican under field conditions. J. Agric. Food Chem. 1998, 46, 4766–4770. [Google Scholar] [CrossRef]
- Dehghani, M.; Nasseri, S.; Karamimanesh, M. Removal of 2, 4-Dichlorophenolyxacetic acid (2, 4-D) herbicide in the aqueous phase using modified granular activated carbon. J. Environ. Health Sci. Eng. 2014, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Van Zwieten, L.; Zhang, P.; McGrath, G.; Seymour, N.; Scanlan, C.; Rose, T. Herbicide Residues in Soil–What is the Scale and Significance? In Grains Research Update; Charles Sturt University: Wagga Wagga, NSW, Australia, 2019; pp. 247–253. Available online: https://researchoutput.csu.edu.au/ws/portalfiles/portal/47904912/46701255_Full_proceedings.pdf (accessed on 10 January 2025).
- Tang, F.H.; Maggi, F. Pesticide mixtures in soil: A global outlook. Environ. Res. Lett. 2021, 16, 044051. [Google Scholar] [CrossRef]
- Riedo, J.; Wettstein, F.E.; Rösch, A.; Herzog, C.; Banerjee, S.; Büchi, L.; van der Heijden, M.G. Widespread occurrence of pesticides in organically managed agricultural soils—The ghost of a conventional agricultural past? Environ. Sci. Technol. 2021, 55, 2919–2928. [Google Scholar] [CrossRef]
- Gianelli, V.R.; Bedmar, F.; Costa, J.L. Persistence and sorption of imazapyr in three Argentinean soils. Environ. Toxicol. Chem. 2014, 33, 29–34. [Google Scholar] [CrossRef]
- Torstensson, L. Microbial decomposition of herbicides in the soil. Outlook Agric. 1988, 17, 120–124. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef]
- Schneider, T.; Gerrits, B.; Gassmann, R.; Schmid, E.; Gessner, M.O.; Richter, A.; Battin, T.; Eberl, L.; Riedel, K. Proteome analysis of fungal and bacterial involvement in leaf litter decomposition. Proteomics 2010, 10, 1819–1830. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Pino, N.; Peñuela, G. Simultaneous degradation of the pesticides methyl parathion and chlorpyrifos by an isolated bacterial consortium from a contaminated site. Int. Biodeterior. Biodegrad. 2011, 65, 827–831. [Google Scholar]
- Sun, Y.; Kumar, M.; Wang, L.; Gupta, J.; Tsang, D.C. Biotechnology for soil decontamination: Opportunity, challenges, and prospects for pesticide biodegradation. In Bio-Based Materials and Biotechnologies for Eco-Efficient Construction; Woodhead Publishing: Cambridge, UK, 2020; pp. 261–283. [Google Scholar]
- Makarani, N.; Kaushal, R.S. Advances in actinobacteria-based bioremediation: Mechanistic insights, genetic regulation, and emerging technologies. Biodegradation 2025, 36, 24. [Google Scholar] [CrossRef] [PubMed]
- Rout, T.; Rout, S.; Kpeno, A.; Nanda, B.; Kar, D.; Kuanar, A. Bioremediation approaches for soil and aquatic environmental restoration to revive ecosystems. In Recent Advances in Bioremediation and Phytoremediation; Springer Nature: Cham, Switzerland, 2025; pp. 123–147. [Google Scholar]
- Vetrivel, A.; Ilango, S.; Ariyamuthu, R.; Devarajan, G.; Nithya, T.G. Innovative approaches in microbial bioremediation: Harnessing synthetic biology for environmental pollutants removal. In Sustainable Environmental Remediation: Avenues in Nano and Biotechnology; Springer Nature: Cham, Switzerland, 2025; pp. 387–407. [Google Scholar]
- Maqsood, Q.; Waseem, R.; Sumrin, A.; Wajid, A.; Tariq, M.R.; Ali, S.W.; Mahnoor, M. Recent trends in bioremediation and bioaugmentation strategies for mitigation of marine based pollutants: Current perspectives and future outlook. Discov. Sustain. 2024, 5, 524. [Google Scholar] [CrossRef]
- Verma, J.P.; Jaiswal, D.K.; Sagar, R. Pesticide relevance and their microbial degradation: A-state-of-art. Rev. Environ. Sci. Biotechnol. 2014, 13, 429–466. [Google Scholar] [CrossRef]
- Marschner, P.; Crowley, D.; Yang, C.H. Development of specific rhizosphere bacterial communities in relation to plant species, nutrition and soil type. Plant Soil 2004, 261, 199–208. [Google Scholar] [CrossRef]
- Foster, R.C. The ultrastructure of the rhizoplane and rhizosphere. Annu. Rev. Phytopathol. 1986, 24, 211–234. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micropolluted constructed wetland. Sci. Total Environ. 2017, 598, 697–703. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.R.; Bunin, E.; Viana, A.T.; Froufe, H.; Munoz-Merida, A.; Pinho, D.; Figueiredo, J.; Barroso, C.; Vaz-Moreira, I.; Bellanger, X.; et al. In silico prediction of the enzymes involved in the degradation of the herbicide molinate by Gulosibacter molinativorax ON4T. Sci. Rep. 2022, 12, 15502. [Google Scholar] [CrossRef]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, R. Factors limiting bioremediation technologies. Bioresour. Technol. 2000, 74, 63–67. [Google Scholar] [CrossRef]
- Copley, S.D. Evolution of efficient pathways for degradation of anthropogenic chemicals. Nat. Chem. Biol. 2009, 5, 559–566. [Google Scholar] [CrossRef]
- Emamalipour, M.; Seidi, K.; Zununi Vahed, S.; Jahanban-Esfahlan, A.; Jaymand, M.; Majdi, H.; Amoozgar, Z.; Chitkushev, L.T.; Javaheri, T.; Jahanban-Esfahlan, R. Horizontal gene transfer: From evolutionary flexibility to disease progression. Front. Cell Dev. Biol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Nayak, S.K.; Dash, B.; Baliyarsingh, B. Microbial remediation of persistent agrochemicals by soil bacteria: An overview. In Microbial Biotechnology; Patra, J., Das, G., Shin, H.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 275–301. [Google Scholar]
- Rancurel, C.; Legrand, L.; Danchin, E.G.J. Alienness: Rapid detection of candidate horizontal gene transfers across the tree of life. Genes 2017, 8, 248. [Google Scholar] [CrossRef]
- Zhao, Q.; Yue, S.; Bilal, M.; Hu, H.; Wang, W.; Zhang, X. Comparative genomic analysis of 26 Sphingomonas and Sphingobium strains: Dissemination of bioremediation capabilities, biodegradation potential and horizontal gene transfer. Sci. Total Environ. 2017, 609, 1238–1247. [Google Scholar] [CrossRef]
- Nguyen, T.P.O.; Hansen, M.A.; Hansen, L.H.; Horemans, B.; Sørensen, S.J.; De Mot, R.; Springael, D. Intra- and inter-field diversity of 2, 4- dichlorophenoxyacetic acid-degradative plasmids and their tfd catabolic genes in rice fields of the Mekong delta in Vietnam. FEMS Microbiol. Ecol. 2018, 95, fiy214. [Google Scholar]
- Ksiazek-Trela, P.; Potocki, L.; Szpyrka, E. The impact of novel bacterial strains and their consortium on diflufenican degradation in the mineral medium and soil. Sci. Rep. 2025, 15, 18051. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, T.K.; Suresh, G.; Bhandi, M.M.; Mudiam, M.K.R.; Mohan, S.V. Microbial degradation of organochlorine pesticide: 2, 4-Dichlorophenoxyacetic acid by axenic and mixed consortium. Bioresour. Technol. 2023, 382, 129031. [Google Scholar] [CrossRef]
- Xiang, S.; Lin, R.; Shang, H.; Xu, Y.; Zhang, Z.; Wu, X.; Zong, F. Efficient degradation of phenoxyalkanoic acid herbicides by the alkali-tolerant Cupriavidus oxalaticus strain X32. J. Agric. Food Chem. 2020, 68, 3786–3795. [Google Scholar] [CrossRef] [PubMed]
