Perinatal Exposure to Heavy Metals and Trace Elements of Preterm Neonates in the NICU: A Toxicological Study Using Multiple Biomatrices
Highlights
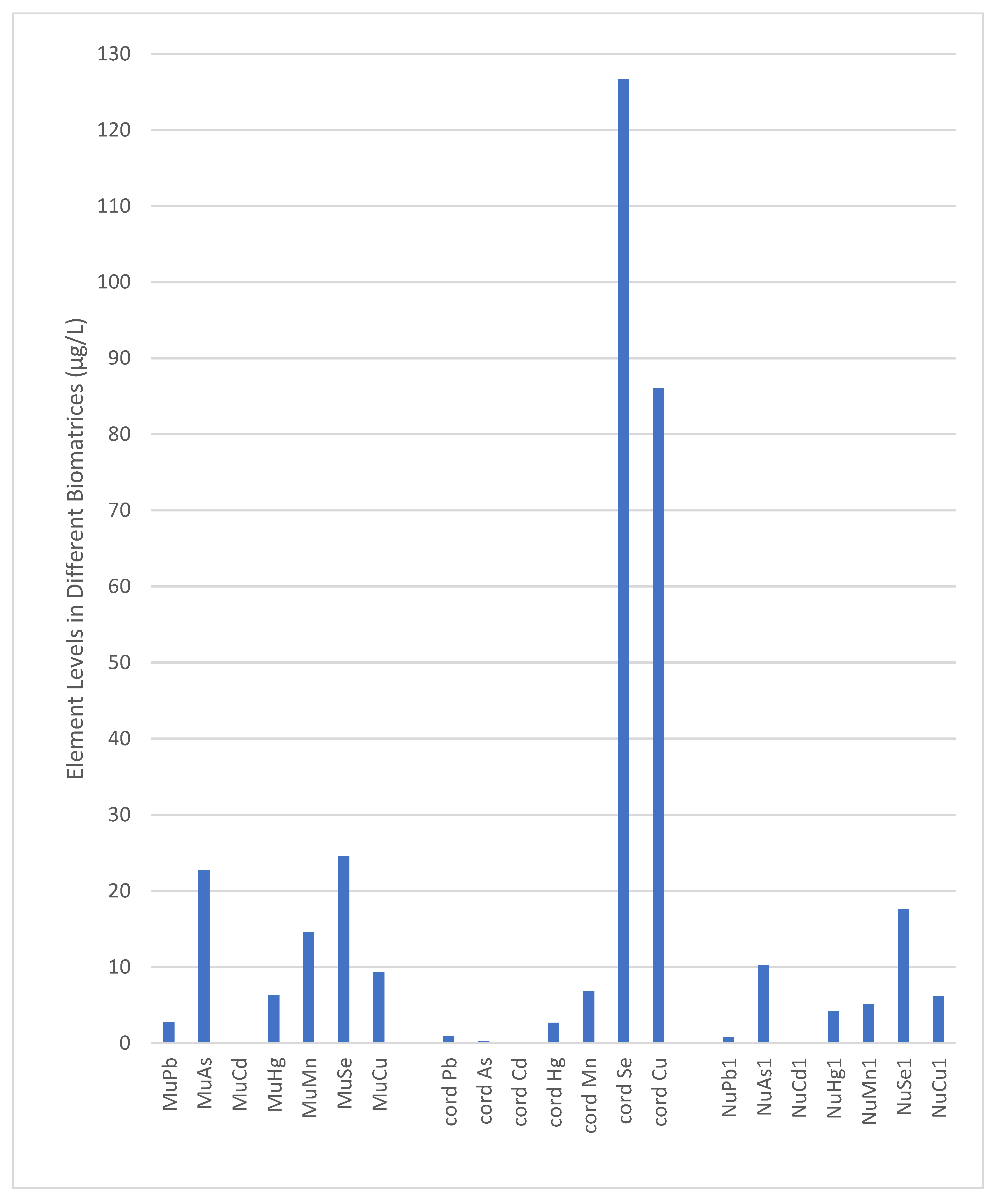
- In preterm infants (born at <35 weeks) in the NICU, cord blood had lower levels of Pb, As, Cd, and Hg and higher levels of Se and Cu compared to maternal urine.
- The excretion of all elements increased in infant urine over time during NICU stay.
- There were negative correlations between maternal urine Mn levels, cord Mn levels, and Nu1Hg levels with penile length or anogenital distance.
- The results highlight the exposure of preterm infants to some heavy metals and trace elements in the NICU, rather than in utero exposure.
- Our results indicate, for the first time in the literature, that Mn and Hg exposure may have a possible endocrine-disrupting effect on preterm infants through penile length and anogenital distance.
Abstract
1. Introduction
2. Materials and Methods
2.1. Working Protocol
2.2. Measurement of Heavy Metals and Trace Elements
2.3. Statistical Analysis
3. Results
3.1. Samples Obtained from Participants
3.2. General Characteristics of Newborns and Exposures in NICU
3.3. Heavy Metal and Trace Element Levels in Different Biomatrices
3.4. Correlations Between Mother–Child Pair Characteristics and Element Levels
4. Discussion
4.1. Comparison of Cord Blood Levels
4.2. Comparison of Maternal Urine Levels
4.3. Placental Modulation of Heavy Metals
4.4. Enhanced Transfer of Essential Trace Elements
4.5. Correlations of Elemental Levels in Different Biomatrices
4.6. Endocrine-Disrupting Effects on Genital Development
4.7. Lead Levels and Birth Weight
4.8. Selenium Levels and Gestational Duration
4.9. Selenium and Maternal Age
4.10. Correlation of Maternal Urine Selenium and Copper Levels
4.11. Postnatal Exposure in the NICU
4.12. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NICU | Neonatal Intensive Care Unit |
| Hg | Mercury |
| Pb | Lead |
| Cd | Cadmium |
| As | Arsenic |
| Mn | Manganese |
| Se | Selenium |
| Cu | Copper |
| ICP-MS | Inductively Coupled Plasma—Mass Spectrometer |
| Mu | Maternal urine |
| Nu | Neonatal urine |
| SPL | Stretched Penile Length |
| AGD | Anogenital Distance |
| TPN | Total Parenteral Nutrition |
| NG | Nasogastric |
| OG | Orogastric |
| nCPAP | nasal Continuous Positive Airway Pressure |
References
- Centers for Disease Control and Prevention (CDC); Agency for Toxic Substances and Disease Registry. ATSDR’s Substance Priority List. Available online: https://www.atsdr.cdc.gov (accessed on 12 October 2025).
