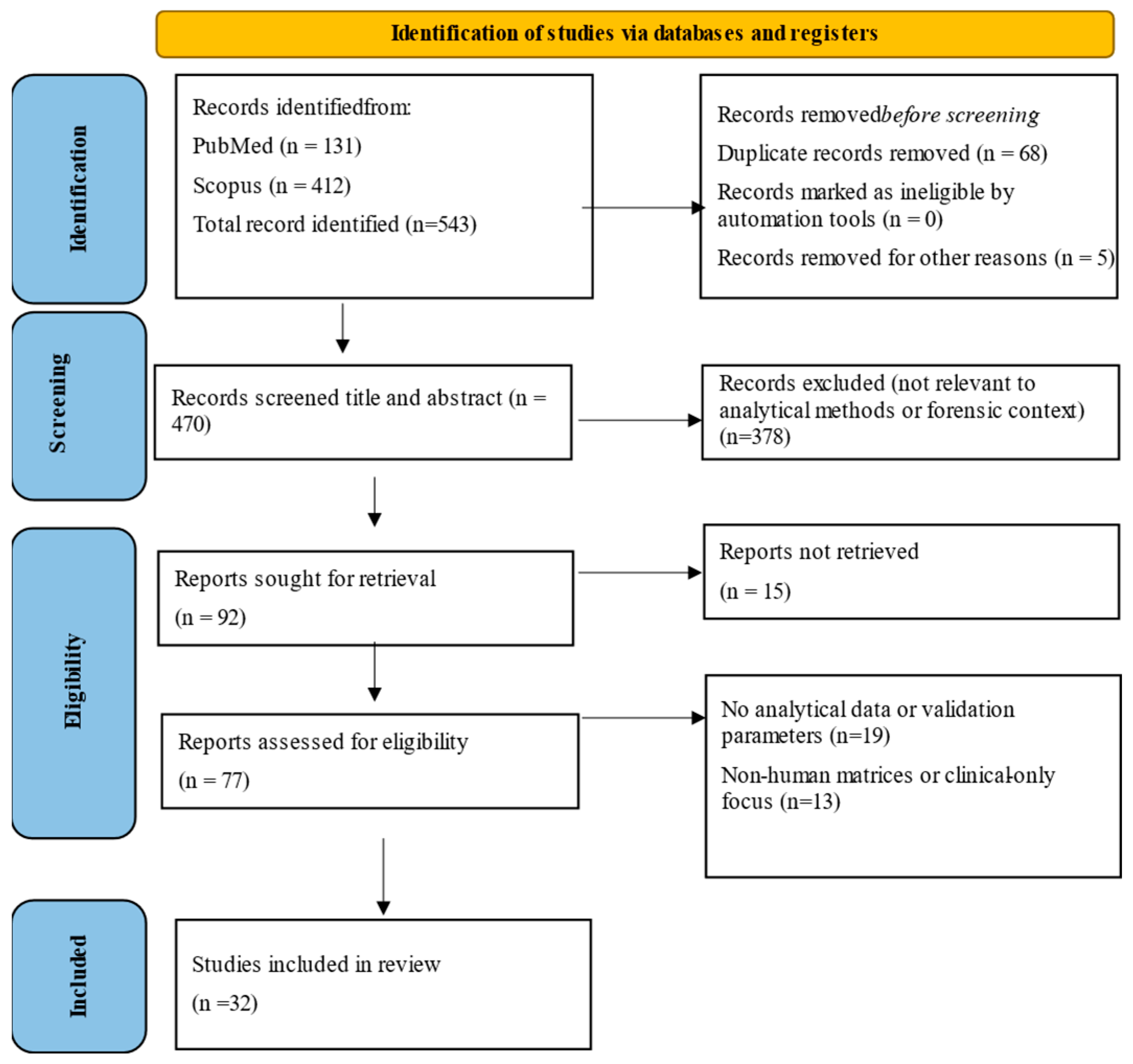
Analytical Methods for the Determination of Diamorphine (Heroin) in Biological Matrices: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Analytical Methods
3.1.1. Detection Approaches and Analytical Families
| Analytes * | Sample Types | Sample Preparation | Analytical Method ## | LOD # /LOQ ** (ng/mL) | Linearity | Recovery (%) | %CV | Stability | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| DIM 6-MAM Morphine | Blood | Rapid freezing → LLE (toluene–n-butanol (7:3, v/v, Burdick & Jackson Labs., Muskegon, MI, USA) → Wash with 0.1 N H2SO4 → pH adjustment to 8–9.0 with 1 N NaOH → Twice 5 mL Toluene-butanol extraction → Centrifuge 7 min → Evaporate under nitrogen at 40 °C to dryness → Reconstitute in 260 µL methanol (Burdick & Jackson Labs., Muskegon, MI, USA) → Inject 200 µL into HPLC | HPLC System → Varian Model 8500 (Varian, Sunnyvale, CA, USA) → Detector: Varichrom VUV-10 (Varian, Sunnyvale, CA, USA) → → Injector: Model 7125 (Rheodyne, Berkeley, CA, USA) → Recorder: Model A-25 (Varian, Sunnyvale, CA, USA) → Column: LiChrosorb Si-60 (30 cm × 4 mm I.D., 5-µm particle size (E. Merck, Darmstadt, Germany)) → Analytical Separation Phase: acetonitrile–methanol (75:25, v/v) → Flow Rate: 80 mL/h → Temp. Control: 30 °C | LOD = 6 LOS = 12.5 ULOQ = 4000 | 12.5–200 | 88 94 92 | 2–4 3.4–5 3–3.5 | Total DIM loss during extraction: 11.2 ± 0.36% | This is the first report of the detection of DAM in human blood | [13] |
| DIM 6-MAM Morphine | Blood | Recruit 11 patients with chronic pain (9 with cancer) → Administer DIM hydrochloride via 4 routes: intravenous injection, intravenous infusion, intramuscular injection, and oral dosing → Collect serial blood samples at multiple time points post-administration → Separate plasma and store appropriately for analysis → Prepare samples for HPLC analysis (as described in Umans et al. [13], without further procedural detail provided in this paper) | Analytes → DIM, 6-MAM, morphine Instrumentation → HPLC with UV detection (Varian Model 8500 (Varian, Sunnyvale, CA, USA), Detection Wavelength → 210 nm Quantification → DIM and 6-MAM: Rapidly detected post-injection, short half-life → Morphine: Detected later, with slower clearance → Pharmacokinetic parameters calculated: Cmax, Tmax, t½, AUC, clearance | LOD = 6 LOS = 12.5 | 12.5–200 | 29–94 | <5.1 | Total DIM loss during extraction: 11.2 ± 0.36% | Pharmacokinetic application | [14] |
| DIM, 6-MAM Morphine Codeine | Hair (20 DIM users) | Collect hair samples (~100 mg) → Cut into ~1 mm segments → Place in fritted reservoirs → Wash with 1 mL methanol (reservoirs ) (30 s vortex) → Dry hair → Add 1 mL methanol + internal standards (100 ng each of D3-morphine, D3-codeine, D3-6-AM) → Seal and incubate at 37 °C for 18 h with stirring → Evaporate methanol under nitrogen at 50 °C → Add 2 mL deionized water + saturated sodium bicarbonate (pH 8.4) (Fisher Scientific, Fair Lawn, NJ, USA) → Extract with 7 mL toluene–heptane–isoamyl alcohol (70:20:10) (Burdick & Jackson Labs., Muskegon, MI, USA) → Shake 15 min → Centrifuge → Transfer organic layer → Evaporate at 60 °C under nitrogen → Add 40 µL N-methyl-bis(trifluoroacetamide) (MBTFA, Pierce Chemical Co., Rockford, IL, USA) → Cap, vortex, heat at 60 °C for 20 min → Transfer to autosampler vial for GC–MS | Instrument → HP 5890A GC + 5970A MSD, (SIM mode) (Hewlett-Packard, Palo Alto, CA, USA) → HP 7673A ALS (autosampler, Hewlett-Packard, Palo Alto, CA, USA) → Column → HP-5 (25 m × 0.32 mm i.d., 0.17 µm film, Hewlett-Packard, Palo Alto, CA, USA) → Carrier Gas → Helium at 2.5 mL/min Injection → Splitless, 250 °C inlet, 280 °C transfer line Oven Program → 200 °C (1 min) → Ramp 10 °C/min to 280 °C → Hold 3 min Detection → SIM of derivatized ions: → DIM: m/z 327 (quant), 204, 268, 369 → 6-AM: m/z 364 (quant), 423 → Morphine: m/z 364 (quant), 477 → Codeine: m/z 282 (quant), 395 → Deuterated IS: m/z 367 (D3-6-AM), 367 (D3-morphine), 285 (D3-codeine) | 0.05 ng/mg hair (with a 100-mg sample). | 5–1000 ng/mg | 56.1 and 77.3% of drug | 7 cases 20 cases 20 cases 15 cases | DIM chronic users. this appears to be the first instance of the detection of DIM and 6-acetylmorphine | [15] | |
