Tobacco Smoke Exposure Biomarker Profiles and Healthcare Utilization Patterns Among U.S. Children
Abstract
1. Introduction
2. Materials and Methods
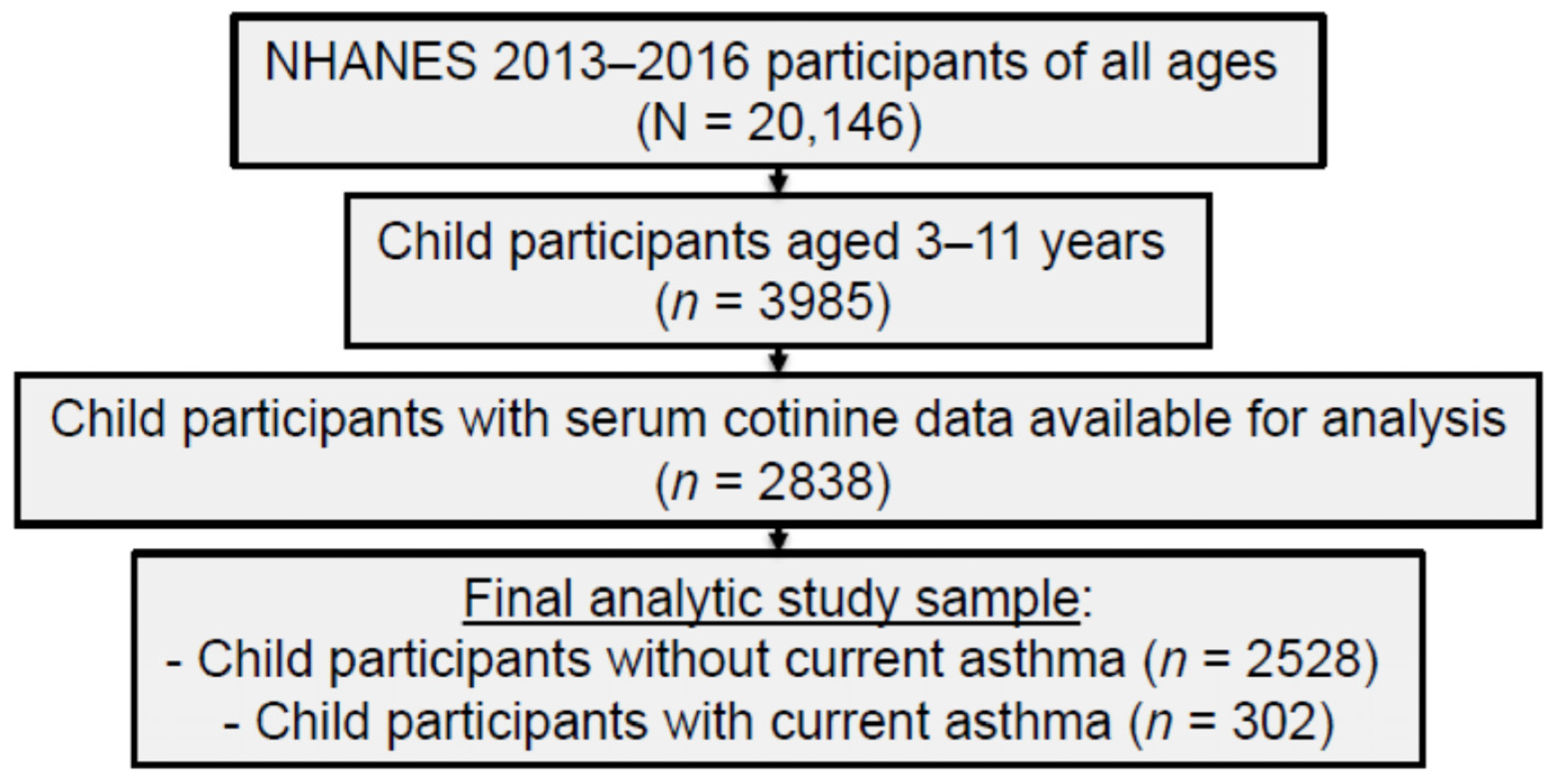
2.1. Study Sample and Procedures
2.2. Measures
2.2.1. TSE Biomarkers
2.2.2. Caregiver-Reported Healthcare Utilization
2.2.3. Current Asthma and Sociodemographic and Home TSE Covariates
2.3. Statistical Analysis
3. Results
3.1. TSE Biomarker Levels in U.S. Children Ages 3–11 Years with and Without Current Asthma
3.2. Child Sociodemographic and Home TSE Characteristics
3.3. Child TSE Biomarker Levels Based on Total Number of Healthcare Visits Within 12 Months
3.4. Child TSE Biomarker Levels Based on Total Number of Overnight Hospital Stays Within 12 Months
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2CyEMA | N-acetyl-S-(2-cyanoethyl)-L-cysteine |
| aIRR | adjusted incidence rate ratio |
| CI | confidence interval |
| GeoM | geometric mean |
| NHANES | National Health and Nutrition Examination Survey |
| NNAL | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol |
| TNE2 | total nicotine equivalents |
| TSE | tobacco smoke exposure |
| TSNA | tobacco-specific nitrosamine |
| VOC | volatile organic compound |
References
- Merianos, A.L.; Jandarov, R.A.; Choi, K.; Mahabee-Gittens, E.M. Tobacco smoke exposure disparities persist in U.S. children: NHANES 1999–2014. Prev. Med. 2019, 123, 138–142. [Google Scholar] [CrossRef]
