Concentration-Dependent Effects of Polyethylene Microplastics on Cadmium and Lead Bioavailability in Soil
Abstract
1. Introduction
2. Material and Methods
2.1. PE MPs and Soils
2.2. Soil Incubation Experiment
2.3. Soil Physicochemical Analysis
2.4. Soil DNA Extraction and 16S rRNA Illumina Sequencing
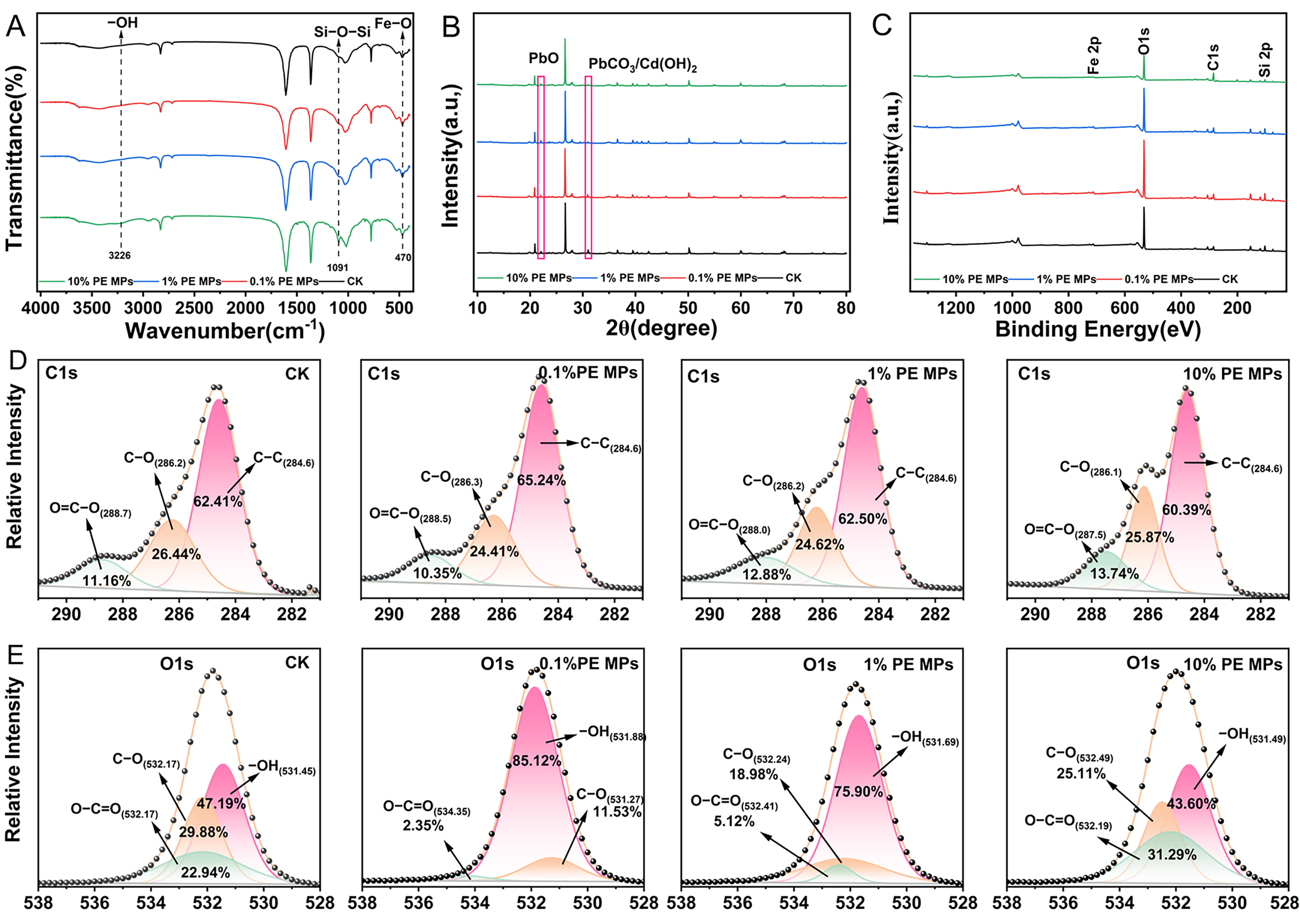
2.5. Characterization of Soil Components
2.6. Statistical Analysis
3. Results
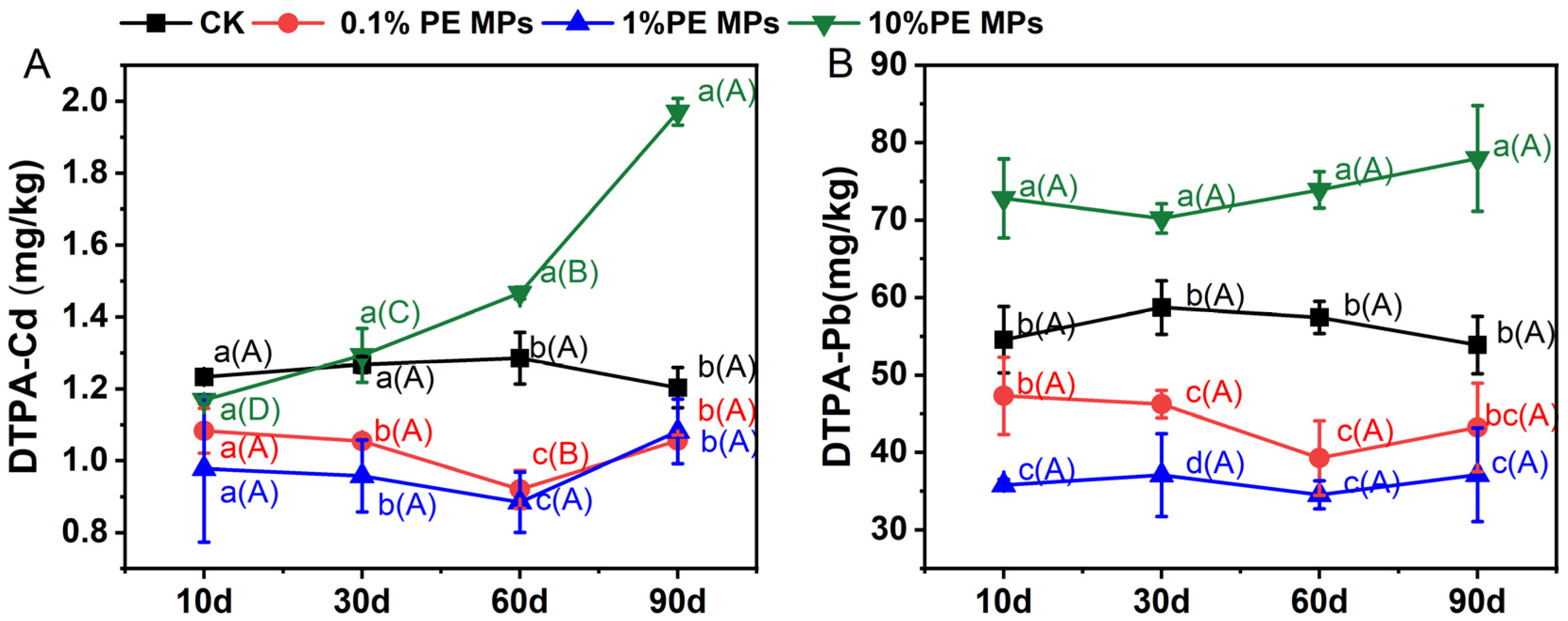
3.1. Effect of PE MPs on Available Cd and Pb
3.2. Effect of PE MPs on the Chemical Speciation of Cd and Pb
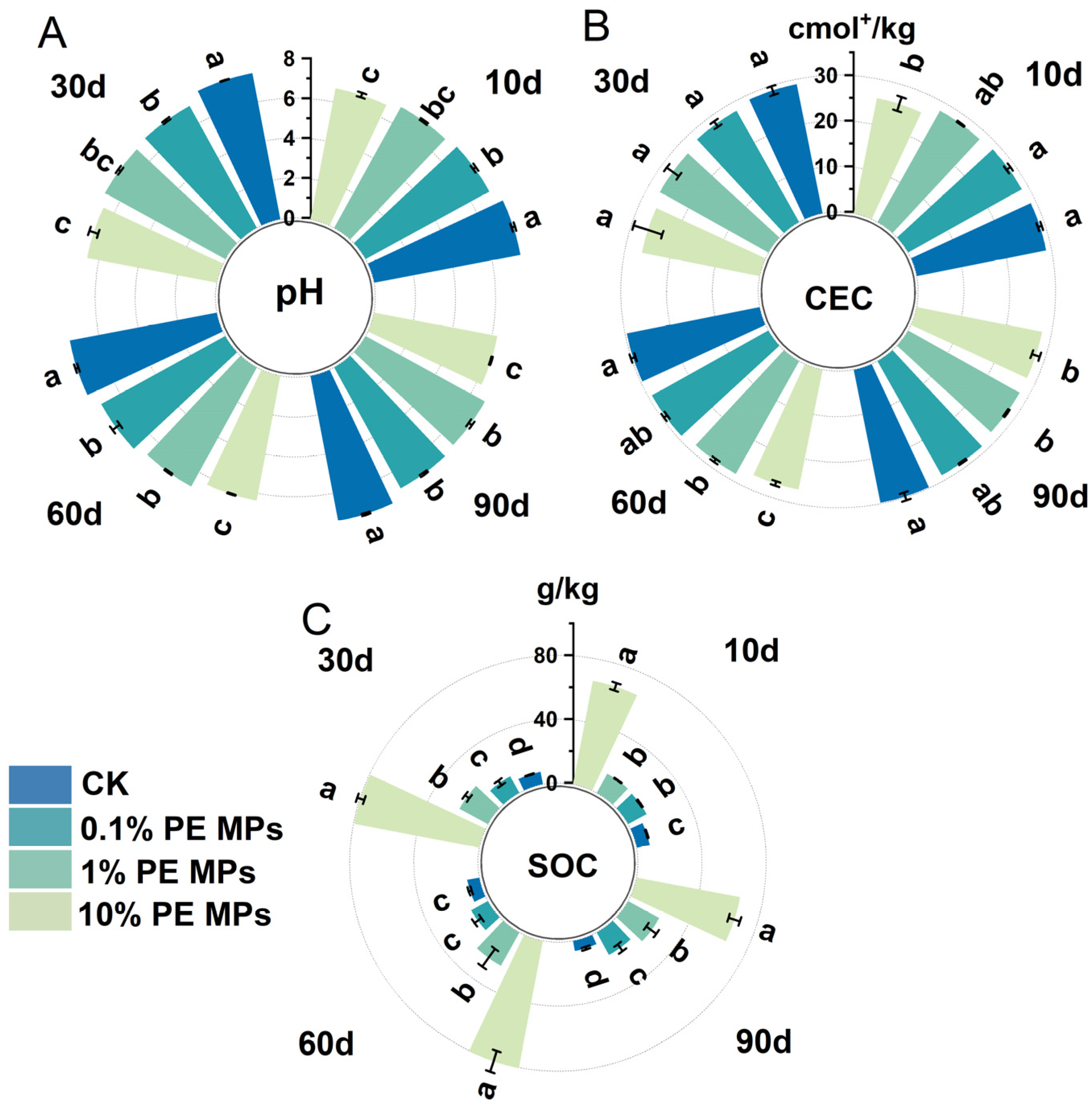
3.3. Effects of PE MPs on Soil Physicochemical Properties
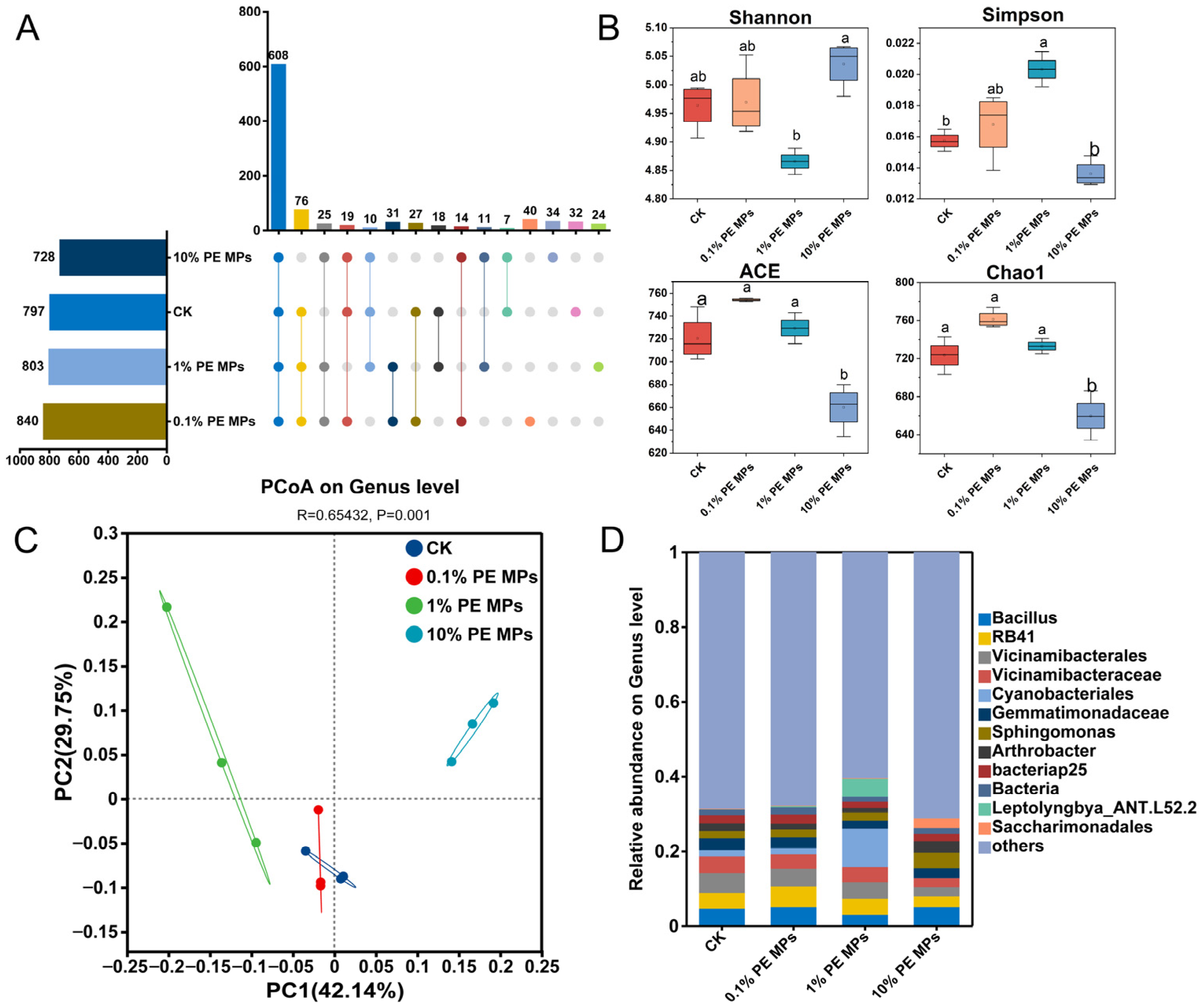
3.4. Effects of PE MPs on the Diversity and Structure of Soil Bacterial Community
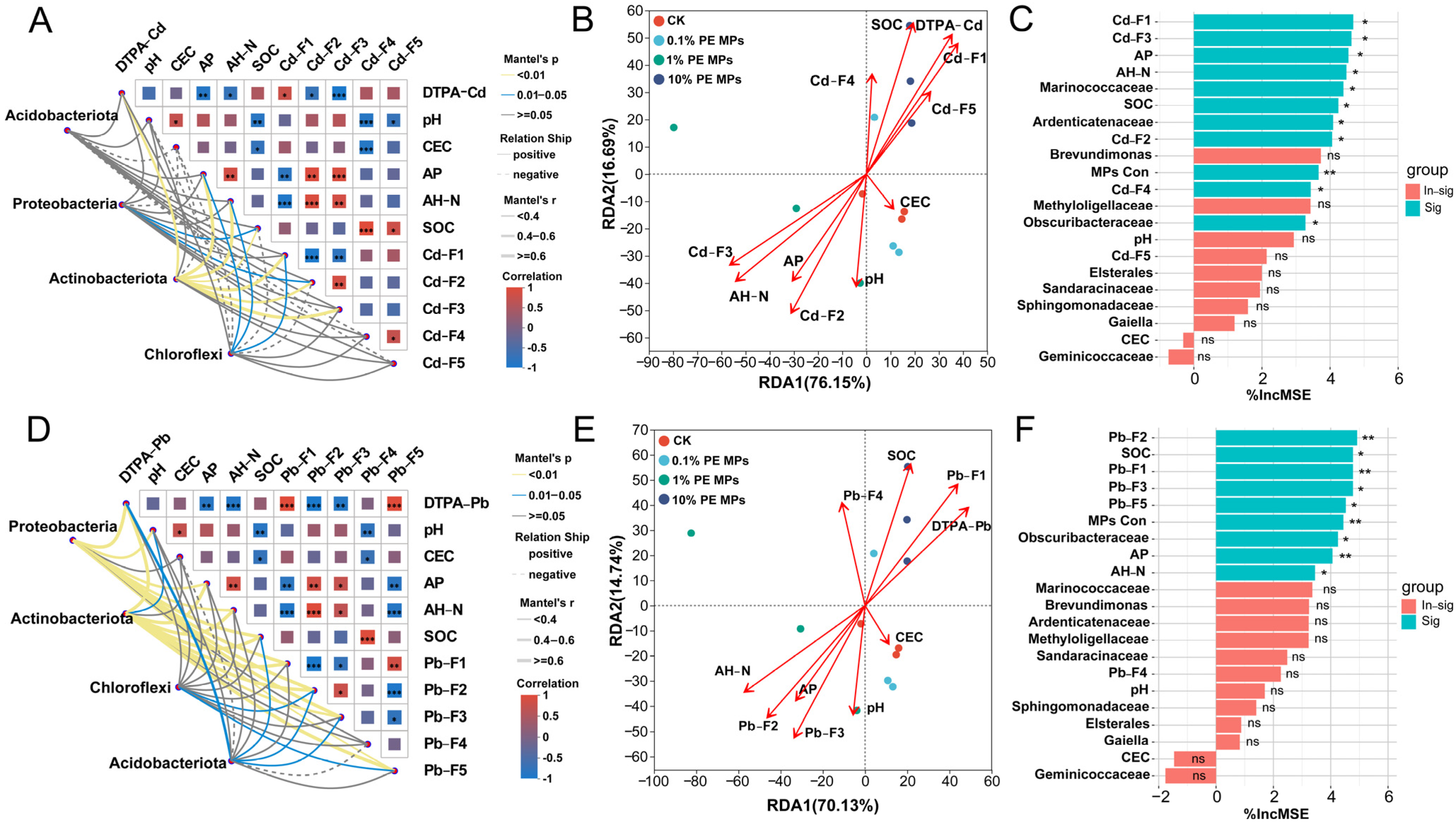
3.5. Correlation Analysis in Soil Physicochemical Properties and Key Bacterial
3.6. Characterization of Soil
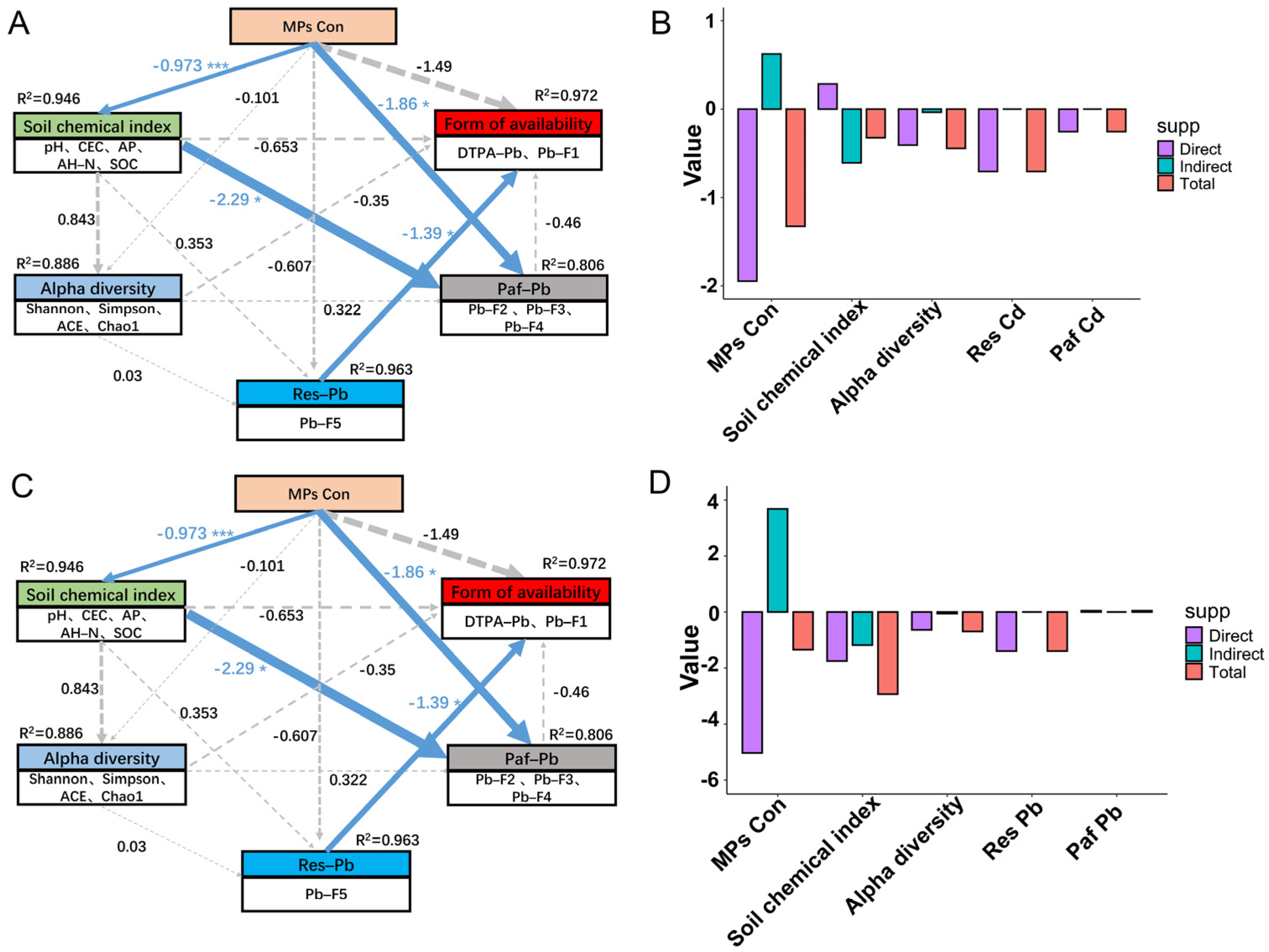
3.7. Analysis of Partial Least Squares Path Modeling
4. Discussion
4.1. PE MPs Altered Properties of the Soil
4.2. Soil Bacterial Community Responses to Combined Exposure of PE MPs and Cd/Pb
4.3. Effects of PE MPs on the Bioavailability and Distribution of Cd and Pb
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, C.; Fang, L.; Yang, C.; Chen, W.; Cui, Y.; Li, S. Reveal the response of enzyme activities to heavy metals through in situ zymography. Ecotoxicol. Environ. Saf. 2018, 156, 106–115. [Google Scholar] [CrossRef]
- Yu, Y.; Rong, K.; Sui, X.; Zhang, L.; Zhang, M.; Hu, H.; Jia, J.; Wu, J.; Li, C. Analysis of NRAMP genes in the Triticeae reveals that TaNRAMP5 positively regulates cadmium (Cd) tolerance in wheat (Triticum aestivum). Plant Physiol. Biochem. 2025, 219, 109321. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Wang, Y.; Wang, J.; Liu, H.; Gao, W.; Qin, S.; Sui, F.; Fu, H.; Zhao, P. Supplementing two wheat genotypes with ZnSO4 and ZnO nanoparticles showed differential mitigation of Cd phytotoxicity by reducing Cd absorption, preserving root cellular ultrastructure, and regulating metal-transporter gene expression. Plant Physiol. Biochem. 2024, 206, 108199. [Google Scholar] [CrossRef] [PubMed]
- Dawar, K.; Mian, I.A.; Khan, S.; Zaman, A.; Danish, S.; Liu, K.; Harrison, M.T.; Saud, S.; Hassan, S.; Nawaz, T.; et al. Alleviation of cadmium toxicity and fortification of zinc in wheat cultivars cultivated in Cd contaminated soil. S. Afr. J. Bot. 2023, 162, 611–621. [Google Scholar] [CrossRef]
- Wu, B.; Liu, Z.; Xu, Y.; Li, D.; Li, M. Combined toxicity of cadmium and lead on the earthworm Eisenia fetida (Annelida, Oligochaeta). Ecotoxicol. Environ. Saf. 2012, 81, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, L. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Qiang, L.; Hu, H.; Li, G.; Xu, J.; Cheng, J.; Wang, J.; Zhang, R. Plastic mulching, and occurrence, incorporation, degradation, and impacts of polyethylene microplastics in agroecosystems. Ecotoxicol. Environ. Saf. 2023, 263, 115274. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Z.; Chen, L.; Cui, Q.; Cui, Y.; Song, D.; Fang, L. Review on migration, transformation and ecological impacts of microplastics in soil. Appl. Soil Ecol. 2022, 176, 104486. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, K.; Dekker, S.C.; Mao, J. Microplastics in soils: A comprehensive review. Sci. Total Environ. 2025, 960, 178298. [Google Scholar] [CrossRef]
- Liu, X.; Shao, J.; Peng, C.; Gong, J. Novel insights related to soil microplastic abundance and vegetable microplastic contamination. J. Hazard. Mater. 2025, 484, 136727. [Google Scholar] [CrossRef]
- An, Q.; Zhou, T.; Wen, C.; Yan, C. The effects of microplastics on heavy metals bioavailability in soils: A meta-analysis. J. Hazard. Mater. 2023, 460, 132369. [Google Scholar] [CrossRef] [PubMed]