| DIM, 6-MAM, Morphine | Blood, Plasma, Saliva, Urine | Solvent: (J.T. Baker, Phillipsburg, NJ, USA) Sample (1 mL) + pH 6.0 acetate buffer (1 mL) + deionized water (6 mL) → Add deuterated IS (Radian Corp., Austin, TX, USA) (25 ng) + acetate buffer → Mix & centrifuge (10 min) → Filter using fritted reservoir → SPE (ZSDAUO2O, (Worldwide Monitoring, Horsham, PA, USA): conditioning (Methanol × 2, Water × 2, Acetate buffer × 2) → Sample loaded, washed (Water × 2, Acetate buffer × 2, Acetonitrile × 4) → Vacuum dry (5 min) → Elution (4 × 1 mL ethyl acetate/diethylamine 98:2) → Evaporate under N2 (50 °C) → Reconstitute (Acetonitrile for DIM, MBTFA (Pierce, Rockford, IL, USA) for 6-MAM & morphine, heat 60 °C for 30 min) → GC–MS Analysis | GC System: (HP 5890A → Autosampler: HP 7673A → Detector: HP 5971A MSD (SIM mode, Hewlett-Packard, Palo Alto, CA, USA) → Column: Rtx-5 capillary (15 m × 25 mm i.d., 0.10 µm film, Restek, Bellefonte, PA, USA)) → Carrier Gas: Helium (1.2 mL/min) → Injection Mode: Splitless (3 µL injection, purge time 0.50 min) → Oven Temp: 150 °C → Ramp to 200 °C → 290 °C → Detector: Mass selective (SIM mode) → Monitored Ions: DIM (m/z 327), 6-MAM (m/z 364), Morphine (m/z 364), Internal Std (m/z 367, 334) → Electron multiplier voltage: 600–800 eV above tune value → Daily tuning with perfluorotributylamine (m/z 219, 414, 502) → Data Acquisition: Selected ion monitoring (SIM) mode | 1.0 ng/mL. | DIM: 1.0–250 ng/mL; 6-AM and Morphine: 1.0–500 ng/mL | Hydrolysis of DIM to 6-acetylmorphine during extraction and analysis was <5% | Within-run: 1.1–8.9% Between-run: 3.7–6.4% | DIM stable for 12–18 h in acetonitrile6-MAM & Morphine stable for 24 h post-derivatization. | corroborate DIM use and to study the pharmacological effects of DIM and its metabolites. | [34] |
| Blood | Recruit 6 healthy male DIM-experienced volunteers → Ensure opioid-free status via urine testing (3 consecutive days negative) → Administer DIM hydrochloride via 3 conditions: 6 mg intranasal (IN), 12 mg IN and 6 mg intramuscular (IM), plus placebo → IN DIM mixed with lactose (total 100 mg) → Subjects inhale equal portions into each nostril using a straw → Blood samples collected pre-dose and at multiple time points post-dose (up to 24 h) → Samples frozen immediately (either plasma harvested or whole blood frozen in dry ice-acetone bath) | GC System: HP 5890A (Hewlett-Packard, Palo Alto, CA, USA) → Column: Rtx-5 capillary (15 m × 0.25 mm i.d., 0.10 µm film, Restek, Bellefonte, PA, USA)) → Carrier Gas: Helium (1.2 mL/min) → Injection Mode: Splitless (3 µL injection, purge time 0.50 min) → Oven Temp: 150 °C → Ramp to 200 °C → 290 °C → Detector: Mass selective (SIM mode) → Monitored Ions: DIM (m/z 327), 6-MAM (m/z 364), Morphine (m/z 364), Internal Std (m/z 367, 334) → Electron multiplier voltage: 600–800 eV above tune value → Daily tuning with perfluorotributylamine (m/z 219, 414, 502) → Data Acquisition: Selected ion monitoring (SIM) mode | 1.0 ng/mL. | DIM: 1.0–250 ng/mL; 6-AM and Morphine: 1.0–500 ng/mL | Hydrolysis of DIM to 6-acetylmorphine during extraction and analysis was <5% | Within-run: 1.1–8.9% Between-run: 3.7–6.4% | 6 healthy males | [11] | ||
| DIM, 6-MAM, Morphine | Hair | Collect hair samples from subjects → Segment hair into 1 mm (or less) sections→ Wash hair with methanol (3× vortex mixing, centrifuge at 1000× g, remove solvent) → Dry hair under nitrogen flow → Weigh 10 mg of hair into reaction vials → Add silylating solution (MSTFA + dithioerythritol ( + ammonium iodide, Pierce Chemical Co., Rockford, IL, USA) with internal standard (nalorphine-TMS, Sigma, St. Louis, MO, USA)) → Vortex mix for 10 s → Heat at 130 °C for 1 h → Cool sample and inject into GC–MS/MS system | GC–MS/MS system → Finnigan TSQ 700 Triple Quadrupole MS (Finnigan MAT, San Jose, CA, USA) coupled with Varian 3400 GC (Varian, Sunnyvale, CA, USA) → Column: J & W DB-5 fused silica capillary (25 m × 0.25 mm i.d., 0.25 µm film thickness, J&W Scientific, Folsom, CA, USA) → Carrier Gas: Helium (1.0 mL/min) → Injection Mode: Splitless (2 min) → Injection Port Temperature: 280 °C Oven Temperature Program → Hold at 150 °C for 2 min → Ramp 38 °C/min to 200 °C → Ramp 20 °C/min to 290 °C, hold for 7 min → Total run time: ~21 min Mass Spectrometry Conditions → Ionization Mode: Electron Impact (EI, 70 eV) → Collision Gas: Argon (0.8 mtorr) → Detection Mode: Multiple Selected Reaction Monitoring (SRM) Monitored Ions (SRM Mode) → DIM (m/z 369 → 204, 310, 327) → 6-MAM-TMS (m/z 399 → 204, 287, 340) → Morphine-2TMS (m/z 429 → 234, 287, 401) → Acetylcodeine (m/z 341 → 162, 229, 282) → Codeine-TMS (m/z 371 → 178, 234, 343) | 25 pg/mg | n.a. ¥ | n.a. | n.a. | Degradation of DIM was reduced to less than lo% | Method optimization for hair testing | [35] |
