Tert-Butylhydroquinone (TBHQ) Suppresses LPS- and Poly (I:C)-Induced RAW 264.7 Macrophage Activation Through Reduced NF-κB/Type 1 Interferon and Enhanced Antioxidant-Related Pathways
Abstract
1. Introduction
2. Methods and Materials
2.1. Cell Culture
2.2. Chemicals/Treatments
2.3. Initial Dose-Dependent Screening Assays to Determine TBHQ Cytotoxicity
2.4. RNA Isolation for Bulk RNAseq Analysis
2.5. Sample Preparation and mRNA Sequencing
2.6. RNAseq Data Analysis
2.7. Western Blotting for Protein Expression in Comparison to RNAseq Data
2.8. Statistical Analysis
3. Results
3.1. Cytotoxicity Assays
3.2. TBHQ Suppresses Proinflammatory Properties of Activated RAW 264.7 Macrophages
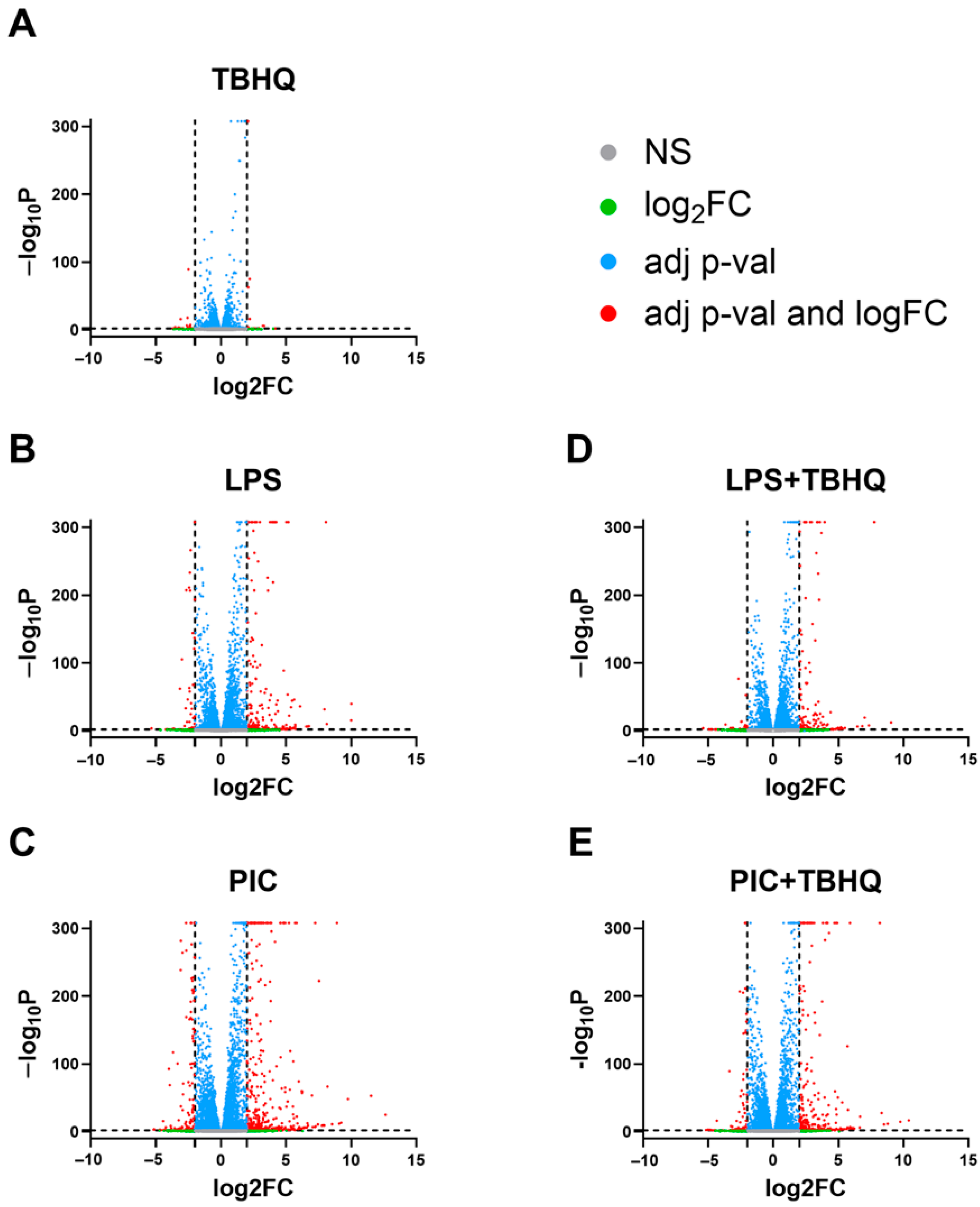
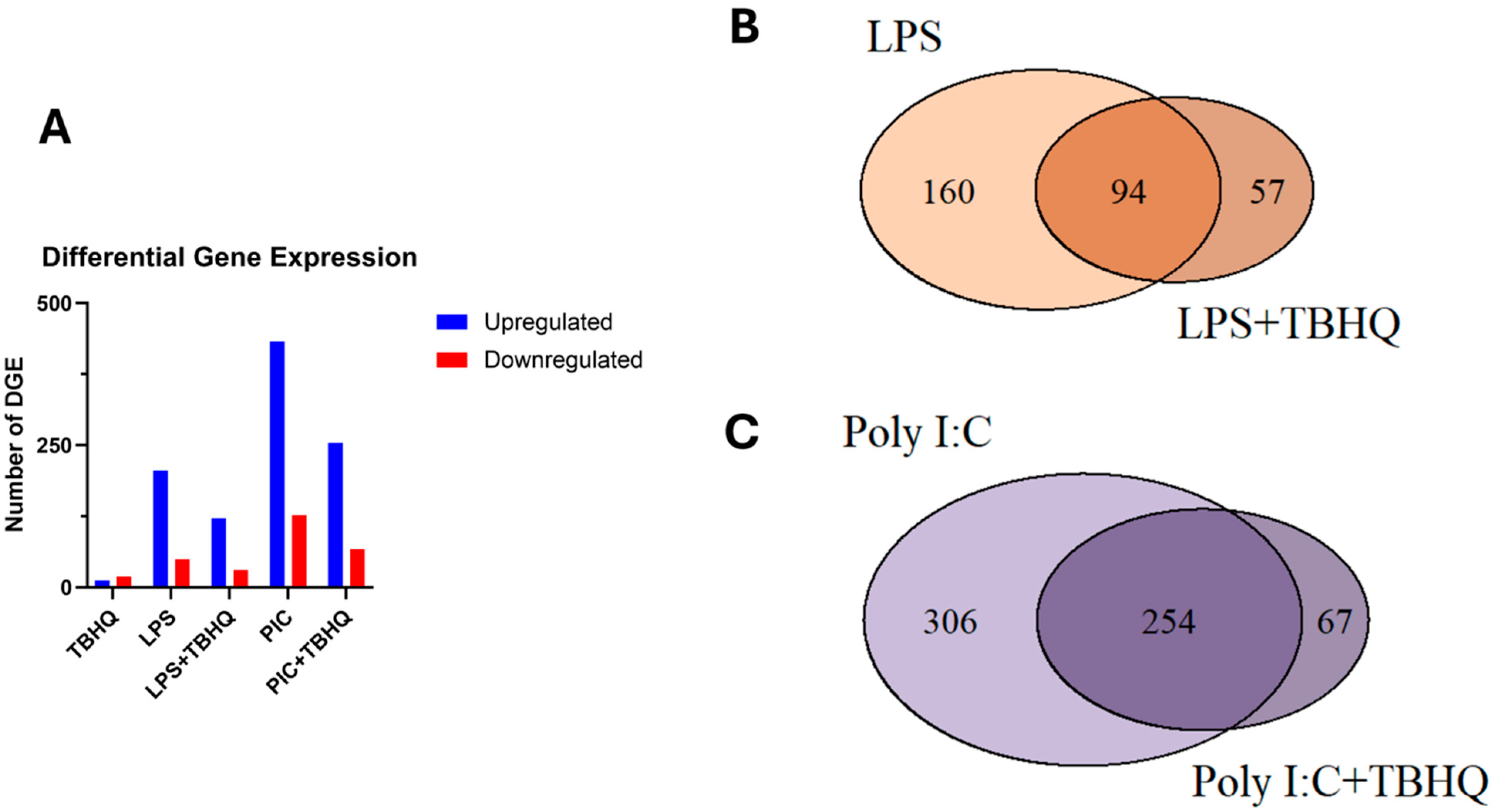
3.3. LPS- and PIC-Stimulated Macrophages Show Reduced Differential Gene Expression When Exposed to TBHQ
3.4. Inflammatory and Cellular Metabolic Profiles Differ Between Treated Macrophages
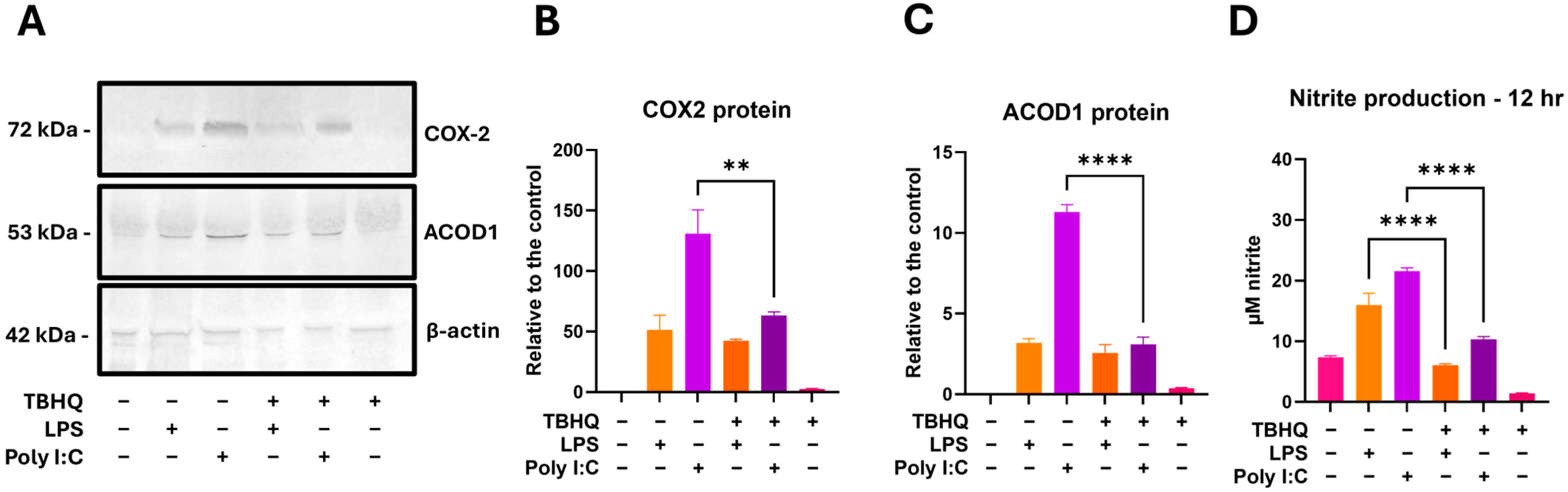
3.5. TBHQ Attenuates Inflammatory Protein Expression and Nitric Oxide Production at 12 H
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175. [Google Scholar] [CrossRef]
- Pressman, P.; Clemens, R.; Hayes, W.; Reddy, C. Food additive safety: A review of toxicologic and regulatory issues. Toxicol. Res. Appl. 2017, 1, 2397847317723572. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Esazadeh, K.; Ezzati Nazhad Dolatabadi, J.; Andishmand, H.; Mohammadzadeh-Aghdash, H.; Mahmoudpour, M.; Naemi Kermanshahi, M.; Roosta, Y. Cytotoxic and genotoxic effects of tert-butylhydroquinone, butylated hydroxyanisole and propyl gallate as synthetic food antioxidants. Food Sci. Nutr. 2024, 12, 7004–7016. [Google Scholar] [CrossRef]
- Khezerlou, A.; Akhlaghi, A.P.; Alizadeh, A.M.; Dehghan, P.; Maleki, P. Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review. Toxicol. Rep. 2022, 9, 1066–1075. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Z.; Liu, Y. Lipophilic antioxidants in edible oils: Mechanisms, applications and interactions. Food Res. Int. 2025, 200, 115423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, Y.; Shi, Y. Antioxidant Consumption Kinetics and Shelf-Life Prediction for Biodiesel Stabilized with Antioxidants Using the Rancimat Method. Energy Fuels 2016, 30, 10534–10542. [Google Scholar] [CrossRef]