- Lozowicka, B.; Wołejko, E.; Kaczyński, P.; Konecki, R.; Iwaniuk, P.; Drągowski, W.; Łozowicki, J.; Tujtebajeva, G.; Wydro, U.; Jablońska-Trypuć, A. Effect of microorganism on behaviour of two commonly used herbicides in wheat/soil system. Appl. Soil Ecol. 2021, 162, 103879. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singh, J. Kinetic study of the biodegradation of glyphosate by indigenous soil bacterial isolates in presence of humic acid, Fe (III) and Cu (II) ions. J. Environ. Chem. Eng. 2019, 7, 103098. [Google Scholar] [CrossRef]
- Ajish, A.; Sukirtha, T.H. Biodegradation and bioremediation of glyphosate herbicide by Nocardia mediterranie THS 1. Int. J. Sci. Res. 2020, 8, 34–36. [Google Scholar]
- Han, L.; Liu, T.; Fang, K.; Li, X.; You, X.; Li, Y.; Wang, X.; Wang, J. Indigenous functional microbial communities for the preferential degradation of chloroacetamide herbicide S-enantiomers in soil. J. Hazard. Mater. 2022, 423, 127135. [Google Scholar] [CrossRef]
- Elarabi, N.I.; Abdelhadi, A.A.; Ahmed, R.H.; Saleh, I.; Arif, I.A.; Osman, G.; Ahmed, D.S. Bacillus aryabhattai FACU: A promising bacterial strain capable of manipulate the glyphosate herbicide residues. Saudi J. Biol. Sci. 2020, 27, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Masotti, F.; Garavaglia, B.S.; Piazza, A.; Burdisso, P.; Altabe, S.; Gottig, N.; Ottado, J. Bacterial isolates from Argentine Pampas and their ability to degrade glyphosate. Sci. Total Environ. 2021, 774, 145761. [Google Scholar] [CrossRef]
- Mousa, N.K.; Ali, A.J.; Hussein, M. Bacillus megaterium biodegradation glyphosate. In Biodegradation Technology of Organic and Inorganic Pollutants; Mendes, K.F., De Sousa, R., Mielke, K.C., Eds.; IntechOpen: London, UK, 2022; pp. 79–88. [Google Scholar]
- Rossi, F.; Carles, L.; Donnadieu, F.; Batisson, I.; Artigas, J. Glyphosate-degrading behavior of five bacterial strains isolated from stream biofilms. J. Hazard. Mater. 2021, 420, 126651. [Google Scholar] [CrossRef]
- Firdous, S.; Iqbal, S.; Anwar, S. Optimization and modeling of glyphosate biodegradation by a novel Comamonas odontotermitis P2 through response surface methodology. Pedosphere 2020, 30, 618–627. [Google Scholar] [CrossRef]
- Góngora-Echeverría, V.R.; García-Escalante, R.; Rojas-Herrera, R.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Pesticide bioremediation in liquid media using a microbial consortium and bacteria-pure strains isolated from a biomixture used in agricultural areas. Ecotoxicol. Environ. Saf. 2020, 200, 110734. [Google Scholar] [CrossRef]
- Pérez Rodríguez, M.; Melo, C.; Jiménez, E.; Dussán, J. Glyphosate bioremediation through the sarcosine oxidase pathway mediated by Lysinibacillus sphaericus in soils cultivated with potatoes. Agriculture 2019, 9, 217. [Google Scholar] [CrossRef]
- Carranza, C.S.; Regñicoli, J.P.; Aluffi, M.E.; Benito, N.; Chiacchiera, S.M.; Barberis, C.L.; Magnoli, C.E. Glyphosate in vitro removal and tolerance by Aspergillus oryzae in soil microcosms. Int. J. Environ. Sci. Technol. 2019, 16, 7673–7682. [Google Scholar] [CrossRef]
- Mirzavand, S.; Aeini, M.; Najafgholi, H.M.; Rezamahalleh, H.M. Identification and characterization of bacterial strains capable of degrading atrazine and metribuzin herbicides in sugarcane fields. Sugar Tech. 2024, 26, 95–105. [Google Scholar] [CrossRef]
- Moretto, J.A.S.; Furlan, J.P.R.; Fernandes, A.F.T.; Bauermeister, A.; Lopes, N.P.; Stehling, E.G. Alternative biodegradation pathway of the herbicide diuron. Int. Biodeterior. Biodegrad. 2019, 143, 104716. [Google Scholar]
- Kaur, R.; Goyal, D. Biodegradation of Butachlor by Bacillus altitudinis and identification of metabolites. Curr. Microbiol. 2020, 77, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.D.; Thuy, N.T.D.; Truc, H.T.T.; Nhu, N.T.H.; Oanh, N.T. Degradation of butachlor and propanil by Pseudomonas sp. strain But2 and Acinetobacter baumannii strain DT. FEMS Microbiol. Lett. 2020, 367, fnaa151. [Google Scholar] [CrossRef]
- Muhammad, J.B.; Jagaba, A.H.; Yusuf, F.; Usman, S.; Yakubu, N.S.; Birniwa, A.H.; Yakasai, H.M.; Shehu, D. Achromobacter xylosoxidans bacteria isolated from contaminated agricultural environment for a sustainable 2, 4-dichlorophenoxyacetic acid herbicide degradation: An experimental study. Case Stud. Chem. Environ. Eng. 2024, 9, 100604. [Google Scholar] [CrossRef]
- Trivedi, N.; Dubey, A. Degradation studies of pendimethalin by indigenous soil bacterium Pseudomonas strain PD1 using spectrophotometric scanning and FTIR. Arch. Microbiol. 2021, 203, 4499–4507. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, G.; Mao, Z.; Song, M.; Zhao, R.; Zhang, X.; Chen, C.; Zhang, H.; Liu, Y.; Wang, G.; et al. Experimental and computational approaches to characterize a novel amidase that initiates the biodegradation of the herbicide propanil in Bosea sp. P5. J. Hazard. Mater. 2023, 451, 131155. [Google Scholar] [CrossRef]
- Ma, Q.; Kong, D.; Zhang, Q.; Li, M.; Han, X.; Che, J.; Zhou, Y.; Zhang, W.; Jiang, X.; Ruan, Z. Microbacterium sulfonylureivorans sp. nov. isolated from sulfonylurea herbicides degrading consortium. Arch. Microbiol. 2022, 204, 136. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Ruiz, A.; Cornejo, P.; Kannan, V.R. Influence of plant beneficial Stenotrophomonas rhizophila strain CASB3 on the degradation of diuron-contaminated saline soil and improvement of Lactuca sativa growth. Environ. Sci. Pollut. Res. 2020, 27, 35195–35207. [Google Scholar] [CrossRef]
- Muendo, B.M.; Shikuku, V.O.; Lalah, J.O.; Getenga, Z.M.; Wandiga, S.O.; Rothballer, M. Enhanced degradation of diuron by two Bacillus species isolated from diuron contaminated sugarcane and pineapple-cultivated soils in Kenya. Appl. Soil Ecol. 2021, 157, 103721. [Google Scholar] [CrossRef]
- Rebai, H.; Sholkamy, E.N.; Abdelhamid, M.A.; Prakasam Thanka, P.; Aly Hassan, A.; Pack, S.P.; Ki, M.R.; Boudemagh, A. Soil actinobacteria exhibit metabolic capabilities for degrading the toxic and persistent herbicide metribuzin. Toxics 2024, 12, 709. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, L.; Jin, C.; Meng, Z.; Yang, N. Study on the isolation of two atrazine-degrading bacteria and the development of a microbial agent. Microorganisms 2019, 7, 80. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Jena, H.M. Degradation kinetics and mechanistic study on herbicide bioremediation using hyper butachlor-tolerant Pseudomonas putida G3. Process Saf. Environ. Prot. 2019, 125, 172–181. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.U. Biodegradation of alachlor by a newly isolated bacterium: Degradation pathway and product analysis. Processes 2022, 10, 2256. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Yao, J.; Sunahara, G.; Duran, R.; Zhang, Q.; Ruan, Z. Transcriptomic response of Pseudomonas nicosulfuronedens LAM1902 to the sulfonylurea herbicide nicosulfuron. Sci. Rep. 2022, 12, 13656. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, Y.; Li, X.; Fu, L.I. Characteristics of two terbutylazine-degrading bacteria and the construction of a live bacterial agent for effective degradation of terbutylazine in soil. An. Acad. Bras. Cienc. 2022, 94, e20200658. [Google Scholar] [CrossRef]