| DIM, 6-MAM, Morphine | Blood, Urine, Tissue | Sample Preparation → Condition SPE cartridge (ZSDAU020, Worldwide Monitoring, Horsham, PA, USA) with elution solvent (Burdick & Jackson Labs., Muskegon, MI, USA), methanol, deionized water, acetate buffer → Add liquid biological specimens (blood, plasma, saliva, urine) → Wash with deionized water, acetate buffer, acetonitrile → Aspirate to dryness → Collect analytes in elution solvent → Divide extract into two equal aliquots → Evaporate aliquots → Add acetonitrile to one set, transfer to GC–MS vials for DIM analysis → Add MBTFA (Pierce Chemical Co., Rockford, IL, USA) to second set, incubate at 60 °C for 30 min → Cool samples, transfer to GC–MS vials for 6-MAM & morphine analysis | GC System: HP 5890A Series II GC + HP 7673A autosampler + HP 5971A MSD (Hewlett-Packard, Palo Alto, CA, USA) → Column: Rtx-5 capillary (15 m × 0.25 mm i.d., 0.10 µm film) → Carrier Gas: Helium (1.2 mL/min) → Injection Mode: Splitless (3 µL injection, purge time 0.50 min) → Oven Temp: 150 °C → Ramp to 200 °C → 290 °C → Detector: Mass selective (SIM mode) → Monitored Ions: DIM (m/z 327), 6-MAM (m/z 364), Morphine (m/z 364), Internal Std (m/z 367, 334) → Electron multiplier voltage: 600–800 eV above tune value → Daily tuning with perfluorotributylamine (m/z 219, 414, 502) → Data Acquisition: Selected ion monitoring (SIM) mode (Hewlett-Packard, Palo Alto, CA, USA). | 1.0 ng/mL | DIM: 2.5–100 ng/mL 6-MAM & Morphine: 10–500 ng/mL | Hydrolysis of DIM to 6-acetylmorphine during extraction and analysis was <5%, | Within-run CV: 1.1–8.9%, Between-run CV: 3.7–6.4% | DIM stable for 12–18 h in acetonitrile 6-MAM & Morphine stable for 24 h post-derivatization | Postmortem Toxicology-DIM related deaths. | [3] |
| DIM, 6-MAM Morphine, Normorphine | Plasma Saliva Urine, Hair | Hair Sample Preparation → Wash hair with methanol & deionized water → Dry & pulverize hair → Digest with acetate buffer (pH 4.0) + enzymatic hydrolysis (β-glucuronidase/arylsulfatase, Boehringer Mannheim, Indianapolis, IN, USA) → SPE ZSDAU020 (Worldwide Monitoring, Horsham, PA, USA): conditioning (Methanol × 2, Water × 2, Acetate buffer × 2) → Load digested sample onto SPE column → Wash (Water × 2, Acetate buffer × 2, Acetonitrile × 4) → Vacuum dry (5 min) → Elution (4 × 1 mL ethyl acetate/diethylamine 98:2) → Evaporate under N2 (50 °C) → Reconstitute (Acetonitrile for DIM, MBTFA (Pierce, Rockford, IL, USA) for 6-MAM & morphine, heat 60 °C for 30 min) → GC–MS Analysis Plasma, Urine and Saliva Sample Preparation → Mix sample with acetate buffer (pH 4.0, J.T. Baker, Phillipsburg, NJ, USA) + deuterated IS (25 ng) → Centrifuge (10 min) → Filter using fritted reservoir → SPE conditioning (Methanol × 2, Water × 2, Acetate buffer × 2) → Load sample onto SPE column → Wash (Water × 2, Acetate buffer × 2, Acetonitrile × 4) → Vacuum dry (5 min) → Elution (4 × 1 mL ethyl acetate/diethylamine 98:2) → Evaporate under N2 (50 °C) → Reconstitute (Acetonitrile for DIM, MBTFA for 6-MAM & morphine, heat 60 °C for 30 min) → GC–MS Analysis | GC–MS Analysis Method → GC System: HP 5890A + HP 7673A autosampler + HP 5970B MSD (Hewlett-Packard, Palo Alto, CA, USA) → Column: HP-1 capillary (12 m × 0.20 mm i.d., 0.33 µm film, Agilent/HP, Palo Alto, CA, USA) → Carrier Gas: Helium (1 mL/min) → Injection Mode: Splitless → Oven Temp: 120 °C → Ramp to 220 °C → 250 °C → 260 °C → Detector: Mass selective (SIM mode) → Monitored Ions: DIM (m/z 327), 6-MAM (m/z 364), Morphine (m/z 364), Internal Std (m/z 367, 334) → Electron multiplier voltage: 600–800 eV above tune value → Data Acquisition: Selected ion monitoring (SIM) mode (Hewlett-Packard, Palo Alto, CA, USA) | Hair Samples: LOD: 0.1 Plasma, Sérum, Urine samples: LOD: 1 | Hair Samples: 0.1–10 ng/mg and 10–100 ng/mg Plasma, Sérum, Urine samples: 2.5–500 ng/mL | >90% | Hair Samples Within-run CV: 3–26.5% Between-run CV: 5–15% Plasma, Serum, Urine samples: Within-run CV: 4–12% Between-run CV: 6–14% | Hydrolysis of DIM to 6-acetylmorphine during extraction and analysis was 10%, | Forensic Toxicology– Hair for long-term DIM use. P.S.U for recent DIM used. | [36] |
| DIM 6-MAM | Sweat | Apply sweat patch (PharmChem Inc., Menlo Park, CA, USA) to subject’s skin → Clean skin with 70% isopropyl alcohol ((Fisher Scientific, Fair Lawn, NJ, USA) → Secure patch firmly → Wear patch for designated period (up to several days) → Remove patch carefully, avoiding contamination → Freeze patch at −30 °C until analysis → Extract sweat from absorbent pad using acetate buffer + Triton X-100 → Centrifuge (10 min at 2000 rpm) → Filter extract through SPE column ((Clean Screen® DAU, 200 mg, 10 mL; United Chemical Technologies, Bristol, PA, USA)) → Wash with methanol (J.T. Baker, Phillipsburg, NJ, USA), deionized water, acetate buffer → Elute analytes (ethyl acetate-diethylamine for DIM metabolites) → Evaporate under nitrogen → Reconstitute (N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA, Pierce Chemical Co., Rockford, IL, USA) for derivatization of 6-MAM & morphine, heat at 60 °C for 30 min) → GC–MS Analysis | GC System: HP 5890A (Hewlett-Packard, Avondale, PA, USA))→ Column: HP-1 capillary (12 m × 0.20 mm i.d., 0.33 µm film, Hewlett-Packard, Avondale, PA, USA) for cocaine, Rtx-5 capillary (15 m × 0.25 mm i.d., 0.10 µm film) for DIM → Carrier Gas: Helium (1 mL/min for cocaine, 1.2 mL/min for DIM) → Injection Mode: Splitless → Oven Temp: Cocaine (120 °C → Ramp to 220 °C → 250 °C → 260 °C), DIM (150 °C → Ramp to 200 °C → 290 °C) → Detector: Mass selective (SIM mode) → Monitored Ions: DIM (m/z 327), 6-MAM (m/z 364), Morphine (m/z 364), Internal Std (m/z 367, 334) → Electron multiplier voltage: 600–800 eV above tune value → Daily tuning with perfluorotributylamine (m/z 219, 414, 502) → Data Acquisition: Selected ion monitoring (SIM) mode. | 1.0 ng per patch | 2.5 to 50 ng per patch | 75–95% | less than 10%, | Not specified | Monitoring DIM and cocaine use in clinical studies | [16] |