- Tsai, J.; Homa, D.M.; Gentzke, A.S.; Mahoney, M.; Sharapova, S.R.; Sosnoff, C.S.; Caron, K.T.; Wang, L.; Melstrom, P.C.; Trivers, K.F. Exposure to secondhand smoke among nonsmokers—United States, 1988–2014. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Brody, D.J.; Lu, Z.; Tsai, J. Secondhand smoke exposure among nonsmoking youth: United States, 2013–2016. NCHS Data Brief 2019, 348, 1–8. [Google Scholar]
- Jacob, P.; Benowitz, N.L.; Destaillats, H.; Gundel, L.; Hang, B.; Martins-Green, M.; Matt, G.E.; Quintana, P.; Samet, J.M.; Schick, S.F.; et al. Thirdhand smoke: New evidence, challenges, and future directions. Chem. Res. Toxicol. 2017, 30, 270–294. [Google Scholar] [CrossRef]
- World Health Organization. Summary of Principles for Evaluating Health Risks in Children Associated with Exposure to Chemicals; World Health Organization: Geneva, Switzerland, 2011; Available online: https://www.who.int/publications/i/item/summary-of-principles-for-evaluating-health-risks-in-children-associated-with-exposure-to-chemicals (accessed on 15 October 2025).
- International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco Smoke and Involuntary Smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer Press: Lyon, France, 2004; Volume 83, Available online: https://publications.iarc.who.int/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Tobacco-Smoke-And-Involuntary-Smoking-2004 (accessed on 15 October 2025).
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf (accessed on 15 October 2025).