- Satapute, P.; De Britto, S.; Hadimani, S.; Abdelrahman, M.; Alarifi, S.; Govind, S.R.; Jogaiah, S. Bacterial chemotaxis of herbicide atrazine provides an insight into the degradation mechanism through intermediates hydroxyatrazine, N–N-isopropylammelide, and cyanuric acid compounds. Environ. Res. 2023, 237, 117017. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Q.; Zhou, Y.; Jiang, X.; Parales, R.E.; Zhao, S.; Zhuang, Y.; Ruan, Z. Isolation, identification, and degradation mechanism by multi-omics of mesotrione-degrading Amycolatopsis nivea La24. J. Hazard. Mater. 2024, 476, 134951. [Google Scholar] [CrossRef]
- Esikova, T.Z.; Anokhina, T.O.; Suzina, N.E.; Shushkova, T.V.; Wu, Y.; Solyanikova, I.P. Characterization of a new Pseudomonas putida strain Ch2, a degrader of toxic anthropogenic compounds epsilon-caprolactam and glyphosate. Microorganisms 2023, 11, 650. [Google Scholar] [CrossRef]
- Muhammad, J.B.; Shehu, D.; Usman, S.; Dankaka, S.M.; Gimba, M.Y.; Jagaba, A.H. Biodegradation potential of 2, 4 dichlorophenoxyacetic acid by Cupriavidus campinensis isolated from rice farm cultivated soil. Case Stud. Chem. Environ. Eng. 2023, 8, 100434. [Google Scholar] [CrossRef]
- Tian, Y.; Zhong, F.; Shang, N.; Yu, H.; Mao, D.; Huang, X. Maize root exudates promote Bacillus sp. Za detoxification of diphenyl ether herbicides by enhancing colonization and biofilm formation. Mol. Plant-Microbe Interact. 2024, 37, 552–560. [Google Scholar] [CrossRef]
- Al-Fathi, H.; Koch, M.; Lorenz, W.G.; Lechner, U. Anaerobic degradation of 2, 4, 5-trichlorophenoxyacetic acid by enrichment cultures from freshwater sediments. Environ. Sci. Pollut. Res. Int. 2019, 26, 34459–34467. [Google Scholar] [CrossRef]
- Lara-Moreno, A.; Morillo, E.; Merchán, F.; Madrid, F.; Villaverde, J. Bioremediation of a trifluralin contaminated soil using bioaugmentation with novel isolated bacterial strains and cyclodextrin. Sci. Total Environ. 2022, 840, 156695. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Li, R.; Pan, X.; Dong, F.; Wu, X. Rapid elimination of butralin residues on tobacco, in water and soils by newly isolated Bacillus sp. LY05. J. Environ. Chem. Eng. 2023, 11, 109213. [Google Scholar] [CrossRef]
- Qin, X.; Shi, C.; Ma, J.; Wu, G.; Qin, D.; He, C.; Gao, X.; Zhu, T.; Jie, W. A Strain of Bacillus subtilis CZ1. Microbial Agent and Application. CN202410311429.6, 24 May 2024. (In Chinese). [Google Scholar]
- Santos, M.I.; Brandao, E.C.; Santos, E.; Batista, M.V.A.; Estevam, C.S.; Alexandre, M.R.; Fernandes, M.F. Pendimethalin biodegradation by soil strains of Burkholderia sp. and Methylobacterium radiotolerans. An. Acad. Bras. Ciênc. 2021, 93 (Suppl. 4), e20210924. [Google Scholar] [CrossRef]
- Han, Y.; Tang, Z.; Bao, H.; Wu, D.; Deng, X.; Guo, G.; Ye, B.C.; Dai, B. Degradation of pendimethalin by the yeast YC2 and determination of its two main metabolites. RSC Adv. 2019, 9, 491–497. [Google Scholar] [CrossRef]
- Correa, L.O.; Bezerra, A.F.M.; Honorato, L.R.S.; Cortêz, A.C.A.; Souza, J.V.B.; Souza, E.S. Amazonian soil fungi are efficient degraders of glyphosate herbicide; novel isolates of Penicillium, Aspergillus, and Trichoderma. Braz. J. Biol. 2021, 83, e242830. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, U.B.; Ilyas, T.; Malviya, D.; Vishwakarma, S.K.; Shafi, Z.; Singh, H.V. Bacillus-mediated degradation of recalcitrant agricultural pesticides: A cutting-edge approach towards the clean-up of environmental contaminants. In Applications of Bacillus and Bacillusderived Genera in Agriculture, Biotechnology and Beyond; Springer Nature: Singapore, 2024; pp. 213–251. [Google Scholar]
- Zhu, J.; Fu, L.; Meng, Z.; Jin, C. Characteristics of an atrazine degrading bacterium and the construction of a microbial agent for effective atrazine degradation. Water Environ. J. 2021, 35, 7–17. [Google Scholar] [CrossRef]
- Suenaga, H.; Mitsuoka, M.; Ura, Y.; Watanabe, T.; Furukawa, K. Directed evolution of biphenyl dioxygenase: Emergence of enhanced degradation capacity for benzene, toluene, and alkylbenzenes. J. Bacteriol. 2001, 183, 5441–5444. [Google Scholar] [CrossRef]
- Laemmli, C.M.; Leveau, J.H.J.; Zehnder, A.J.B.; van der Meer, J.R. Characterization of a second tfd gene cluster for chlorophenol and chlorocatechol metabolism on plasmid pJP4 in Ralstonia eutropha JMP134 (pJP4). J. Bacteriol. 2000, 182, 4165–4172. [Google Scholar] [CrossRef]
- Sayler, G.S.; Hooper, S.W.; Layton, A.C.; King, J.H. Catabolic plasmids of environmental and ecological significance. Microb. Ecol. 1990, 19, 1–20. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Bastos, A.C.; Magan, N. Trametes versicolor: Potential for atrazine bioremediation in calcareous clay soil, under low water availability conditions. Int. Biodeterior. Biodegrad. 2009, 63, 389–394. [Google Scholar] [CrossRef]
- Yang, L.; Chen, S.; Hu, M.; Hao, W.; Geng, P.; Zhang, Y. Biodegradation of carbofuran by Pichia anomala strain HQ-C-01 and its application for bioremediation of contaminated soils. Biol. Fertility Soils 2011, 47, 917. [Google Scholar] [CrossRef]
- Barros do Nascimento, A.C.; Nhampossa, N.A.; Félix, T.P.; Itabaiana, I., Jr.; Nascimento, R.P.D. Exploring fungal biodegradation pathways of 2, 4-D: Enzymatic mechanisms, synergistic actions, and environmental applications. ACS Omega 2025, 10, 39398–39414. [Google Scholar] [CrossRef]
- Hussain, S.; Arshad, M.; Saleem, M.; Khalid, A. Biodegradation of α-and β endosulfan by soil bacteria. Biodegradation 2007, 18, 731–740. [Google Scholar] [CrossRef]
- Kataoka, R.; Takagi, K.; Kamei, I.; Kiyota, H.; Sato, Y. Biodegradation of dieldrin by a soil fungus isolated from a soil with annual endosulfan applications. Environ. Sci. Technol. 2010, 44, 6343–6349. [Google Scholar] [CrossRef]
- Sasek, V.; Cajthaml, T. Mycoremediation: Current state and perspectives. Int. J. Med. Mushrooms 2005, 7, 360–361. [Google Scholar] [CrossRef][Green Version]
- Charles, S.; Ratier, A.; Baudrot, V.; Multari, G.; Siberchicot, A.; Wu, D.; Lopes, C. Taking full advantage of modelling to better assess environmental risk due to xenobiotics—The all-in-one facility MOSAIC. Environ. Sci. Pollut. Res. Int. 2022, 29, 29244–29257. [Google Scholar] [CrossRef] [PubMed]
- van Bruggen, A.H.; Goss, E.M.; Havelaar, A.; van Diepeningen, A.D.; Finckh, M.R.; Morris, J.G., Jr. One Health-Cycling of diverse microbial communities as a connecting force for soil, plant, animal, human and ecosystem health. Sci. Total Environ. 2019, 664, 927–937. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Fang, H.; Lian, J.; Wang, H.; Cai, L.; Yu, Y. Exploring bacterial community structure and function associated with atrazine biodegradation in repeatedly treated soils. J. Hazard. Mater. 2015, 286, 457–465. [Google Scholar] [CrossRef]