- Li, Z.J.; Yang, F.J.; Yang, L.; Zu, Y.G. Comparison of the antioxidant effects of carnosic acid and synthetic antioxidants on tara seed oil. Chem. Cent. J. 2018, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, N.; Haggarty, S.; El-Kadi, A.O. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr. Drug Metab. 2007, 8, 1–7. [Google Scholar] [CrossRef]
- World Health Organization. Safety Evaluation of Certain Food Additive and Contaminants; World Health Organization: Geneva, Switzerland, 1999; Volume 42, pp. 453–459. [Google Scholar]
- Botterweck, A.A.M.; Verhagen, H.; Goldbohm, R.A.; Kleinjans, J.; van den Brandt, P.A. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: Results from analyses in The Netherlands Cohort Study. Food Chem. Toxicol. 2000, 38, 599–605. [Google Scholar] [CrossRef]
- Engelhardt, J.A.; Athanassiadis, I.; Leonards, P.E.G.; Weiss, J.M. Multi-target analysis of synthetic phenolic compounds in human blood. Talanta 2025, 292, 127899. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Shi, X.; Zhang, Q.; Ren, Y.; Chen, J.; Wang, H.; Wang, W.; Wang, Q. The reaction laws and toxicity effects of synthetic phenolic antioxidants (SPAs) in wastewater and atmosphere: A theoretical investigation. Process Saf. Environ. Prot. 2025, 200, 107324. [Google Scholar] [CrossRef]
- Australian Industrial Chemicals Introduction Scheme. Butylated Hydroxyanisole and Related Antioxidants; Australian Industrial Chemicals Introduction Scheme: Sydney, NSW, Australia, 2022; pp. 1–25. [Google Scholar]
- Hoang, N.M.H.; Park, K. Applications of Tert-Butyl-Phenolic Antioxidants in Consumer Products and Their Potential Toxicities in Humans. Toxics 2024, 12, 869. [Google Scholar] [CrossRef] [PubMed]
- van Esch, G.J. Toxicology of tert-Butylhydroquinone (TBHQ). Food Chem. Toxicol. 1986, 24, 1063–1065. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of t-Butylhydroquinone in F344/N Rats and B6C3F1 Mice; National Institutes of Health: Research Triangle Park, NC, USA, 1997. [Google Scholar]
