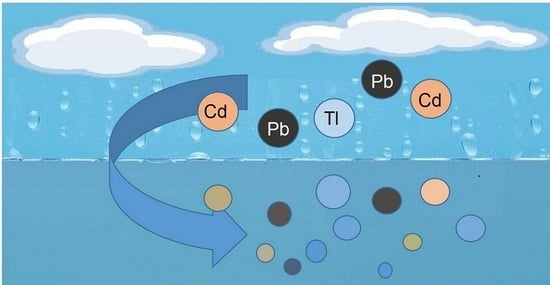
Dissolution of Microparticles of Cadmium, Lead and Thallium in Water
Abstract
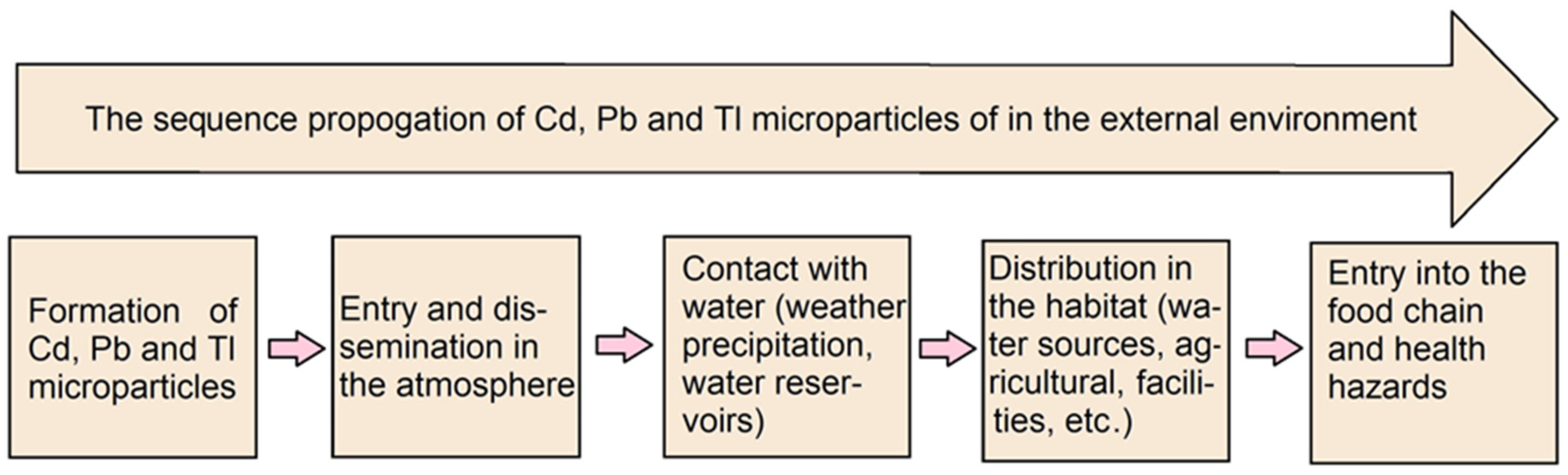
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Metal Dispersions
2.3. Study of Characteristics of Metal Particles
3. Results
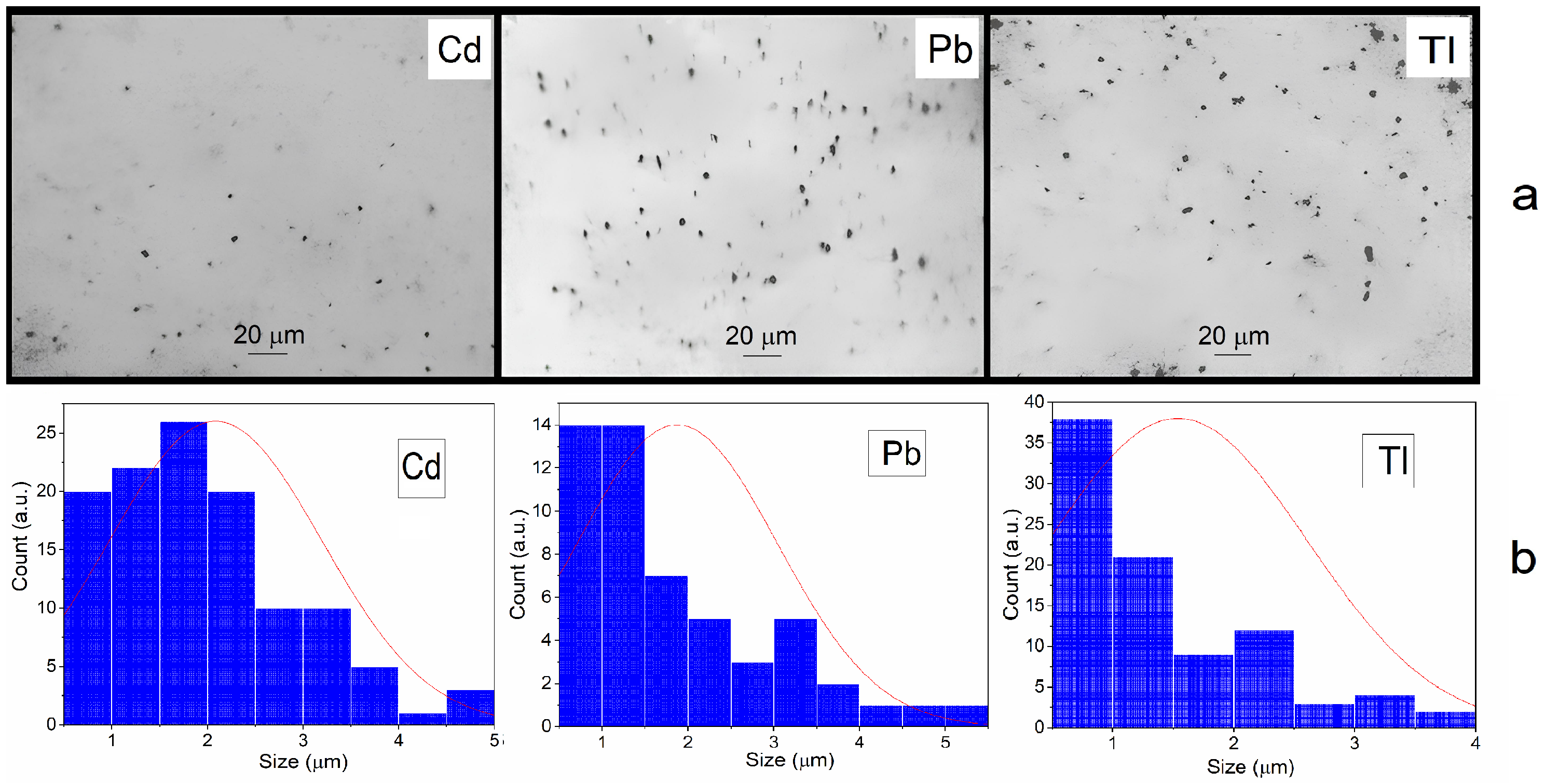
3.1. Formation and Characteristics of the Metal Dispersions in Water
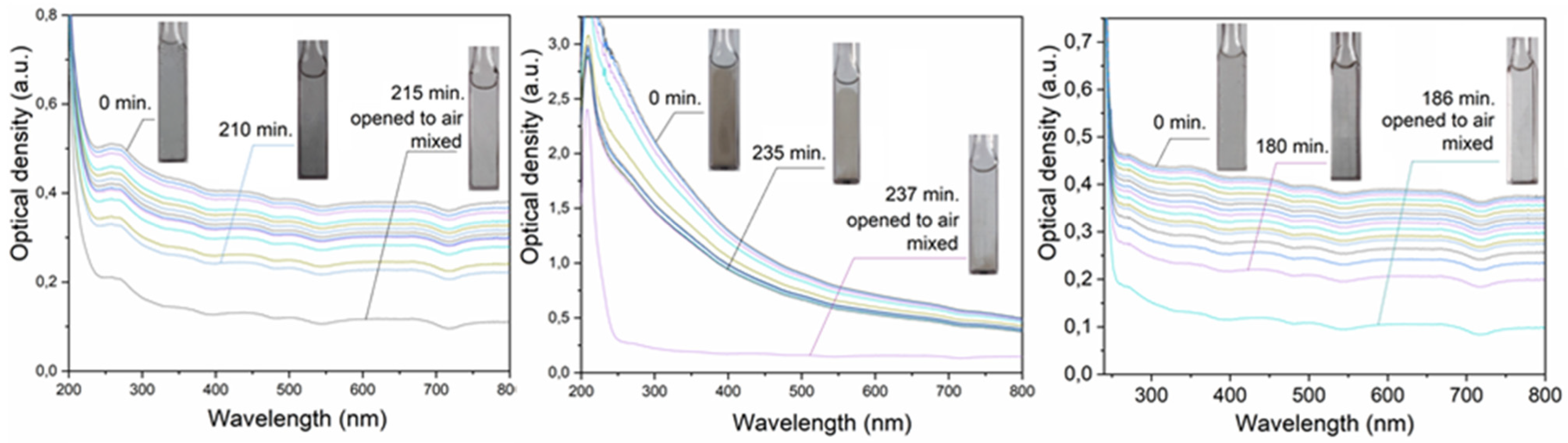
3.2. Oxidative Dissolution of the Metal Microparticles in Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cha, Z.; Zhang, X.; Zhang, K.; Zhou, G.; Gao, J.; Sun, S.; Gao, Y.; Liu, H. Atmospheric Heavy Metal Pollution Characteristics and Health Risk Assessment Across Various Type of Cities in China. Toxics 2025, 13, 220. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Popoola, L.T.; Adebanjo, S.A.; Adeoye, B.K. Assessment of atmospheric particulate matter and heavy metals: A critical review. Int. J. Environ. Sci. Technol. 2018, 15, 935–948. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Yu, Y.; Zhang, M.; Kim, K. Concentration, Source, and Total Health Risks of Cadmium in Multiple Media in Densely Populated Areas, China. Int. J. Environ. Res. Public Health 2019, 16, 2269. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.R.; Harrison, R.M. Cadmium in the atmosphere. Experientia 1984, 40, 29–36. [Google Scholar] [CrossRef]