- Abreo, A.; Gebretsadik, T.; Stone, C.A.; Hartert, T.V. The impact of modifiable risk factor reduction on childhood asthma development. Clin. Transl. Med. 2018, 7, e15. [Google Scholar] [CrossRef]
- Tinuoye, O.; Pell, J.P.; Mackay, D.F. Meta-analysis of the association between secondhand smoke exposure and physician-diagnosed childhood asthma. Nicotine Tob. Res. 2013, 15, 1475–1483. [Google Scholar] [CrossRef]
- Heshmat, R.; Qorbani, M.; Safiri, S.; Eslami-Shahr Babaki, A.; Matin, N.; Motamed-Gorji, N.; Motlagh, M.-E.; Djalalinia, S.; Ardalan, G.; Mansourian, M.; et al. Association of passive and active smoking with self-rated health and life satisfaction in Iranian children and adolescents: The CASPIAN IV study. BMJ Open 2017, 7, e012694. [Google Scholar] [CrossRef]
- Merianos, A.L.; Jandarov, R.A.; Mahabee-Gittens, E.M. Adolescent tobacco smoke exposure, respiratory symptoms, and emergency department use. Pediatrics 2018, 142, e20180266. [Google Scholar] [CrossRef] [PubMed]
- Merianos, A.L.; Jandarov, R.A.; Mahabee-Gittens, E.M. Secondhand smoke exposure and pediatric healthcare visits and hospitalizations. Am. J. Prev. Med. 2017, 53, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Merianos, A.L.; Jandarov, R.A.; Mahabee-Gittens, E.M. High cotinine and healthcare utilization disparities among low-income children. Am. J. Prev. Med. 2021, 60, 267–275. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Bernert, J.T.; Foulds, J.; Hecht, S.S.; Jacob III, P.; Jarvis, M.J.; Joseph, A.; Oncken, C.; Piper, M.E. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob. Res. 2019, 22, 1086–1097. [Google Scholar] [CrossRef]
- Benowitz, N.; Goniewicz, M.L.; Eisner, M.D.; Lazcano-Ponce, E.; Zielinska-Danch, W.; Koszowski, B.; Sobczak, A.; Havel, C.; Jacob III, P. Urine cotinine underestimates exposure to the tobacco-derived lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in passive compared with active smokers. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 2795–2800. [Google Scholar] [CrossRef]
- Lei, X.; Wen, H.; Xu, Z. Relationship between urinary tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and lung function: Evidence from NHANES 2007–2012. Tob. Induc. Dis. 2023, 21, 165. [Google Scholar] [CrossRef]
- Park, E.Y.; Lim, M.K.; Park, E.; Oh, J.-K.; Lee, D.-H. Relationship between urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and lung cancer risk in the general population: A community-based prospective cohort study. Front. Oncol. 2021, 11, 611674. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Tang, H.; Robbins, J.A.; Tong, E.K. Biomarkers of tobacco smoke exposure and asthma severity in adults. Am. J. Prev. Med. 2013, 45, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Tobacco and Volatiles Branch. Laboratory Procedures Manual: Cotinine and Hydroxycotinine—Serum and Saliva; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2015/labmethods/COT_I_MET.pdf (accessed on 15 October 2025).
- Goniewicz, M.L.; Havel, C.M.; Peng, M.W.; Jacob III, P.; Dempsey, D.; Yu, L.; Zielinska-Danch, W.; Koszowski, B.; Czogala, J.; Sobczak, A.; et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 3421–3425. [Google Scholar] [CrossRef]
- Hecht, S.S.; Stepanov, I.; Carmella, S.G. Exposure and metabolic activation biomarkers of carcinogenic tobacco-specific nitrosamines. Acc. Chem. Res. 2016, 49, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Schick, S.F.; Glantz, S. Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in sidestream cigarette smoke increase after release into indoor air: Results from unpublished tobacco industry research. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1547–1553. [Google Scholar] [CrossRef]
- Merianos, A.L.; Jandarov, R.A.; Mahabee-Gittens, E.M. Carcinogenic and tobacco smoke-derived particulate matter biomarker uptake and associated healthcare patterns among children. Pediatr. Res. 2023, 93, 143–153. [Google Scholar] [CrossRef]
- Hecht, S.S.; Ye, M.; Carmella, S.G.; Fredrickson, A.; Adgate, J.L.; Greaves, I.A.; Church, T.R.; Ryan, A.D.; Mongin, S.J.; Sexton, K. Metabolites of a tobacco-specific lung carcinogen in the urine of elementary school-aged children. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1109–1116. Available online: https://www.scopus.com/pages/publications/0035170583 (accessed on 15 October 2025).
