Protective Role of Gallic Acid Against Corticosterone-Induced Hepatic Toxicity: Modulation of Oxidative Stress and Inflammatory Pathways in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Habitat of the Animal
2.3. Ethical Clearance
2.4. Experimental Protocol
2.5. Behavioral Analysis and Body Weight Monitoring
2.6. Collection of Blood Serum
2.7. Preparation of Tissue Homogenate
2.8. Protein Assessment
2.9. Assessment of Nonenzymatic Antioxidant Status in Rat Liver
2.9.1. Glutathione Content (GSH) in Rat Liver
2.9.2. MDA Analysis in Rat Liver
2.9.3. Estimation of Protein Carbonyl Content in Rat Liver
2.10. Determination of Oxidative Stress Status in Rat Liver
2.10.1. Estimation of Superoxide Dismutase (SOD) Activity in Rat Liver
2.10.2. Estimation of Catalase (CAT) Activity in Rat Liver
2.10.3. Estimation of Glutathione S-Transferase (GST) Activity in Rat Liver
2.11. Oxidative Stress Index (OSI)
2.12. Quantification of ATP in the Liver of Rats
2.13. Determination of the Level of Hepatic Function Enzymes in Rat Blood Serum
2.13.1. Assays for the Activities of Liver Transaminases Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST)
2.13.2. Assays for the Estimation of Alkaline Phosphatase (ALP) and Acid Phosphatase (ACP) Activity in the Serum
2.14. Estimation of Lactate Dehydrogenase (LDH) Activity in the Serum
2.15. Estimation of Glucose Concentration in Serum and Evaluation of Body Weight Change
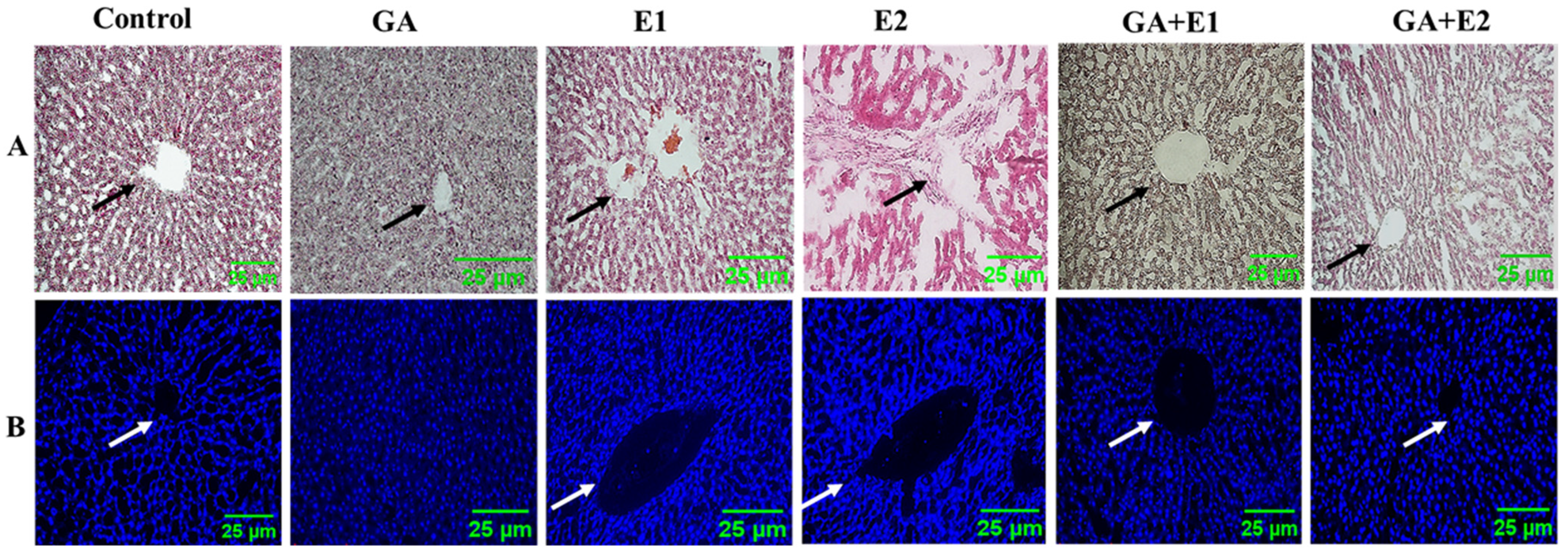
2.16. Analysis of Histological Parameters Using H&E and DAPI Staining
2.17. Analysis of Immunohistochemical Properties
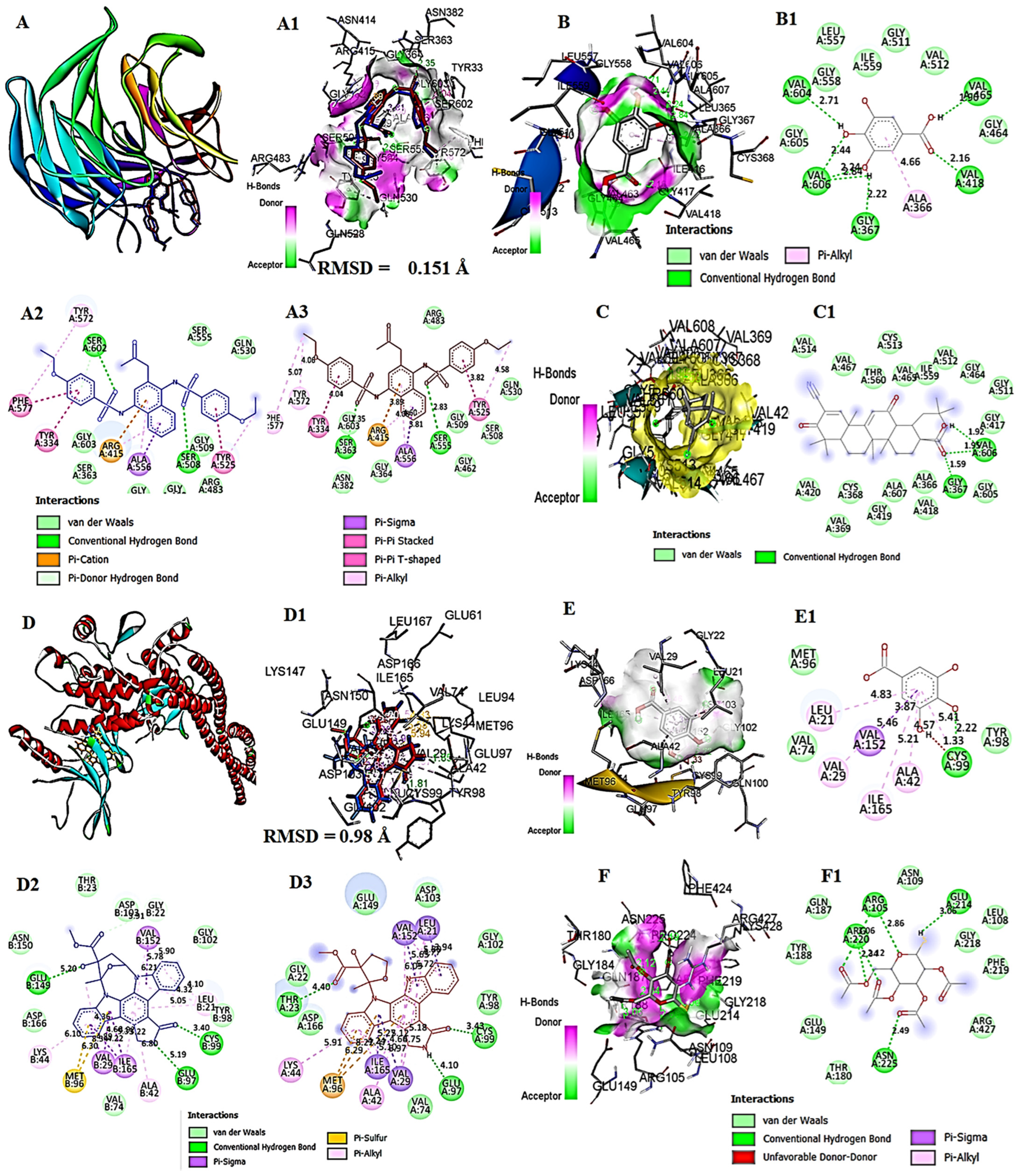
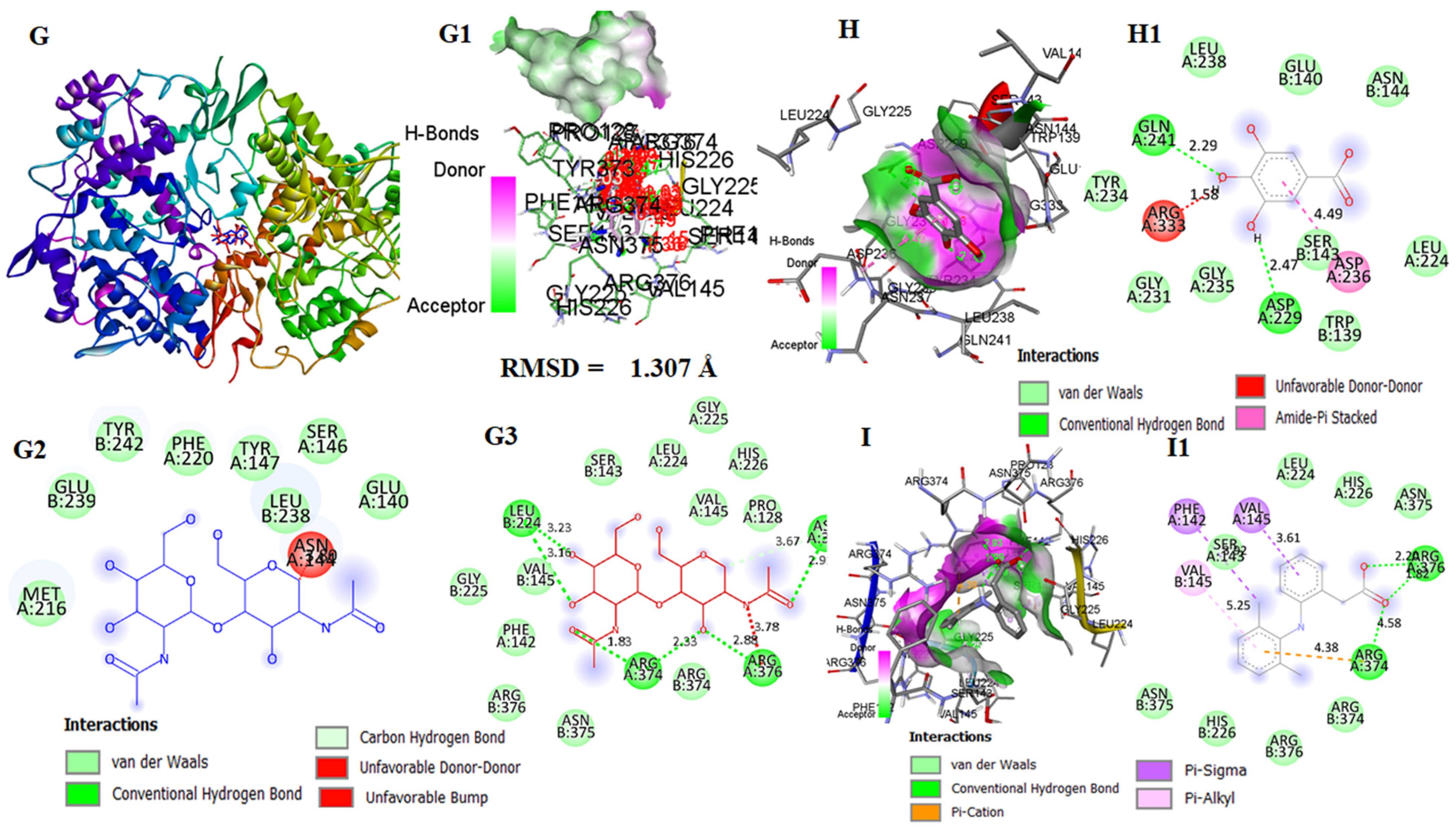
2.18. In Silico Study on Hepatotoxicity of the Liver with CORT and Possible Protection by GA
2.19. Statistical Investigation
3. Results
3.1. Impact of CORT and GA on the Physical and Behavioral Parameters of Experimental Rats
3.2. Evaluation of CORT-Induced Oxidative Stress in Rats and the Protective Effect of GA
3.2.1. Impact of CORT on the Levels of Protein and Nonenzymatic Antioxidants
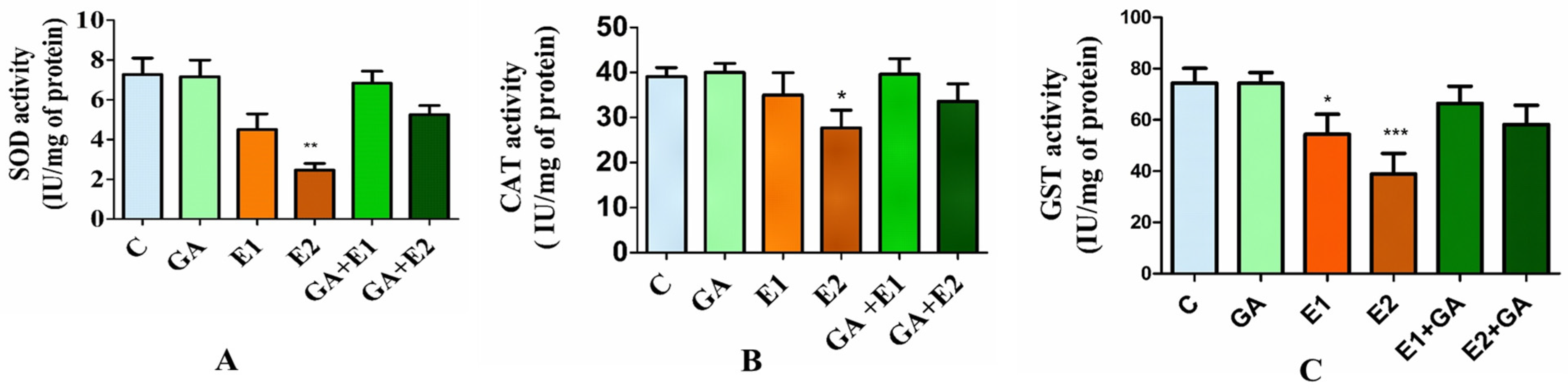
3.2.2. Impact of CORT and GA on the Activities of Antioxidative Enzymes in Rat Liver
3.3. Impact of CORT and GA on the Oxidative Stress Index (OSI) of the Rat Liver
3.4. Effect of CORT and GA on the Serum Cortisol Level and Serum Glucose
3.5. Impact of CORT and GA on the ATP Level in the Rat Liver
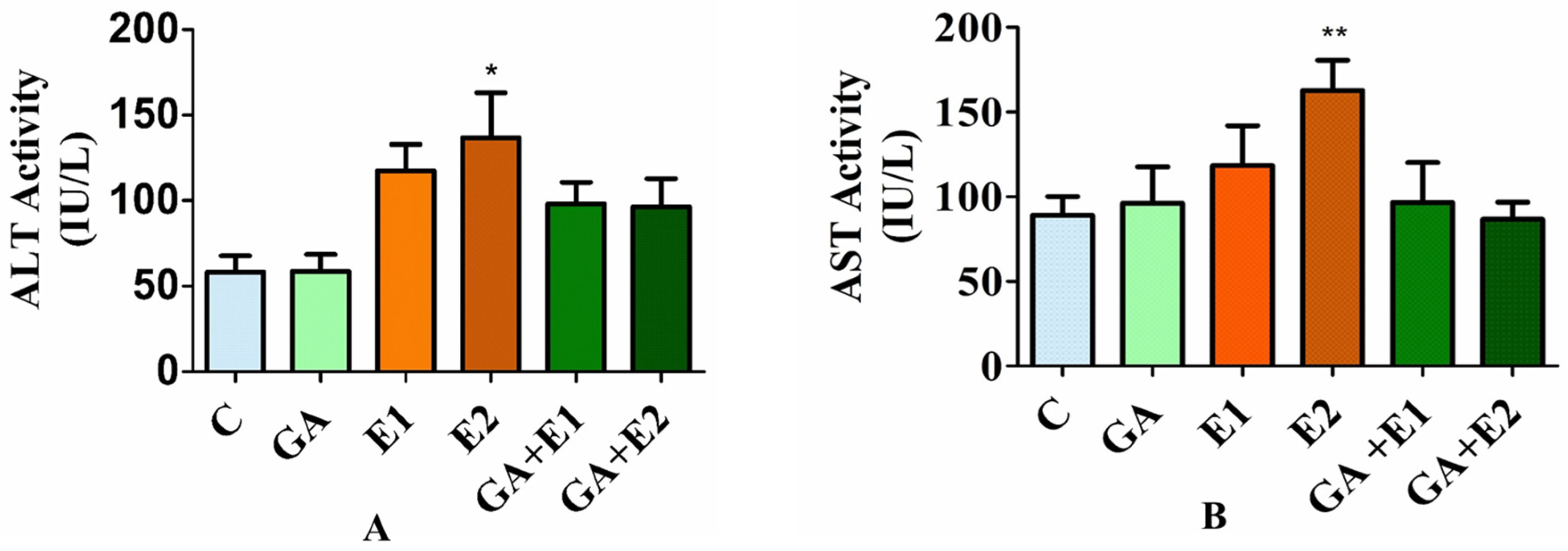
3.6. Effect of CORT on the Levels of Serum ALT and AST and the Ameliorative Effect of GA
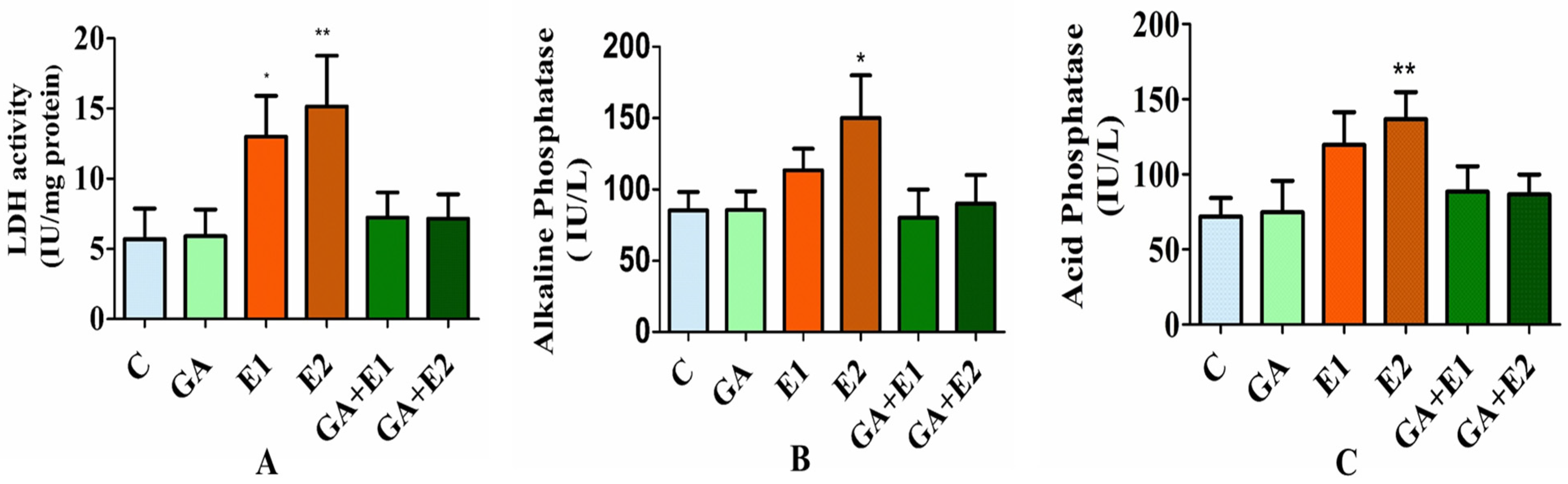
3.7. Impact of CORT and GA on the Activities of LDH, ALP, and ACP in Rat Blood Serum
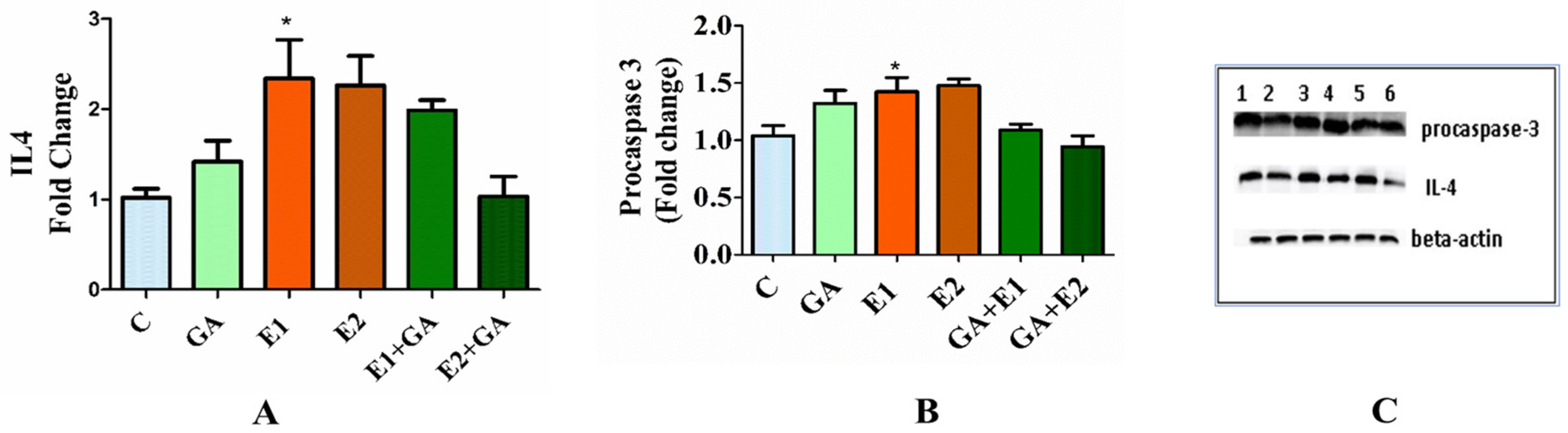
3.8. Western Analysis of the Expression Levels of Procaspase-3 and Interleukin-4 (IL-4) in the Animals Treated with CORT and GA
3.9. Immunohistochemical Analysis of the Impact of CORT and GA on IL-4