| DIM, 6-MAM, Morphine | Blood | Prepare DIM base solution in acetonitrile (Burdick & Jackson, Muskegon, MI, USA) → Apply precise dose to nichrome wire coil → Air-dry overnight → Verify weight within ±20% of target → Load coil into smoking device → Activate heating element (≤200 °C) to volatilize DIM → Inhale single puff, hold for 15 s → Collect blood samples at specific time intervals → Store blood at −30 °C until analysis → Extract DIM and metabolites using SPE (ZSDAU020, (Worldwide Monitoring, Horsham, PA, USA)) → Elute analytes with ethyl acetate-2% diethylamine → Evaporate under nitrogen → Reconstitute (Acetonitrile for DIM, MBTFA (Pierce Chemical Co., Rockford, IL, USA) for 6-MAM & morphine, heat at 60 °C for 30 min) → GC–MS Analysis | GC System: HP 5890A (Hewlett–Packard, Avondale, PA, USA) → Column: Rtx-5 capillary (15 m × 25 mm i.d., 0.10 µm film, Restek, Bellefonte, PA, USA) → Carrier Gas: Helium (1.2 mL/min) → Injection Mode: Splitless (3 µL injection, purge time 0.50 min) → Oven Temp: 150 °C → Ramp to 200 °C → 290 °C → Detector: Mass selective (SIM mode) → Monitored Ions: DIM (m/z 327), 6-MAM (m/z 364), Morphine (m/z 364), Internal Std (m/z 367, 334) → Electron multiplier voltage: 600–800 eV above tune value → Daily tuning with perfluorotributylamine (m/z 219, 414, 502) → Data Acquisition: Selected ion monitoring (SIM) mode | LOD: 1 ng/mL for DIM and metabolites. | LOD: 1.0 ng/mL DIM: 1.0–250 ng/mL, 6-MAM & Morphine: 1.0–500 ng/mL | Hydrolysis of DIM to 6-acetylmorphine during extraction and analysis was <5% | Within-run: 1.1–8.9% Between-run: 3.7–6.4% | Recovery studies indicated that smoking device delivered approximately 89% of DIM | Comparison of smoked vs. intravenous DIM pharmacokinetics in experienced DIM users | [12] |
| DIM, 6-MAM Morphine | Blood Urine Vitreous humor | Biological fluid (1 mL) + Internal standard (Diethylnalorphine, 100 µL, 10 mg/L) → Add sodium carbonate-sodium bicarbonate buffer (~10 mg) + sodium chloride (~250 mg) → Liquid–liquid extraction with ethyl acetate–chloroform–hexane (7:2:1, 20 mL) (Fisher Scientific, Ottawa, Canada) → Shake (10 min) + Centrifuge (5 min, 2000 rpm) → Transfer organic phase (10 mL) to round-bottom flask → Evaporate under nitrogen (70 °C sand bath) → Add catalyst solution (4-dimethylaminopyridine, pyridine, chloroform, Aldrich, Milwaukee, WI, USA)) → Propionylation with propionic anhydride (50 µL, vortex 10 sec, react 30 min at room temp, (Aldrich, Milwaukee, WI, USA) → Quench reaction with methanol (50 µL, vortex 15 s, stand 1 min) → Eliminate propionic acid via hexane azeotrope (2 × 500 µL, nitrogen stream, 80 °C sand bath) → Reconstitute in chloroform-0.1% pyridine (250 µL) → GC–MS Analysis | GC System: Varian 3400 gas chromatograph + Saturn II ion trap detector ((Varian, Mississauga, ON, Canada) + Varian 8100 Autosampler (Varian, Mississauga, ON, Canada) → Column: DB-1 fused silica capillary (30 m × 0.32 mm i.d., 0.25 µm film thickness, Chromatographic Specialties, Brockville, ON, Canada) → Carrier Gas: Helium (99.999%, 16 psi, 3.6 mL/min) → Injection Mode: Programmable temperature injector (SPI) → Oven Temp: 40 °C (3 min) → Ramp 20 °C/min to 200 °C (1 min) → Ramp 5 °C/min to 260 °C → MS Transfer Line Temp: 300 °C → Monitored Ions: DIM (m/z 310, 327, 369), 6-AM (m/z 327, 382, 383), Morphine (m/z 324, 341, 396), Internal Std (m/z 322, 338, 367) → Electron impact mode (23 Pa filament emission current) → Data Acquisition: Full-scan (250–405 amu), 8 scans/s → Daily tuning with perfluorotributylamine (50–650 amu range) | 2 (2–5) 2 (50) 2 (100) | 10–50 50–250 100–500 | >95% ~80% ~80% | 5–12%. (4–15%) | DIM (Stable for 12–18 h in chloroform) → 6-MAM (Stable for 24 h post-derivatization) → Morphine (Stable for 24 h post-derivatization) | Forensic toxicology—postmortem DIM detection | [37] |