- Wang, K.; Ren, Y.; Pan, X.; Wu, X.; Xu, J.; Zheng, Y.; Dong, F. Insights on persistent herbicides in cropland soils in northern China: Occurrence, ecological risks, and phytotoxicity to subsequent crops. J. Hazard. Mater. 2025, 490, 137794. [Google Scholar] [CrossRef]
- Horvath, R.S. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol. Rev. 1972, 36, 146–155. [Google Scholar] [CrossRef]
- Pratap, B.; Tiwari, H.; Singh, R.S. Optimization of atrazine degradation process by Bacillus paramycoides strain in a batch system, and assessment of growth kinetics, bacterial toxicity, phytotoxicity and chlorophyll: Optimization and assessment of atrazine degradation process. Indian J. Exp. Biol. 2024, 62, 560–570. [Google Scholar]
- Horvath, R.S.; Alexander, M. Cometabolism of m-chlorobenzoate by an Arthrobacter. Appl. Microbiol. 1970, 20, 254–258. [Google Scholar] [CrossRef]
- Zablotowicz, R.M.; Weaver, M.A.; Locke, M.A. Microbial adaptation for accelerated atrazine mineralization/degradation in Mississippi Delta soils. Weed Sci. 2006, 54, 538–547. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate: Uses other than in glyphosate-resistant crops, mode of action, degradation in plants, and effects on non-target plants and agricultural microbes. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 255, pp. 1–65. [Google Scholar]
- Fox, J.L. Soil microbes pose problems for pesticides. Science 1983, 221, 1029–1031. [Google Scholar] [CrossRef]
- Kaufman, D.D.; Katan, J.; Edwards, D.F.; Jordan, E.G. Microbial adaptation and metabolism of pesticides. In Agricultural Chemicals of the Future; Hilton, J.L., Ed.; BARC Symposium no. 8. Rowman and Allanheld: Totowa, NJ, USA, 1984; pp. 437–451. [Google Scholar]
- Huang, X.; He, J.; Yan, X.; Hong, Q.; Chen, K.; He, Q.; Zhang, L.; Liu, X.; Chuang, S.; Li, S.; et al. Microbial catabolism of chemical herbicides: Microbial resources, metabolic pathways and catabolic genes. Pestic. Biochem. Physiol. 2017, 143, 272–297. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Dao, A.T.N.; Dang, H.T.C.; Koekkoek, J.; Brouwer, A.; de Boer, T.E.; van Spanning, R.J. Degradation of 2, 4-dichlorophenoxyacetic acid (2, 4-D) and 2, 4, 5-trichlorophenoxyacetic acid (2, 4, 5-T) by fungi originating from Vietnam. Biodegradation 2022, 33, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Yadav, A.N.; Saxena, R.; Paul, D.; Tomar, R.S. Biodiversity of pesticides degrading microbial communities and their environmental impact. Biocatal. Agric. Biotechnol. 2021, 31, 101883. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Ma, F.; Yang, J. Characterisation of an efficient atrazine degrading bacterium, Arthrobacter sp. ZXY-2: An attempt to lay the foundation for potential bioaugmentation applications. Biotechnol. Biofuels 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Top, E.M.; Springael, D. The role of mobile genetic elements in bacterial adaptation to xenobiotic organic compounds. Curr. Opin. Biotechnol. 2003, 14, 262–269. [Google Scholar] [CrossRef]
- Hossain, M.S.; Iken, B.; Iyer, R. Whole genome analysis of 26 bacterial strains reveals aromatic and hydrocarbon degrading enzymes from diverse environmental soil samples. Sci. Rep. 2024, 14, 30685. [Google Scholar] [CrossRef]
- Boettcher, G.; Nyer, E.K.; Palmer, P.L.; Carman, E.P.; Bedessem, J.; Kidd, D.F.; Lenzo, F.; Rorech, G.J.; Crossman, T.L. In situ bioremediation. In In Situ Treatment Technology, 2nd ed.; Nyer, E.K., Palmer, P.L., Carman, E.P., Boettcher, G., Bedessem, J.M., Lenzo, F., Crossman, T.L., Rorech, G.J., Kidd, D.F., Eds.; Lewis Publishers: London, UK, 2001; pp. 259–325. [Google Scholar]
- Tran, N.H.; Urase, T.; Ngo, H.H.; Hu, J.; Ong, S.L. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour. Technol. 2013, 146, 721–731. [Google Scholar] [CrossRef]
- Hazen, T.C. Cometabolic bioremediation. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer Nature: Singapore, 2010; pp. 2505–2514. [Google Scholar]
- Powell, C.L.; Nogaro, G.; Agrawal, A. Aerobic cometabolic degradation of trichloroethene by methane and ammonia oxidizing microorganisms naturally associated with Carex comosa roots. Biodegradation 2011, 22, 527–538. [Google Scholar] [CrossRef]
- Moreira, I.S.; Amorim, C.L.; Carvalho, M.F.; Castro, P.M.L. Co-metabolic degradation of chlorobenzene by the fluorobenzene degrading wild strain Labrys portucalensis. Int. Biodeterior. Biodegr. 2012, 72, 76–81. [Google Scholar] [CrossRef]
- Janke, D.; Fritsche, W. Nature and significance of microbial cometabolism of xenobiotics. J. Basic Microbiol. 1985, 25, 603–619. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Naidu, R. Bioremediation of high molecular weight polycyclic aromatic hydrocarbons: A review of the microbial degradation of benzo [a] pyrene. Int. Biodeterior. Biodegrad. 2000, 45, 57–88. [Google Scholar] [CrossRef]
- Robador, A.; Müller, A.L.; Sawicka, J.E.; Berry, D.; Hubert, C.R.J.; Loy, A.; Jørgensen, B.B.; Brüchert, V. Activity and community structures of sulfate-reducing microorganisms in polar, temperate and tropical marine sediments. ISME J. 2016, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I. Microbial Degradation of Trifluralin and Atrazine Residues in Soil. Ph.D. Thesis, Charles Sturt University, Bathurst, Australia, 2022. [Google Scholar]
- Pertile, M.; Antunes, J.E.L.; Araujo, F.F.; Mendes, L.W.; Van den Brink, P.J.; Araujo, A.S.F. Responses of soil microbial biomass and enzyme activity to herbicides imazethapyr and flumioxazin. Sci. Rep. 2020, 10, 7694. [Google Scholar] [CrossRef] [PubMed]
- Racke, K.; Skidmore, M.; Hamilton, D.; Unsworth, J.; Miyamoto, J.; Cohen, S. Pesticide fate in tropical soils. Pest Manage. Sci. 1999, 55, 219–220. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, S. Isolation and characterization of a high-efficiency soil atrazine-degrading Arthrobacter sp. strain. Int. Biodeterior. Biodegrad. 2012, 71, 61–66. [Google Scholar] [CrossRef]
- Ma, J.; Hung, H.; Tian, C.; Kallenborn, R. Revolatilization of persistent organic pollutants in the Arctic induced by climate change. Nat. Clim. Change 2011, 1, 255–260. [Google Scholar] [CrossRef]
- Ramanathan, S.S.; Gannon, T.W.; Everman, W.J.; Locke, A.M. Atrazine, mesosulfuron-methyl, and topramezone persistence in North Carolina soils. Agron. J. 2022, 114, 1068–1079. [Google Scholar] [CrossRef]
- Levy, W.; Radl, V.; Ruth, B.; Schmid, M.; Munch, J.C.; Schroll, R. Harsh summer conditions caused structural and specific functional changes of microbial communities in an arable soil. Eur. J. Soil Sci. 2007, 58, 736–745. [Google Scholar] [CrossRef]
- Tomco, P.L.; Duddleston, K.N.; Schultz, E.J.; Hagedorn, B.; Stevenson, T.J.; Seefeldt, S.S. Field degradation of aminopyralid and clopyralid and microbial community response to application in Alaskan soils. Environ. Toxicol. Chem. 2016, 35, 485–493. [Google Scholar] [CrossRef]
