1. Introduction
Envenomation by venomous animals is a global public health issue [
1,
2]. Beyond the acute, lethal toxicity [
3] resulting from the direct action of venom, the majority of severely envenomated patients experience secondary complications, such as cytokine storms [
4,
5] and multi-organ dysfunction [
6,
7], which can culminate in death. The time from envenomation to death, often spanning from tens of minutes to several hours, defines a critical therapeutic window [
8]. Within this period, accurate detection of residual jellyfish venom (venom proteins, peptides or other antigenic components) is crucial for species identification, venom typing, injury assessment, and prognostic [
9,
10]. This provides a critical foundation for selecting the optimal antivenom or targeted therapies and for implementing precision treatment. Nevertheless, there is currently a widespread lack of clinical recognition of this therapeutic window, coupled with a scarcity of effective diagnostic tools, which severely impedes improvements in treatment outcomes for envenomation [
11].
Our understanding of marine envenomation significantly lags behind that of terrestrial envenomation, a knowledge gap compounded by a scarcity of effective diagnostic tools. For instance, jellyfish, which represent one of the greatest marine threats to humans, typically appear in seasonal blooms (e.g., summer) [
1,
12], presenting a common, widespread, and substantial threat. They are a paradigmatic example of marine envenomation. Jellyfish envenomation can occur without direct contact with the animal, which complicates the timely and accurate identification of the causative species. Furthermore, jellyfish species exhibit marked differences in venom toxicity; stings from most do not cause severe complications and are managed with established treatment protocols [
13]. For lethal species, however, misidentification can lead to missing the critical 2- to 48-h therapeutic window, allowing the condition to progress to a severe systemic inflammatory response, multi-organ dysfunction, and potentially death [
4,
14]. Therefore, the development of diagnostic assays for the venom of medically significant jellyfish, such as
Nemopilema nomurai [
15,
16], the primary species responsible for envenomation in Chinese coastal waters, is of critical importance for the diagnosis and clinical management of marine envenomation.