- Sanders, A.P.; Flood, K.; Chiang, S.; Herring, A.H.; Wolf, L.; Fry, R.C. Towards prenatal biomonitoring in North Carolina: Assessing arsenic, cadmium, mercury, and lead levels in pregnant women. PLoS ONE 2012, 7, e31354. [Google Scholar] [CrossRef]
- Liu, X.W.; Hu, Q.Y.; Fang, Z.; Zhang, X.J.; Zhang, B.B. Magnetic Chitosan Nanocomposites: A Useful Recyclable Tool for Heavy Metal Ion Removal. Langmuir 2009, 25, 3–8. [Google Scholar] [CrossRef]
- Issah, I.; Duah, M.S.; Arko-Mensah, J.; Bawua, S.A.; Agyekum, T.P.; Fobil, J.N. Exposure to metal mixtures and adverse pregnancy and birth outcomes: A systematic review. Sci. Total Environ. 2024, 908, 168380. [Google Scholar] [CrossRef]
- Sanyal, A.; Rautaray, D.; Bansal, V.; Ahmad, A.; Sastry, M. Heavy-metal remediation by a fungus as a means of production of lead and cadmium carbonate crystals. Langmuir 2005, 21, 7220–7224. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.G.; Lee, C.J.; McCallum, G.P.; Perstin, J.; Harper, P.A. Receptor- and reactive intermediate-mediated mechanisms of teratogenesis. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 131–162. [Google Scholar] [CrossRef]
- Huang, N.; Wang, B.; Liu, S.; Wang, K.; Wang, R.; Liu, F.; Chen, C. Cadmium exposure in infants and children: Toxicity, health effects, dietary risk assessment and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2025, 65, 5085–5107. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, H.; Zhang, B.; Zheng, T.; Li, Y.; Zhou, A.; Du, X.; Pan, X.; Yang, J.; Wu, C.; et al. Prenatal cadmium exposure and preterm low birth weight in China. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Graziano, A.; Lo Monte, G.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2198–2206. [Google Scholar]
- Iyengar, G.V.; Rapp, A. Human placenta as a ‘dual’ biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 3: Toxic trace elements in placenta and placenta as a biomarker for these elements. Sci. Total Environ. 2001, 280, 221–238. [Google Scholar] [CrossRef]
- Amegah, A.K.; Sewor, C.; Jaakkola, J.J.K. Cadmium exposure and risk of adverse pregnancy and birth outcomes: A systematic review and dose-response meta-analysis of cohort and cohort-based case-control studies. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 299–317. [Google Scholar] [CrossRef]
- Sun, X.; Liu, W.; Zhang, B.; Shen, X.; Hu, C.; Chen, X.; Jin, S.; Jiang, Y.; Liu, H.; Cao, Z.; et al. Maternal Heavy Metal Exposure, Thyroid Hormones, and Birth Outcomes: A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 5043–5052. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, S.; Zhang, W.; Zeng, X.; Chu, C.; Li, Q.; Cui, X.; Wu, Q.; Dong, G.; Huang, J.; et al. Chemical element concentrations in cord whole blood and the risk of preterm birth for pregnant women in Guangdong, China. Ecotoxicol. Environ. Saf. 2022, 247, 114228. [Google Scholar] [CrossRef]
- Wai, K.M.; Mar, O.; Kosaka, S.; Umemura, M.; Watanabe, C. Prenatal Heavy Metal Exposure and Adverse Birth Outcomes in Myanmar: A Birth-Cohort Study. Int. J. Environ. Res. Public. Health 2017, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Khanam, R.; Kumar, I.; Oladapo-Shittu, O.; Twose, C.; Islam, A.A.; Biswal, S.S.; Raqib, R.; Baqui, A.H. Prenatal Environmental Metal Exposure and Preterm Birth: A Scoping Review. Int. J. Environ. Res. Public. Health 2021, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- Quansah, R.; Armah, F.A.; Essumang, D.K.; Luginaah, I.; Clarke, E.; Marfoh, K.; Cobbina, S.J.; Nketiah-Amponsah, E.; Namujju, P.B.; Obiri, S.; et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: A systematic review and meta-analysis. Environ. Health Perspect. 2015, 123, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Milton, A.H.; Hussain, S.; Akter, S.; Rahman, M.; Mouly, T.A.; Mitchell, K. A Review of the Effects of Chronic Arsenic Exposure on Adverse Pregnancy Outcomes. Int. J. Environ. Res. Public. Health 2017, 14, 556. [Google Scholar] [CrossRef]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Mirabella, F.; Martinez, M.A.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part B: Predictors of exposure. Environ. Res. 2020, 182, 109108. [Google Scholar] [CrossRef]
- Sun, H.; Chen, W.; Wang, D.; Jin, Y.; Chen, X.; Xu, Y. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere 2014, 108, 33–39. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Morimoto, N.; Hosokawa, S.; Matsushita, T. Associations of maternal and neonatal serum trace element concentrations with neonatal birth weight. PLoS ONE 2013, 8, e75627. [Google Scholar] [CrossRef]
- Afeiche, M.; Peterson, K.E.; Sanchez, B.N.; Cantonwine, D.; Lamadrid-Figueroa, H.; Schnaas, L.; Ettinger, A.S.; Hernandez-Avila, M.; Hu, H.; Tellez-Rojo, M.M. Prenatal lead exposure and weight of 0- to 5-year-old children in Mexico city. Environ. Health Perspect. 2011, 119, 1436–1441. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public. Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Zeng, T.; Liang, Y.; Chen, J.; Cao, G.; Yang, Z.; Zhao, X.; Tian, J.; Xin, X.; Lei, B.; Cai, Z. Urinary metabolic characterization with nephrotoxicity for residents under cadmium exposure. Environ. Int. 2021, 154, 106646. [Google Scholar] [CrossRef]