- Stepanov, I.; Hecht, S.S.; Duca, G.; Mardari, I. Uptake of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by Moldovan children. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 7–11. [Google Scholar] [CrossRef]
- St Helen, G.; Jacob III, P.; Peng, M.; Dempsey, D.A.; Hammond, S.K.; Benowitz, N.L. Intake of toxic and carcinogenic volatile organic compounds from secondhand smoke in motor vehicles. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2774–2782. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, D.; Zhang, L.; Zhu, W.; De Jesús, V.R.; Blount, B.C. Optimal cutoff concentration of urinary cyanoethyl mercapturic acid for differentiating cigarette smokers from nonsmokers. Nicotine Tob. Res. 2021, 24, 761–767. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Acrylonitrile; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2025.
- International Agency for Research on Cancer. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide. Proceedings of the IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer Press: Lyon, France, 1998. IARC Monogr. Eval. Carcinog. Risks Hum. 1999, 71 Pt 1, 1–315. [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2014. Available online: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2013 (accessed on 15 October 2025).
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey Data; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2016. Available online: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2015 (accessed on 15 October 2025).
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National Health and Nutrition Examination Survey: Sample design, 2011–2014. Vital Health Stat. 2 2014, 2, 1–25. [Google Scholar]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2014 and 2015–2016; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/analyticguidelines/11-16-analytic-guidelines.pdf (accessed on 15 October 2025).
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): MEC Laboratory Procedures Manual; National Center for Health Statistics: Atlanta, GA, USA, 2013. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2013/manuals/2013_MEC_Laboratory_Procedures_Manual.pdf (accessed on 15 October 2025).
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES): MEC Laboratory Procedures Manual; National Center for Health Statistics: Atlanta, GA, USA, 2016. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2015/manuals/2016_MEC_Laboratory_Procedures_Manual.pdf (accessed on 15 October 2025).
- Bernert, J.T.; Harmon, T.L.; Sosnoff, C.S.; McGuffey, J.E. Use of continine immunoassay test strips for preclassifying urine samples from smokers and nonsmokers prior to analysis by LC-MS-MS. J. Anal. Toxicol. 2005, 29, 814–818. [Google Scholar] [CrossRef]
- Wei, B.; Feng, J.; Rehmani, I.J.; Miller, S.; McGuffey, J.E.; Blount, B.C.; Wang, L.A. High-throughput robotic sample preparation system and HPLC-MS/MS for measuring urinary anatabine, anabasine, nicotine and major nicotine metabolites. Clin. Chim. Acta 2014, 436, 290–297. [Google Scholar] [CrossRef] [PubMed]
- National Health and Nutrition Examination Survey. 2013–2014 Data Documentation, Codebook, and Frequencies: Cotinine, Hydroxycotinine, & Other Nicotine Metabolites and Analogs—Urine (UCOT_H); National Center for Health Statistics: Hyattsville, MD, USA, 2019. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2013/DataFiles/UCOT_H.htm (accessed on 15 October 2025).
- National Health and Nutrition Examination Survey. 2015–2016 Data Documentation, Codebook, and Frequencies: Cotinine, Hydroxycotinine, & Other Nicotine Metabolites and Analogs—Urine (UCOT_I); National Center for Health Statistics: Hyattsville, MD, USA, 2019. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2015/DataFiles/UCOT_I.htm (accessed on 15 October 2025).
- Tobacco and Volatiles Branch. Tobacco-Specific Nitrosamines; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2013/labmethods/TSNA_H_MET.pdf (accessed on 15 October 2025).
- Xia, B.; Xia, Y.; Wong, J.; Nicodemus, K.J.; Xu, M.; Lee, J.; Guillot, T.; Li, J. Quantitative analysis of five tobacco-specific N-nitrosamines in urine by liquid chromatography-atmospheric pressure ionization tandem mass spectrometry. Biomed. Chromatogr. 2014, 28, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Tobacco and Volatiles Branch. Volatile Organic Compounds (VOCs) Metabolites; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2013/labmethods/UVOC_H_MET.pdf (accessed on 15 October 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org (accessed on 9 October 2025).