3.10. Impact of CORT and the Ameliorative Effect of GA on the Histological Status of Rat Liver as Observed by DAPI and H&E Staining
3.11. In Silico Molecular Docking Analysis of GA with Keap1, IKKβ, and COX-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCORT | Corticosterone |
| GGA | Gallic acid |
| IIL-4 | Interleukin-4 |
| kKeap-1 | Kelch-like ECH-associated protein 1 |
| IIKKβ | IκB kinase β |
| CCOX-1 | Cyclooxygenase-1 |
| GGCs | Glucocorticoids |
References
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Chalitsios, C.V.; Shaw, D.E.; McKeever, T.M. A retrospective database study of oral corticosteroid and bisphosphonate prescribing patterns in England. NPJ Prim. Care Respir. Med. 2020, 30, 5. [Google Scholar] [CrossRef]
- Burén, J.; Lai, Y.-C.; Lundgren, M.; Eriksson, J.W.; Jensen, J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch. Biochem. Biophys. 2008, 474, 91–101. [Google Scholar] [CrossRef]
- Movahedi, M.; Beauchamp, M.E.; Abrahamowicz, M.; Ray, D.W.; Michaud, K.; Pedro, S.; Dixon, W.G. Risk of incident diabetes mellitus associated with the dosage and duration of oral glucocorticoid therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 1089–1098. [Google Scholar] [CrossRef]
- Dos Santos, C.; da Silva, J.S.; Brunetta, H.S.; Chagas, T.R.; Zoccal, D.B.; Nunes, E.A.; Rafacho, A. Impact of combined long-term fructose and prednisolone intake on glucose and lipid homeostasis in rats: Benefits of intake interruption or fish oil administration. J. Nutr. Biochem. 2021, 90, 108572. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Z.; Dong, Y.; Ni, Y.; Zhao, R.; Ma, W. Chronic corticosterone exposure suppresses copper transport through GR-mediated intestinal CTR1 pathway in mice. Biology 2023, 12, 197. [Google Scholar] [CrossRef]
- Tiwari, P.; Singh, N.; Sharma, B. Long term treatment of corticostreroids may cause hepatotoxicity and oxidative damage: A case controlled study. Indian J. Clin. Biochem. 2024, 39, 179–187. [Google Scholar] [CrossRef]
- Wang, G.; Li, S.; Li, Y.; Zhang, M.; Xu, T.; Li, T.; Cao, L.; Lu, J. Corticosterone induces obesity partly via promoting intestinal cell proliferation and survival. Front. Endocrinol. 2023, 13, 1052487. [Google Scholar] [CrossRef] [PubMed]
- Chimin, P.; Farias, T.d.S.; Torres-Leal, F.; Bolsoni-Lopes, A.; Campaña, A.; Andreotti, S.; Lima, F. Chronic glucocorticoid treatment enhances lipogenic activity in visceral adipocytes of male Wistar rats. Acta Physiol. 2014, 211, 409–420. [Google Scholar] [CrossRef]
- Harno, E.; Sefton, C.; Wray, J.R.; Allen, T.-J.; Davies, A.; Coll, A.P.; White, A. Chronic glucocorticoid treatment induces hepatic lipid accumulation and hyperinsulinaemia in part through actions on AgRP neurons. Sci. Rep. 2021, 11, 13776. [Google Scholar] [CrossRef]