| DIM 6-MAM Morphine M3G M6G | Serum | Collect blood samples at specific time intervals → Store plasma at −30 °C until analysis → Thaw plasma before extraction → Add ammonium bicarbonate buffer (500 µL, 0.1 M, pH 9.2, Merck, Darmstadt, Germany) to 250 µL plasma → Solid-phase extraction (SPE) using C8, 50 mg columns (Varian, Harbor City, CA, USA) → Reconstitute residue with 100 µL methanol → Inject 80 µL into system. | HPLC Analysis Method → HPLC System: Hewlett-Packard 1050 series LC pump (Waldbronn, Germany) → Column: Nucleosil 100 5 C18 AB reversed-phase (250 mm × 4 mm, 5 µm, (Macherey-Nagel, Düren, Germany) → Mobile Phase: Triethylammonium phosphate (TEAP) buffer, pH 7.0; prepared from 1 M stock and diluted 1:40 with distilled water → Detection: Fluorescence (Excitation: 220 nm, Emission: 340 nm) → Injection Volume: 80 µL → Analytes: Morphine-3-glucuronide, Morphine-6-glucuronide → Calibration Range: Morphine-3-glucuronide (5–500 ng/mL), Morphine & Morphine-6-glucuronide (15–500 ng/mL) GC–MS Analysis Method: [34] | n.d. n.d. 3 10 10 | n.a. n.a. 15–500 5–500 15–500 | n.a. n.a. 71 71 62 | n.a. n.a. 3–6 4–6 4–7 | DIM, 6-MAM (GC–MS), MOR, M3G, M6G (HPLC) | Postmortem toxicology application for DIM-related death investigations | [30] |
| DIM 6-MAM Morphine M3G M6G Normorphine C6G Codeine | Plasma | Plasma sample (1.5 mL) + Internal standard (Ethylmorphine, 150 µL, 0.1% aqueous solution, Fluka, Buchs, Switzerland) → Add phosphate buffer (4.5 mL, 0.01 M, pH 2.1, Merck, Darmstadt, Germany), vortex for 10 sec → Load onto Nucleosil C18 ODS-2 SPE column (Macherey-Nagel, Düren, Germany) → 0% acetonitrile in phosphate buffer (pH 2.1) → Bi-distilled water (0.5 mL) → Acetonitrile-phosphate buffer (0.35 mL, 40%) → Elute analytes with acetonitrile-phosphate buffer (2 × 0.6 mL, 40%) → Evaporate eluate to dryness → Reconstitute in bi-distilled water (100 µL) → Inject 2 µL into HPLC system | HPLC System: Hewlett-Packard 1090 Series II (Hewlett-Packard, Waldbronn, Germany) → Column: Spherisorb C18 ODS-2 (125 × 2 mm, 3 µm, Stagroma, Wallisellen, Switzerland) + Guard column (20 × 2 mm, Stagroma, Wallisellen, Switzerland) → Mobile Phase: Multi-step gradient (all solvents HPLC-grade from Merck, Basel, Switzerland and Fluka, Buchs, Switzerland) (Water–phosphoric acid–hexylamine + Acetonitrile–water–phosphoric acid–hexylamine) → Flow Rate: 0.25 mL/min → Column Temperature: 30 °C → Detection: Diode-array (DAD, Hewlett-Packard, Waldbronn, Germany) at 210 nm → Online UV spectra recorded (192–350 nm) for peak purity and assignment | (25) (25) (25) (25) (25) (25) (25) | 25–5000 | 88 94 98 91 90 88 100 91 | 3.1–4.8 2.5–4.4 1.6–4 2.5–5 2.2–4.7 2–4.6 3.2–4.8 2.2–4.4 | Forensic toxicology—monitoring DIM and metabolite concentrations in plasma samples | [31] | |
| DIM 6-MAM Morphine M3G M6G Codeine | Serum | Serum sample (1 mL) + Internal standard (Nalorphine, 200 µL, 1 µg/mL aqueous solution, Sigma, St. Louis, MO, USA) → SPE using ethyl SPE columns (1 mL, 100 mg sorbent, J.T. Baker, Phillipsburg, NJ, USA) → Condition SPE column (Methanol × 2, Water × 1, Ammonium hydrogen carbonate buffer (pH 9.3, 2 mL), Merck, Darmstadt, Germany) → Load serum sample onto SPE column → Wash (1 mL Ammonium Hydrogen Carbonate buffer → Elute analytes with 1 mL methanol → Evaporate eluate under nitrogen at room temperature → Reconstitute in mobile phase (100 µL methanol–acetonitrile–water–formic acid) → Inject 5 µL into LC–MS system | LC System: Applied Biosystems 140B dual-syringe solvent-delivery pump (Applied Biosystems, Foster City, CA, USA)→ Column: Supelcosil LC–Si (25 cm × 2.1 mm, 5 µm particle size, Supelco Inc., Bellefonte, PA, USA) → Mobile Phase: Methanol-acetonitrile-water-formic acid (59.8:5.2:34.65:0.35, v/v/v/v) → Flow Rate: 230 µL/min (split to 46 µL/min before MS inlet) → Ionization Source: Atmospheric pressure chemical ionization (API I single quadrupole mass spectrometer (Thornhill, Ontario, Canada) equipped with an atmospheric pressure ionspray (API) interface) → Capillary Tip Voltage: 5000 V → Orifice Voltage: 50 V (M3G, M6G), 70 V (Nalorphine, Morphine, DIM, Codeine) → Vacuum Pressure: 1.8 × 10−5 torr → Source Temperature: 60 °C → Carrier Gas: Nitrogen → Detection Mode: Selected-ion monitoring (SIM) → Monitored Ions: DIM (m/z 370), 6-MAM (m/z 328), Morphine (m/z 286), M3G (m/z 462), M6G (m/z 462), Codeine (m/z 300), Internal Std (m/z 312) | (0.5) (4) (4) (1) (4) (4) | 0.5–10 4–10 4–10 1–10 4–10 4–10 | 72–75 99–100 99 77–80 44–46 99–100 | 3.6–5.3 1–1.5 1.1–2.2 3.6–6.7 4–6.2 1–1.6 | DIM-related pharmacokinetic studies | [38] | |
| DIM, 6-MAM, Morphine | Sweat | Apply PharmChek sweat patch (PharmChem, Menlo Park, CA, USA) to upper back → Clean skin with 70% isopropanol before application → Wear patch for 24 h before removal → Store absorbent pad at −20 °C in sealed plastic tubes → Extract target drugs using 5 mL acetonitrile (Merck, Darmstadt, Germany) with internal standards (DIM-d9, 6-MAM-d3, morphine-d3) → Agitate for 30 min at 200 rpm → Divide extract into two portions (2 mL for DIM, remainder for other compounds) → Evaporate acetonitrile to dryness → Reconstitute DIM fraction in acetonitrile → Derivatise remaining fraction with BSTFA + TMCS (60 °C, 20 min, (Interchim, Montluçon, France) → Inject into GC–MS system | GC System → Hewlett-Packard 5890 GC coupled with HP 5989B MSD (Hewlett-Packard, Les