- Hahn, M.E.; McArthur, A.G.; Karchner, S.I.; Franks, D.G.; Jenny, M.J.; Timme-Laragy, A.R.; Stegeman, J.J.; Woodin, B.R.; Cipriano, M.J.; Linney, E. The transcriptional response to oxidative stress during vertebrate development: Effects of tert-butylhydroquinone and 2,3,7,8-tetrachlorodibenzo-p-dioxin. PLoS ONE 2014, 9, e113158. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Yildirim, S.; Ghosigharehaghaji, A.; Bolat, İ.; Sulukan, E.; Ceyhun, S.B. An approach to evaluating the potential teratogenic and neurotoxic mechanism of BHA based on apoptosis induced by oxidative stress in zebrafish embryo (Danio rerio). Hum. Exp. Toxicol. 2021, 40, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Leonard, E.R.; Marques, E.S.; Roy, M.A.; Conlin, S.M.; Ranjan, R.; Timme-Laragy, A.R. Dietary exposure to the food preservative tert-Butylhydroquinone (tBHQ) impairs zebrafish (Danio rerio) survival, growth, organ development, and gene expression in Nrf2a-dependent and independent ways. Food Chem. Toxicol. 2023, 176, 113788. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Wang, W.; Zhou, Q.; Shi, G.; Wei, F.; Jiang, G. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total Environ. 2018, 643, 559–568. [Google Scholar] [CrossRef]
- Wufuer, R.; Fan, Z.; Liu, K.; Zhang, Y. Differential Yet Integral Contributions of Nrf1 and Nrf2 in the Human HepG2 Cells on Antioxidant Cytoprotective Response against Tert-Butylhydroquinone as a Pro-Oxidative Stressor. Antioxidants 2021, 10, 1610. [Google Scholar] [CrossRef]
- Zagorski, J.W.; Turley, A.E.; Dover, H.E.; VanDenBerg, K.R.; Compton, J.R.; Rockwell, C.E. The Nrf2 Activator, tBHQ, Differentially Affects Early Events Following Stimulation of Jurkat Cells. Toxicol. Sci. 2013, 136, 63–71. [Google Scholar] [CrossRef]
- Lazaro, I.; Lopez-Sanz, L.; Bernal, S.; Oguiza, A.; Recio, C.; Melgar, A.; Jimenez-Castilla, L.; Egido, J.; Madrigal-Matute, J.; Gomez-Guerrero, C. Nrf2 Activation Provides Atheroprotection in Diabetic Mice Through Concerted Upregulation of Antioxidant, Anti-inflammatory, and Autophagy Mechanisms. Front. Pharmacol. 2018, 9, 2018. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, B.R.; Hansen, J.M. Tert-butylhydroquinone induces mitochondrial oxidative stress causing Nrf2 activation. Cell Biol. Toxicol. 2010, 26, 541–551. [Google Scholar] [CrossRef]
