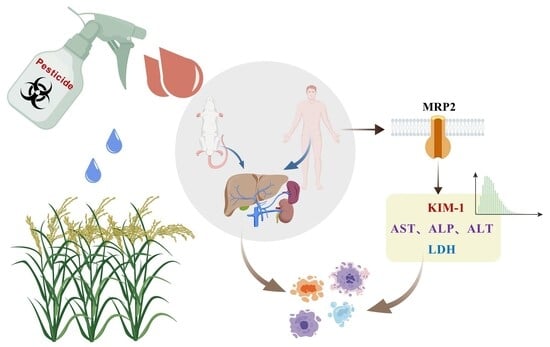
Specific Hepatorenal Toxicity and Cross-Species Susceptibility of Eight Representative Pesticides
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Cell Culture and Chemicals
2.3. Cell Culture and Exposure
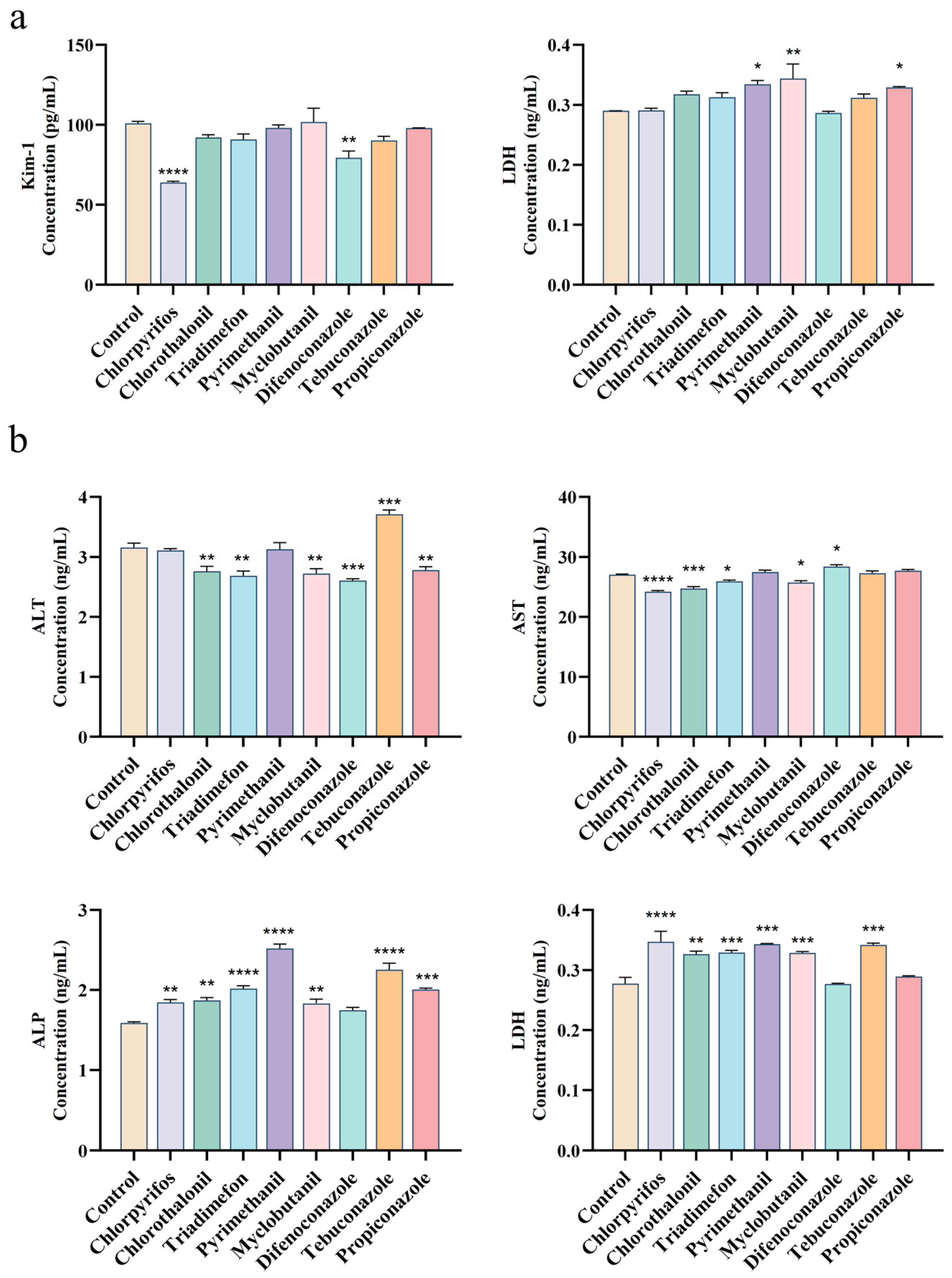
2.4. ELISA for Hepatorenal Toxicity Biomarkers
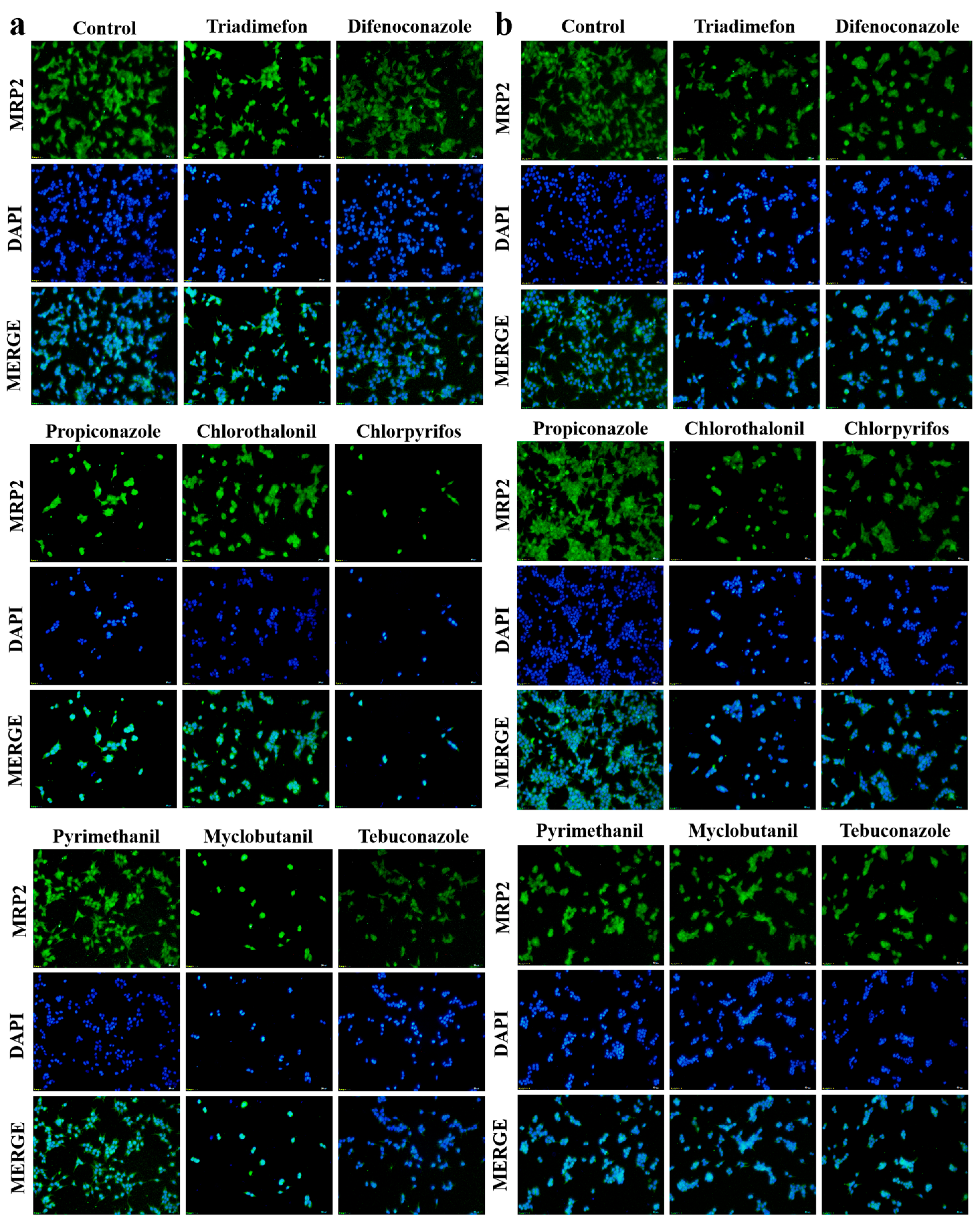
2.5. Immunofluorescence Staining
2.6. Statistical Analysis
3. Results
3.1. Analysis of Pesticide Toxicity Variations
3.2. Cross-Cell Line and Species-Specific Toxicity Profiling
3.3. Comparative Analysis of Hepatotoxicity and Nephrotoxicity Biomarkers
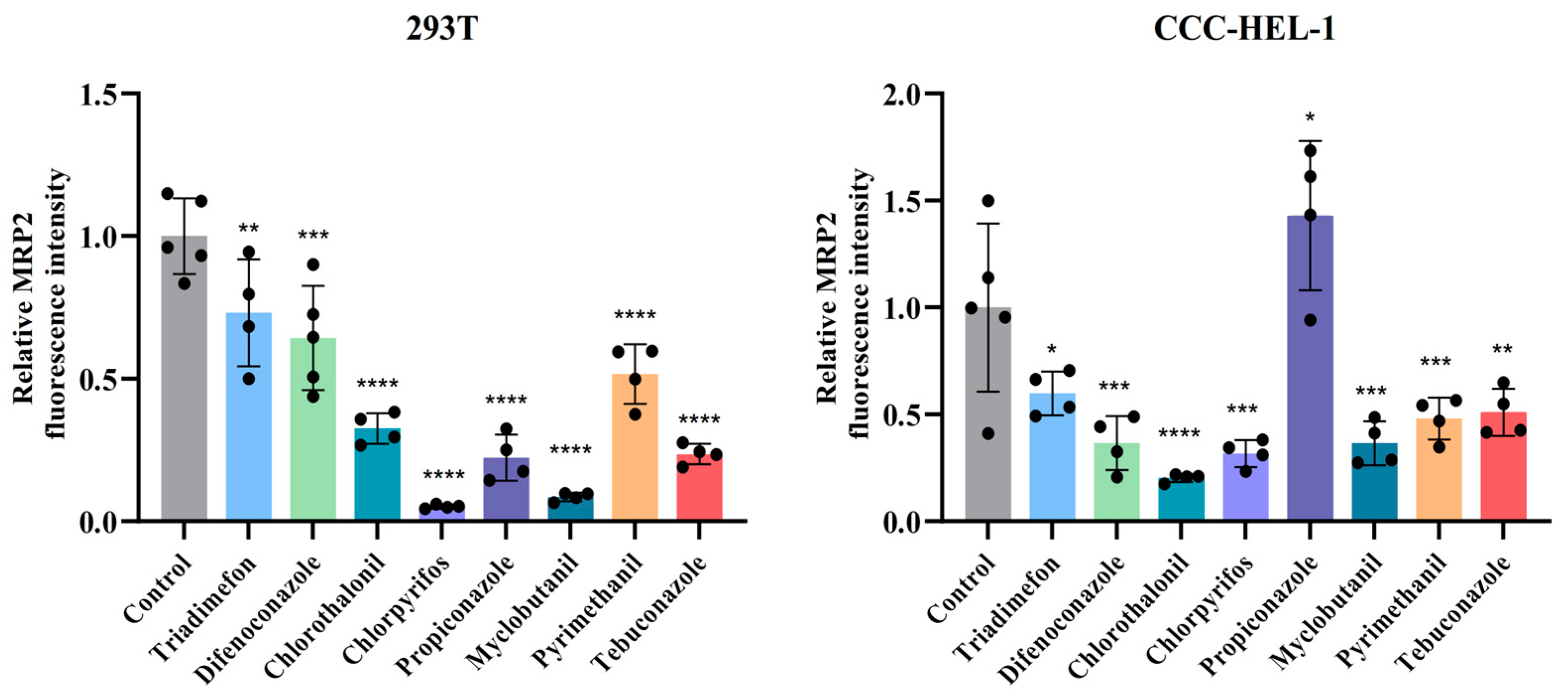
3.4. Multidrug Resistance-Associated Protein 2 (MRP2) Protein Expression Analysis in Liver and Kidney Cells Following Pesticide Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monticelli Barizon, R.R.; Kummrow, F.; Fernandes de Albuquerque, A.; Assalin, M.R.; Rosa, M.A.; Cassoli de Souza Dutra, D.R.; Almeida Pazianotto, R.A. Surface water contamination from pesticide mixtures and risks to aquatic life in a high-input agricultural region of Brazil. Chemosphere 2022, 308 Pt 3, 136400. [Google Scholar] [CrossRef]
- Brown, J.B.; Langley, S.A.; Snijders, A.M.; Wan, K.H.; Morris, S.N.S.; Booth, B.W.; Fisher, W.W.; Hammonds, A.S.; Park, S.; Weiszmann, R.; et al. An integrated host-microbiome response to atrazine exposure mediates toxicity in Drosophila. Commun. Biol. 2021, 4, 1324. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, A.; Werner, M.; Mempel, F.; Dunivin, Z.; Galt, R. Global pesticide use and trade database (GloPUT): New estimates show pesticide use trends in low-income countries substantially underestimated. Glob. Environ. Change 2023, 81, 102693. [Google Scholar] [CrossRef]
- Ruomeng, B.; Meihao, O.; Siru, Z.; Shichen, G.; Yixian, Z.; Junhong, C.; Ruijie, M.; Yuan, L.; Gezhi, X.; Xingyu, C.; et al. Degradation strategies of pesticide residue: From chemicals to synthetic biology. Synth. Syst. Biotechnol. 2023, 8, 302–313. [Google Scholar] [CrossRef]
- Fernández-Martínez, N.F.; Ching-López, A.; Olry de Labry Lima, A.; Salamanca-Fernández, E.; Pérez-Gómez, B.; Jiménez-Moleón, J.J.; Sánchez, M.J.; Rodríguez-Barranco, M. Relationship between exposure to mixtures of persistent, bioaccumulative, and toxic chemicals and cancer risk: A systematic review. Environ. Res. 2020, 188, 109787. [Google Scholar] [CrossRef]
- Ying, Y.; Pan, P.; Zou, C.; Wang, Y.; Tang, Y.; Hou, X.; Li, Y.; Xu, Q.; Lin, L.; Lu, J.; et al. Tebuconazole exposure disrupts placental function and causes fetal low birth weight in rats. Chemosphere 2021, 264 Pt 2, 128432. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, D.; Rao, H.; Liu, X. Potential neurotoxicity, immunotoxicity, and carcinogenicity induced by metribuzin and tebuconazole exposure in earthworms (Eisenia fetida) revealed by transcriptome analysis. Sci. Total Environ. 2022, 807 Pt 1, 150760. [Google Scholar] [CrossRef]