- Paccou, J.; Yavropoulou, M.P.; Naciu, A.M.; Chandran, M.; Messina, O.D.; Rolvien, T.; Carey, J.J.; D’oronzo, S.; Anastasilakis, A.D.; Saag, K.G. Prevention and treatment of glucocorticoid-induced osteoporosis in adults: Recommendations from the European Calcified Tissue Society. Eur. J. Endocrinol. 2024, 191, G1–G17. [Google Scholar] [CrossRef]
- de Souza, L.B.; Brandão, A.B.P.; Ferreira, F.G.; Guzzoni, V.; Albuquerque, R.C.; Mendes, L.A.; Yokota, R.; Porto, F.G.; Casarini, D.E.; Aimbire, F. Lacticaseibacillus rhamnosus and Limosilactobacillus reuteri probiotics modulate inflammation, attenuating chronic stress-induced liver injury and behavior in rats. J. Nutr. 2025. [Google Scholar] [CrossRef]
- Xi, H.; Chen, H.; Fu, J.; He, S.; Liu, X.; Sun, G.; Du, B. Traditional Chinese medicine Youguiyin decoction ameliorate glucocorticoid-induced osteonecrosis in rat by modulating ROS/PHD2/HIF-1α oxidative stress signaling pathway in bone marrow mesenchymal stem cells. Chin. Med. 2025, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.; Ferreira, F.B.D.; Goncalves-Neto, L.M.; Taboga, S.R.; Boschero, A.C.; Rafacho, A. Age-and gender-related changes in glucose homeostasis in glucocorticoid-treated rats. Can. J. Physiol. Pharmacol. 2014, 92, 867–878. [Google Scholar] [CrossRef]
- Suh, S.; Park, M.K. Glucocorticoid-induced diabetes mellitus: An important but overlooked problem. Endocrinol. Metab. 2017, 32, 180–189. [Google Scholar] [CrossRef]
- Vander Kooi, B.T.; Onuma, H.; Oeser, J.K.; Svitek, C.A.; Allen, S.R.; Vander Kooi, C.W.; Chazin, W.J.; O’Brien, R.M. The glucose-6-phosphatase catalytic subunit gene promoter contains both positive and negative glucocorticoid response elements. Mol. Endocrinol. 2005, 19, 3001–3022. [Google Scholar] [CrossRef] [PubMed]
- Aponte, Y.; Atasoy, D.; Sternson, S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011, 14, 351–355. [Google Scholar] [CrossRef]
- Andrews, R.C.; Walker, B.R. Glucocorticoids and insulin resistance: Old hormones, new targets. Clin. Sci. 1999, 96, 513–523. [Google Scholar] [CrossRef]
- Pasieka, A.M.; Rafacho, A. Impact of glucocorticoid excess on glucose tolerance: Clinical and preclinical evidence. Metabolites 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Rafacho, A.; Ortsäter, H.; Nadal, A.; Quesada, I. Author copy only. J. Endocrinol. 2014, 223, R49–R62. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Bassil, K.; Krontira, A.C.; Leroy, T.; Escoto, A.I.; Snijders, C.; Pernia, C.D.; Pasterkamp, R.J.; de Nijs, L.; van den Hove, D.; Kenis, G. In vitro modeling of the neurobiological effects of glucocorticoids: A review. Neurobiol. Stress 2023, 23, 100530. [Google Scholar] [CrossRef]
- Shirazi, S.N.; Friedman, A.R.; Kaufer, D.; Sakhai, S.A. Glucocorticoids and the brain: Neural mechanisms regulating the stress response. Glucocorticoid Signal. Mol. Mice Man 2015, 872, 235–252. [Google Scholar]