- Freire, C.; Amaya, E.; Gil, F.; Murcia, M.; S, L.L.; Casas, M.; Vrijheid, M.; Lertxundi, A.; Irizar, A.; Fernandez-Tardon, G.; et al. Placental metal concentrations and birth outcomes: The Environment and Childhood (INMA) project. Int. J. Hyg. Environ. Health 2019, 222, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.X.; Wang, L. Cadmium: Toxic effects on placental and embryonic development. Environ. Toxicol. Pharmacol. 2019, 67, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental toxicity of cadmium in infants and children: A review. Environ. Anal. Health Toxicol. 2021, 36, e2021003. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.V.; Lewin, M.; Ragin-Wilson, A.; Jones, R.; Jarrett, J.M.; Wallon, K.; Ward, C.; Hilliard, N.; Irvin-Barnwell, E. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999–2016. Environ. Res. 2020, 183, 109208. [Google Scholar] [CrossRef]
- Kippler, M.; Engstrom, K.; Mlakar, S.J.; Bottai, M.; Ahmed, S.; Hossain, M.B.; Raqib, R.; Vahter, M.; Broberg, K. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics 2013, 8, 494–503. [Google Scholar] [CrossRef]
- Concha, G.; Vogler, G.; Lezcano, D.; Nermell, B.; Vahter, M. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. 1998, 44, 185–190. [Google Scholar] [CrossRef]
- Nyanza, E.C.; Dewey, D.; Manyama, M.; Martin, J.W.; Hatfield, J.; Bernier, F.P. Maternal exposure to arsenic and mercury and associated risk of adverse birth outcomes in small-scale gold mining communities in Northern Tanzania. Environ. Int. 2020, 137, 105450. [Google Scholar] [CrossRef]
- Fei, D.L.; Koestler, D.C.; Li, Z.; Giambelli, C.; Sanchez-Mejias, A.; Gosse, J.A.; Marsit, C.J.; Karagas, M.R.; Robbins, D.J. Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: A US birth cohort study. Environ. Health 2013, 12, 58. [Google Scholar] [CrossRef]
- Liu, H.; Lu, S.; Zhang, B.; Xia, W.; Liu, W.; Peng, Y.; Zhang, H.; Wu, K.; Xu, S.; Li, Y. Maternal arsenic exposure and birth outcomes: A birth cohort study in Wuhan, China. Environ. Pollut. 2018, 236, 817–823. [Google Scholar] [CrossRef]
- Fry, R.C.; Navasumrit, P.; Valiathan, C.; Svensson, J.P.; Hogan, B.J.; Luo, M.; Bhattacharya, S.; Kandjanapa, K.; Soontararuks, S.; Nookabkaew, S.; et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007, 3, e207. [Google Scholar] [CrossRef]
- Smith, A.H.; Steinmaus, C.M. Health effects of arsenic and chromium in drinking water: Recent human findings. Annu. Rev. Public. Health 2009, 30, 107–122. [Google Scholar] [CrossRef]
- Kim, B.; Shah, S.; Park, H.S.; Hong, Y.C.; Ha, M.; Kim, Y.; Kim, B.N.; Kim, Y.; Ha, E.H. Adverse effects of prenatal mercury exposure on neurodevelopment during the first 3 years of life modified by early growth velocity and prenatal maternal folate level. Environ. Res. 2020, 191, 109909. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Al-Rouqi, R.; Obsum, C.A.; Shinwari, N.; Mashhour, A.; Billedo, G.; Al-Sarraj, Y.; Rabbah, A. Mercury (Hg) and oxidative stress status in healthy mothers and its effect on birth anthropometric measures. Int. J. Hyg. Environ. Health 2014, 217, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Holzman, C.; Rahbar, M.H.; Trosko, K.; Fischer, L. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ. Health Perspect. 2007, 115, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sattler, B.; Randall, K.S.; Choiniere, D. Reducing hazardous chemical exposures in the neonatal intensive care unit: A new role for nurses. Crit. Care Nurs. Q. 2012, 35, 102–112. [Google Scholar] [CrossRef]
- Boucher, O.; Muckle, G.; Jacobson, J.L.; Carter, R.C.; Kaplan-Estrin, M.; Ayotte, P.; Dewailly, E.; Jacobson, S.W. Domain-specific effects of prenatal exposure to PCBs, mercury, and lead on infant cognition: Results from the Environmental Contaminants and Child Development Study in Nunavik. Environ. Health Perspect. 2014, 122, 310–316. [Google Scholar] [CrossRef]
- Chen, Z.; Myers, R.; Wei, T.; Bind, E.; Kassim, P.; Wang, G.; Ji, Y.; Hong, X.; Caruso, D.; Bartell, T.; et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 537–544. [Google Scholar] [CrossRef]
- Chuang, H.Y.; Schwartz, J.; Gonzales-Cossio, T.; Lugo, M.C.; Palazuelos, E.; Aro, A.; Hu, H.; Hernandez-Avila, M. Interrelations of lead levels in bone, venous blood, and umbilical cord blood with exogenous lead exposure through maternal plasma lead in peripartum women. Environ. Health Perspect. 2001, 109, 527–532. [Google Scholar] [CrossRef]
- Jelliffe-Pawlowski, L.L.; Miles, S.Q.; Courtney, J.G.; Materna, B.; Charlton, V. Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. J. Perinatol. 2006, 26, 154–162. [Google Scholar] [CrossRef]
- Perkins, M.; Wright, R.O.; Amarasiriwardena, C.J.; Jayawardene, I.; Rifas-Shiman, S.L.; Oken, E. Very low maternal lead level in pregnancy and birth outcomes in an eastern Massachusetts population. Ann. Epidemiol. 2014, 24, 915–919. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Gao, D.; Jing, J.; Hu, Q. Prenatal and postnatal lead exposure and cognitive development of infants followed over the first three years of life: A prospective birth study in the Pearl River Delta region, China. Neurotoxicology 2014, 44, 326–334. [Google Scholar] [CrossRef]