- Saka, W.A.; Adeogun, A.E.; Adisa, V.I.; Olayioye, A.; Igbayilola, Y.D.; Akhigbe, R.E. L-arginine attenuates dichlorvos-induced testicular toxicity in male Wistar rats by suppressing oxidative stress-dependent activation of caspase 3-mediated apoptosis. Biomed. Pharmacother. 2024, 178, 117136. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, R.; Joshi, A.; Vamadeva, S.; Rajini, P.S. Effect of chronic exposure to monocrotophos on white adipose tissue in rats and its association with metabolic dyshomeostasis. Hum. Exp. Toxicol. 2020, 39, 1190–1199. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Jiang, F.; Peng, Y.; Sun, Q. Pesticides exposure induced obesity and its associated diseases: Recent progress and challenges. J. Future Foods 2022, 2, 119–124. [Google Scholar] [CrossRef]
- Wu, C.; He, K.; Li, H.; Zhang, L.; Mao, L.; Zhu, L.; Jiang, J.; Liu, X. Transgenerational combined toxicity effects of neonicotinoids and triazole pesticides at environmentally relevant concentrations on D. magna: From individual to population level. J. Hazard. Mater. 2025, 486, 137023. [Google Scholar] [CrossRef]
- Banaee, M.; Zeidi, A.; Haghi, B.N.; Beitsayah, A. The toxicity effects of imidacloprid and chlorpyrifos on oxidative stress and blood biochemistry in Cyprinus carpio. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 284, 109979. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Dang, F.; Yu, L.; Zhao, H.; Wei, T.; An, F. Soil residues and crop accumulation of organophosphorus and pyrethroid pesticides in agricultural fields in Shaanxi, China. J. Soils Sediments 2024, 24, 2713–2723. [Google Scholar] [CrossRef]
- Morais, L.G.; Gusso-Choueri, P.K.; Abreu, F.E.L.; Castro, Í.B.; Abessa, D.M.; Choueri, R.B. Multilevel assessment of chlorothalonil sediment toxicity to Latin American estuarine biota: Effects on biomarkers, reproduction and survival in different benthic organisms. Sci. Total Environ. 2023, 872, 162215. [Google Scholar] [CrossRef]
- Campos-Mañas, M.C.; Plaza-Bolaños, P.; Martínez-Piernas, A.B.; Sánchez-Pérez, J.A.; Agüera, A. Determination of pesticide levels in wastewater from an agro-food industry: Target, suspect and transformation product analysis. Chemosphere 2019, 232, 152–163. [Google Scholar] [CrossRef]
- Tust, M.; Kohler, M.; Lagojda, A.; Lamshoeft, M. Comparison of the in vitro assays to investigate the hepatic metabolism of seven pesticides in Cyprinus carpio and Oncorhynchus mykiss. Chemosphere 2021, 277, 130254. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, J.; Su, G.; Wang, R.; Zhai, Z.; Yu, F.; Li, Y. A comparative study of the established methods and evaluation of rat trauma models. Anim. Model Exp. Med. 2025, 8, 501–510. [Google Scholar] [CrossRef]