Compared with conventional laboratory detection methods such as high-performance liquid chromatography and mass spectrometry, immunodiagnostic techniques [
17,
18] based on antigen–antibody interactions offer practical advantages for clinical and field settings, including high sensitivity, specificity, and throughput, and can be readily adapted for rapid on-site detection platforms such as colloidal gold test strips [
19]. In this study, using
N. nomurai as the target species, we first generated polyclonal antibodies against
N. nomurai venom and subsequently established an indirect enzyme-linked immunosorbent assay (i-ELISA) for the detection of its venom. This assay exhibited strong specificity, high sensitivity, and good stability, which were validated in skin and serum residues after venom exposure. This work provides a methodological foundation for the development of detection kits for jellyfish and other venomous marine organisms.
2. Materials and Methods
2.1. Jellyfish Collection and Venom Preparation
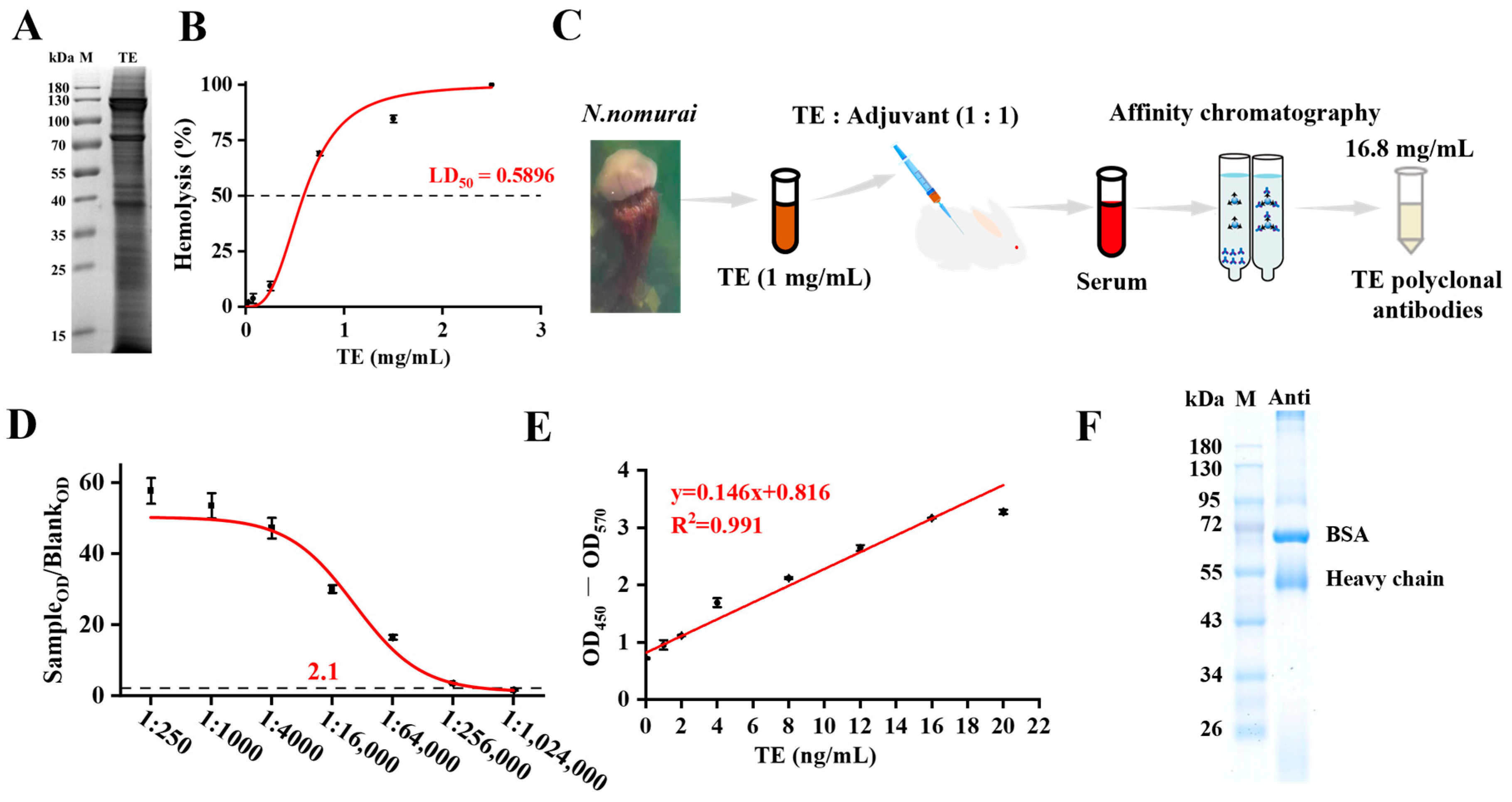
N. nomurai specimens were collected from the Longwangtang area of the Bohai Sea, Dalian, China, collected during summer months (July–August) of 2023, and the tentacles were immediately frozen at –80 °C and transported to the laboratory. According to the previously reported method [
20], the tentacles were stirred at 4 °C for 72 h, followed by filtration through a 200-mesh sieve. The filtrate was centrifuged at 4 °C, 1000×
g for 10 min, and the supernatant was transferred to activated dialysis bags (MD34 MW, 6000–12,000 Da). Dialysis was performed using 1× PBS at 4 °C for 8 h to obtain the
N. nomurai tentacle extract (TE). The TE concentration was measured as 5 mg/mL by BCA protein concentration assay kit.
2.2. Animal Husbandry
Male Institute of Cancer Rsearch (ICR) mice with an average weight of 25 ± 2 g were obtained from Shanghai Jiesijie Experimental Animal Co., Ltd, Shanghai, China. Mice were housed in ventilated cages at 22–23 °C with 55–60% relative humidity and a 12-h light/dark cycle. Water and standard chow pellets were available ad libitum. All animal care and experimental procedures strictly followed the guidelines of the Institutional Animal Care and Use Committee of the Institute of Nutrition and Food Safety, Chinese Academy of Sciences, and were approved by the Institutional Animal Ethics Committee of the Naval Medical University.