- Virgolini, M.B.; Rossi-George, A.; Weston, D.; Cory-Slechta, D.A. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicology 2008, 29, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Ritz, B.; Heinrich, J.; Hoelscher, B.; Wichmann, H.E. The effect of low-level blood lead on hematologic parameters in children. Environ. Res. 2000, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Serrani, R.E.; Gioia, I.A.; Corchs, J.L. Lead effects on structural and functional cellular parameters in human red cells from a prenatal hematopoiesis stage. Biometals 1997, 10, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, J.L.; Brody, D.J.; Gunter, E.W.; Kramer, R.A.; Paschal, D.C.; Flegal, K.M.; Matte, T.D. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 1994, 272, 284–291. [Google Scholar] [CrossRef]
- Berkowitz, Z.; Price-Green, P.; Bove, F.J.; Kaye, W.E. Lead exposure and birth outcomes in five communities in Shoshone County, Idaho. Int. J. Hyg. Environ. Health 2006, 209, 123–132. [Google Scholar] [CrossRef]
- Sowers, M.; Jannausch, M.; Scholl, T.; Li, W.; Kemp, F.W.; Bogden, J.D. Blood lead concentrations and pregnancy outcomes. Arch. Environ. Health 2002, 57, 489–495. [Google Scholar] [CrossRef]
- Satin, K.P.; Neutra, R.R.; Guirguis, G.; Flessel, P. Umbilical cord blood lead levels in California. Arch. Environ. Health 1991, 46, 167–173. [Google Scholar] [CrossRef]
- Dettwiler, M.; Flynn, A.C.; Rigutto-Farebrother, J. Effects of Non-Essential “Toxic” Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses. Int. J. Environ. Res. Public. Health 2023, 20, 5536. [Google Scholar] [CrossRef]
- Mertz, W. The essential trace elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Aspects Med. 2005, 26, 235–244. [Google Scholar] [CrossRef]
- Jariwala, M.; Suvarna, S.; Kumar, G.K.; Amin, A.; Udas, A.C. Study of the Concentration of Trace Elements Fe, Zn, Cu, Se and Their Correlation in Maternal Serum, Cord Serum and Colostrums. Indian. J. Clin. Bioche 2014, 29, 181–188. [Google Scholar] [CrossRef]
- Claus Henn, B.; Ettinger, A.S.; Schwartz, J.; Tellez-Rojo, M.M.; Lamadrid-Figueroa, H.; Hernandez-Avila, M.; Schnaas, L.; Amarasiriwardena, C.; Bellinger, D.C.; Hu, H.; et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology 2010, 21, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Boskabadi, H.; Maamouri, G.; Rezagholizade Omran, F.; Mafinejad, S.; Tara, F.; Rayman, M.P.; Ghayour-Mobarhan, M.; Sahebkar, A.; Tavallaie, S.; Shakeri, M.T.; et al. Effect of prenatal selenium supplementation on cord blood selenium and lipid profile. Pediatr. Neonatol. 2012, 53, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.H.; Samms-Vaughan, M.; Dickerson, A.S.; Hessabi, M.; Bressler, J.; Desai, C.C.; Shakespeare-Pellington, S.; Reece, J.A.; Morgan, R.; Loveland, K.A.; et al. Concentration of lead, mercury, cadmium, aluminum, arsenic and manganese in umbilical cord blood of Jamaican newborns. Int. J. Environ. Res. Public. Health 2015, 12, 4481–4501. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, J.; Qi, X.; Wang, Z.; Zheng, M.; Liu, P.; Jiang, S.; Guo, J.; Wu, C.; Zhou, Z. Cord Blood Manganese Concentrations in Relation to Birth Outcomes and Childhood Physical Growth: A Prospective Birth Cohort Study. Nutrients 2021, 13, 4304. [Google Scholar] [CrossRef]
- Yamamoto, M.; Eguchi, A.; Sakurai, K.; Nakayama, S.F.; Sekiyama, M.; Mori, C.; Kamijima, M.; Japan Environment, C.s.S.G. Longitudinal analyses of maternal and cord blood manganese levels and neurodevelopment in children up to 3 years of age: The Japan Environment and Children’s Study (JECS). Environ. Int. 2022, 161, 107126. [Google Scholar] [CrossRef]
- Lorenzo Alonso, M.J.; Bermejo Barrera, A.; Cocho de Juan, J.A.; Fraga Bermudez, J.M.; Bermejo Barrera, P. Selenium levels in related biological samples: Human placenta, maternal and umbilical cord blood, hair and nails. J. Trace Elem. Med. Biol. 2005, 19, 49–54. [Google Scholar] [CrossRef]
- Gilman, C.L.; Soon, R.; Sauvage, L.; Ralston, N.V.; Berry, M.J. Umbilical cord blood and placental mercury, selenium and selenoprotein expression in relation to maternal fish consumption. J. Trace Elem. Med. Biol. 2015, 30, 17–24. [Google Scholar] [CrossRef]
- Archimbaud, Y.; Grillon, G.; Poncy, J.L.; Masse, R. Se-75 Transfer Via Placenta and Milk, Distribution and Retention in Fetal, Young and Adult-Rat. Radiat. Prot. Dosim. 1992, 41, 147–151. [Google Scholar] [CrossRef]
- Jandial, V.; Henderson, P.; Macgillivray, I. Placental-Transfer of Radioactive Selenomethionine in Late Pregnancy. Eur. J. Obstet. Gyn R. B 1976, 6, 295–300. [Google Scholar] [CrossRef]
- Cerna, M.; Spevackova, V.; Cejchanova, M.; Benes, B.; Rossner, P.; Bavorova, H.; Ocadlikova, D.; Smid, J.; Kubinova, R. Population-based biomonitoring in the Czech Republic--the system and selected results. Sci. Total Environ. 1997, 204, 263–270. [Google Scholar] [CrossRef]