- Rockwell, C.E.; Zhang, M.; Fields, P.E.; Klaassen, C.D. Th2 Skewing by Activation of Nrf2 in CD4+ T Cells. J. Immunol. 2012, 188, 1630–1637. [Google Scholar] [CrossRef]
- Jin, Y.; Boss, A.P.; Bursley, J.K.; Wilson, C.; Gangur, V.; Rockwell, C.E. The transcription factor Nrf2 links Th2-mediated experimental allergy to food preservatives. Front. Immunol. 2025, 15, 2024. [Google Scholar] [CrossRef]
- Boss, A.P.; Freeborn, R.A.; Duriancik, D.M.; Kennedy, R.C.; Gardner, E.M.; Rockwell, C.E. The Nrf2 activator tBHQ inhibits the activation of primary murine natural killer cells. Food Chem. Toxicol. 2018, 121, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, N.; El-Kadi, A.O. tert-Butylhydroquinone is a novel aryl hydrocarbon receptor ligand. Drug Metab. Dispos. 2005, 33, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Zhao, W.; Liu, M.; Liu, L.; Wang, Y. TBHQ-Overview of Multiple Mechanisms against Oxidative Stress for Attenuating Methamphetamine-Induced Neurotoxicity. Oxidative Med. Cell. Longev. 2020, 2020, 8874304. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct Cysteine Residues in Keap1 Are Required for Keap1-Dependent Ubiquitination of Nrf2 and for Stabilization of Nrf2 by Chemopreventive Agents and Oxidative Stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef]
- Zagorski, J.W.; Turley, A.E.; Freeborn, R.A.; VanDenBerg, K.R.; Dover, H.E.; Kardell, B.R.; Liby, K.T.; Rockwell, C.E. Differential effects of the Nrf2 activators tBHQ and CDDO-Im on the early events of T cell activation. Biochem. Pharmacol. 2018, 147, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Janeway, C.A. How the immune system protects the host from infection. Microbes Infect. 2001, 3, 1167–1171. [Google Scholar] [CrossRef]
- Moratilla-Rivera, I.; Sánchez, M.; Valdés-González, J.A.; Gómez-Serranillos, M.P. Natural Products as Modulators of Nrf2 Signaling Pathway in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 3748. [Google Scholar] [CrossRef]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Babcock, A.S.; Anderson, A.L.; Rice, C.D. Indirubin-3′-(2,3 dihydroxypropyl)-oximether (E804) is a potent modulator of LPS-stimulated macrophage functions. Toxicol. Appl. Pharmacol. 2013, 266, 157–166. [Google Scholar] [CrossRef]