- An, X.; Wang, R.; Cao, C.; Wang, D.; Chen, C.; Wang, Y. Synergistic risk in the gut and liver: Insights into the toxic mechanisms and molecular interactions of combined exposure to triazophos and fenvalerate in zebrafish. Sci. Total Environ. 2024, 948, 174710. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Zhang, T.; Zhou, M.; Li, X.; Ping, K.; Ji, X.; Yang, H.; Dong, J. Hepatotoxicity of the Pesticide Avermectin Exposure to Freshwater-Farmed Carp: Evidence from In Vivo and In Vitro Research. J. Agric. Food Chem. 2023, 71, 20654–20670. [Google Scholar] [CrossRef] [PubMed]
- Alehashem, M.; Mamet, S.; Hogan, N.; Hecker, M.; Florou, D.; Tsatsakis, A.; Siciliano, S. Correlation between in vitro toxicity of pesticides and in vivo risk guidelines in support of complex operating site risk management: A meta-analysis. Food Chem. Toxicol. 2022, 170, 113502. [Google Scholar] [CrossRef]
- Wang, C.; Liang, D.; Shen, X.; Chen, X.; Lai, L.; Hou, H. Compound 4a induces paraptosis in liver cancer through endoplasmic reticulum stress mediated by the calreticulin protein. Br. J. Pharmacol. 2024; early view. [Google Scholar] [CrossRef]
- Deng, J.; Wei, W.; Chen, Z.; Lin, B.; Zhao, W.; Luo, Y.; Zhang, X. Engineered Liver-on-a-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines 2019, 10, 676. [Google Scholar] [CrossRef]
- Qin, S.S.; Wang, J.; Yuan, H.Q.; He, J.Z.; Luan, S.J.; Deng, Y. Liver function indicators and risk of hepatocellular carcinoma: A bidirectional mendelian randomization study. Front. Genet. 2024, 14, 1260352. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Paniagua, D.; Parrón, T.; Alarcón, R.; Requena, M.; López-Guarnido, O.; Lacasaña, M.; Hernández, A.F. Evaluation of conventional and non-conventional biomarkers of liver toxicity in greenhouse workers occupationally exposed to pesticides. Food Chem. Toxicol. 2021, 151, 112127. [Google Scholar] [CrossRef]
- Lash, L.H. Trichloroethylene: An Update on an Environmental Contaminant with Multiple Health Effects. Annu. Rev. Pharmacol. Toxicol. 2025, 65, 507–527. [Google Scholar] [CrossRef]
- Wan, E.T.; Darssan, D.; Karatela, S.; Reid, S.A.; Osborne, N.J. Association of Pesticides and Kidney Function among Adults in the US Population 2001-2010. Int. J. Environ. Res. Public Health 2021, 18, 10249. [Google Scholar] [CrossRef]
- Schmidt, I.M.; Srivastava, A.; Sabbisetti, V.; McMahon, G.M.; He, J.; Chen, J.; Kusek, J.W.; Taliercio, J.; Ricardo, A.C.; Hsu, C.Y.; et al. Plasma Kidney Injury Molecule 1 in CKD: Findings from the Boston Kidney Biopsy Cohort and CRIC Studies. Am. J. Kidney Dis. 2022, 79, 231–243.e1. [Google Scholar] [CrossRef]