2.3. Hemolytic Activity Assay
After anesthesia, blood was collected from the orbital sinus of mice using heparinized glass capillary tubes into 1.5 mL heparin-treated centrifuge tubes. The blood was diluted with an equal volume of PBS, centrifuged at 3000× g for 5 min at room temperature, and washed repeatedly until the supernatant was clear. One hundred microliters of pelleted erythrocytes were added to 22.2 mL of PBS to prepare a red blood cell suspension. Subsequently, 100 μL of graded concentrations of TE (final concentrations: 2.5, 1.5, 0.75, 0.25, 0.15, or 0.075 mg/mL) were mixed with 100 μL of red blood cell suspension and equal volumes of PBS and saponin (25 g/mL) served as the negative and positive controls, respectively. Following incubation at 37 °C for 30 min, the mixtures were centrifuged at 2000× g for 5 min at 4 °C. A 100 μL aliquot of the supernatant was collected, and its absorbance at 415 nm (OD415) was measured using a microplate reader. The percentage of hemolytic activity was calculated using the following formula: Hemolytic activity (%) = [(OD415_experimental group − OD415_blank group)/(OD415_control group − OD415_blank group)] × 100%.
2.4. Polyclonal Antibody Production
The prepared TE was diluted to 1 mg/mL and emulsified with an equal volume of Complete Freund’s Adjuvant (CFA; Sigma, St. Louis, MO, USA). Male New Zealand White (NZW) rabbits (2.0 ± 0.5 kg) were immunized via subcutaneous injection at multiple sites (0.2 mL per site). A second immunization was administered two weeks later, followed by booster immunizations every 7 days. For these boosters, TE was emulsified with Incomplete Freund’s Adjuvant (IFA; Sigma). Blood was collected from the central ear artery to monitor the antiserum titer using an indirect ELISA. Once the titer exceeded 1:50,000, a final booster was given. Seven days post-boost, blood was collected for antiserum preparation. For purification, 10 mL of the serum sample was filtered through a 0.45 μm membrane and loaded onto an affinity chromatography column at a flow rate of 40 mL/h; this process was repeated once. The column was then washed with 20 mL of 1× PBS (pH 7.4) at 70 mL/h. After 10 min, a protein detector (HD-4, Shanghai Qingpu Huxi, Shanghai, China) was connected, and the transmittance (T%) was adjusted to 100% during the wash. Finally, the antibody was eluted with 0.2 M glycine solution (pH 2.7) at 40 mL/h and collected. The final concentration of the purified TE polyclonal antibody was determined to be 16.8 mg/mL.
2.5. SDS-PAGE Protein Electrophoresis Assay
SDS-PAGE was performed using a 10% resolving gel prepared with the PAGE Gel Fast Preparation Kit (Yamei, Shanghai, China) in accordance with the manufacturer’s instructions. An aliquot of TE (15 μg) was loaded into each well. Protein maker (Beyotime, Shanghai, China) add 10 uL. Electrophoresis was carried out at a constant voltage of 80 V for 30 min, and subsequently at 120 V until the bromophenol blue dye front migrated to approximately 1 cm from the bottom of the gel. Following the run, the gel was carefully removed from the glass plates and stained with Coomassie Brilliant Blue staining solution (Yamei) for 60 min on a horizontal shaker. The gel was then destained with deionized water until a clear background was achieved. Finally, the gel was scanned using a G:BOX Chemi XX6 High-Performance Fluorescence imaging system (Gene Company Limited, Hong Kong, China).