- Robkin, M.A.; Swanson, D.R.; Shepard, T.H. Trace Metal Concentrations in Human Fetal Livers. T Am. Nucl. Soc. 1973, 17, 97–98. [Google Scholar]
- Kim, H.; Harrison, F.E.; Aschner, M.; Bowman, A.B. Exposing the role of metals in neurological disorders: A focus on manganese. Trends Mol. Med. 2022, 28, 555–568. [Google Scholar] [CrossRef]
- Amoros, R.; Murcia, M.; Gonzalez, L.; Soler-Blasco, R.; Rebagliato, M.; Iniguez, C.; Carrasco, P.; Vioque, J.; Broberg, K.; Levi, M.; et al. Maternal copper status and neuropsychological development in infants and preschool children. Int. J. Hyg. Environ. Health 2019, 222, 503–512. [Google Scholar] [CrossRef]
- Weyde, K.V.F.; Winterton, A.; Suren, P.; Andersen, G.L.; Vik, T.; Biele, G.; Knutsen, H.K.; Thomsen, C.; Meltzer, H.M.; Skogheim, T.S.; et al. Association between gestational levels of toxic metals and essential elements and cerebral palsy in children. Front. Neurol. 2023, 14, 1124943. [Google Scholar] [CrossRef]
- Kumar, V.; Kalita, J.; Misra, U.K.; Bora, H.K. A study of dose response and organ susceptibility of copper toxicity in a rat model. J. Trace Elem. Med. Biol. 2015, 29, 269–274. [Google Scholar] [CrossRef]
- Kumar, V.; Kalita, J.; Bora, H.K.; Misra, U.K. Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol. Appl. Pharmacol. 2016, 293, 37–43. [Google Scholar] [CrossRef]
- Chen, J.; Gao, X.; Zheng, C.; Zhang, C.; Li, P.; He, K.; Liu, G.; Huang, X.; Liu, J.; Xie, Y.; et al. Low-dose Cu exposure enhanced alpha-synuclein accumulation associates with mitochondrial impairments in mice model of Parkinson’s disease. Toxicol. Lett. 2023, 387, 14–27. [Google Scholar] [CrossRef]
- Monangi, N.K.; Xu, H.; Fan, Y.M.; Khanam, R.; Khan, W.; Deb, S.; Pervin, J.; Price, J.T.; Kaur, L.; INTERBIO-21st Study Consortium; et al. Association of maternal prenatal copper concentration with gestational duration and preterm birth: A multicountry meta-analysis. Am. J. Clin. Nutr. 2024, 119, 221–231. [Google Scholar] [CrossRef]
- Cengiz, B.; Soylemez, F.; Ozturk, E.; Cavdar, A.O. Serum zinc, selenium, copper, and lead levels in women with second-trimester induced abortion resulting from neural tube defects: A preliminary study. Biol. Trace Elem. Res. 2004, 97, 225–235. [Google Scholar] [CrossRef]
- Leotsinidis, M.; Alexopoulos, A.; Kostopoulou-Farri, E. Toxic and essential trace elements in human milk from Greek lactating women: Association with dietary habits and other factors. Chemosphere 2005, 61, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.W.; Palumbo, D.; Myers, G.J.; Cox, C.; Shamlaye, C.F.; Sloane-Reeves, J.; Cernichiari, E.; Wilding, G.E.; Clarkson, T.W. Neurodevelopmental outcomes of Seychellois children from the pilot cohort at 108 months following prenatal exposure to methylmercury from a maternal fish diet. Environ. Res. 2000, 84, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dorea, J.G.; Donangelo, C.M. Early (in uterus and infant) exposure to mercury and lead. Clin. Nutr. 2006, 25, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Dursun, A.; Yurdakok, K.; Yalcin, S.S.; Tekinalp, G.; Aykut, O.; Orhan, G.; Morgil, G.K. Maternal risk factors associated with lead, mercury and cadmium levels in umbilical cord blood, breast milk and newborn hair. J. Matern. Fetal Neonatal Med. 2016, 29, 954–961. [Google Scholar] [CrossRef]
- Yalçin, S.S.; Yurdakök, K.; Yalçin, S.; Engür-Karasimav, D.; Coşkun, T. Maternal and environmental determinants of breast-milk mercury concentrations. Turk. J. Pediatr. 2010, 52, 1–9. [Google Scholar]
- Yang, W.; Han, N.; Jiao, M.; Chang, X.; Liu, J.; Zhou, Q.; Wang, H.J. Maternal diet quality during pregnancy and its influence on low birth weight and small for gestational age: A birth cohort in Beijing, China. Br. J. Nutr. 2023, 129, 1360–1369. [Google Scholar] [CrossRef]
- Stone, J.; Sutrave, P.; Gascoigne, E.; Givens, M.B.; Fry, R.C.; Manuck, T.A. Exposure to toxic metals and per- and polyfluoroalkyl substances and the risk of preeclampsia and preterm birth in the United States: A review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100308. [Google Scholar] [CrossRef]
- Yang, J.; Huo, W.; Zhang, B.; Zheng, T.; Li, Y.; Pan, X.; Liu, W.; Chang, H.; Jiang, M.; Zhou, A.; et al. Maternal urinary cadmium concentrations in relation to preterm birth in the Healthy Baby Cohort Study in China. Environ. Int. 2016, 94, 300–306. [Google Scholar] [CrossRef]
- Takci, S.; Asci, A.; Erkekoglu, P.; Yiğit, S.; Kocer-Gumusel, B.; Yurdakök, M. Lead and Mercury Levels in Preterm Infants Before and After Blood Transfusions. Biol. Trace Elem. Res. 2019, 188, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Bearer, C.F.; O’Riordan, M.A.; Powers, R. Lead exposure from blood transfusion to premature infants. J. Pediatr. 2000, 137, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Martinez, E.; Romano-Riquer, P.; Yanez-Marquez, E.; Longnecker, M.P.; Hernandez-Avila, M. Anogenital distance in human male and female newborns: A descriptive, cross-sectional study. Environ. Health 2004, 3, 8. [Google Scholar] [CrossRef]