- Griffin, B.R.; Faubel, S.; Edelstein, C.L. Biomarkers of Drug-Induced Kidney Toxicity. Ther. Drug Monit. 2019, 41, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, M.; Meng, J.; Xue, Y.; Huang, Q.; Zheng, Y.; Wu, Y.; Chen, Z.; Yu, J.; Zhong, D.; et al. Mechanistic Study on the Species Differences in Excretion Pathway of HR011303 in Humans and Rats. Drug Metab. Dispos. 2022, 50, 809–818. [Google Scholar] [CrossRef]
- Li, J.; Dawson, P.A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Adigbli, G.; Ménoret, S.; Cross, A.R.; Hester, J.; Issa, F.; Anegon, I. Humanization of Immunodeficient Animals for the Modeling of Transplantation, Graft Versus Host Disease, and Regenerative Medicine. Transplantation 2020, 104, 2290–2306. [Google Scholar] [CrossRef]
- Pecquet, A.M.; Bridgwood, K.; Cowie, D.; Hofstra, A.; Wu, Y.; Whalley, S.; Webb, S.D. Data derived extrapolation factors (DDEFs) for rat to human interspecies extrapolation for the HPPD inhibitor mesotrione. Crit. Rev. Toxicol. 2024, 54, 418–429. [Google Scholar] [CrossRef]
- Golden, E.; Macmillan, D.S.; Dameron, G.; Kern, P.; Hartung, T.; Maertens, A. Evaluation of the global performance of eight in silico skin sensitization models using human data. Altex 2021, 38, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vizcaíno, E.; Ortiz-Santaliestra, M.E.; Fernández-Tizón, M.; Mateo, R.; Camarero, P.R.; Mougeot, F. Bird exposure to fungicides through the consumption of treated seeds: A study of wild red-legged partridges in central Spain. Environ. Pollut. 2022, 292, 118335. [Google Scholar] [CrossRef] [PubMed]
- Aouzal, B.; Slimani, S.; Kamah, F.; Ounissi, I.; Bouacha, A.; Samira, E. LC MS/MS Phytochemical analysis of Ruta montana and the Hepatic Preventive Effects in male Rats exposed to Tebuconazole. Glob. NEST J. 2024, 26, 06877. [Google Scholar] [CrossRef]
- Han, L.; Kong, X.; Xu, M.; Nie, J. Repeated exposure to fungicide tebuconazole alters the degradation characteristics, soil microbial community and functional profiles. Environ. Pollut. 2021, 287, 117660. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Zhang, Y. Tubeimoside-1 Protects Against Renal Ischemia Reperfusion Injury In Vivo and In Vitro. Nat. Prod. Commun. 2020, 15, 1934578X20977647. [Google Scholar] [CrossRef]
- Zheng, S.; Lavrenyuk, K.; Lamson, N.G.; Fein, K.C.; Whitehead, K.A.; Dahl, K.N. Piperazine Derivatives Enhance Epithelial Cell Monolayer Permeability by Increased Cell Force Generation and Loss of Cadherin Structures. ACS Biomater. Sci. Eng. 2020, 6, 367–374. [Google Scholar] [CrossRef]