- Lesuis, S.L.; Weggen, S.; Baches, S.; Lucassen, P.J.; Krugers, H.J. Targeting glucocorticoid receptors prevents the effects of early life stress on amyloid pathology and cognitive performance in APP/PS1 mice. Transl. Psychiatry 2018, 8, 53. [Google Scholar] [CrossRef]
- Zafir, A.; Banu, N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress 2009, 12, 167–177. [Google Scholar] [CrossRef]
- Joung, J.Y.; Cho, J.H.; Kim, Y.H.; Choi, S.H.; Son, C.G. A literature review for the mechanisms of stress-induced liver injury. Brain Behav. 2019, 9, e01235. [Google Scholar] [CrossRef] [PubMed]
- Fouad, D.; Shuker, E.; Farhood, M. Renal toxicity of methylprednisolone in male Wistar rats and the potential protective effect by boldine supplementation. J. King Saud Univ.-Sci. 2023, 35, 102381. [Google Scholar] [CrossRef]
- Ohtsuka, A.; Kojima, H.; Ohtani, T.; HAYASHI, K. Vitamin E reduces glucocorticoid-induced oxidative stress in rat skeletal muscle. J. Nutr. Sci. Vitaminol. 1998, 44, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Rajashree, S.; Puvanakrishnan, R. Dexamethasone induced alterations in enzymatic and nonenzymatic antioxidant status in heart and kidney of rats. Mol. Cell. Biochem. 1998, 181, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Orzechowski, A.; Jank, M.; Gajkowska, B.; Sadkowski, T.; Godlewski, M.M.; Ostaszewski, P. Delineation of signalling pathway leading to antioxidant-dependent inhibition of dexamethasone-mediated muscle cell death. J. Muscle Res. Cell Motil. 2003, 24, 33–53. [Google Scholar] [CrossRef]
- Pereira, B.; Bechara, E.J.H.; Mendonca, J.; Curi, R. Superoxide dismutase, catalase and glutathione peroxidase activities in the lymphoid organs and skeletal muscles of rats treated with dexamethasone. Cell Biochem. Funct. 1999, 17, 15–19. [Google Scholar] [CrossRef]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef]
- Cippitelli, M.; Sica, A.; Viggiano, V.; Ye, J.; Ghosh, P.; Birrer, M.J.; Young, H.A. Negative transcriptional regulation of the interferon-γ promoter by glucocorticoids and dominant negative mutants of c-Jun. J. Biol. Chem. 1995, 270, 12548–12556. [Google Scholar] [CrossRef]
- Vandevyver, S.; Dejager, L.; Libert, C. Comprehensive overview of the structure and regulation of the glucocorticoid receptor. Endocr. Rev. 2014, 35, 671–693. [Google Scholar] [CrossRef] [PubMed]
- Smoak, K.; Cidlowski, J.A. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol. Cell. Biol. 2006, 26, 9126–9135. [Google Scholar] [CrossRef] [PubMed]
- Wiegers, G.J.; Reul, J.M. Induction of cytokine receptors by glucocorticoids: Functional and pathological significance. Trends Pharmacol. Sci. 1998, 19, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Aoudjehane, L.; Pissaia, A., Jr.; Scatton, O.; Podevin, P.; Massault, P.-P.; Chouzenoux, S.; Soubrane, O.; Calmus, Y.; Conti, F. Interleukin-4 induces the activation and collagen production of cultured human intrahepatic fibroblasts via the STAT-6 pathway. Lab. Investig. 2008, 88, 973–985. [Google Scholar] [CrossRef]