- Daunoras, J.; Kacergius, A.; Gudiukaite, R. Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.; Allison, S.D.; Ernakovich, J.; Steinweg, J.M.; Sinsabaugh, R. Controls on the temperature sensitivity of soil enzymes: A key driver of in situ enzyme activity rates. In Soil Enzymology; Springer Nature: Berlin/Heidelberg, Germany, 2010; pp. 245–258. [Google Scholar]
- Jordan, L.S. Degradation of Herbicides in Soils. Presented at the California Weed Conference (USA), San Jose, CA, USA, 15–17 January 1990. [Google Scholar]
- Dong, X.; Sun, H. Effect of temperature and moisture on degradation of herbicide atrazine in agricultural soil. Int. J. Environ. Agric. Res. 2016, 2, 150–157. [Google Scholar]
- Speight, J.G.; El-Gendy, N.S. Bioremediation of contaminated soil. In Introduction to Petroleum Biotechnology; Speight, J.G., El-Gendy, N.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 361–417. [Google Scholar]
- Avila, L.A.; Zemolin, C.R.; Fipke, M.V.; Cassol, G.V.; Cassol, L.L.; Cassol, A.P.; Zanella, R.; Camargo, E.R. Persistence of S-metolachlor in the soil as affected by moisture content. Adv. Weed Sci. 2024, 42, e020240042. [Google Scholar] [CrossRef]
- Miles, J.R.W.; Harris, C.R.; Tu, C.M. Influence of moisture on the persistence of chlorpyrifos and chlorfenvinphos in sterile and natural mineral and organic soils. J. Environ. Sci. Health B 1984, 19, 237–243. [Google Scholar] [CrossRef]
- UfaqNabi, A.; Shajar, F.; UlRehman, R. Microbe assisted remediation of xenobiotics: A sustainable solution. In Microbes Based Approaches for the Management of Hazardous Contaminants; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2024; pp. 20–41. [Google Scholar]
- Zhao, P.; Wang, L.; Chen, L.; Pan, C. Residue dynamics of clopyralid and picloram in rape plant rapeseed and field soil. Bull. Environ. Contam. Toxicol. 2011, 86, 78–82. [Google Scholar] [CrossRef]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science: Principles and Practices, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Kaur, L.; Kaur, P. Degradation of imazethapyr in soil: Impact of application rate, soil physicochemical properties and temperature. Int. J. Environ. Sci. Technol. 2022, 19, 1877–1892. [Google Scholar] [CrossRef]
- Ross, M.; Lembi, C. Herbicide incorporation techniques and equipment. In Applied Weed Science, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1999; pp. 371–375. [Google Scholar]
- Poiger, T.; Muller, J.; Kasteel, R.; Buerge, I.J. Degradation and sorption of the herbicide pelargonic acid in subsoils below railway tracks compared to a range of topsoils. Environ. Sci. Eur. 2024, 36, 4. [Google Scholar] [CrossRef]
- Reid, B.J.; Jones, K.C.; Semple, K.T. Bioavailability of persistent organic pollutants in soils and sediments—A perspective on mechanisms, consequences and assessment. Environ. Pollut. 2000, 108, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.R.; Hutson, D.H.; Jewess, P.J.; Lee, P.; Nicholls, P.H.; Plimmer, J. Metabolic pathways of agrochemicals. In Insecticides and Fungicides; Plimmer, J., Ed.; Royal Society of Chemistry: Cambridge, UK, 1999; Volume 1, p. 1475. [Google Scholar]
- Raeder, A.J.; Lyon, D.J.; Harsh, J.; Burke, I.; Kowalewski, A.; Stahnke, G.K.; Cook, T.W.; Goss, R.L.; Kennedy, N.; Yorgey, G. How Soil pH Affects the Activity and Persistence of Herbicides? Washington State University Extension: Pullman, WA, USA, 2015. [Google Scholar]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Mohapatra, D.; Rath, S.K.; Mohapatra, P.K. Soil fungi for bioremediation of pesticide toxicants: A perspective. Geomicrobiol. J. 2022, 39, 352–372. [Google Scholar] [CrossRef]
- Arshad, M.; Hussain, S.; Saleem, M. Optimization of environmental parameters for biodegradation of alpha and beta endosulfan in soil slurry by Pseudomonas aeruginosa. J. Appl. Microbiol. 2008, 104, 364–370. [Google Scholar] [CrossRef]
- Camenzind, T.; Mason-Jones, K.; Mansour, I.; Rillig, M.C.; Lehmann, J. Formation of necromass-derived soil organic carbon determined by microbial death pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Sarathchandra, S.; Perrott, K.; Boase, M.; Waller, J. Seasonal changes and the effects of fertiliser on some chemical, biochemical and microbiological characteristics of high-producing pastoral soil. Biol. Fertil. Soils 1988, 6, 328–335. [Google Scholar] [CrossRef]
- Mielnik, L.; Bernacki, B.; Weber, J. The influence of selected herbicides on soil organic matter: Determining the sustainable development of agroecosystems. Sustainability 2025, 17, 1376. [Google Scholar] [CrossRef]
- Mamy, L.; Barriuso, E. Desorption and time-dependent sorption of herbicides in soils. Eur. J. Soil Sci. 2007, 58, 174–187. [Google Scholar] [CrossRef]
- Wu, G.; Ling, J.; Xu, Y.P.; Zhao, D.Q.; Liu, Z.X.; Wen, Y.; Zhou, S.L. Effects of soil warming and straw return on soil organic matter and greenhouse gas fluxes in winter wheat seasons in the North China Plain. J. Clean. Prod. 2022, 356, 131810. [Google Scholar] [CrossRef]
- Burns, R.G. Factors affecting pesticide loss from soil. In Soil Biochemistry; Paul, E.A., Mclaren, A.D., Eds.; Marcel Dekker: New York, NY, USA, 1975; Volume 4, pp. 103–141. [Google Scholar]
- Fu, S.; Wu, Y.; He, S.; Yao, J.; Han, Z.; Zhao, J.; Wang, G.; Li, T. Combining organic amendments and enhanced efficiency fertilizers to improve the quality and nutrient use efficiency of pineapple. Sci. Hortic. 2025, 339, 113839. [Google Scholar] [CrossRef]
- Xiao, M.; Jiang, S.; Li, J.; Li, W.; Fu, P.; Liu, G.; Chen, J. Synergistic effects of bio-organic fertilizer and different soil amendments on salt reduction, soil fertility, and yield enhancement in salt-affected coastal soils. Soil Tillage Res. 2025, 248, 106433. [Google Scholar] [CrossRef]
- Karimi, S.; Raza, T.; Mechri, M. Composting and vermitechnology in organic waste management. In Environmental Engineering and Waste Management: Recent Trends and Perspectives; Springer Nature: Cham, Switzerland, 2024; pp. 449–470. [Google Scholar]
- Sinatra, M.; Giannetta, B.; Plaza, C.; Galluzzi, G.; Squartini, A.; Zaccone, C. Anaerobic digestate influences the carbon distribution in soil organic matter pools after six months from its application. Soil Tillage Res. 2024, 239, 106049. [Google Scholar] [CrossRef]
- Doyle, R.C.; Kaufman, D.D.; Burt, G.W. Effect of dairy manure and sewage sludge on 14C-pesticide degradation in soil. J. Agric. Food Chem. 1978, 26, 987–989. [Google Scholar] [CrossRef]
- Hance, R.J. The effect of nutrients on the decomposition of the herbicides atrazine and linuron incubated with soil. Pest Manag. Sci. 1973, 4, 817–822. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.; Xi, H.; Zhu, Y. Co-remediation of PTEs contaminated soil in mining area by heat modified sawdust and herb. Chemosphere 2021, 281, 130908. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Perez-Lucas, G.; Martinez-Zapata, A.; Navarro, S. Valorization of agro-industrial wastes as organic amendments to reduce herbicide leaching into soil. J. Xenobiot. 2025, 15, 100. [Google Scholar] [CrossRef]