- Merianos, A.L.; Jandarov, R.A.; Gordon, J.S.; Lyons, M.S.; Mahabee-Gittens, E.M. Child tobacco smoke exposure and healthcare resource utilization patterns. Pediatr. Res. 2020, 88, 571–579. [Google Scholar] [CrossRef]
- Merianos, A.L.; Jandarov, R.A.; Mahabee-Gittens, E.M. Tobacco smoke exposure, respiratory health, and health care utilization among US adolescents. Chest 2020, 158, 1975–1983. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. America’s Children and the Environment: Biomonitoring—Cotinine; U.S. Environmental Protection Agency: Washington, DC, USA, 2025. Available online: https://www.epa.gov/americaschildrenenvironment/biomonitoring-cotinine (accessed on 15 October 2025).


| Overall (N = 2838) | No Current Asthma ((n = 2528) | Current Asthma ((n = 302) | ||
|---|---|---|---|---|
| Biomarker Variable | n (Imputed %) a | GeoM (95% CI) b | GeoM (95% CI) b | GeoM (95% CI) b |
| Serum Nicotine Metabolite | ||||
| Serum Cotinine (ng/mL) | 2838 (0.0) | 0.05 (0.04, 0.05) | 0.05 (0.04, 0.05) | 0.08 (0.06, 0.10) |
| Urinary Nicotine Metabolite | ||||
| Urinary TNE2 (nmol/mL) | 919 (68.0) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) | 0.01 (0.01, 0.01) |
| Urinary TSNA | ||||
| NNAL (pg/mL) | 1001 (65.4) | 1.63 (1.54, 1.73) | 1.53 (1.45, 1.62) | 2.32 (1.92, 2.8) |
| NNAL/TNE2 | 301 (89.5) | 169.76 (162.35, 177.51) | 179.46 (170.93, 188.42) | 132.04 (120.11, 145.16) |
| Urinary VOC | ||||
| 2CyEMA/TNE2 | 913 (68.3) | 129.21 (120.9, 138.08) | 131.05 (122.33, 140.40) | 91.64 (74.48, 112.75) |
| Overall (N = 2838) | No Current Asthma (n = 2528) | Current Asthma (n = 302) | |
|---|---|---|---|
| Characteristic | n (%) a | n (%) a | n (%) a |
| Child Age, M (SD) | 7.3 (2.6) | 7.2 (2.5) | 7.7 (2.6) |
| Child Sex | |||
| Male | 1455 (51.0) | 1270 (50.1) | 181 (59.3) |
| Female | 1383 (49.0) | 1258 (49.9) | 121 (40.7) |
| Child Race and/or Ethnicity | |||
| Non-Hispanic White | 717 (48.1) | 648 (48.6) | 68 (43.1) |
| Non-Hispanic Black | 681 (13.7) | 582 (13.1) | 95 (19.3) |
| Non-Hispanic Other/Multiracial | 410 (10.2) | 367 (10.2) | 43 (10.9) |
| Hispanic | 1030 (28.0) | 931 (28.1) | 96 (26.7) |
| Caregiver Education Level | |||
| ≤High school graduate/equivalent | 1300 (40.4) | 1169 (40.7) | 127 (37.0) |
| Some college | 879 (32.0) | 768 (31.3) | 109 (38.5) |
| ≥College graduate | 571 (27.6) | 517 (28.0) | 52 (24.5) |
| FPL | |||
| <185% | 1608 (49.2) | 1427 (48.8) | 178 (52.9) |
| 185–349% | 539 (24.6) | 479 (24.8) | 59 (22.9) |
| ≥350% | 478 (25.7) | 430 (25.9) | 47 (24.2) |
| Unspecified | 9 (0.5) | 9 (0.5) | 0 (0) |
| Child Home TSE Status | |||
| No home TSE | 2087 (75.9) | 1879 (76.5) | 203 (70.9) |
| Home thirdhand smoke exposure only | 450 (16.1) | 382 (15.6) | 65 (19.9) |