- Sungur, A.; Soylak, M.; Ozcan, H. Investigation of heavy metal mobility and availability by the BCR sequential extraction procedure: Relationship between soil properties and heavy metals availability. Chem. Spec. Bioavailab. 2014, 26, 219–230. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Adams, C.A.; Sun, Y. Effects of Co-Contamination of Microplastics and Cd on Plant Growth and Cd Accumulation. Toxics 2020, 8, 36. [Google Scholar] [CrossRef]
- Halim, M.; Conte, P.; Piccolo, A. Potential availability of heavy metals to phytoextraction from contaminated soils induced by exogenous humic substances. Chemosphere 2003, 52, 265–275. [Google Scholar] [CrossRef]
- Semenov, V.M.; Tulina, A.S.; Semenova, N.A.; Ivannikova, L.A. Humification and Nonhumification Pathways of the Organic Matter Stabilization in Soil: A Review. Eurasian Soil Sci. 2013, 46, 355–368. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Diao, T.; Yan, L.; Sun, Y. Effects of single and combined contamination of microplastics and cadmium on soil organic carbon and microbial community structural: A comparison with different types of soil. Appl. Soil Ecol. 2023, 183, 104763. [Google Scholar] [CrossRef]
- Zeb, A.; Liu, W.; Meng, L.; Lian, J.; Wang, Q.; Lian, Y.; Chen, C.; Wu, J. Effects of polyester microfibers (PMFs) and cadmium on lettuce (Lactuca sativa) and the rhizospheric microbial communities: A study involving physio-biochemical properties and metabolomic profiles. J. Hazard. Mater. 2022, 424, 127405. [Google Scholar] [CrossRef]
- Tang, Y.; Xing, Y.; Wang, X.; Ya, H.; Zhang, T.; Lv, M.; Wang, J.; Zhang, H.; Dai, W.; Zhang, D.; et al. PET microplastics influenced microbial community and heavy metal speciation in heavy-metal contaminated soils. Appl. Soil Ecol. 2024, 201, 105488. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Wang, Q.; Liu, N.; Li, M. Seasonal variations and feedback from microplastics and cadmium on soil organisms in agricultural fields. Environ. Int. 2022, 161, 107096. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, H.; Shi, Y.; Zhao, Z.; Zhang, Z.; Zhao, P.; Gao, Q.; Zhang, X.; Chen, B.; Li, Y.; et al. Polyethylene and poly (butyleneadipate-co-terephthalate)-based biodegradable microplastics modulate the bioavailability and speciation of Cd and As in soil: Insights into transformation mechanisms. J. Hazard. Mater. 2023, 445, 130638. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.B.; Pereao, O.; Sparks, C.; Opeolu, B. Assessing microplastic characteristics and abundance in the sediment and surface water of the Diep River, Western Cape, South Africa. Environ. Pollut. 2025, 381, 126555. [Google Scholar] [CrossRef]
- Li, Q.; Yan, J.; Li, Y.; Liu, Y.; Andom, O.; Li, Z. Microplastics alter cadmium accumulation in different soil-plant systems: Revealing the crucial roles of soil bacteria and metabolism. J. Hazard. Mater. 2024, 474, 134768. [Google Scholar] [CrossRef]
- Niu, R.; Zhu, C.; Jiang, G.; Yang, J.; Zhu, X.; Li, L.; Shen, F.; Jie, X.; Liu, S. Variations in Soil Nitrogen Availability and Crop Yields under a Three-Year Annual Wheat and Maize Rotation in a Fluvo-Aquic Soil. Plants 2023, 12, 808. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, L.; Qin, Q.; Sun, Y.; Yang, S.; Wang, J.; Yang, Y.; Gao, G.; Xue, Y. The Adsorption Process and Mechanism of Benzo[a]pyrene in Agricultural Soil Mediated by Microplastics. Toxics 2024, 12, 922. [Google Scholar] [CrossRef]
- Guo, J.-J.; Li, F.; Xiao, H.-C.; Liu, B.-L.; Feng, L.-N.; Yu, P.-F.; Meng, C.; Zhao, H.-M.; Feng, N.-X.; Li, Y.-W.; et al. Polyethylene and polypropylene microplastics reduce chemisorption of cadmium in paddy soil and increase its bioaccessibility and bioavailability. J. Hazard. Mater. 2023, 449, 130994. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, X.; Xu, J.; Selim, H.M. Heavy metal contaminations in a soil-rice system: Identification of spatial dependence in relation to soil properties of paddy fields. J. Hazard. Mater. 2010, 181, 778–787. [Google Scholar] [CrossRef]