- Akin, Y.; Ercan, O.; Telatar, B.; Tarhan, F. Penile size in term newborn infants. Turk. J. Pediatr. 2011, 53, 301–307. [Google Scholar]
- Lozano, M.; Murcia, M.; Soler-Blasco, R.; Casas, M.; Zubero, B.; Riutort-Mayol, G.; Gil, F.; Olmedo, P.; Grimalt, J.O.; Amoros, R.; et al. Exposure to metals and metalloids among pregnant women from Spain: Levels and associated factors. Chemosphere 2022, 286, 131809. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Huang, Q.; Wei, C.; Yang, D.; Wang, C.; Fan, K.; Cheng, S.; Guo, X.; Wang, J. Predictors of urinary heavy metal concentrations among pregnant women in Jinan, China. J. Trace Elem. Med. Biol. 2024, 84, 127444. [Google Scholar] [CrossRef]
- Rudge, C.V.; Rollin, H.B.; Nogueira, C.M.; Thomassen, Y.; Rudge, M.C.; Odland, J.O. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J. Environ. Monit. 2009, 11, 1322–1330. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Al-Rouqi, R.; Alnuwaysir, H.; Aldhalaan, H.; Alismail, E.; Binmanee, A.; Hawari, A.; Alhazzani, F.; Bin Jabr, M. Exposure of preterm neonates to toxic metals during their stay in the Neonatal Intensive Care Unit and its impact on neurodevelopment at 2 months of age. J. Trace Elem. Med. Biol. 2023, 78, 127173. [Google Scholar] [CrossRef]
- Iwai-Shimada, M.; Kameo, S.; Nakai, K.; Yaginuma-Sakurai, K.; Tatsuta, N.; Kurokawa, N.; Nakayama, S.F.; Satoh, H. Exposure profile of mercury, lead, cadmium, arsenic, antimony, copper, selenium and zinc in maternal blood, cord blood and placenta: The Tohoku Study of Child Development in Japan. Environ. Health Prev. Med. 2019, 24, 35. [Google Scholar] [CrossRef]
- Kopp, R.S.; Kumbartski, M.; Harth, V.; Bruning, T.; Kafferlein, H.U. Partition of metals in the maternal/fetal unit and lead-associated decreases of fetal iron and manganese: An observational biomonitoring approach. Arch. Toxicol. 2012, 86, 1571–1581. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Liang, C.L.; Morisset, A.S.; Fisher, M.; Weiler, H.; Cirtiu, C.M.; Legrand, M.; Davis, K.; Ettinger, A.S.; Fraser, W.D. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 2016, 163, 270–282. [Google Scholar] [CrossRef]
- Garí, M.; Grzesiak, M.; Krekora, M.; Kaczmarek, P.; Jankowska, A.; Król, A.; Kaleta, D.; Jerzyńska, J.; Janasik, B.; Kuraś, R.; et al. Prenatal exposure to neurotoxic metals and micronutrients and neurodevelopmental outcomes in early school age children from Poland. Environ. Res. 2022, 204, 112049. [Google Scholar] [CrossRef]
- Dahiri, B.; Martín-Carrasco, I.; Carbonero-Aguilar, P.; Cerrillos, L.; Ostos, R.; Fernández-Palacín, A.; Bautista, J.; Moreno, I. Monitoring of metals and metalloids from maternal and cord blood samples in a population from Seville (Spain). Sci. Total Environ. 2023, 854, 158687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, X.; Luo, Z.C.; Liu, J.; Chen, Y.; Fan, P.; Ma, R.; Ma, J.; Luo, K.; Yan, C.H.; et al. Maternal blood concentrations of toxic metal(loid)s and trace elements from preconception to pregnancy and transplacental passage to fetuses. Ecotoxicol. Environ. Saf. 2023, 264, 115394. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Esquinas, E.; Perez-Gomez, B.; Fernandez-Navarro, P.; Fernandez, M.A.; de Paz, C.; Perez-Meixeira, A.M.; Gil, E.; Iriso, A.; Sanz, J.C.; Astray, J.; et al. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public. Health 2013, 13, 841. [Google Scholar] [CrossRef] [PubMed]
- Grzesik-Gasior, J.; Sawicki, J.; Pieczykolan, A.; Bien, A. Content of selected heavy metals in the umbilical cord blood and anthropometric data of mothers and newborns in Poland: Preliminary data. Sci. Rep. 2023, 13, 14077. [Google Scholar] [CrossRef]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Martinez, M.A.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Environ. Res. 2019, 177, 108599. [Google Scholar] [CrossRef]
- Guo, X.; Song, J.; Wang, X.; Huang, Q.; Wei, C.; Yang, Y.; Li, N.; Cheng, S.; Li, J.; Li, Q.; et al. Urinary concentrations of mineral elements and their predictors in pregnant women in Jinan, China. J. Trace Elem. Med. Biol. 2024, 85, 127496. [Google Scholar] [CrossRef]
- Gundacker, C.; Frohlich, S.; Graf-Rohrmeister, K.; Eibenberger, B.; Jessenig, V.; Gicic, D.; Prinz, S.; Wittmann, K.J.; Zeisler, H.; Vallant, B.; et al. Perinatal lead and mercury exposure in Austria. Sci. Total Environ. 2010, 408, 5744–5749. [Google Scholar] [CrossRef]
- Lagerkvist, B.I.; Sandberg, S.; Frech, W.; Jin, T.; Nordberg, G.F. Is placenta a good indicator of cadmium and lead exposure? Arch. Environ. Health 1996, 51, 389–394. [Google Scholar] [CrossRef]
- Gundacker, C.; Hengstschlager, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, L.; Shariat, M.; Chamari, M.; Chahardoli, R.; Asgarzadeh, L.; Seighali, F. Comparison of Maternal and Umbilical Cord Blood Selenium Levels in Low and Normal Birth Weight Neonates. J. Family Reprod. Health 2015, 9, 125–128. [Google Scholar] [PubMed]