- He, Q.; Yang, Q.; Liu, Q.; Hu, Z.; Gao, Q.; Dong, Y.; Xiao, J.; Yu, L.; Cao, H. The effects of beta-cypermethrin, chlorbenzuron, chlorothalonil, and pendimethalin on Apis mellifera ligustica and Apis cerana cerana larvae reared in vitro. Pest Manag. Sci. 2022, 78, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Bao, Z.; Jin, Y. Chlorothalonil exposure induces “liver-gut axis” disorder in mice. Acta Biochim. Biophys. Sin. 2022, 54, 1030–1033. [Google Scholar] [CrossRef]
- Li, X.; Yao, Y.; Wang, S.; Xu, S. Resveratrol relieves chlorothalonil-induced apoptosis and necroptosis through miR-15a/Bcl2-A20 axis in fish kidney cells. Fish Shellfish Immunol. 2020, 107 Pt B, 427–434. [Google Scholar] [CrossRef] [PubMed]
- AlKahtane, A.A.; Ghanem, E.; Bungau, S.G.; Alarifi, S.; Ali, D.; AlBasher, G.; Alkahtani, S.; Aleya, L.; Abdel-Daim, M.M. Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues. Environ. Sci. Pollut. Res. Int. 2020, 27, 11663–11670. [Google Scholar] [CrossRef]
- Bai, J.; Deng, S.; Fu, H.; Yang, Q.; Ren, F.; Zeng, S.; Chen, Z.; Yang, Y.; Wu, Z. Chlorpyrifos induces placental oxidative stress and barrier dysfunction by inducing mitochondrial apoptosis through the ERK/MAPK signaling pathway: In vitro and in vivo studies. Sci. Total Environ. 2023, 903, 166449. [Google Scholar] [CrossRef]
- Weis, G.C.C.; Assmann, C.E.; Mostardeiro, V.B.; Alves, A.O.; da Rosa, J.R.; Pillat, M.M.; de Andrade, C.M.; Schetinger, M.R.C.; Morsch, V.M.M.; da Cruz, I.B.M.; et al. Chlorpyrifos pesticide promotes oxidative stress and increases inflammatory states in BV-2 microglial cells: A role in neuroinflammation. Chemosphere 2021, 278, 130417. [Google Scholar] [CrossRef]
- Stocco, M.R.; Tyndale, R.F. Cytochrome P450 enzymes and metabolism of drugs and neurotoxins within the mammalian brain. Adv. Pharmacol. 2022, 95, 73–106. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.A.; Bromek, E.; Danek, P.J.; Haduch, A. The mechanisms of interactions of psychotropic drugs with liver and brain cytochrome P450 and their significance for drug effect and drug-drug interactions. Biochem. Pharmacol. 2022, 199, 115006. [Google Scholar] [CrossRef]
- Zhang, M.; Du, P.; Xiao, Y.; Liu, H.; Wang, M.; Zhang, Y.; Chen, X. Sex differences in CYP450-based sodium dehydroacetate metabolism and its metabolites in rats. npj Sci. Food 2024, 8, 110. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, S.; Qin, X.; Li, F.; Guo, L. Study of the roles of cytochrome P450 (CYPs) in the metabolism and cytotoxicity of perhexiline. Arch. Toxicol. 2022, 96, 3219–3231. [Google Scholar] [CrossRef]
- Wang, X.; Fa, J.; Zhang, Y.; Huang, S.; Liu, J.; Gao, J.; Xing, L.; Liu, Z.; Wang, X. Evaluation of Herb-Drug Interaction Between Danshen and Rivaroxaban in Rat and Human Liver Microsomes. Front. Pharmacol. 2022, 13, 950525. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Xu, Y.; Liu, J.; Huang, S.; Li, G.; Yao, B.; Sun, Z.; Wang, X. Investigation of cytochrome P450 inhibitory properties of deoxyshikonin, a bioactive compound from Lithospermum erythrorhizon Sieb. et Zucc. Phytother. Res. 2024, 38, 4855–4864. [Google Scholar] [CrossRef]