2.6. Immunohistochemistry
The dorsal skin of male ICR mice was depilated. Mice in the
N. nomurai group (
n = 4) were placed in disposable containers filled with
N. nomurai tentacle autolysis solution to a depth of approximately 1 cm. To simulate natural envenomation, the skin was repeatedly bathed with the solution every 30 min using a plastic pipette. For the negative control (NC) group (
n = 4), the same procedure was performed using seawater. After 8 h, the mice were anesthetized in a sealed chamber containing isoflurane (Merck, Darmstadt, Germany) and subsequently euthanized by cervical dislocation. Dorsal skin tissue samples were excised and fixed in paraformaldehyde. The fixed tissues were then sequentially dehydrated, embedded, sectioned, and baked for 2 h at 60 °C. The resulting sections were deparaffinized in xylene and rehydrated through a graded series of ethanol. To block non-specific background staining, endogenous peroxidase activity was quenched by incubating the sections with Endogenous Peroxidase Blocking Buffer (Beyotime, Shanghai, China) for 10 min, followed by three washes in PBS. The TE polyclonal antibody was diluted 1:1000 in Universal Blocking and Antibody Dilution Buffer (Beyotime). The sections were incubated with the primary antibody solution for 2 h at 37 °C. After washing, they were incubated with a goat anti-rabbit IgG-HRP secondary antibody (1:10,000) for 30 min at 37 °C. The sections were then visualized with a DAB kit (Beyotime) for 5 min, thoroughly rinsed with tap water, and counterstained with hematoxylin for 2 min. Finally, the sections were dehydrated and mounted with Neutral Balsam (Beyotime, Shanghai, China). Images were captured using a fluorescence microscope (Zeiss, Oberkochen, Germany) and analyzed with ImageJ software (v1.51,
https://imagej.nih.gov/ij/, accessed on 8 February 2025).
2.7. Establishment of i-ELISA Assay
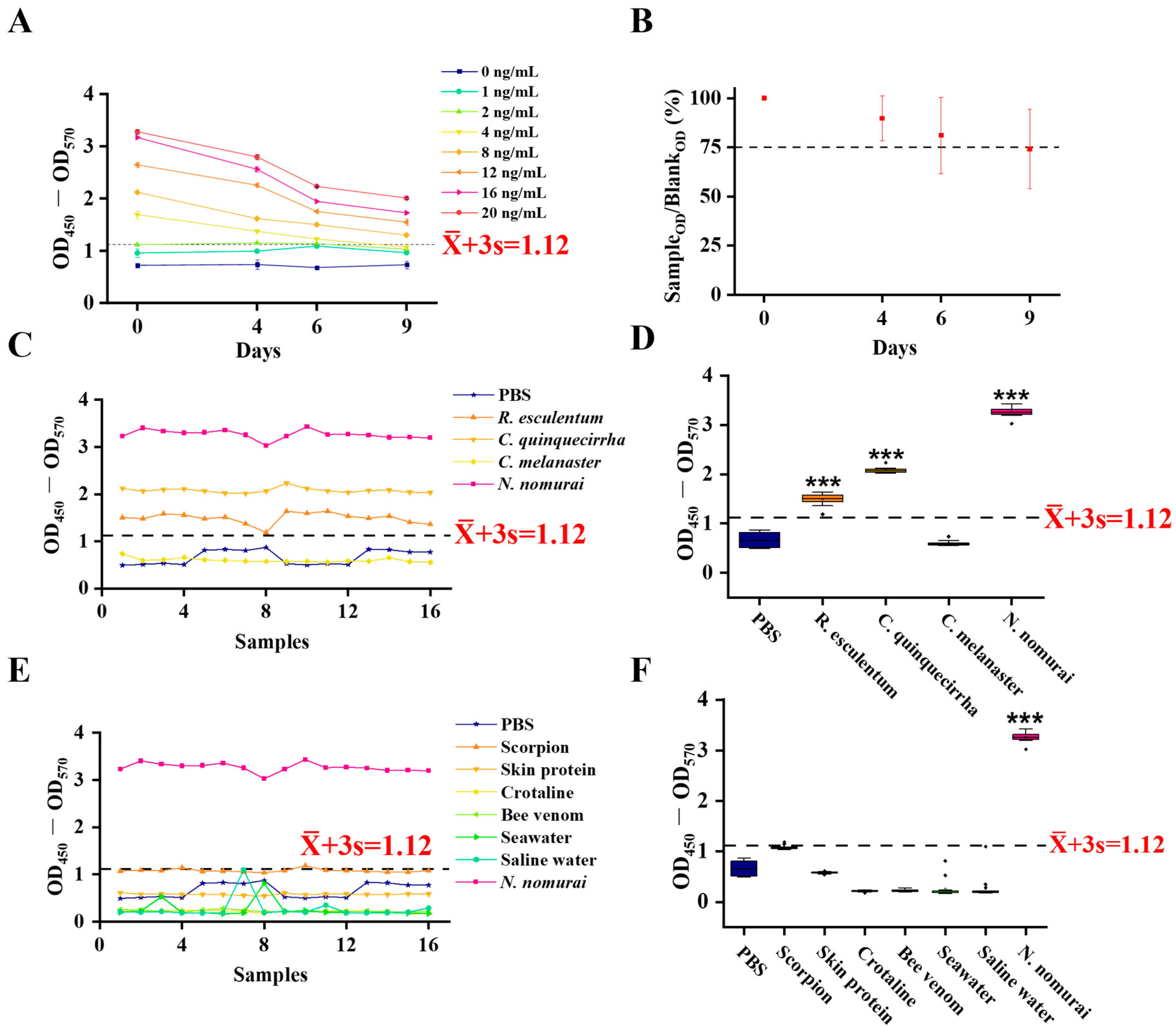
An indirect ELISA was developed to detect the TE antigen using the ELISA Basic Kit (Lianke Bio, Hangzhou, China). Briefly, 96-well polystyrene microplates (Corning, Shanghai, China) were coated with 100 μL/well of TE, which was serially diluted in coating buffer (Lianke Bio) to create a standard curve (0, 1, 2, 4, 8, 12, 16, and 20 ng/mL), and incubated overnight at 4 °C. The plates were then incubated for 3 h at room temperature. After washing three times with 300 μL/well of wash buffer (Lianke Bio), the plates were blocked by adding 250 μL/well of assay buffer (Lianke Bio) and incubating for 3 h at