- Bandow, N.; Will, V.; Wachtendorf, V.; Simon, F.-G. Contaminant release from aged microplastic. Environ. Chem. 2017, 14, 394–405. [Google Scholar] [CrossRef]
- Binda, G.; Kalcikova, G.; Allan, I.J.; Hurley, R.; Rodland, E.; Spanu, D.; Nizzetto, L. Microplastic aging processes: Environmental relevance and analytical implications. TrAC Trends Anal. Chem. 2024, 172, 117566. [Google Scholar] [CrossRef]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef]
- Peng, H.; Lin, Z.; Lu, D.; Yu, B.; Li, H.; Yao, J. How do polystyrene microplastics affect the adsorption of copper in soil? Sci. Total Environ. 2024, 924, 171545. [Google Scholar] [CrossRef]
- Zhai, Y.; Bai, J.; Chang, P.; Liu, Z.; Wang, Y.; Liu, G.; Cui, B.; Peijnenburg, W.; Vijver, M.G. Microplastics in terrestrial ecosystem: Exploring the menace to the soil-plant-microbe interactions. TrAC Trends Anal. Chem. 2024, 174, 117667. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Zhang, C.; Li, S.; Li, J. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. J. Environ. Manag. 2022, 302, 113995. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, S.; Zhang, C.; Zhou, Y.; Qin, W. Soil microplastic characteristics and the effects on soil properties and biota: A systematic review and meta-analysis. Environ. Pollut. 2022, 313, 120183. [Google Scholar] [CrossRef]