- Zheng, D.; Ge, K.; Qu, C.; Sun, T.; Wang, J.; Jia, W.; Zhao, A. Comparative profiling of serum, urine, and feces bile acids in humans, rats, and mice. Commun. Biol. 2024, 7, 641. [Google Scholar] [CrossRef]
- Adachi, K.; Hosoi, M.; Shimura, Y.; Shimizu, M.; Yamazaki, H. Reported liver toxicity of food chemicals in rats extrapolated to humans using virtual human-to-rat hepatic concentration ratios generated by pharmacokinetic modeling with machine learning-derived parameters. J. Toxicol. Sci. 2025, 50, 205–213. [Google Scholar] [CrossRef]
- Asare, E.; Emmanuel, S.; Du, T.; Xie, H.; Liang, D.; Gao, S. Impact of Species and Tissue Differences on In Vitro Glucuronidation of Diclofenac. Molecules 2024, 29, 5867. [Google Scholar] [CrossRef]
- Tian, T.; Song, D.; Zhen, L.; Bi, Z.; Zhang, L.; Huang, H.; Li, Y. Colorimetric-Fluorescence-Photothermal tri-mode sensor array combining the machine learning method for the selective identification of sulfonylurea pesticides. Biosens. Bioelectron. 2025, 277, 117286. [Google Scholar] [CrossRef]
- Sándor, Z.; Katics, D.; Varga, Á.; Kalmár Nagy, K.; Szakály, P. Interpretation of LDH Values after Kidney Transplantation. J. Clin. Med. 2024, 13, 485. [Google Scholar] [CrossRef]
- Stellavato, A.; Lamberti, M.; Pirozzi, A.V.A.; de Novellis, F.; Schiraldi, C. Myclobutanil worsens nonalcoholic fatty liver disease: An in vitro study of toxicity and apoptosis on HepG2 cells. Toxicol. Lett. 2016, 262, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Šimečková, P.; Marvanová, S.; Kulich, P.; Králiková, L.; Neča, J.; Procházková, J.; Machala, M. Screening of Cellular Stress Responses Induced by Ambient Aerosol Ultrafine Particle Fraction PM0.5 in A549 Cells. Int. J. Mol. Sci. 2019, 20, 6310. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.K.; Kong, X.Y.; Zhang, C.; He, C.Q.; Cai, J.L.; Lu, R.Q.; Zhang, B.S.; Lu, L.; Yang, Y.L. Free fatty acids and triglyceride change in the gallbladder bile of gallstone patients with pancreaticobiliary reflux. Lipids Health Dis. 2021, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Liu, Y.; Shang, J.; Xie, Q.; Li, J.; Yan, M.; Xu, J.; Niu, J.; Liu, J.; Watkins, P.B.; et al. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology 2019, 156, 2230–2241.e11. [Google Scholar] [CrossRef]
- Xia, S.; Hu, Z.Y.; Cao, R.; Guo, L.; Ma, J.T.; Xiao, M.X.; Liu, J.Y.; Zhai, B.W.; Fu, R.; Jiang, Z.C.; et al. Magnesium isoglycyrrhizinate prevented the liver injury of acetaminophen by promoting mitochondrial biogenesis. Toxicol. Res. 2025, 14, tfaf024. [Google Scholar] [CrossRef]