room temperature. Following another wash step, 100 μL of TE polyclonal antibody, diluted 1:10 in PBST (Yamei), was added to each well, and the plates were incubated for 23 h at 37 °C. After three washes, 100 μL of HRP-conjugated goat anti-rabbit IgG antibody (Lianke Bio), diluted 1:10,000 in dilution buffer (Lianke Bio), was added and incubated for 1 h at 37 °C. After a final series of three washes, the reaction was developed by adding 100 μL of TMB (3,3′,5,5′-Tetramethylbenzidine) substrate (Lianke Bio) and incubating for 15 min at room temperature in the dark. The reaction was terminated with stop solution (Lianke Bio). The absorbance was measured at 450 nm with a reference wavelength of 570 nm (OD450–570) using a microplate reader. The cut-off value for positivity was calculated as the mean (
) plus three times the standard deviation (s) of the negative samples. A sample was considered positive if its OD450–570 value was ≥
+ 3 s [
21].
2.8. Precision Assessment of i-ELISA
To evaluate the precision of the established i-ELISA, eight different batches of TE were first quantified using a BCA Protein Assay Kit. The samples were then diluted to a final concentration of 10 ng/mL with coating buffer (Lianke Bio). The assay was performed according to the i-ELISA protocol described above. For intra-assay precision, each of the eight batches was assayed in quadruplicate on a single microplate. For inter-assay precision, the assay was conducted on four different microplates. The mean () and standard deviation (s) of the OD450–570 values were calculated for both the intra- and inter-assay measurements. The coefficient of variation (CV) was determined using the formula: CV (%) = (s/) × 100. Both intra-assay and inter-assay CVs were calculated accordingly.
2.9. Detection of Residual Venom on Skin
To more closely simulate a natural sting event, 32 male ICR mice with depilated dorsal skin were divided into a negative control (NC) group and a Sting group. The skin was topically treated with seawater (NC group) or TE (Sting group) for 15 min. Subsequently, the residual liquid on the skin surface was collected by a cell scraper into 100 μL of coating buffer (Lianke Bio). The collected samples were then analyzed by the i-ELISA. Similarly, seawater and TE were applied to human skin for 15 min, and the residues were collected using the same scraping method for analysis. This human experiment, which posed no significant risk to the subject, was performed on one of the authors who had provided written informed consent.