Ulis, France) → Column: HP-5MS fused-silica capillary (30 m × 0.25 mm i.d., 0.25 µm film thickness, Hewlett-Packard, Avondale, PA, USA) → Carrier Gas: Helium (1.0 mL/min) → Injection Mode: Splitless (1.5 µL sample) → Injection Port Temperature: 260 °C Oven Temperature Program → Hold at 60 °C for 1 min → Ramp 30 °C/min to 290 °C, hold for 6 min → Total run time: ~12 min Mass Spectrometry Conditions → Ion Source Temperature: 230 °C → Ionization Mode: Electron Impact (EI, 70 eV) → Detection Mode: Selected-ion monitoring (SIM) Monitored Ions (SIM Mode) → DIM (m/z 310, 327, 369), HER-d9 (m/z 378) → 6-MAM-TMS (m/z 340, 399), 6-MAM-d3-TMS (m/z 402) → Morphine-TMS (m/z 401, 414, 429), Morphine-d3-TMS (m/z 432) | 0.5 1.0 1.0 | 2.1–96.3 (DIM), 0–24.6 (6–MAM), 0–11.2 (morphine) | 71 76 71 | <13% | hydrolysis of DIM and 6-acetylmorphine was, 4% over that period of one month. | Forensic toxicology drug monitoring using sweat patches | [17] |
| DIM 6-MAM Morphine M3G M6G | Plasma Urine | Recruit 2 opioid-dependent patients enrolled in a DIM-assisted treatment program → Administer diacetylmorphine (DAM) via three routes: → Intravenous (IV): 200 mg bolus → Oral: Capsules (2 × 400 mg) and controlled-release tablets (460 mg + 690 mg) → Rectal: Suppositories (2 × 400 mg) → Collect blood samples at multiple time points (pre-dose to 720 min post-dose) via peripheral venous catheter → Collect urine samples for metabolite profiling → Store samples appropriately for chromatographic analysis. Analytical methods (HPLC–DAD for plasma and GC–MS for urine) were performed according to the validated procedures previously described by Inturrisi et al [14]. | Analytes → DIM, 6- 6-MAM, morphine, M3G, and M6G→ Instrumentation → Plasma analysis: HPLC, DAD, → Urine analysis: GC–MS Detection → Quantification of DIM and metabolites in plasma and urine AS previously described by Inturrisi et al [14]. | n.a. | n.a. | n.a. | n.a. | Pharmacodynamics and pharmacokinetics | [39] | |
| DIM, 6-MAM, Morphine, M3G, M6G Codeine C6G | Blood, Plasma | Collect arterial and venous blood samples at specific time intervals → Store plasma at −20 °C until analysis → Thaw plasma before extraction → Add fluoride and heparin to vacuum tubes for stabilization →Dilute 1 mL plasma with 2 mL 0.01 M ammonium carbonate buffer (pH 9.3; Merck, Darmstadt, Germany) → Solid-phase extraction (SPE, Bond Elut C18 (200 mg, (Varian, Harbor City, CA, USA) using reversed-phase cartridges → Wash with 0.01 M ammonium carbonate buffer (pH 9.3) → Elute analytes with 0.5 mL methanol—0.5 M acetic acid (9:1 v/v; Merck, Darmstadt, Germany) → Evaporate under nitrogen → Reconstitute in mobile phase (100 µL methanol–acetonitrile–water–formic acid) → Inject into LC–MS system | LC System →Instrument: Merck–Hitachi Model 6200 gradient pump (Merck–Hitachi, Darmstadt, Germany) → Injection Valve: Rheodyne Type 7125 (20 µL loop) (Rheodyne, Berkeley, CA, USA) → Column: Superspher RP 18 (125 × 3 mm I.D., 4 µm particle size, Merck, Darmstadt, Germany) → Mobile Phase: Acetonitrile–50 mM ammonium formate buffer, pH 3.0 (5:95, v/v) → Flow Rate Program: 0.6 mL/min for 4 min → Increase to 1.1 mL/min over 3 min → Hold at 1.1 mL/min for 10 min ESI (+)–MS Analysis →Instrument: Finnigan MAT SSQ 7000 single quadrupole (Finnigan MAT, San Jose, CA, USA) → Ionization Source: APCI → Sheath Gas: Nitrogen (70 psi) → Auxiliary Gas: Nitrogen (20 mL/min) → Heated Vaporizer Temperature: 450 °C → Heated Capillary Temperature: 180 °C → Corona Current: 5 µA → Mass Spectra Range: 100–500 u → Octapole Offset: 10 V (positive-ion mode) → Detection Mode: Selected-ion monitoring (SIM) → SIM Time Windows & Monitored Ions → 0–5 min → m/z 286, 289, 462, 465 5–11 min → m/z 300, 303, 306, 476, 479 11–17 min → m/z 328, 334 Scan Time → 0.5 s | 0.5–100 | n.a. | n.a. | n.a. | Linear up to high doses | Arterial and venous pharmacokinetics of DIM metabolism in addicts | [8] |
| DIM 6-MAM Morphine | Urine | Collect urine samples from suspected DIM users → Directly inject into column-switching HPLC system → Use strong cation-exchange (SCX, Capcell Pak MF SCX 2 × 10 mm, 5 µm (Shiseido, Tokyo, Japan) column for on-line SPE → Remove endogenous urinary constituents during trapping phase → Enrich DIM and metabolites on SCX column (Capcell Pak MF SCX (2.0 × 10 mm, 5 µm; Shiseido, Japan) → Transfer enriched analytes to analytical column via backflush mode → Elute with ammonium acetate–acetonitrile mobile phase (Merck, Darmstadt, Germany) → Introduce entire eluent into electrospray ionization mass spectrometry (ESI–MS) without splitting | Instrumentation → Column-switching HPLC (Shiseido, Tokyo, Japan) coupled with ESI–MS (Platform quadrupole, Micromass, Manchester, UK) → Columns: Trapping column: CAPCELL PAK MF SCX (2.0 mm × 10 mm, 5 µm, Shiseido, Japan)) → Analytical column: CAPCELL PAK SCX (1.5 mm × 150 mm, 5 µm, Shiseido, Japan) Mobile Phase → Main separation: 10 mM ammonium acetate (pH 6.0)–acetonitrile (30:70, v/v) Flow Rates → Trapping: 200 µL/min → Main separation: 120 µL/min ESI–MS Conditions → Ion source temperature: 70 °C → Capillary voltage: +4.5 kV → Cone voltage: 50 V → Detection Mode → Selected Ion Monitoring (SIM) → Target Ions → DAM: m/z 370 → 6-MAM: m/z 328 → Morphine: m/z 286 | 0.1 0.5 3 | 1–100 1–100 10–1000 | 99 101 104 | 3.7 4.1 3.4 | opiates in users’ urine samples | [18] | |