- Bunsri, S.; Muenchamnan, N.; Naksen, W.; Ong-Artborirak, P. The Hematological and Biochemical Effects from Pesticide Exposure on Thai Vegetable Farmers. Toxics 2023, 11, 707. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Wu, Y.; Liu, M.; Kannan, K.; Li, A.J.; Robinson, M.; Warady, B.A.; Furth, S.; Trachtman, H.; Trasande, L. Organophosphate pesticides and progression of chronic kidney disease among children: A prospective cohort study. Environ. Int. 2021, 155, 106597. [Google Scholar] [CrossRef]
- Shearer, J.J.; Sandler, D.P.; Andreotti, G.; Murata, K.; Shrestha, S.; Parks, C.G.; Liu, D.; Alavanja, M.C.; Landgren, O.; Beane Freeman, L.E.; et al. Pesticide use and kidney function among farmers in the Biomarkers of Exposure and Effect in Agriculture study. Environ. Res. 2021, 199, 111276. [Google Scholar] [CrossRef]
- Casanova, A.G.; Hinojosa, M.G.; Chamorro-López, C.; Martín-Reina, J.; Aguilera-Velázquez, R.; Bautista, J.D.; Morales, A.I.; Moreno, I.M. Oxidative stress and renal status of farmers exposed to pesticides in Seville (Spain). Sci. Total Environ. 2024, 951, 175180. [Google Scholar] [CrossRef] [PubMed]
- Jetter, A.; Kullak-Ublick, G.A. Drugs and hepatic transporters: A review. Pharmacol. Res. 2020, 154, 104234. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, A.; Bruneau, A.; Mareux, E.; Lapalus, M.; Delaunay, J.L.; Gonzales, E.; Jacquemin, E.; Aït-Slimane, T.; Falguières, T. Molecular Regulation of Canalicular ABC Transporters. Int. J. Mol. Sci. 2021, 22, 2113. [Google Scholar] [CrossRef]
- Liu, J.; Pang, Y.; Li, W.; Sun, J.; He, Y.; Guo, Y.; Dong, J. Impact of hepatic impairment and renal failure on the pharmacokinetics of linezolid and its metabolites: Contribution of hepatic metabolism and renal excretion. Antimicrob. Agents Chemother. 2025, 69, e0189224. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, R.; Wang, S.S.; Liu, H.; Shao, C.; Li, Y.; Link, F.; Munker, S.; Liebe, R.; Meyer, C.; et al. FOXA2 prevents hyperbilirubinaemia in acute liver failure by maintaining apical MRP2 expression. Gut 2023, 72, 549–559. [Google Scholar] [CrossRef]
- Morais, M.B.; Machado, M.V. Benign inheritable disorders of bilirubin metabolism manifested by conjugated hyperbilirubinemia-A narrative review. United Eur. Gastroenterol. J. 2022, 10, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Cai, S.Y.; Liu, X.; Lian, W.; Chen, S.; Zhang, L.; Feng, X.; Cheng, Y.; He, X.; He, Y.; et al. Canalicular membrane MRP2/ABCC2 internalization is determined by Ezrin Thr567 phosphorylation in human obstructive cholestasis. J. Hepatol. 2015, 63, 1440–1448. [Google Scholar] [CrossRef]