- Wauchope, R.D.; Yeh, S.; Linders, J.B.H.J.; Kloskowski, R.; Tanaka, K.; Rubin, B.; Katayama, A.; Kordel, W.; Gerstl, Z.; Lane, M.; et al. Pesticide soil sorption parameters: Theory, measurement, uses, limitations and reliability. Pest Manag. Sci. 2002, 58, 419–445. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.; Barba, V.; Ordax, J.; Andrades, M.; Sánchez-Martín, M.; Rodríguez-Cruz, M. Application of green compost as amendment in an agricultural soil: Effect on the behaviour of triasulfuron and prosulfocarb under field conditions. J. Environ. Manage. 2018, 207, 180–191. [Google Scholar] [CrossRef]
- Luan, T.G.; Keith, S.H.; Zhong, Y.; Zhou, H.W.; Lan, C.Y.; Tam, N.F. Study of metabolites from the degradation of polycyclic aromatic hydrocarbons (PAHs) by bacterial consortium enriched from mangrove sediments. Chemosphere 2006, 65, 2289–2296. [Google Scholar] [CrossRef]
- Chowdhury, A.; Pradhan, S.; Saha, M.; Sanyal, N. Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Indian J. Microbiol. 2008, 48, 114–127. [Google Scholar] [CrossRef]
- Cork, D.J.; Krueger, J.P. Microbial transformations of herbicides and pesticides. Adv. Appl. Microbiol. 1991, 36, 1–66. [Google Scholar]
- Kaur, R.; Singh, D.; Kumari, A.; Sharma, G.; Rajput, S.; Arora, S.; Kaur, R. Pesticide residues degradation strategies in soil and water: A review. Int. J. Environ. Sci. Technol. 2023, 20, 3537–3560. [Google Scholar] [CrossRef]
- Wang, M.; Orr, A.A.; He, S.; Dalaijamts, C.; Chiu, W.A.; Tamamis, P.; Phillips, T.D. Montmorillonites can tightly bind glyphosate and paraquat reducing toxin exposures and toxicity. ACS Omega 2019, 4, 17702–17713. [Google Scholar] [CrossRef]
- Smreczak, B.; Ukalska-Jaruga, A. Dissolved organic matter in agricultural soils. Soil Sci. Annu. 2021, 72, 132234. [Google Scholar] [CrossRef]
- Guggenberger, G.; Zech, W.; Schulten, H.-R. Formation and mobilization pathways of dissolved organic matter: Evidence from chemical structural studies of organic matter fractions in acid forest floor solutions. Org. Geochem. 1994, 21, 51–66. [Google Scholar] [CrossRef]
- McDowell, W.H.; Likens, G.E. Origin, composition, and flux of dissolved organic carbon in the Hubbard Brook Valley. Ecol. Monogr. 1988, 58, 177–195. [Google Scholar] [CrossRef]
- Freeman, E.C.; Emilson, E.J.; Dittmar, T.; Braga, L.P.; Emilson, C.E.; Goldhammer, T.; Martineau, C.; Singer, G.; Tanentzap, A.J. Universal microbial reworking of dissolved organic matter along environmental gradients. Nat. Commun. 2024, 15, 187. [Google Scholar] [CrossRef]
- Luo, Y.; Atashgahi, S.; Rijnaarts, H.H.; Comans, R.N.; Sutton, N.B. Influence of different redox conditions and dissolved organic matter on pesticide biodegradation in simulated groundwater systems. Sci. Total Environ. 2019, 677, 692–699. [Google Scholar] [CrossRef]
- Coquille, N.; Menard, D.; Rouxel, J.; Dupraz, V.; Eon, M.; Pardon, P.; Budzinski, H.; Morin, S.; Parlanti, E.; Stachowski-Haberkorn, S. The influence of natural dissolved organic matter on herbicide toxicity to marine microalgae is species-dependent. Aquat. Toxicol. 2018, 198, 103–117. [Google Scholar] [CrossRef]
- Wu, X.; Wu, L.; Liu, Y.; Zhang, P.; Li, Q.; Zhou, J.; Hess, N.J.; Hazen, T.C.; Yang, W.; Chakraborty, R. Microbial interactions with dissolved organic matter drive carbon dynamics and community succession. Front. Microbiol. 2018, 9, 1234. [Google Scholar] [CrossRef]
- Horemans, B.; Vandermaesen, J.; Vanhaecke, L.; Smolders, E.; Springael, D. Variovorax sp. -mediated biodegradation of the phenyl urea herbicide linuron at micropollutant concentrations and effects of natural dissolved organic matter as supplementary carbon source. Appl. Microbiol. Biotechnol. 2013, 97, 9837–9846. [Google Scholar] [CrossRef]
- Artigas, J.; Batisson, I.; Carles, L. Dissolved organic matter does not promote glyphosate degradation in auto-heterotrophic aquatic microbial communities. Environ. Pollut. 2020, 259, 113951. [Google Scholar] [CrossRef] [PubMed]
- Said-Pullicino, D.; Gigliotti, G.; Vella, A.J. Environmental fate of triasulfuron in soils amended with municipal waste compost. J. Environ. Qual. 2004, 33, 1743–1751. [Google Scholar] [CrossRef]
- Tian, B.B.; Zhou, J.H.; Xie, F.; Guo, Q.N.; Zhang, A.P.; Wang, X.Q.; Yu, Q.Q.; Li, N.; Yang, H. Impact of surfactant and dissolved organic matter on uptake of atrazine in maize and its mobility in soil. J. Soils Sediments 2019, 19, 599–608. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Sharma, K. Microbial biodiversity and bioremediation assessment through omics approaches. Front. Environ. Chem. 2020, 1, 570326. [Google Scholar] [CrossRef]
- Rodriguez, A.; Castrejon-Godínez, M.L.; Salazar-Bustamante, E.; Gama-Martínez, Y.; Sánchez-Salinas, E.; Mussali-Galante, P.; Tovar-Sánchez, E.; Ortiz-Hernández, M.L. Omics approaches to pesticide biodegradation. Curr. Microbiol. 2020, 77, 545–563. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Singh, R.; Gong, Q.; Wu, Q.; Shi, J. Omics approaches in understanding the benefits of plant-microbe interactions. Front. Microbiol. 2024, 15, 1391059. [Google Scholar] [CrossRef]
- Alidoosti, F.; Giyahchi, M.; Moien, S.; Moghimi, H. Unlocking the potential of soil microbial communities for bioremediation of emerging organic contaminants: Omics-based approaches. Microb. Cell Fact. 2024, 23, 210. [Google Scholar] [CrossRef]
- Sibalekile, A.; Araya, T.; Castillo Hernandez, J.; Kotze, E. Glyphosate-microbial interactions: Metagenomic insights and future directions. Front. Microbiol. 2025, 16, 1570235. [Google Scholar] [CrossRef]
- Pongsupasa, V.; Watthaisong, P.; Treesukkasem, N.; Naramittanakul, A.; Tirapanampai, C.; Weeranoppanant, N.; Chaiyen, P.; Wongnate, T. Sustainable pesticide degradation using esterase and coimmobilized cells in Agriculture. Biotechnol. J. 2025, 20, e70034. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shen, S.; Feng, J.; Deng, S.; Lu, E.; Gao, C.; Zhang, Y.; Chen, H.; Li, W. Efficient biodegradation of flurochloridone herbicide by Streptomyces parvus strain FG-2 and its degradation mechanism. Bioresour. Technol. 2025, 421, 132158. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, B.; Liu, J.; Wang, Q.; Tian, B. Biodegradation of dinitroaniline herbicides: A comprehensive review. Environ. Technol. Innov. 2025, 39, 104225. [Google Scholar] [CrossRef]
- Droz, B.; Drouin, G.; Lohmann, J.; Guyot, B.; Imfeld, G.; Payraudeau, S. How combining multi-scale monitoring and compound-specific isotope analysis helps to evaluate degradation of the herbicide S-metolachlor in agro-ecosystems? EGUsphere 2025, 1–27. [Google Scholar] [CrossRef]
- Reed, K.; Dang, C.; Walkup, J.; Purcell, A.; Hungate, B.; Morrissey, E. Comparing field and lab quantitative stable isotope probing for nitrogen assimilation in soil microbes. Appl. Environ. Microbiol. 2025, 91, e01849-24. [Google Scholar] [CrossRef]