- Wu, C.; Ma, Y.; Wang, D.; Shan, Y.; Song, X.; Hu, H.; Ren, X.; Ma, X.; Cui, J.; Ma, Y. Integrated microbiology and metabolomics analysis reveal plastic mulch film residue affects soil microorganisms and their metabolic functions. J. Hazard. Mater. 2022, 423, 127258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.; Ru, J.; Zhao, J.; Xiao, S. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci. Total Environ. 2018, 637, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Diao, C.; Cui, Z.; Li, Z.; Zhao, J.; Zhang, H.; Hu, M.; Xu, J.; Jiang, Y.; Haider, G.; et al. Unravelling the influence of microplastics with/without additives on radish (Raphanus sativus) and microbiota in two agricultural soils differing in pH. J. Hazard. Mater. 2024, 478, 135535. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Withana, P.A.; Jeong, Y.; Sang, M.K.; Cho, Y.; Hwang, G.; Chang, S.X.; Ok, Y.S. Contrasting effects of food waste and its biochar on soil properties and lettuce growth in a microplastic-contaminated soil. Appl. Biol. Chem. 2024, 67, 3. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shi, L.; Sarkar, B.; Parikh, S.J.; Sang, M.K.; Lee, S.-R.; Ok, Y.S. Effect of LDPE microplastics on chemical properties and microbial communities in soil. Soil Use Manag. 2022, 38, 1481–1492. [Google Scholar] [CrossRef]
- Xiang, Y.; Lan, J.; Dong, Y.; Zhou, M.; Hou, H.; Huang, B.-T. Pollution control performance of solidified nickel-cobalt tailings on site: Bioavailability of heavy metals and microbial response. J. Hazard. Mater. 2024, 471, 134295. [Google Scholar] [CrossRef]
- Sun, T.; Li, G.; Mazarji, M.; Delaplace, P.; Yang, X.; Zhang, J.; Pan, J. Heavy metals drive microbial community assembly process in farmland with long-term biosolids application. J. Hazard. Mater. 2024, 468, 133845. [Google Scholar] [CrossRef]
- Yan, C.; Wang, F.; Geng, H.; Liu, H.; Pu, S.; Tian, Z.; Chen, H.; Zhou, B.; Yuan, R.; Yao, J. Integrating high-throughput sequencing and metagenome analysis to reveal the characteristic and resistance mechanism of microbial community in metal contaminated sediments. Sci. Total Environ. 2020, 707, 136116. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, A.; Chen, X.; Cheng, X.; Lai, Y.; Li, C.; Ji, Q.; Ji, Q.; Kong, J.; Ding, Y.; et al. Insight into the adsorption behaviors and bioaccessibility of three altered microplastics through three types of advanced oxidation processes. Sci. Total Environ. 2024, 917, 170420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Z.; Zhang, Y.; Fan, P.; Xi, B.; Tan, W. Metal type and aggregate microenvironment govern the response sequence of speciation transformation of different heavy metals to microplastics in soil. Sci. Total Environ. 2021, 752, 141956. [Google Scholar] [CrossRef]
- Yu, H.; Hou, J.; Dang, Q.; Cui, D.; Xi, B.; Tan, W. Decrease in bioavailability of soil heavy metals caused by the presence of microplastics varies across aggregate levels. J. Hazard. Mater. 2020, 395, 122690. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Shen, B.; Wang, X.; Zhang, Y.; Zhang, M.; Wang, K.; Xie, P.; Ji, H. The optimum pH and Eh for simultaneously minimizing bioavailable cadmium and arsenic contents in soils under the organic fertilizer application. Sci. Total Environ. 2020, 711, 135229. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Jones, D.L.; Tang, Y.; Gao, F.; Li, J.; Chi, Y.; Yan, C. Regulatory path for soil microbial communities depends on the type and dose of microplastics. J. Hazard. Mater. 2024, 473, 134702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Peng, C.; Li, H.; Zhang, P.; Liu, X. The impact of microplastic-microbe interactions on animal health and biogeochemical cycles: A mini-review. Sci. Total Environ. 2021, 773, 145697. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, R.; Wang, X.; Zhang, J.; Wang, J.; Cao, B.; Zhao, Y.; Xu, L.; Chen, Y.; Zou, G. How do controlled-release fertilizer coated microplastics dynamically affect Cd availability by regulating Fe species and DOC content in soil? Sci. Total Environ. 2022, 850, 157886. [Google Scholar] [CrossRef]