- Kast, H.R.; Goodwin, B.; Tarr, P.T.; Jones, S.A.; Anisfeld, A.M.; Stoltz, C.M.; Tontonoz, P.; Kliewer, S.; Willson, T.M.; Edwards, P.A. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 2002, 277, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Li, W.; Zhou, F.; Zhao, Y.; Yu, M.; Tong, L.; Xin, H.; Yu, A. Cyclosporine-A induced cytotoxicity within HepG2 cells by inhibiting PXR mediated CYP3A4/CYP3A5/MRP2 pathway. Drug Chem. Toxicol. 2024, 47, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sun, Y.; Mortimer, M.; Guo, L.H.; Yang, F. Enantiomer-specific burden of metalaxyl and myclobutanil in non-occupationally exposed population with evidence from dietary intake and urinary excretion. Ecotoxicol. Environ. Saf. 2023, 267, 115623. [Google Scholar] [CrossRef]
- Šulc, L.; Janoš, T.; Figueiredo, D.; Ottenbros, I.; Šenk, P.; Mikeš, O.; Huss, A.; Čupr, P. Pesticide exposure among Czech adults and children from the CELSPAC-SPECIMEn cohort: Urinary biomarker levels and associated health risks. Environ. Res. 2022, 214 Pt 3, 114002. [Google Scholar] [CrossRef]
- Huen, K.; Bradman, A.; Harley, K.; Yousefi, P.; Barr, D.B.; Eskenazi, B.; Holland, N. Organophosphate pesticide levels in blood and urine of women and newborns living in an agricultural community. Environ. Res. 2012, 117, 8–16. [Google Scholar] [CrossRef] [PubMed]

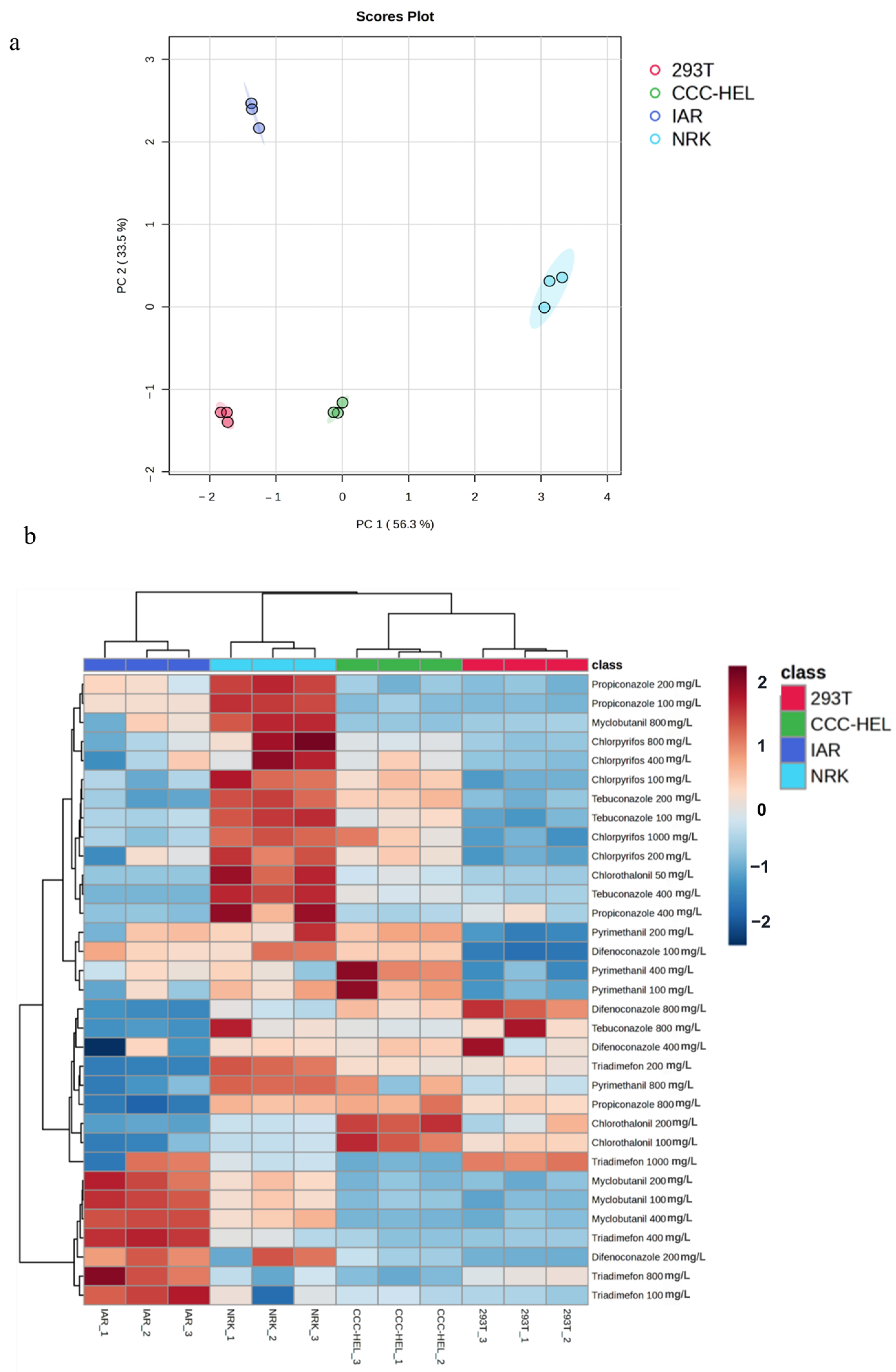
| Pesticide | Purity/% | CCC-HEL-1 IC50 (mg/L)) | IAR IC50 (mg/L) | 293T IC50 (mg/L) | NRK IC50 (mg/L) |
|---|---|---|---|---|---|
| Myclobutanil | 98.0% | 455.2 | 567.0 | 458.5 | 588.0 |
| Triadimefon | 99.1% | 219.2 | 878.0 | 106.6 | 976.8 |
| Propiconazole | 98.3% | 72.6 | 188.9 | 27.2 | 203.1 |
| Difenoconazole | 98.7% | 374.0 | 132.4 | 208.9 | 498.0 |
| Tebuconazole | 99.7% | 134.7 | 188.1 | 41.5 | 171.4 |
| Chlorpyrifos | 98.2% | 632.7 | 897.6 | 444.5 | 989.5 |
| Chlorothalonil | 99.1% | 48.1 | 21.3 | 32.6 | 55.6 |
| Pyrimethanil | 98.3% | 504.8 | 361.7 | 111.3 | 191.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, N.; Song, X.; Deng, M.; Sun, R.; Wang, P.; Cao, L. Specific Hepatorenal Toxicity and Cross-Species Susceptibility of Eight Representative Pesticides. Toxics 2025, 13, 911. https://doi.org/10.3390/toxics13110911
Liu Y, Xu N, Song X, Deng M, Sun R, Wang P, Cao L. Specific Hepatorenal Toxicity and Cross-Species Susceptibility of Eight Representative Pesticides. Toxics. 2025; 13(11):911. https://doi.org/10.3390/toxics13110911
Chicago/Turabian StyleLiu, Yue, Ning Xu, Xinyu Song, Muchen Deng, Ranfeng Sun, Peilong Wang, and Lidong Cao. 2025. "Specific Hepatorenal Toxicity and Cross-Species Susceptibility of Eight Representative Pesticides" Toxics 13, no. 11: 911. https://doi.org/10.3390/toxics13110911
APA StyleLiu, Y., Xu, N., Song, X., Deng, M., Sun, R., Wang, P., & Cao, L. (2025). Specific Hepatorenal Toxicity and Cross-Species Susceptibility of Eight Representative Pesticides. Toxics, 13(11), 911. https://doi.org/10.3390/toxics13110911