| DIM, 6-MAM Morphine Normorphine | Urine | Urine sample (100 µL) + Internal standard (Cerilliant, Austin, TX, USA)) (10 µL working solution) → Vortex-mix briefly → Centrifuge (5 min at 510 g) to remove large particles → Inject 10 µL supernatant directly into LC–MS/MS system | LC System: Surveyor HPLC system, LCQ Deca XP ion trap with orthogonal APCI source (positive ion mode) (ThermoFinnigan, San Jose, CA, USA) → Column: Synergi Polar RP (150 × 2.0 mm, 4 µm, Phenomenex, Torrance, CA, USA) + guard column (4 × 2 mm) (Phenomenex, Torrance, CA, USA) → Mobile Phase: Gradient elution (A: 10 mM ammonium formate in water, 0.001% formic acid, pH 4.5; B: Acetonitrile, Sigma, St. Louis, MO, USA) → Flow Rate: 300 µL/min → Gradient Program: 0–13 min (5% B → 26%B ) → 13–22 min (26% B → 90% B) → 22–24 min (90% B) → 24–27 min (90% B → 5% B) → 27–35 min (5% B) → Column Oven Temp: 25 °C → Auto-injector Tray Temp: 4 °C → Detection Mode: Selected reaction monitoring (SRM) → Ionization Source: Atmospheric pressure chemical ionization (APCI) → Corona Discharge Needle Voltage: 4.7 kV → Vaporizer Temp: 450 °C → Sheath Gas: Nitrogen (70 psi) → Transfer Capillary Temp: 220 °C → Electron Multiplier Voltage: 850 eV → Monitored Ions: DIM (m/z 370 → 310, 328, 268), 6-MAM (m/z 328 → 268, 211, 193), Morphine (m/z 286 → 268, 229, 211), Internal Std (m/z varies per analyte) | Between 10–100 | 10–10,000 | >80 | 3.1–16 | Analyte stability was accepted with a recovery greater than 80%. | Applicable in drug abuse screening and methadone treatment monitoring | [9] |
| DIM, 6-MAM, Morphine, M3G, M6G | Plasma | Collect arterial and venous blood samples at specific time intervals → Store plasma at −20 °C until analysis → Thaw plasma before extraction → Add fluoride and heparin to vacuum tubes for stabilization → Solid-phase extraction (SPE, Bond Elut C18 (200 mg, Varian, Harbor City, CA, USA) using reversed-phase cartridges → Condition cartridges with methanol, water, and ammonium formate buffer (pH 3.0) → Wash with ammonium formate buffer (pH 3), methanol and acetonitrile → Elute analytes with methanol-acetonitrile gradient → Evaporate under nitrogen → Reconstitute in mobile phase (100 µL methanol–acetonitrile–water–formic acid) → Inject into LC–MS system | LC System →Instrument: Merck–Hitachi Model L2000 gradient pump (Merck, Darmstadt, Germany) → Injection Valve: Rheodyne Type 8125 (20 µL loop, Cotati, CA, USA) → Column: Superspher RP 18 (125 × 3 mm i.d., 4 µm particle size, Merck, Darmstadt, Germany) → Mobile Phase: Acetonitrile–50 mM ammonium formate buffer, pH 3.0 (5:95, v/v) → Flow Rate Program: 0.6 mL/min for 4 min → Increase to 1.1 mL/min over 3 min → Hold at 1.1 mL/min for 10 min APCI–MS Analysis →Instrument: Finnigan MAT SSQ 7000 single quadrupole (Finnigan MAT, San Jose, CA, USA) → Ionization Source: Atmospheric Pressure Chemical Ionization (APCI) → Sheath Gas: Nitrogen (70 psi) → Auxiliary Gas: Nitrogen (20 mL/min) → Heated Vaporizer Temperature: 400 °C → Heated Capillary Temperature: 170 °C → Corona Current: 5 µA → Mass Spectra Range: 100–500 u → Octapole Offset: 10 V (positive-ion mode) → Detection Mode: Selected-ion monitoring (SIM) → SIM Time Windows & Monitored Ions → 0–5 min → m/z 286, 289, 462, 465 5–11 min → m/z 300, 303, 306, 476, 479 11–17 min → m/z 328, 334 Scan Time → 0.5 s | LLQ: 1 nmol/L (DIM, 6-MAM), 10 nmol/L (Morphine, M3G, M6G) | Linear pharmacokinetics observed for intramuscular and oral DIM administration | n.a. | n.a. | n.a. | Comparison of intramuscular vs. oral DIM pharmacokinetics in opioid-dependent patients receiving high-dose maintenance therapy | [40] |