- Zulet-Gonzalez, A.; Gorzolka, K.; Doll, S.; Gil-Monreal, M.; Royuela, M.; Zabalza, A. Unravelling the phytotoxic effects of glyphosate on sensitive and resistant Amaranthus palmeri populations by GC–MS and LC–MS metabolic profiling. Plants 2023, 12, 1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gilbert, J.A.; Liu, X.; Nie, L.; Xu, X.; Gao, G.; Lyu, L.; Ma, Y.; Fan, K.; Yang, T.; et al. SynCom-mediated herbicide degradation activates microbial carbon metabolism in soils. iMeta 2025, 4, e70058. [Google Scholar] [CrossRef]
- Dhakar, K.; Eizenberg, H.; Ronen, Z.; Zarecki, R.; Freilich, S. Microbial degradation of herbicides in contaminated soils by following computational approaches. In Bioinformatics in Agriculture; Academic Press: Cambridge, MA, USA, 2022; pp. 399–417. [Google Scholar]
- Helvig, C.; Kariyawasam, T.; Vriens, B.; Petkovich, M. Genetically engineered bacteria and microalgae expressing a mutant of cytochrome P450 BM3 for efficient diuron degradation in wastewater treatment. Microbiol. Spectr. 2025, 13, e02905-24. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Tao, B. Cloning of cyp57A1 gene from Fusarium verticillioides for degradation of herbicide fomesafen. Environ. Technol. Innov. 2024, 36, 103822. [Google Scholar] [CrossRef]
- Widada, J.; Nojiri, H.; Omori, T. Recent developments in molecular techniques for identification and monitoring of xenobiotic-degrading bacteria and their catabolic genes in bioremediation. Appl. Microbiol. Biotechnol. 2002, 60, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Gangola, S.; Chaudhary, P.; Khati, P.; Kumar, G.; Sharma, A.; Srivastava, A. Pesticide induced up-regulation of esterase and aldehyde dehydrogenase in indigenous Bacillus spp. Bioremediat. J. 2019, 23, 42–52. [Google Scholar] [CrossRef]
- Zang, H.; Wang, H.; Miao, L.; Cheng, Y.; Zhang, Y.; Liu, Y.; Sun, S.; Wang, Y.; Li, C. Carboxylesterase, a de-esterification enzyme, catalyzes the degradation of chlorimuron-ethyl in Rhodococcus erythropolis D310-1. J. Hazard. Mater. 2020, 387, 121684. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Liu, W.; Hou, N.; Zhao, F.; Han, P.; Tan, H. Biodegradation properties and mechanism of triketone herbicide mesotrione via newly isolated bacterium Klebsiella pasteurii CM-1. Int. Biodeterior. Biodegrad. 2024, 187, 105727. [Google Scholar] [CrossRef]
- Sun, S.; Chen, W.; Peng, K.; Chen, X.; Chen, J. Characterization of a novel amidohydrolase with promiscuous esterase activity from a soil metagenomic library and its application in degradation of amide herbicides. Environ. Sci. Pollut. Res. 2024, 31, 20970–20982. [Google Scholar] [CrossRef]
- Jadeja, N.B.; Kapley, A. Designing knowledge-based bioremediation strategies using metagenomics. In Metagenomic Data Analysis; Springer: New York, NY, USA, 2023; pp. 195–208. [Google Scholar]
- Bharagava, R.N.; Purchase, D.; Saxena, G.; Mulla, S.I. Applications of metagenomics in microbial bioremediation of pollutants: From genomics to environmental cleanup. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 459–477. [Google Scholar]
- Hussain, I.; Aleti, G.; Naidu, R.; Puschenreiter, M.; Mahmood, Q.; Rahman, M.M.; Wang, F.; Shaheen, S.; Syed, J.H.; Reichenauer, T.G. Microbe and plant assisted-remediation of organic xenobiotics and its enhancement by genetically modified organisms and recombinant technology: A review. Sci. Total Environ. 2018, 628–629, 1582–1599. [Google Scholar] [CrossRef] [PubMed]

| Isolated Strains | Herbicide(s) Degraded | Degradation Rate (%) | Location/Source of Isolation | Reference(s) |
|---|---|---|---|---|
| Bacteria | ||||
| Arthrobacter sp. SVMIICT25, Sphingomonas sp. SVMIICT11 and Stenotrophomonas sp. SVMIICT13. | 2,4-D | 81–90% after 12 days | India | [66] |
| Cupriavidus oxalaticus strain X32 | 2,4-D | 100% in 3 days | China | [67] |
| Bacillus cereus | MCPA | 99.70% on 31 days | Poland | [68] |
| Pseudomonas fluorescens | Sulfosulfuron | 97% on 46 days | Poland | [68] |
| Bacillus subtilis, Rhizobium leguminosarum, Streptomyces sp. | Glyphosate | 85–90% in 30 days | India | [69] |
| Nocardia mediterranie THS 1 | Glyphosate | ND | India | [70] |
| Amycolatopsis sp. and Saccharomonospora sp. | Acetochlor, S-metolachlor | ND | China | [71] |
| Bacillus aryabhattai FACU3 | Glyphosate | ND | Egypt | [72] |
| Achromobacter denitrificans SOS5 | Glyphosate | 56% after 96 h | Argentina | [73] |
| Achromobacter insolitus SOR2 | Glyphosate | 47% after 96 h | Argentina | [73] |
| Achromobacter xylosoxidans SOS3 | Glyphosate | 37% after 96 h | Argentina | [73] |
| Agrobacterium tumefaciens CHLDO | Glyphosate | 40% after 96 h | Argentina | [73] |
| Ochrobactrum haematophilum SR | Glyphosate | 41% after 96 h | Argentina | [73] |
| Bacillus megaterium | Glyphosate | 70–71% after 60 days | Iraq | [74] |
| Acidovorax sp. CNI26 | Glyphosate | 100% after 125–400 h | France | [75] |
| Agrobacterium tumefaciens CNI28 | Glyphosate | 100% after 125–400 h | France | [75] |
| Ensifer sp. CNI115 | Glyphosate | 100% after 125–400 h | France | [75] |
| Novosphingobium sp. CNI35 | Glyphosate | 100% after 125–400 h | France | [75] |
| Ochrobactrum pituitosum CNI52 | Glyphosate | 100% after 125–400 h | France | [75] |
| Comamonas odontotermitis P2 | Glyphosate | 90% after 104 h | Australia | [76] |
| Ochrobactrum sp. | Glyphosate | 60% after 15 days | Mexico | [77] |
| Pseudomonas citronelloli | Glyphosate | 60% after 15 days | Mexico | [77] |
| Lysinibacillus sphaericus | Glyphosate | 79% after 30 days | Colombia | [78] |
| Aspergillus oryzae AM1 | Glyphosate | 57% after 15 days | Argentina | [79] |
| Stenotrophomonas sp., Brucella sp., Ensifer adhaerens | Atrazine and metribuzin | ND | Iran | [80] |
| Escherichia fergusonii | Diuron | ND | Brazil | [81] |
| Bacillus altitudinis A16 | Butachlor | 90% in 5 days | India | [82] |
| Pseudomonas sp. But2 | Butachlor | 100% after 30 h | Vietnam | [83] |
| Acinetobacter baumannii DT | Propanil | 100% after 48 h | Vietnam | [83] |
| Achromobacter xylosoxidans | 2,4-D | 95.38% after 96 h | Nigeria | [84] |
| Pseudomonas strain PD1 | Pendimethalin | 77.05% in 30 h | India | [85] |
| Bosea sp. strain P5 and Alicycliphilus sp. PH-34 | Propanil | 100% in 144 h | China | [86] |
| Arthrobacter sp. SVMIICT25, Sphingomonas sp. SVMIICT11 and Stenotrophomonas sp. SVMIICT13. | 2,4-D | 81–90% in 12 days | India | [66] |
| Microbacterium sulfonylureivorans sp. nov. | Nicosulfuron | 69.56% within 7 days | China | [87] |
| Stenotrophomonas rhizophila CASB3 | Diuron | 94% in 42 days | Chile | [88] |
| Bacillus pseudomycoides D/T, Bacillus simplex/Bacillus muralis D/N | Diuron | 54% and 51% in 46 days, respectively | Kenya | [89] |
| Streptomyces heliomycini C1 | Metribuzin | 73.06% after 15 days | Algeria | [90] |
| Bacillus licheniformis and Bacillus megaterium | Atrazine | 98.60 and 99.60% after 7 days, respectively | China | [91] |
| Pseudomonas putida | Butachlor | 100% in 15 days | India | [92] |
| Acinetobacter sp. GC-A6 | Alachlor | 100% in 48 h | South Korea | [93] |
| Pseudomonas nicosulfuronedens LAM1902 | Nicosulfuron | 99% after 6 days | China | [94] |