- Keegan, A.D.; Leonard, W.J.; Zhu, J. Recent advances in understanding the role of IL-4 signaling. Fac. Rev. 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.A.; Kanagawa, O.; Sleckman, B.P.; Muglia, L.J. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J. Immunol. 2002, 169, 1837–1843. [Google Scholar] [CrossRef]
- Siegel, R.M.; Katsumata, M.; Miyashita, T.; LouIE, D.C.; Greene, M.I.; Reed, J.C. Inhibition of thymocyte apoptosis and negative antigenic selection in bcl-2 transgenic mice. Proc. Natl. Acad. Sci. USA 1992, 89, 7003–7007. [Google Scholar] [CrossRef]
- Boudreau, M.W.; Peh, J.; Hergenrother, P.J. Procaspase-3 overexpression in cancer: A paradoxical observation with therapeutic potential. ACS Chem. Biol. 2019, 14, 2335–2348. [Google Scholar] [CrossRef]
- Gupta, V.K.; Park, U.; Kim, E.; Kim, S.; Siddiqi, N.J.; Huh, Y.S.; Sharma, B. Antioxidative effect of Aloe vera against malathion induced neurotoxic response in Wistar rats. Arab. J. Chem. 2023, 16, 105169. [Google Scholar] [CrossRef]
- Keyvani-Ghamsari, S.; Rahimi, M.; Khorsandi, K. An update on the potential mechanism of gallic acid as an antibacterial and anticancer agent. Food Sci. Nutr. 2023, 11, 5856–5872. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Deng, X.; Jian, S.; Zhang, M.; Wen, C.; Xin, Z.; Zhang, L.; Tong, A.; Ye, S.; Liao, P. Gallic acid alleviates gut dysfunction and boosts immune and antioxidant activities in puppies under environmental stress based on microbiome–metabolomics analysis. Front. Immunol. 2022, 12, 813890. [Google Scholar] [CrossRef] [PubMed]
- Latief, U.; Husain, H.; Mukherjee, D.; Ahmad, R. Hepatoprotective efficacy of gallic acid during Nitrosodiethylamine-induced liver inflammation in Wistar rats. J. Basic Appl. Zool. 2016, 76, 31–41. [Google Scholar] [CrossRef]
- Feng, R.-B.; Wang, Y.; He, C.; Yang, Y.; Wan, J.-B. Gallic acid, a natural polyphenol, protects against tert-butyl hydroperoxide-induced hepatotoxicity by activating ERK-Nrf2-Keap1-mediated antioxidative response. Food Chem. Toxicol. 2018, 119, 479–488. [Google Scholar] [CrossRef]
- Bolat, M.; Tekin, S.; Bolat, İ.; Atasever, A.; Çinar, B.; Dağ, Y.; Şengül, E.; Yildirim, S.; Warda, M.; Çelebi, F. Gallic acid’s protective mechanisms against acrylamide-induced pulmonary injury: In vivo and in silico insights into the Nrf-2/HO-1/NFκB pathway modulation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 11821–11837. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gl, E. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar]