- Sa’adeh, H.; Chiari, M.; Pollastri, S.; Aquilanti, G. Quantification and speciation of lead in air particulate matter collected from an urban area in Amman, Jordan. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2023, 539, 108–112. [Google Scholar] [CrossRef]
- Pakkanen, T.A.; Kerminen, V.-M.; Korhonen, C.H.; Hillamo, R.E.; Aarnio, P.; Koskentalo, T.; Maenhaut, W. Urban and rural ultrafine (PM0.1) particles in the Helsinki area. Atmos. Environ. 2001, 35, 4593–4607. [Google Scholar] [CrossRef]
- Convention on Long-Range Transboundary Air Pollution; United Nations Economic Commission for Europe: Geneva, Switzerland, 1979; Available online: https://unece.org/environmental-policy/air/convention-and-its-achievements (accessed on 16 October 2025).
- Bykov, G.L.; Bludenko, A.V.; Ponomarev, A.V.; Ershov, B.G. Dynamics of metal formation upon radiation-chemical reduction of Cd2+ ions in aqueous solutions: Morphology of cadmium precipitate. Radiat. Phys. Chem. 2024, 225, 112115. [Google Scholar] [CrossRef]
- Bykov, G.L.; Ershov, B.G. Effect of Oxygen and Solution Composition on the Oxidative Dissolution of Cadmium Microparticles in Water. ACS ES&T Water 2024, 4, 5669–5677. [Google Scholar] [CrossRef]
- Spinks, J.W.T.; Woods, R.J. An Introduction to Radiation Chemistry; Wiley-Interscience: Hoboken, HJ, USA, 1990. [Google Scholar]