- Manceau, A.; Charlet, L.; Boisset, M.; Didier, B.; Spadini, L. Sorption and speciation of heavy metals on hydrous Fe and Mn oxides. From microscopic to macroscopic. Appl. Clay Sci. 1992, 7, 201–223. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Z.; Yan, B.; Zhao, L.; Chen, T.; Yang, X. Effects of active silicon amendment on Pb(II)/Cd(II) adsorption: Performance evaluation and mechanism. J. Hazard. Mater. 2024, 478, 135614. [Google Scholar] [CrossRef]
- Guo, X.; Du, B.; Wei, Q.; Yang, J.; Hu, L.; Yan, L.; Xu, W. Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J. Hazard. Mater. 2014, 278, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, K.; Dötterl, S.; Xu, J.; Garland, G.; Liu, X. The critical role of organic matter for cadmium-lead interactions in soil: Mechanisms and risks. J. Hazard. Mater. 2024, 476, 135123. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, S.; Zhao, P.; Li, G.; Duan, R.; Li, C.; Fu, H. Concentration-Dependent Effects of Polyethylene Microplastics on Cadmium and Lead Bioavailability in Soil. Toxics 2025, 13, 901. https://doi.org/10.3390/toxics13100901
Wang Z, Liu S, Zhao P, Li G, Duan R, Li C, Fu H. Concentration-Dependent Effects of Polyethylene Microplastics on Cadmium and Lead Bioavailability in Soil. Toxics. 2025; 13(10):901. https://doi.org/10.3390/toxics13100901
Chicago/Turabian StyleWang, Zhenbo, Sihan Liu, Peng Zhao, Guangxin Li, Ran Duan, Chang Li, and Haichao Fu. 2025. "Concentration-Dependent Effects of Polyethylene Microplastics on Cadmium and Lead Bioavailability in Soil" Toxics 13, no. 10: 901. https://doi.org/10.3390/toxics13100901
APA StyleWang, Z., Liu, S., Zhao, P., Li, G., Duan, R., Li, C., & Fu, H. (2025). Concentration-Dependent Effects of Polyethylene Microplastics on Cadmium and Lead Bioavailability in Soil. Toxics, 13(10), 901. https://doi.org/10.3390/toxics13100901