| Bacillus pumilus and Bacillus subtilis | Terbutylazine | 95 and 98% within 6 days, respectively | China | [95] |
| Pseudomonas stutzeri | Atrazine | 87% after 3 days | India | [96] |
| Cupriavidus oxalaticus strain X32 | 2,4-D | 100% in 3 days | China | [67] |
| Amycolatosis nivea La24 | Mesotrione | 100% within 48 h | China | [97] |
| Pseudomonas putida Strain Ch2 | Glyphosate | ND | Russia | [98] |
| Cupriavidus campinensis | 2,4-D | 94.69% after 6 days | Nigeria | [99] |
| Bacillus sp. Za | Fluoroglycofen | 100% in 48 h | China | [100] |
| Bacillus sp. Za | Lactofen | 100% in 36 h | China | [100] |
| Dehalococcoides mccartyi and Desulfitobacterium hafniense | 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) | ND | Germany | [101] |
| Arthrobacter aurescens CTFL7 | Trifluralin | 92% after 60 days | Spain | [102] |
| Bacillus sp. LY05 | Butralin | 85.43% after 30 days | China | [103] |
| Bacillus subtilis CZ1 | Pendimethalin | 58.85% | China | [104] |
| Burkholderia sp. F7G4PR33–4 | Pendimethalin | 65% in 15 days | Brazil | [105] |
| Pseudomonas strain PD1 | Pendimethalin | 77.05% in 1.25 day | India | [85] |
| Fungi | ||||
| Clavispora lusitaniae YC2 | Pendimethalin | 74% in 8 days | China | [106] |
| Aspergillus 2B112 | Glyphosate | 36.48 ± 0.01 in 14 days | Brazil | [107] |
| Penicillium 4A21 | Glyphosate | 42.72 ± 0.02 in 14 days | Brazil | [107] |
| Penicillium 2A31 | Glyphosate | 34.91 ± 0.02 in 14 days | Brazil | [107] |
| Degradation Mechanism | Outcome |
|---|---|
| 1. Direct decomposition of herbicides through adaptation where herbicide compounds serve as energy sources (catabolism). | Repeated application of same herbicide results in faster degradation, such as atrazine [123]. Serious consequences may also arise such as persistence of some specific herbicides, e.g., atrazine and nicosulfuron residues in soil [124]. |
| 2. Accidental transformation through peripheral metabolic process (co-metabolism). | All herbicides may be degraded by this mechanism [125]. |
| 3. General activities by microorganisms such as modification of pH, production of different free radicals and other reactive compounds. | Degradation of herbicides due to the influence of microorganisms on biological and non-biological reactions [126]. |
| Factors | Properties | Degradation Rate | |
|---|---|---|---|
| Rapid Degradation | Slow Degradation | ||
| Physical | Water solubility | Soluble | Insoluble |
| Size | Relatively small | Relatively large | |
| Soil adsorption | Lower | Higher | |
| Soil moisture and temperature | Optimum | Both higher and lower values | |
| Origin | Biological | Synthetic or artificial | |
| Structural | Functional group substitutions | Few | More |
| Molecular weight and size | Low-molecular-weight and simple compounds | High-molecular-weight and complex compounds | |
| Substitutions on organic molecules | Alcohols, aldehydes, acids, esters, amides, amino acids | Alkanes, olefins, ethers, ketones, dicarboxylic acids, nitriles, amines, chloroalkanes | |
| Presence of polar functional groups | Presence | Absence | |
| Chemical | Chemical reactivity | Presence of reactive groups (amines, esters) | Absence |
| Volatility | low volatility | High volatility | |
| Microbial | Microbial density and diversity | Higher microbial population | Lower microbial population |
| Co-metabolism and nutrient availability | Sufficient nutrient available | Not available | |
| Environmental | Oxygen availability | Aerobic conditions | Anaerobic conditions |
| Soil amendments | Application of soil amendments (compost, biochar, etc.) | No application | |
| Name of Approach | Techniques | Objective | Outcome | References |
|---|---|---|---|---|
| Identification and Characterization | Culture methods, 16S rRNA sequencing, biodegradation trials | Isolation of novel bacterial strains and consortia capable of degrading specific herbicide | Identification of potential degraders (microorganisms) | [65] |
| Metagenomics and Next-Generation Sequencing (NGS) | Shotgun sequencing, Oxford Nanopore, PacBio, single-cell genomics | Analysis of the total microbial communities present in soil post herbicide application | Identification of non-culturable microorganisms, microbial diversity, and functional profiling | [210] |
| Biochemical and Enzymatic assays | Enzyme isolation, spectrophotometry, chromatography, mass spectrometry | Identification of enzymes responsible for degradation of herbicides | Understanding enzymatic pathways for microbial degradation | [211,212,213] |
| Stable Isotope Probing (SIP) | Isotopic labelling (13C, 15N), DNA/RNA extraction, sequencing | Detecting herbicide-derived isotopes in microbial DNA/RNA to identify potential degraders | Direct relation of microorganisms to degradation mechanism | [214,215] |
| Metabolomics and Mass Spectrometry | LC-MS, GC-MS, NMR spectroscopy | Characterization of herbicide metabolites to understand degradation pathways | Pathway analysis and identification of herbicide metabolites | [65,216] |
| Microcosm and Field Experiments | Herbicide residue analysis, microbial community profiling | Controlled laboratory or field experiments to monitor herbicide degradation and microbial response upon exposure | Microbial degradation processes under various environmental conditions | [146,217] |
| Bioinformatics and Systemic Biology | Genomic/transcriptomic data analysis, metabolic modelling | Integration and modelling of multi-omics data to understand degradation networks | Predictive insights for improving microbial degradation strategies | [29,218] |
| Transgenic microorganisms | Cloning and expression of the degradation gene into the engineered bacterium | Development of engineered bacteria for the degradation of highly persistent herbicides | Engineered bacteria degraded herbicides faster under optimized degradation conditions | [219,220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, I.F.; Doran, G.S.; Stodart, B.J.; Chen, C.; Wu, H. Microbial Degradation of Herbicide Residues in Australian Soil: An Overview of Mechanistic Insights and Recent Advancements. Toxics 2025, 13, 949. https://doi.org/10.3390/toxics13110949
Chowdhury IF, Doran GS, Stodart BJ, Chen C, Wu H. Microbial Degradation of Herbicide Residues in Australian Soil: An Overview of Mechanistic Insights and Recent Advancements. Toxics. 2025; 13(11):949. https://doi.org/10.3390/toxics13110949
Chicago/Turabian StyleChowdhury, Imtiaz Faruk, Gregory S. Doran, Benjamin J. Stodart, Chengrong Chen, and Hanwen Wu. 2025. "Microbial Degradation of Herbicide Residues in Australian Soil: An Overview of Mechanistic Insights and Recent Advancements" Toxics 13, no. 11: 949. https://doi.org/10.3390/toxics13110949
APA StyleChowdhury, I. F., Doran, G. S., Stodart, B. J., Chen, C., & Wu, H. (2025). Microbial Degradation of Herbicide Residues in Australian Soil: An Overview of Mechanistic Insights and Recent Advancements. Toxics, 13(11), 949. https://doi.org/10.3390/toxics13110949