- Sanchez, C.; Lopez-Jurado, M.; Aranda, P.; Llopis, J. Plasma levels of copper, manganese and selenium in an adult population in southern Spain: Influence of age, obesity and lifestyle factors. Sci. Total Environ. 2010, 408, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Tessier, B.; Paris, F.; Bergougnoux, A.; Hamamah, S.; Sultan, C.; Kalfa, N. Endocrine-Disrupting Chemicals and Disorders of Penile Development in Humans. Sex. Dev. 2021, 15, 213–228. [Google Scholar] [CrossRef]
- Gaspari, L.; Sampaio, D.R.; Paris, F.; Audran, F.; Orsini, M.; Neto, J.B.; Sultan, C. High prevalence of micropenis in 2710 male newborns from an intensive-use pesticide area of Northeastern Brazil. Int. J. Androl. 2012, 35, 253–264. [Google Scholar] [CrossRef]
- Nelson, C.P.; Park, J.M.; Wan, J.; Bloom, D.A.; Dunn, R.L.; Wei, J.T. The increasing incidence of congenital penile anomalies in the United States. J. Urol. 2005, 174, 1573–1576. [Google Scholar] [CrossRef]
- Martin, M.B.; Reiter, R.; Johnson, M.; Shah, M.S.; Iann, M.C.; Singh, B.; Richards, J.K.; Wang, A.; Stoica, A. Effects of tobacco smoke condensate on estrogen receptor-alpha gene expression and activity. Endocrinology 2007, 148, 4676–4686. [Google Scholar] [CrossRef]
- dos Santos, N.R.; Rodrigues, J.L.G.; Bandeira, M.J.; Anjos, A.L.d.S.; Araújo, C.d.F.S.; Adan, L.F.F.; Menezes-Filho, J.A. Manganese exposure and association with hormone imbalance in children living near a ferro-manganese alloy plant. Environ. Res. 2019, 172, 166–174. [Google Scholar] [CrossRef]
- Dos Santos, N.R.; Rodrigues, J.L.G.; Bandeira, M.J.; Anjos, A.; Araújo, C.; Adan, L.F.F.; Menezes-Filho, J.A. Manganese and Lead Exposure and Early Puberty Onset in Children Living near a Ferromanganese Alloy Plant. Int. J. Environ. Res. Public. Health 2022, 19, 7158. [Google Scholar] [CrossRef]
- Gomes-Silva, A.P.; Medeiros, P.D.C.; Silva, L.N.D.; Santiago, M.; Trevizani, T.H.; Figueira, R.C.L.; Moreira, L.B.; Perobelli, J.E. Exposure to manganese during late intrauterine development and lactation: Long-term effects on male reproductive function. Environ. Toxicol. Pharmacol. 2025, 118, 104784. [Google Scholar] [CrossRef]
- Zheng, G.; Zhong, H.; Guo, Z.; Wu, Z.; Zhang, H.; Wang, C.; Zhou, Y.; Zuo, Z. Levels of heavy metals and trace elements in umbilical cord blood and the risk of adverse pregnancy outcomes: A population-based study. Biol. Trace Elem. Res. 2014, 160, 437–444. [Google Scholar] [CrossRef]
- Zinia, S.S.; Yang, K.H.; Lee, E.J.; Lim, M.N.; Kim, J.; Kim, W.J. Effects of heavy metal exposure during pregnancy on birth outcomes. Sci. Rep. 2023, 13, 18990. [Google Scholar] [CrossRef]
- Rahman, A.; Al-Rashidi, H.A.; Khan, A.R. Association of maternal blood lead level during pregnancy with child blood lead level and pregnancy outcome in Kuwait. Ecol. Food Nutr. 2012, 51, 40–57. [Google Scholar] [CrossRef]
- Rahman, A.; Kumarathasan, P.; Gomes, J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci. Total Environ. 2016, 569–570, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ding, G.; Cui, C.; Chen, L.; Gao, Y.; Zhou, Y.; Shi, R.; Tian, Y. The effects of low-level prenatal lead exposure on birth outcomes. Environ. Pollut. 2013, 175, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Angouria-Tsorochidou, E.; Caro, D.; Thomsen, M. Daily intake of heavy metals and minerals in food–A case study of four Danish dietary profiles. J. Clean. Prod. 2021, 280, 124279. [Google Scholar] [CrossRef]
- Muntau, A.C.; Streiter, M.; Kappler, M.; Röschinger, W.; Schmid, I.; Rehnert, A.; Schramel, P.; Roscher, A.A. Age-related reference values for serum selenium concentrations in infants and children. Clin. Chem. 2002, 48, 555–560. [Google Scholar] [CrossRef]
- Bebars, G.; Kamel, B.; Allam, E. Comparison between preterm and full term neonatal cord selenium in correlation to maternal serum selenium levels. Egypt. Pediatr. Assoc. Gaz. 2018, 66, 96–99. [Google Scholar] [CrossRef]
- Monangi, N.; Xu, H.; Khanam, R.; Khan, W.; Deb, S.; Pervin, J.; Price, J.T.; Kennedy, S.H.; Al Mahmud, A.; Fan, Y.; et al. Association of maternal prenatal selenium concentration and preterm birth: A multicountry meta-analysis. BMJ Glob. Health 2021, 6, e005856. [Google Scholar] [CrossRef]
- Lewandowska, M.; Sajdak, S.; Lubiński, J. The Role of Early Pregnancy Maternal Selenium Levels on the Risk for Small-for-Gestational Age Newborns. Nutrients 2019, 11, 2298. [Google Scholar] [CrossRef]
- Lyons, G.H.; Judson, G.J.; Stangoulis, J.C.; Palmer, L.T.; Jones, J.A.; Graham, R.D. Trends in selenium status of South Australians. Med. J. Aust. 2004, 180, 383–386. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Piao, J.; Mao, D.; Li, Y.; Li, W.; Yang, L.; Yang, X. Reference Values of 14 Serum Trace Elements for Pregnant Chinese Women: A Cross-Sectional Study in the China Nutrition and Health Survey 2010–2012. Nutrients 2017, 9, 309. [Google Scholar] [CrossRef]
| Mother (N = 40) | Mean ± SD (Min–Max) |
|---|---|
| Mother’s age, years | 29.9 ± 5.2 (19–39) |
| Mother’s height | 162.7 ± 5.8 |
| Mother’s BMI (preconceptional) | 25.4 ± 5.0 |
| Pregnancy and Birth | |
| Parity | 2.3 ± 1.1 (1–5) |
| Birth order, 1st | 15 (32.5) |