- Dietert, R.R.; Hotchkiss, J.H.; Austic, R.E.; Sung, Y.-J. Production of reactive nitrogen intermediates by macrophages. In Methods in Immunotoxicology; Burleson, G.R., Dean, J.H., Munson, A.E., Eds.; Wiley-Liss, Inc.: New York, NY, USA, 1995; Volume 2, pp. 99–117. [Google Scholar]
- Nguyen, T.; Sherratt, P.J.; Nioi, P.; Yang, C.S.; Pickett, C.B. Nrf2 Controls Constitutive and Inducible Expression of ARE-driven Genes through a Dynamic Pathway Involving Nucleocytoplasmic Shuttling by Keap1*. J. Biol. Chem. 2005, 280, 32485–32492. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Q.; Kim, M.Y.; Godoy, L.C.; Thiantanawat, A.; Trudel, L.J.; Wogan, G.N. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14547–14551. [Google Scholar] [CrossRef]
- Anderson, A.L.; Dubanksy, B.D.; Wilson, L.B.; Tanguay, R.L.; Rice, C.D. Development and Applications of a Zebrafish (Danio rerio) CYP1A-Targeted Monoclonal Antibody (CRC4) with Reactivity across Vertebrate Taxa: Evidence for a Conserved CYP1A Epitope. Toxics 2022, 10, 404. [Google Scholar] [CrossRef]
- Margiotta, A.L.; Bain, L.J.; Rice, C.D. Expression of the Major Vault Protein (MVP) and Cellular Vault Particles in Fish. Anat. Rec. 2017, 300, 1981–1992. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species 2019, 8, 312–322. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Trask, O.J., Jr. Translocation Assay Development and Validation for High Content Screening; Markossian, S., Grossman, A., Baskir, H., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2012. [Google Scholar]
- Reimer, T.; Brcic, M.; Schweizer, M.; Jungi, T.W. poly(I:C) and LPS induce distinct IRF3 and NF-κB signaling during type-I IFN and TNF responses in human macrophages. J. Leukoc. Biol. 2008, 83, 1249–1257. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Yang, Z.-X.; Guo, D.-Y.; Shen, L.-Z.; Mao, G.-X.; Dai, J.-H.; Chen, S.-S.; Yan, J. Transcriptome profiling of poly(I:C)-induced RAW 264.7 mouse macrophages in response to panaxadiol. Biologia 2019, 74, 1385–1394. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, H.-J.; Lee, J.Y.; Kim, D.-H.; Kang, M.S.; Park, W. Anti-Inflammatory Effect of Baicalein on Polyinosinic–Polycytidylic Acid-Induced RAW 264.7 Mouse Macrophages. Viruses 2018, 10, 224. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Pradhan, P.; Vijayan, V.; Liu, B.; Martinez-Delgado, B.; Matamala, N.; Nikolin, C.; Greite, R.; DeLuca, D.S.; Janciauskiene, S.; Motterlini, R.; et al. Distinct metabolic responses to heme in inflammatory human and mouse macrophages—Role of nitric oxide. Redox Biol. 2024, 73, 103191. [Google Scholar] [CrossRef]
- Ye, Q.; Gao, Z.; Yan, H.; Zhang, H.; Meng, X.; Xiao, H. Transcriptomic analysis reveals significant changes in gene expression and a potential mitochondria-mediated apoptotic pathway in murine RAW 264.7 macrophages exposed to tert-butylquinone. Food Biosci. 2025, 64, 105891. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Artyomov, M.N. Itaconate: The poster child of metabolic reprogramming in macrophage function. Nat. Rev. Immunol. 2019, 19, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Chen, F.; Wang, N.; Tang, D.; Kang, R. ACOD1 in immunometabolism and disease. Cell. Mol. Immunol. 2020, 17, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2022, 9, 2021. [Google Scholar] [CrossRef]
- Taciak, B.; Bialasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, L.; Rygiel, T.; Krol, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Gordon, S.; Plüddemann, A. Tissue macrophages: Heterogeneity and functions. BMC Biol. 2017, 15, 53. [Google Scholar] [CrossRef]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue macrophages: Origin, heterogenity, biological functions, diseases and therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Meng, X.; Jiang, L. Identification and assessment of residual levels of the main oxidation product of tert-butylhydroquinone in frying oils after heating and its cytotoxicity to RAW 264.7 cells. Food Chem. 2018, 264, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Yokoyama, Y.; Kano, K.; Kano, I. Cell death induced by the phenolic antioxidant tert-butylhydroquinone and its metabolite tert-butylquinone in human monocytic leukemia U937 cells. Food Chem. Toxicol. 2003, 41, 679–688. [Google Scholar] [CrossRef] [PubMed]