- Niehaus, W., Jr.; Samuelsson, B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968, 6, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Siddiqi, N.J.; Alrashood, S.T.; Khan, H.A.; Dubey, A.; Sharma, B. Protective effect of eugenol on hepatic inflammation and oxidative stress induced by cadmium in male rats. Biomed. Pharmacother. 2021, 139, 111588. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative stress indexes for diagnosis of health or disease in humans. Oxidative Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Goldberg, A.F.; Barka, T. Acid phosphatase activity in human blood cells. Nature 1962, 195, 297. [Google Scholar] [CrossRef]
- Kornberg, A.; Horecker, B.; Smyrniotis, P. [42] Glucose-6-phosphate dehydrogenase 6-phosphogluconic dehydrogenase. Methods Enzymol. 1955, 1, 323–327. [Google Scholar]
- Giordano, R.; Guaraldi, F.; Berardelli, R.; Karamouzis, I.; D’Angelo, V.; Marinazzo, E.; Picu, A.; Ghigo, E.; Arvat, E. Glucose metabolism in patients with subclinical Cushing’s syndrome. Endocrine 2012, 41, 415–423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Esmaeilzadeh, M.; Heidarian, E.; Shaghaghi, M.; Roshanmehr, H.; Najafi, M.; Moradi, A.; Nouri, A. Gallic acid mitigates diclofenac-induced liver toxicity by modulating oxidative stress and suppressing IL-1β gene expression in male rats. Pharm. Biol. 2020, 58, 590–596. [Google Scholar] [CrossRef]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1α interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Lazar, M.A.; Birnbaum, M.J. Unraveling the regulation of hepatic metabolism by insulin. Trends Endocrinol. Metab. 2017, 28, 497–505. [Google Scholar] [CrossRef]
- Nakamura, H.; Fujieda, Y.; Nakamura, A.; Atsumi, T. How should rheumatologists manage glucocorticoid-induced hyperglycemia? Mod. Rheumatol. 2021, 31, 519–528. [Google Scholar] [CrossRef]
- Vegiopoulos, A.; Herzig, S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007, 275, 43–61. [Google Scholar] [CrossRef]
- Li, J.-X.; Cummins, C.L. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat. Rev. Endocrinol. 2022, 18, 540–557. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Rahman, M.; Anjum, F. Fludrocortisone. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef]
- Sun, X.; Luo, W.; Tan, X.; Li, Q.; Zhao, Y.; Zhong, W.; Sun, X.; Brouwer, C.; Zhou, Z. Increased plasma corticosterone contributes to the development of alcoholic fatty liver in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 305, G849–G861. [Google Scholar] [CrossRef]
- Fujita, C.; Ichikawa, F.; Teratani, T.; Murakami, G.; Okada, T.; Shinohara, M.; Kawato, S.; Ohta, Y. Direct effects of corticosterone on ATP production by mitochondria from immortalized hypothalamic GT1-7 neurons. J. Steroid Biochem. Mol. Biol. 2009, 117, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, H.; Li, D.; Chen, X.; Ao, M.; Jin, W.; Yu, L. N-(3-Methozybenzyl)-(9Z, 12Z, 15Z)-octadecatrienamide from maca (Lepidium meyenii Walp.) ameliorates corticosterone-induced testicular toxicity in rats. Food Funct. 2020, 11, 7762–7774. [Google Scholar] [CrossRef]