| Twin pregnancy | 13 (32.5) |
| Newborn (N = 40) | |
| Gestational duration, weeks | 31.9 ± 2.0 (27.6–34.5) |
| Sex, male | 25 (62.5) |
| Birth weight, percentile | 40.2 ± 28.5 |
| Head circumference, percentile | 52.5 ± 28.8 |
| Anogenital distance, cm | 1.78 ± 0.41 |
| Stretched penile length (n = 25), cm | 2.36 ± 0.33 |
| TPN use | 27 (67.5) |
| Formula intake | 22 (55.0) |
| GM | Mean | SD | Min | 25 p | 50 p | 75 p | 90 p | Max | |
|---|---|---|---|---|---|---|---|---|---|
| Pb | |||||||||
| MuPb | 2.80 | 2.91 | 0.81 | 1.41 | 2.36 | 2.81 | 3.41 | 4.08 | 4.78 |
| Cord Pb | 0.85 | 1.00 | 0.54 | 0.21 | 0.60 | 0.98 | 1.30 | 1.55 | 2.65 |
| NuPb1 | 0.80 | 0.83 | 0.23 | 0.33 | 0.72 | 0.78 | 0.96 | 1.12 | 1.41 |
| NuPb2 | 0.86 | 0.90 | 0.26 | 0.41 | 0.75 | 0.88 | 1.04 | 1.26 | 1.56 |
| As | |||||||||
| MuAs | 21.85 | 22.33 | 4.48 | 14.27 | 18.77 | 22.76 | 26.15 | 27.44 | 31.04 |
| CordAs | 0.24 | 0.26 | 0.13 | 0.11 | 0.17 | 0.23 | 0.32 | 0.45 | 0.63 |
| NuAs1 | 9.97 | 10.23 | 2.37 | 6.11 | 8.29 | 10.24 | 11.24 | 14.50 | 16.20 |
| NuAs2 | 10.54 | 10.76 | 2.22 | 7.52 | 8.94 | 10.26 | 12.34 | 14.13 | 16.23 |
| Cd | |||||||||
| MuCd | 0.12 | 0.13 | 0.03 | 0.08 | 0.11 | 0.12 | 0.14 | 0.17 | 0.25 |
| Cord Cd | 0.17 | 0.18 | 0.07 | 0.10 | 0.13 | 0.17 | 0.23 | 0.31 | 0.41 |
| NuCd1 | 0.08 | 0.09 | 0.02 | 0.05 | 0.07 | 0.08 | 0.10 | 0.12 | 0.16 |
| NuCd2 | 0.09 | 0.09 | 0.03 | 0.02 | 0.08 | 0.09 | 0.12 | 0.13 | 0.14 |
| Hg | |||||||||
| MuHg | 6.01 | 6.26 | 1.77 | 3.28 | 4.65 | 6.38 | 7.36 | 8.51 | 10.62 |
| Cord Hg | 2.74 | 3.13 | 1.68 | 1.08 | 1.96 | 2.67 | 4.27 | 5.40 | 8.77 |
| NuHg1 | 4.27 | 4.35 | 0.86 | 3.01 | 3.67 | 4.20 | 4.76 | 5.98 | 6.37 |
| NuHg2 | 4.86 | 4.98 | 1.13 | 3.24 | 4.18 | 4.95 | 5.67 | 6.79 | 7.99 |
| Mn | |||||||||
| MuMn | 11.38 | 12.37 | 4.76 | 4.24 | 7.62 | 14.61 | 15.81 | 17.50 | 21.18 |
| Cord Mn | 6.63 | 6.76 | 1.31 | 4.21 | 5.78 | 6.86 | 7.97 | 8.27 | 9.21 |
| NuMn1 | 5.02 | 5.26 | 1.59 | 2.33 | 4.02 | 5.10 | 6.30 | 7.45 | 8.45 |
| NuMn2 | 5.65 | 5.86 | 1.54 | 2.36 | 4.89 | 5.86 | 7.02 | 8.08 | 9.06 |
| Se | |||||||||
| MuSe | 24.43 | 24.55 | 2.42 | 19.87 | 22.43 | 24.61 | 26.43 | 27.45 | 28.17 |
| Cord Se | 121.43 | 123.25 | 20.61 | 78.56 | 108.98 | 126.67 | 140.21 | 149.88 | 156.71 |
| NuSe1 | 17.63 | 17.82 | 2.67 | 13.21 | 15.65 | 17.58 | 20.04 | 21.72 | 24.12 |
| NuSe2 | 18.57 | 18.86 | 3.45 | 13.27 | 16.29 | 18.18 | 21.28 | 23.60 | 28.75 |
| Cu | |||||||||
| MuCu | 9.47 | 9.81 | 2.74 | 5.66 | 7.83 | 9.35 | 10.87 | 14.49 | 17.45 |
| Cord Cu | 86.97 | 87.49 | 9.76 | 70.54 | 79.66 | 86.11 | 94.45 | 100.15 | 110.21 |
| NuCu1 | 5.75 | 5.93 | 1.35 | 2.23 | 4.94 | 6.15 | 6.98 | 7.45 | 8.54 |
| NuCu2 | 6.14 | 6.31 | 1.40 | 2.65 | 5.34 | 6.25 | 7.47 | 7.83 | 9.02 |
| Mu & Cord | Mu & Nu1 | Cord & Nu1 | Nu1 & Nu2 | ||
|---|---|---|---|---|---|
| Pb * | rs | 0.08 | 0.11 | −0.01 | 0.94 |
| p | 0.627 | 0.485 | 0.969 | <0.001 | |
| As ** | rs | −0.02 | 0.09 | 0.22 | 0.68 |
| p | 0.928 | 0.583 | 0.171 | <0.001 | |
| Cd ** | rs | −0.29 | −0.11 | −0.09 | 0.52 |
| p | 0.074 | 0.509 | 0.573 | 0.001 | |
| Hg ** | rs | −0.19 | −0.17 | −0.20 | 0.85 |
| p | 0.254 | 0.298 | 0.210 | <0.001 | |
| Mn * | rs | 0.13 | 0.07 | −0.05 | 0.81 |
| p | 0.421 | 0.666 | 0.782 | <0.001 | |
| Se * | rs | −0.04 | −0.20 | 0.21 | 0.73 |
| p | 0.831 | 0.224 | 0.201 | <0.001 | |
| Cu * | rs | −0.07 | 0.35 | 0.04 | 0.65 |
| p | 0.669 | 0.026 | 0.789 | <0.001 |
| Nu1 | Nu2 | Sign & | |
|---|---|---|---|
| Pb | 0.82 ± 0.23 | 0.90 ± 0.26 | <0.001 |
| As | 10.24 [8.28–11.24] | 10.26 [8.93–12.33] | 0.003 |
| Cd | 0.08 [0.07–0.10] | 0.09 [0.08–0.12] | 0.024 |
| Hg | 4.19 [3.67–4.75] | 4.95 [4.18–5.67] | <0.001 |
| Mn | 5.26 ± 1.59 | 5.85 ± 1.54 | <0.001 |
| Se | 17.81 ± 2.67 | 18.86 ± 3.45 | 0.008 |
| Cu | 5.93 ± 1.35 | 6.31 ± 1.40 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celik, M.; Iyigun, I.; Yalcin, S.S.; Cagan, M.; Cakir, D.A.; Celik, H.T.; Deren, O.; Erkekoglu, P. Perinatal Exposure to Heavy Metals and Trace Elements of Preterm Neonates in the NICU: A Toxicological Study Using Multiple Biomatrices. Toxics 2025, 13, 898. https://doi.org/10.3390/toxics13100898
Celik M, Iyigun I, Yalcin SS, Cagan M, Cakir DA, Celik HT, Deren O, Erkekoglu P. Perinatal Exposure to Heavy Metals and Trace Elements of Preterm Neonates in the NICU: A Toxicological Study Using Multiple Biomatrices. Toxics. 2025; 13(10):898. https://doi.org/10.3390/toxics13100898
Chicago/Turabian StyleCelik, Melda, Irem Iyigun, Siddika Songül Yalcin, Murat Cagan, Deniz Arca Cakir, Hasan Tolga Celik, Ozgur Deren, and Pinar Erkekoglu. 2025. "Perinatal Exposure to Heavy Metals and Trace Elements of Preterm Neonates in the NICU: A Toxicological Study Using Multiple Biomatrices" Toxics 13, no. 10: 898. https://doi.org/10.3390/toxics13100898
APA StyleCelik, M., Iyigun, I., Yalcin, S. S., Cagan, M., Cakir, D. A., Celik, H. T., Deren, O., & Erkekoglu, P. (2025). Perinatal Exposure to Heavy Metals and Trace Elements of Preterm Neonates in the NICU: A Toxicological Study Using Multiple Biomatrices. Toxics, 13(10), 898. https://doi.org/10.3390/toxics13100898