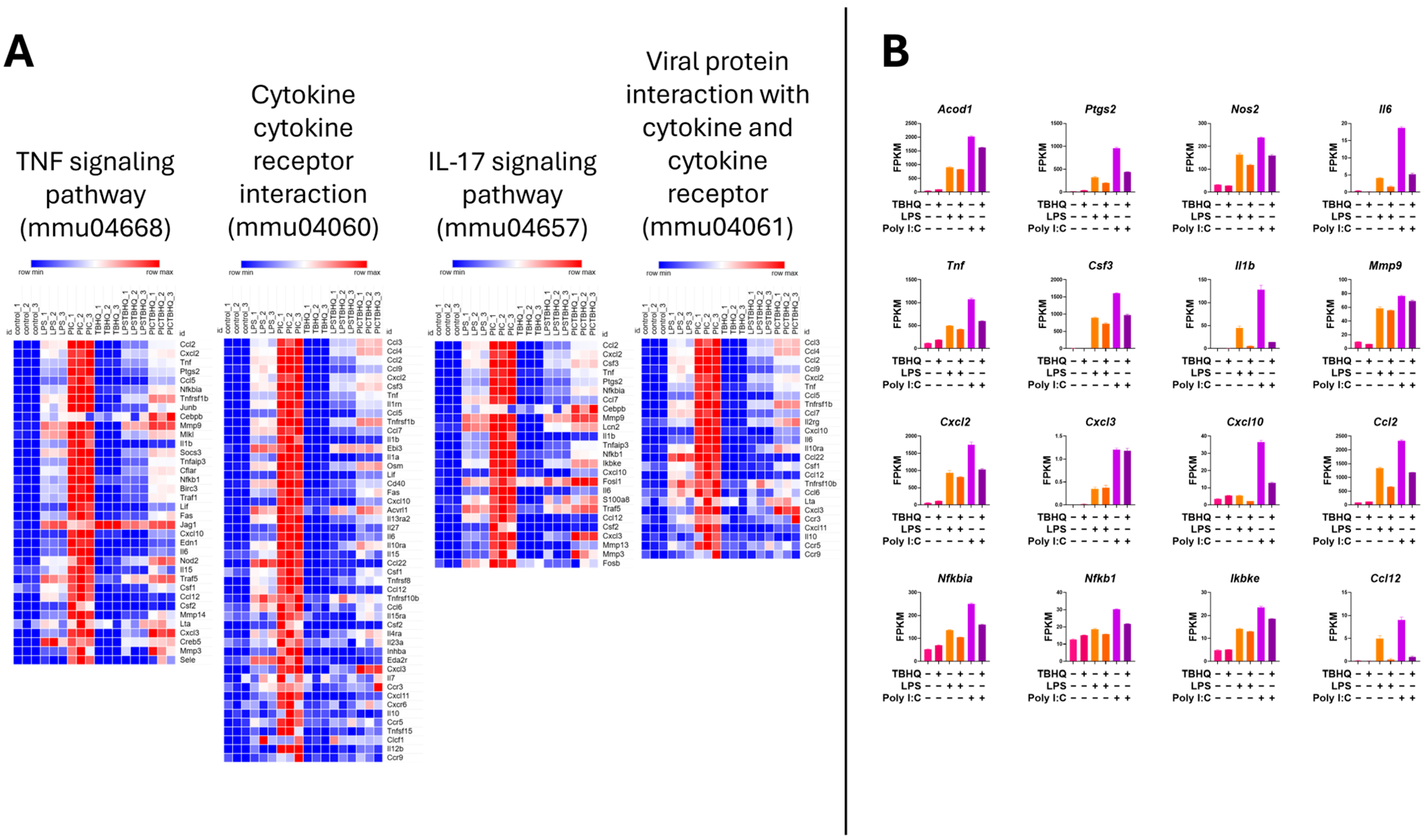
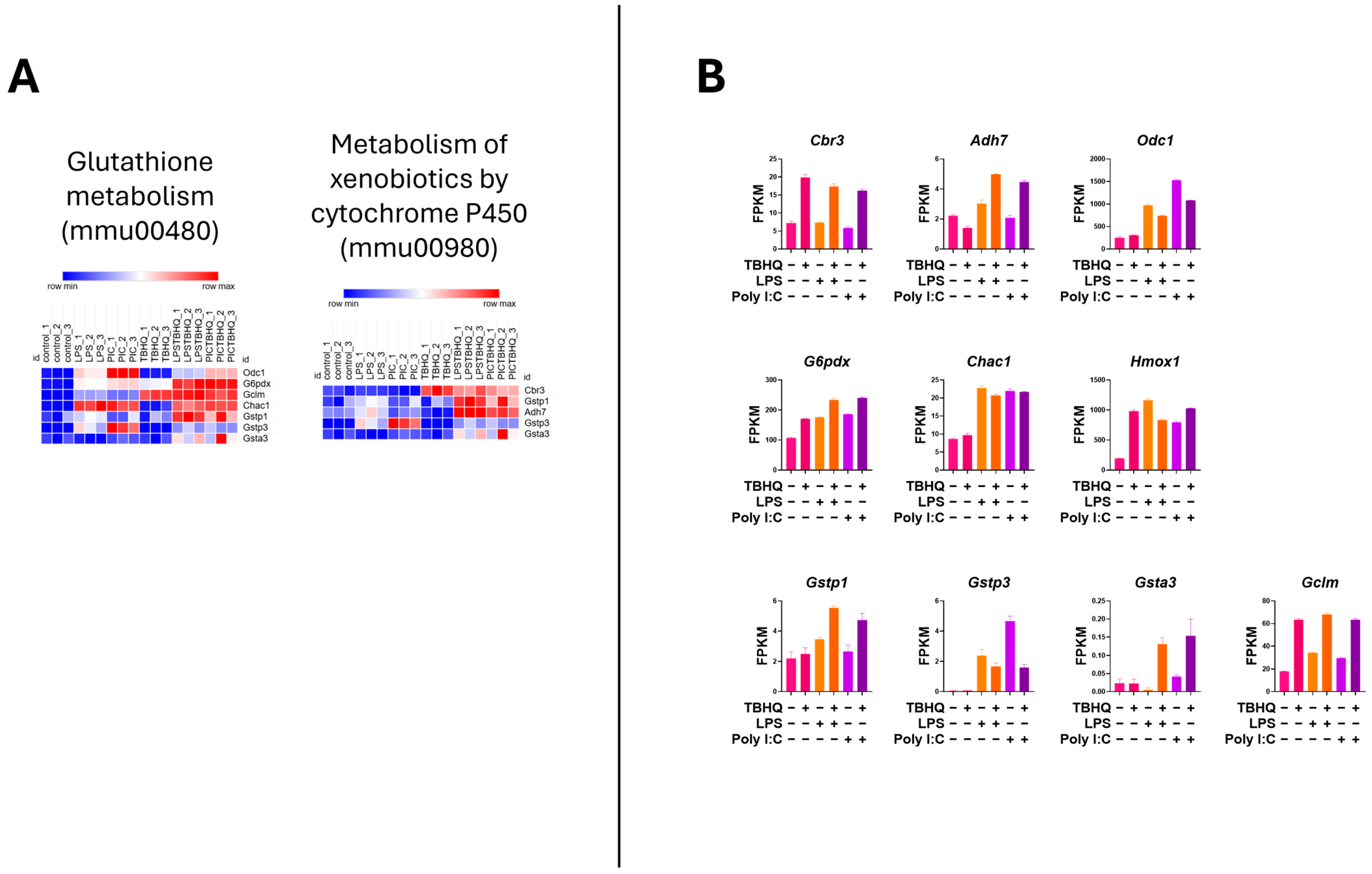

| Gene Name | Gene ID | Genbank NO. | Symbol | TBHQ * | LPS | LPS + TBHQ | PIC | PIC + TBHQ |
|---|---|---|---|---|---|---|---|---|
| Inflammation | ||||||||
| Tumor necrosis factor | 21926 | NM_013693.3 | Tnf | +0.69 | +2.05 | +1.81 | +3.16 | +2.32 |
| Interleukin 6 | 16193 | NM_031168.2 | Il6 | −1.44 | +3.14 | +1.80 | +5.32 | +3.47 |
| C-C motif chemokine ligand 12 | 20293 | NM_011331.3 | Ccl12 | - | +5.02 | - | +5.88 | +2.67 |
| C-C motif chemokine ligand 2 | 20296 | NM_011333.3 | Ccl2 | +0.55 | +4.00 | +2.97 | +4.80 | +3.82 |
| C-X-C motif chemokine ligand 2 | 20310 | NM_009140.2 | Cxcl2 | +0.93 | +3.91 | +3.70 | +4.82 | +4.06 |
| C-X-C motif chemokine ligand 3 | 330122 | NM_203320.3 | Cxcl3 | - | +6.76 | +6.84 | +8.53 | +8.51 |
| C-X-C motif chemokine ligand 10 | 15945 | NM_021274.2 | Cxcl10 | +0.65 | +0.68 | −0.71 | +3.41 | +1.89 |
| Nitric oxide synthase 2, inducible | 18126 | NM_010927.4 | Nos2 | - | +2.36 | +1.89 | +2.90 | +2.32 |
| Prostaglandin-endoperoxide synthase 2 | 19225 | NM_011198.5 | Ptgs2 | +1.36 | +4.26 | +3.57 | +5.82 | +4.70 |
| Aconitate decarboxylase 1 | 16365 | NM_008392.1 | Acod1 | +0.88 | +4.08 | +3.95 | +5.24 | +4.91 |
| Nuclear factor of kappa light polypeptide gene enhancer in B cells 1, p105 | 18033 | NM_008689.3 | Nfkb1 | +0.27 | +0.57 | +0.32 | +1.27 | +0.79 |
| Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor, alpha | 18035 | NM_010907.2 | Nfkbia | +0.41 | +1.36 | +0.99 | +2.25 | +1.61 |
| Inhibitor of kappaB kinase epsilon | 56489 | NM_019777.3 | Ikbke | - | +1.57 | +1.43 | +2.29 | +1.94 |
| Interleukin 1 beta | 16176 | NM_008361.4 | Il1b | - | +10.02 | +7.03 | +11.54 | +8.33 |
| Colony stimulating factor 3 | 12985 | NM_009971.1 | Csf3 | +2.22 | +8.07 | +7.76 | +8.91 | +8.19 |
| Matrix metallopeptidase 9 | 17395 | NM_013599.5 | Mmp9 | +0.61 | +2.56 | +2.49 | +2.95 | +2.81 |
| Cellular Metabolism | ||||||||
| Glutathione S-transferase, pi 1 | 14870 | NM_013541.1 | Gstp1 | - | +0.64 | +1.32 | - | +1.09 |