- Dirks-Naylor, A.J.; Griffiths, C.L. Glucocorticoid-induced apoptosis and cellular mechanisms of myopathy. J. Steroid Biochem. Mol. Biol. 2009, 117, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scheinman, R.I.; Cogswell, P.C.; Lofquist, A.K.; Baldwin, A.S., Jr. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science 1995, 270, 283–286. [Google Scholar] [CrossRef]
- Schakman, O.; Gilson, H.; Thissen, J.-P. Mechanisms of glucocorticoid-induced myopathy. J. Endocrinol. 2008, 197, 1–10. [Google Scholar] [CrossRef]
- Vojnović Milutinović, D.; Teofilović, A.; Veličković, N.; Brkljačić, J.; Jelača, S.; Djordjevic, A.; Macut, D. Glucocorticoid signaling and lipid metabolism disturbances in the liver of rats treated with 5α-dihydrotestosterone in an animal model of polycystic ovary syndrome. Endocrine 2021, 72, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, A.; Popov, V. Eukaryotic cell membranes: Structure, composition, research methods and computational modelling. Int. J. Mol. Sci. 2023, 24, 11226. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Meena, A.S.; Dalal, K.; Canelas, C.; Samak, G.; Pierre, J.F.; Rao, R. Chronic stress and corticosterone exacerbate alcohol-induced tissue injury in the gut-liver-brain axis. Sci. Rep. 2021, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, L.; Rajpal, A.; Ismail-Beigi, F. Glucocorticoid-induced fatty liver disease. Diabetes Metab. Syndr. Obes. 2020, 13, 1133–1145. [Google Scholar] [CrossRef]
- Goh, Y.S.; Henderson, N.C.; Heredia, J.E.; Red Eagle, A.; Odegaard, J.I.; Lehwald, N.; Nguyen, K.D.; Sheppard, D.; Mukundan, L.; Locksley, R.M. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9914–9919. [Google Scholar] [CrossRef]




| S. No. | Parameters | Control | GA | E1 | E2 | GA + E1 | GA + E2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Days | 7 | 14 | 21 | 7 | 14 | 21 | 7 | 14 | 21 | 7 | 14 | 21 | 7 | 14 | 21 | 7 | 14 | 21 |
| 2. | Weight (g) | 160 | 170 | 178 | 150 | 165 | 155 | 175 | 185 | 200 | 175 | 200 | 215 | 160 | 170 | 180 | 150 | 158 | 165 |
| 3. | Temperature (°C) | 98 | 97 | 97 | 98 | 97 | 98 | 98 | 98 | 99 | 98 | 99 | 98 | 97 | 99 | 98 | 97 | 98 | 98 |
| 4. | Locomotion | + | ++ | ++ | + | ++ | +++ | ++ | + | + | ++ | + | + | ++ | + | ++ | + | + | ++ |
| 5. | Water intake | + | ++ | ++ | + | ++ | ++ | + | ++ | +++ | + | ++ | +++ | + | + | ++ | + | + | ++ |
| 6. | Thigmotactic Response | + | ++ | ++ | + | ++ | ++ | + | + | + | + | + | + | + | ++ | ++ | + | ++ | ++ |
| 7. | Food intake | + | ++ | ++ | + | ++ | ++ | + | ++ | +++ | + | ++ | +++ | + | ++ | ++ | + | ++ | ++ |
| 8. | Rest and sleep | + | ++ | ++ | + | ++ | ++ | + | + | +++ | + | + | +++ | + | ++ | ++ | + | ++ | ++ |
| 9. | Hyperactivity | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - |
| 10. | Aggression | - | - | - | - | - | - | - | - | + | - | + | - | - | - | - | - | - | - |
| 11. | Biting | - | - | - | - | - | - | + | + | + | + | + | + | + | + | - | + | + | - |
| 12. | Rubbing | + | + | + | + | + | + | + | + | ++ | + | + | ++ | + | + | + | + | + | + |
| 13. | Alertness | + | + | + | + | + | + | - | - | - | - | - | - | - | - | + | - | - | + |
| Groups | C | GA | E1 | E2 | GA + E1 | GA + E2 |
|---|---|---|---|---|---|---|
| Protein (mg g1 tissue) | 70 ± 3.73 | 72 ± 2.27 ns | 75 ± 3.53 ns | 75 ± 3.97 ns | 74 ± 2.18 ns | 74 ± 2.32 ns |
| GSH (µg mg−1 of protein) | 1.86 ± 0.15 | 1.81 ± 0.23 | 1.09 ± 0.25 | 0.89 ± 0.32 ** | 1.76 ± 0.20 | 1.20 ± 0.39 |
| MDA (n mol mg−1 protein) | 18.25 ± 1.40 | 17.90 ± 1.90 | 22.10 ± 2.30 | 25.01 ± 3.21 * | 19.17 ± 2.31 | 20.10 ± 2.90 |
| PCO (nmol mg−1 of protein) | 0.45 ± 0.15 | 0.40 ± 0.14 | 0.57 ± 0.15 | 0.79 ± 0.19 * | 0.43 ± 0.17 | 0.51 ± 0.14 |
| Parameter | C (×10−3) | GA (×10−3) | E1 (×10−3) | E2 (×10−3) | GA + E1 (×10−3) | GA + E2 (×10−3) |
|---|---|---|---|---|---|---|
| OSI | 0.150 | 0.147 | 0.255 (+70%) | 0.357 (+122%) | 0.163 (+8%) | 0.200 (+33%) |
| Parameter | C | GA | E1 | E2 | E1 + GA | E1 + GA |
|---|---|---|---|---|---|---|
| Intensity of IL-4 antibody | - | + | ++ | +++ | + | + |
| Liver Architectural Parameters | C | GA | E1 | E2 | GA + E1 | GA + E2 |
|---|---|---|---|---|---|---|
| CNH | - | - | ++ | +++ | + | ++ |
| CCv | - | - | ++ | +++ | - | + |
| DH | - | - | ++ | +++ | + | + |
| DHt | - | - | ++ | ++ | + | + |
| H | - | - | + | ++ | - | - |
| ND | - | - | ++ | +++ | + | + |
| VD | - | - | ++ | +++ | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, P.; Kumar, P.; Srikrishna, S.; Siddiqi, N.J.; Sharma, B. Protective Role of Gallic Acid Against Corticosterone-Induced Hepatic Toxicity: Modulation of Oxidative Stress and Inflammatory Pathways in Wistar Rats. Toxics 2025, 13, 897. https://doi.org/10.3390/toxics13100897
Tiwari P, Kumar P, Srikrishna S, Siddiqi NJ, Sharma B. Protective Role of Gallic Acid Against Corticosterone-Induced Hepatic Toxicity: Modulation of Oxidative Stress and Inflammatory Pathways in Wistar Rats. Toxics. 2025; 13(10):897. https://doi.org/10.3390/toxics13100897
Chicago/Turabian StyleTiwari, Priyanka, Prabhat Kumar, Saripella Srikrishna, Nikhat Jamal Siddiqi, and Bechan Sharma. 2025. "Protective Role of Gallic Acid Against Corticosterone-Induced Hepatic Toxicity: Modulation of Oxidative Stress and Inflammatory Pathways in Wistar Rats" Toxics 13, no. 10: 897. https://doi.org/10.3390/toxics13100897
APA StyleTiwari, P., Kumar, P., Srikrishna, S., Siddiqi, N. J., & Sharma, B. (2025). Protective Role of Gallic Acid Against Corticosterone-Induced Hepatic Toxicity: Modulation of Oxidative Stress and Inflammatory Pathways in Wistar Rats. Toxics, 13(10), 897. https://doi.org/10.3390/toxics13100897