2.10. Optimization of i-ELISA Conditions for Serum Residual Venom Detection
Serum dilution: Serum samples were collected from mice in the NC group (administered PBS via tail vein injection) and the N. nomurai group (treated with N. nomurai tentacle lysate for 8 h) and subsequently diluted with coating buffer (Lianke Bio) at ratios of 1:10, 1:100, 1:1000, and 1:10,000. 100 μL was added per well, and OD450–OD570 values for N. nomurai (P) and control group (N) serum were measured. The dilution with the highest P/N ratio was chosen as optimal.
Serum coating time: Using the optimal dilution, NC and N. nomurai group serum samples were coated at 4 °C for 1 h, 3 h, or 12 h. P and N values were measured, and the condition with the highest P/N ratio was selected.
Incubation time of goat anti-rabbit IgG-HRP: Under the previously optimized conditions, various incubation times (30 min, 60 min, and 120 min at 37 °C) were tested. P and N values were compared, and the time with the highest P/N ratio was defined as optimal.
Color development time: Under the optimized conditions above, tetramethylbenzidine (TMB) color development times of 5 min, 15 min, and 30 min were tested, followed by stop solution addition. OD450–OD570 was measured, and the optimal color development time was selected.
2.11. Sensitivity and Specificity of i-ELISA for Detecting Residual Venom in Serum
To evaluate the sensitivity and specificity of the established i-ELISA, mice were divided into four groups: a negative control (NC) group (administered PBS via tail vein injection, n = 72), a TE-IV group (intravenous injection of TE, n = 48), an R. esculentum-IV group (intravenous injection of R. esculentum venom, n = 21), and an N. nomurai group (topical envenomation with N. nomurai tentacle autolysis solution, n = 100). At 8 h post-treatment, serum samples were collected from all groups. The presence of residual venom (antigen) in the sera was then determined using the i-ELISA protocol with the anti-TE polyclonal antibody. Samples with OD450–OD570 ≥ + 3s were considered positive. Sensitivity for the N. nomurai group was calculated as (number of positive serum samples in the N. nomurai group/total serum samples in the N. nomurai group) × 100%; TE-IV group serum sensitivity = (number of positive serum samples in the TE-IV group/total TE-IV group samples) × 100%; specificity = (number of negative samples in R. esculentum-IV and NC serum samples/total number of serum samples from R. esculentum-IV and NC groups) × 100%.
2.12. Statistical Analyses
All data are presented as mean ± SEM. Data were analyzed by one-way ANOVA, and the differences in p < 0.05 between groups were considered significant.
4. Discussion
Fatal envenomation by toxic jellyfish represents a growing global marine public health concern, with the rapid and accurate etiological diagnosis constituting a major bottleneck for effective clinical intervention and epidemiological surveillance. Currently, the diagnosis of jellyfish stings relies primarily on indirect evidence such as patient self-reporting, clinical symptomatology, and geographical information, lacking objective standards that directly detect the culpable agent, jellyfish venom, an issue similarly observed with snake envenomations [
22]. In this study, we sought to establish a highly sensitive and specific immunoassay for the detection of
N. nomurai venom, providing a crucial diagnostic tool for
N. nomurai envenomation. The generation of high-affinity polyclonal antibodies was a key technical prerequisite [
23]. The resulting antisera exhibited high titers (1:256,000), strong affinity, and high purity. Immunogenicity assessment via SDS-PAGE revealed multiple prominent venom protein bands, particularly at 125, 75, and 38 kDa, suggesting the presence of conserved or major antigenic components within the venom that could represent important targets for future antibody specificity improvement. The high-titer polyclonal antibodies demonstrated remarkable specificity for jellyfish venom, indicating the feasibility of antivenom development via host immunization as well as broader applications in rapid diagnostic kit design. Moreover, this study optimized and established an indirect ELISA (i-ELISA) for the detection of jellyfish venom. This assay allows for the detection of
N. nomurai venom at concentrations as low as 4 ng/mL and maintains sensitivity after prolonged storage (at least 9 days at 37 °C), with projected stability for up to one year at 4 °C [
24]. These findings highlight the practicality and portability of this method for clinical or environmental surveillance. The assay exhibited excellent reproducibility, with intra- and inter-assay coefficients of variation (CV) well within the acceptable range for clinical applications (CV < 10%) [
25], ensuring reliability and batch-to-batch consistency. Importantly, the method distinctly differentiates
N. nomurai venom from those of related species (e.g.,
Rhopilema esculentum,
Chrysaora quinquecirrha, and
Cyanea melanaster) as well as from various unrelated biological and environmental proteins. Indeed, Future research should certainly broaden the specificity testing to include a more comprehensive set of sympatric cnidarians, such as
Cyanea capillata and
Aurelia aurita, to fully validate its clinical utility in areas with greater species diversity. This specificity is critical for the accurate identification and diagnosis of
N. nomurai stings, given the sympatric distribution and morphological similarities of jellyfish species in Chinese and global coastal waters.