| Home secondhand and thirdhand smoke exposure | 261 (8.0) | 230 (7.9) | 31 (9.2) |
| Adjusted GeoM (95% CI) | Total Healthcare Visits | p-Value | Adjusted GeoM (95% CI) | Total Overnight Hospital Stays | p-Value | |
|---|---|---|---|---|---|---|
| aIRR (95% CI) a | aIRR (95% CI) a | |||||
| No Current Asthma | ||||||
| Serum Nicotine Metabolite | ||||||
| Serum Cotinine (ng/mL) | 0.37 (0.34, 0.40) | 0.97 (0.96, 0.99) | <0.001 | 0.33 (0.22, 0.50) | 1.21 (1.07, 1.37) | 0.002 |
| Urinary Nicotine Metabolite | ||||||
| Urinary TNE2 (nmol/mL) | 0.15 (0.13, 0.17) | 1.03 (1.02, 1.04) | <0.001 | 0.23 (0.11, 0.46) | 1.03 (0.96, 1.11) | 0.369 |
| Urinary TSNA | ||||||
| NNAL (pg/mL) | 1.38 (1.25, 1.52) | 1.00 (0.99, 1.01) | 0.916 | 1.13 (0.81, 1.59) | 1.11 (0.95, 1.29) | 0.189 |
| NNAL/TNE2 | 9.51 (8.72, 10.37) | 1.00 (0.99, 1.02) | 0.470 | 8.71 (6.40, 11.84) | 1.16 (0.99, 1.36) | 0.075 |
| Urinary VOC | ||||||
| 2CyEMA/TNE2 | 7.41 (6.58, 8.34) | 1.01 (1.00, 1.02) | 0.064 | 5.46 (3.20, 9.30) | 1.25 (1.14,1.37) | <0.001 |
| Current Asthma | ||||||
| Serum Nicotine Metabolite | ||||||
| Serum Cotinine (ng/mL) | 0.43 (0.39, 0.48) | 1.00 (0.97, 1.03) | 0.897 | - | 0.88 (0.71, 1.10) | 0.277 |
| Urinary Nicotine Metabolite | ||||||
| Urinary TNE2 (nmol/mL) | 0.14 (0.12, 0.16) | 1.02 (1.00, 1.04) | 0.050 | - | 0.91 (0.77, 1.08) | 0.279 |
| Urinary TSNA | ||||||
| NNAL (pg/mL) | 1.63 (1.41, 1.88) | 0.93 (0.90, 0.95) | <0.001 | - | 0.98 (0.80, 1.20) | 0.845 |
| NNAL/TNE2 | 8.23 (7.63, 8.87) | 1.04 (1.00, 1.09) | 0.069 | - | 1.52 (1.11, 2.09) | 0.009 |
| Urinary VOC | ||||||
| 2CyEMA/TNE2 | 6.64 (5.66, 7.79) | 1.05 (1.03, 1.07) | <0.001 | - | 1.08 (0.90, 1.29) | 0.408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merianos, A.L.; Matt, G.E.; Jandarov, R.A.; Mahabee-Gittens, E.M. Tobacco Smoke Exposure Biomarker Profiles and Healthcare Utilization Patterns Among U.S. Children. Toxics 2025, 13, 909. https://doi.org/10.3390/toxics13110909
Merianos AL, Matt GE, Jandarov RA, Mahabee-Gittens EM. Tobacco Smoke Exposure Biomarker Profiles and Healthcare Utilization Patterns Among U.S. Children. Toxics. 2025; 13(11):909. https://doi.org/10.3390/toxics13110909
Chicago/Turabian StyleMerianos, Ashley L., Georg E. Matt, Roman A. Jandarov, and E. Melinda Mahabee-Gittens. 2025. "Tobacco Smoke Exposure Biomarker Profiles and Healthcare Utilization Patterns Among U.S. Children" Toxics 13, no. 11: 909. https://doi.org/10.3390/toxics13110909
APA StyleMerianos, A. L., Matt, G. E., Jandarov, R. A., & Mahabee-Gittens, E. M. (2025). Tobacco Smoke Exposure Biomarker Profiles and Healthcare Utilization Patterns Among U.S. Children. Toxics, 13(11), 909. https://doi.org/10.3390/toxics13110909