- Schwarz, H.A.; Dodson, R.W. Reduction potentials of CO2− and the alcohol radicals. J. Phys. Chem. 1989, 93, 409–414. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- Ershov, B.G.; Bykov, G.L. Formation of porous metals upon radiation chemical reduction of Cd2+ and Pb2+ ions in aqueous solution in the presence of formate ions. Radiat. Phys. Chem. 2022, 201, 110460. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: Hoboken, HJ, USA, 1998. [Google Scholar] [CrossRef]
- Henglein, A. Chemisorption Effects on Colloidal Lead Nanoparticles. J. Phys. Chem. B 1999, 103, 9302–9305. [Google Scholar] [CrossRef]
- Guha, A. Transport and Deposition of Particles in Turbulent and Laminar Flow. Annu. Rev. Fluid Mech. 2008, 40, 311–341. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bykov, G.L.; Ershov, B.G. Dissolution of Microparticles of Cadmium, Lead and Thallium in Water. Toxics 2025, 13, 904. https://doi.org/10.3390/toxics13110904
Bykov GL, Ershov BG. Dissolution of Microparticles of Cadmium, Lead and Thallium in Water. Toxics. 2025; 13(11):904. https://doi.org/10.3390/toxics13110904
Chicago/Turabian StyleBykov, Gennadii L., and Boris G. Ershov. 2025. "Dissolution of Microparticles of Cadmium, Lead and Thallium in Water" Toxics 13, no. 11: 904. https://doi.org/10.3390/toxics13110904
APA StyleBykov, G. L., & Ershov, B. G. (2025). Dissolution of Microparticles of Cadmium, Lead and Thallium in Water. Toxics, 13(11), 904. https://doi.org/10.3390/toxics13110904