Importantly, toxic antigens may persist on the skin following contact with venom [
22]. Our assay demonstrated clear detection of venom residues on mammalian (murine and human) skin, with results highly consistent with those obtained via immunohistochemistry, further underscoring its practical utility in both clinical and experimental settings. In addition, effective detection of venom in serum represents a significant diagnostic approach [
26]. Our assay discriminated between sera from animals systemically exposed to
N. nomurai venom and control sera from unexposed animals, with an area under the curve (AUC) of 0.8596, indicating reliable diagnostic performance. The method achieved 100% sensitivity in the intravenous injection model and exhibited high specificity in the cutaneous envenomation model, further supporting its translational potential for clinical diagnostics. Collectively, these findings demonstrate the feasibility and analytical advantages of this immunoassay as an innovative platform for jellyfish venom exposure detection, addressing a critical need for objective diagnostic standards in jellyfish stings.
Certainly, several aspects remain open for future investigation. Firstly, while the polyclonal antibodies demonstrated excellent performance and reproducibility in the current study, the development of monoclonal antibodies targeting specific, stable, and abundant protein components within the venom—such as the 125 kDa or 75 kDa proteins identified herein—will undoubtedly enhance assay specificity and eliminate inter-batch variability. This will pave the way for the development of more robust and standardized diagnostic tools. Second, the reduced sensitivity of serum samples from the dermal envenomation model (68%) compared to the intravenous injection model (100%) likely reflects two key factors. Firstly, a high local retention of jellyfish venom within the cutaneous tissue upon administration may result in a diminished quantity of specific analytes entering the systemic circulation. Concurrently, venom components that enter the bloodstream undergo rapid dilution, systemic distribution, and subsequent metabolic clearance by organs such as the liver and kidneys. The 8-h exposure period in our experimental model provides an ample timeframe for the substantial clearance of venom from the circulation, thereby contributing to the lower detection sensitivity in the dermal model. Prospectively, the detection rate could be enhanced by adopting signal amplification technologies, such as the biotin-avidin system [
27,
28], or by developing assays that target more stable venom metabolites. This constitutes a critical direction for our subsequent investigations. Moreover, it is essential to recognize that this study was limited to simulated venom models. To translate this detection method from the laboratory to clinical practice, rigorous validation using a broad range of human clinical samples from actual sting cases is required. This poses ethical and logistical challenges but is an imperative next step. Additionally, the platform could be adapted into formats more suitable for point-of-care testing, such as lateral flow assays (LFA) [
29], enabling real-time diagnosis of
N. nomurai stings. In summary, this study systematically established and validated a highly sensitive and specific i-ELISA for the detection of
N. nomurai venom. The assay demonstrated outstanding analytical performance in vitro, and its potential utility was further confirmed in simulated clinical scenarios using skin and serum samples.
This work provides a promising and reliable tool for the clinical diagnosis, forensic identification, and epidemiological study of N. nomurai envenomation, thereby laying a solid methodological foundation for the development of related diagnostic products and the optimization of future treatment strategies.