| Glutathione S-transferase pi 3 | 225884 | NM_144869.4 | Gstp3 | - | +5.72 | +5.20 | +6.68 | +5.16 |
| Glutathion S-transferase, alpha 3 | 14859 | NM_010365.5 | Gsta3 | - | - | +2.47 | - | +2.68 |
| Carbonyl reductase 3 | 109857 | NM_173047.3 | Cbr3 | +1.46 | - | +1.27 | - | +1.17 |
| Alcohol dehydrogenase 7 (class IV), mu or sigma polypeptide | 11529 | NM_009626.4 | Adh7 | −0.67 | +0.45 | +1.16 | - | +1.00 |
| Ornithine decarboxylase, structural 1 | 18263 | NM_013614.3 | Odc1 | +0.29 | +1.93 | +1.55 | +2.59 | +2.09 |
| Glucose-6-phosphate dehydrogenase X-linked | 14381 | NM_008062.3 | G6pdx | +0.67 | +0.71 | +1.12 | +0.80 | +1.17 |
| Glutamate-cysteine ligase, modifer subunit | 14630 | NM_008129.4 | Gclm | +1.82 | +0.94 | +1.92 | +0.73 | +1.83 |
| Chac, cation transport regulator 1 | 69065 | NM_026929.4 | Chac1 | - | +1.39 | +1.26 | +1.35 | +1.33 |
| Heme oxygenase 1 | 15368 | NM_010442.2 | Hmox1 | +2.10 | +2.34 | +2.04 | +2.58 | +2.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whisel, A.M.; Rice, C.D. Tert-Butylhydroquinone (TBHQ) Suppresses LPS- and Poly (I:C)-Induced RAW 264.7 Macrophage Activation Through Reduced NF-κB/Type 1 Interferon and Enhanced Antioxidant-Related Pathways. Toxics 2025, 13, 883. https://doi.org/10.3390/toxics13100883
Whisel AM, Rice CD. Tert-Butylhydroquinone (TBHQ) Suppresses LPS- and Poly (I:C)-Induced RAW 264.7 Macrophage Activation Through Reduced NF-κB/Type 1 Interferon and Enhanced Antioxidant-Related Pathways. Toxics. 2025; 13(10):883. https://doi.org/10.3390/toxics13100883
Chicago/Turabian StyleWhisel, Alyssa M., and Charles D. Rice. 2025. "Tert-Butylhydroquinone (TBHQ) Suppresses LPS- and Poly (I:C)-Induced RAW 264.7 Macrophage Activation Through Reduced NF-κB/Type 1 Interferon and Enhanced Antioxidant-Related Pathways" Toxics 13, no. 10: 883. https://doi.org/10.3390/toxics13100883
APA StyleWhisel, A. M., & Rice, C. D. (2025). Tert-Butylhydroquinone (TBHQ) Suppresses LPS- and Poly (I:C)-Induced RAW 264.7 Macrophage Activation Through Reduced NF-κB/Type 1 Interferon and Enhanced Antioxidant-Related Pathways. Toxics, 13(10), 883. https://doi.org/10.3390/toxics13100883