| DIM 6-MAM Morphine | Hair | Collect hair samples from subjects in DIM-maintenance program and opiate-associated fatalities → Segment hair into 1 cm sections for analysis → all chemical obtained from (Merck, Darmstadt, Germany): Wash hair samples with dichloromethane (2×, 5 mL, 2 min each) → Dry and cut into small pieces (~30 mg) → Incubate in methanol (1 mL, 45 °C, 16 h) with internal standards (DIM-d3, 6-MAM-d3, morphine-d3) → Evaporate methanol extract to dryness → Derivatise residue with N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA), pyridine and iso-octane at 90 °C for 15 min, Düren, Germany) → Inject into GC–MS system | GC System → Agilent 6890 Plus GC coupled with Agilent 5973N MSD (Chromtech, Idstein, Germany) → Column: HP-5MS fused-silica capillary (30 m × 0.25 mm i.d., 0.25 µm film thickness, Agilent Technologies, Palo Alto, CA, USA)) → Carrier Gas: Helium (1.0 mL/min) → Injection Mode: Splitless (1 µL sample) → Injection Port Temperature: 280 °C Oven Temperature Program → Hold at 180 °C for 1 min → Ramp 15 °C/min to 190 °C, hold for 10 min → Ramp 5 °C/min to 250 °C → Increase at 30 °C/min to 290 °C, hold for 2 min → Total run time: 21.28 min Mass Spectrometry Conditions → Ion Source Temperature: 230 °C → Quadrupole Temperature: 150 °C → Transfer Line Temperature: 290 °C → Ionization Mode: Electron Impact (EI, 70 eV) → Detection Mode: Selected-ion monitoring (SIM) Monitored Ions (SIM Mode) → DIM (m/z 369, 327, 268), HER-d9 (m/z 378, 334, 272) → 6-MAM-TMS (m/z 399, 340, 287), 6-MAM-d3-TMS (m/z 402, 343, 290) → Morphine-TMS (m/z 429, 414, 236), Morphine-d3-TMS (m/z 432, 417, 239) → Codeine-TMS (m/z 371, 234, 196), Codeine-d3-TMS (m/z 374, 237, 199) → Acetylcodeine (m/z 229, 341, 282), Acetylcodeine-d3 (m/z 232, 344, 285) | 0.04 (0.21) 0.02 (0.15) 0.03 (0.11) 0.02 (0.04) 0.02 (0.13) | 0.5–25 0.5–25 0.5–25 0.5–25 0.5–25 | 79–95 91–96 92–95 80–85 62–86 | <15% | Forensic toxicology—hair analysis for long-term DIM use verification | [27] | |
| DIM, 6-MAM Morphine, M3G M6G | Plasma | Collect human plasma sample(0.25 mL) → Add internal standards: DIM-d6, Morphine-d3, Morphine-3-glucuronide-d3, Methadone-d9 (Cerilliant, Round Rock, TX, USA) → Acidify with 0.15 N HCl (VWR International, Leuven, Belgium) and add sodium fluoride (Merck, Darmstadt, Germany→ Perform SPE: Use mixed-mode sorbent columns (MCX Oasis, Waters Corporation, Milford, MA, USA) to extract analytes → all solvent were obtained from Merck, Darmstadt, Germany): Condition SPE column with methanol and aqueous buffer (Merck, Darmstadt, Germany) → Load plasma onto SPE column → Wash (Aqueous buffer × 1, Methanol × 1) → Elute analytes with 0.5% ammonium acetate in methanol (v/v 1:20) → Evaporate eluate under nitrogen at room temperature → Reconstitute in mobile phase (e.g., 100 µL methanol–acetonitrile–water–formic acid) → Inject defined volume into LC–MS/MS system | LC-MS/MS Analysis Method → LC System → Instrument: Agilent 1290 Infinity II LC (Agilent Technologies, Santa Clara, CA, USA) → Column: Zorbax Eclipse Plus C18 (2.1 × 100 mm, 1.8 µm particle size, Agilent Technologies, Santa Clara, CA, USA)→ Column: Zorbax Bonus-RP (150 × 4.6 mm, 5 µm particle size, Agilent Technologies, Palo Alto, CA, USA) →Temperature: 40 °C → Mobile Phase: Gradient elution using 5 mM ammonium formate buffer (pH 4.0, Fluka, Buchs, Switzerland) and acetonitrile (Merck, Darmstadt, Germany) → Flow Rate: 1.0 mL/min → Run Time: 15 min Mass Spectrometry Conditions → Ionization Source: Electrospray ionization (Turbo Ionspray, ESI) in positive-ion mode → Mass Spectrometer: API 3000 Triple Quadrupole (PE Sciex, Concord, ON, Canada) → Detection Mode: Multiple Reaction Monitoring (MRM) → Monitored Analytes: DIM, methadone and their metabolites (including 6-MAM, morphine, morphine-3-glucuronide, morphine-6-glucuronide), as well as cocaine, acetylcodeine and their metabolites | 5 | 5–500 | 86–96 86–94 83–101 79–80 79–81 | 2.4–11 4.3–9.5 2.9–11 2.8–7.4 6.4–10 | Short term, long-term, freeze and thaw were less than 10% | Validated method for forensic toxicology and clinical drug monitoring | [32] |
| DIM 6-MAM Morphine | Serum | Collect serum sample → Dilute 1:10 with micellar mobile phase (0.1 M sodium dodecyl sulfate + 4% (v/v) 1-butanol in 0.01 M phosphate buffer, pH 7.0) → Inject diluted serum (100 µL) directly into chromatographic system without extraction or derivatization. | HPLC System → Konik 500 HPLC system (Konik Instruments, Barcelona, Spain) → Column: C18 reversed-phase (250 × 4.0 mm, 5 µm particle size, Scharlab, Barcelona, Spain)) → Mobile Phase: 0.1 M sodium dodecyl sulfate-4% butanol (pH 7.0) → Flow Rate: 1.0 mL/min → Run Time: <18 min Detection → UV detection at 230 nm → Retention times: DIM (~15 min), 6-MAM (~13 min), Morphine (~11 min), Benzoylecgonine (~7 min). | 23 (36) 15 (28) 11 (23) | 0.02–10 | 90–98 98–101 99–100 | 1.1–2.4 0.7–1.9 0.4–1.6 | forensic toxicology and clinical drug monitoring | [41] | |
| DIM, 6-MAM, Morphine, M3G, M6G | Collect plasma samples from DIM-dependent patients → Add sodium fluoride and potassium oxalate immediately to prevent hydrolysis → Centrifuge and freeze plasma at −70 °C → Pre-treat plasma samples via SPE → Use Oasis MCX sorbent columns (Waters Corporation, Milford, MA, USA) → Apply deuterated internal standards for DAM, 6-AM, morphine and glucuronides → Follow validated procedure based on [32] → Inject extracts into HPLC–MS/MS system | [32] | 5 ng/mL | [32] | [32] | 5–11 | n.a. | [19] | ||
| DIM, 6-MAM, Morphine M3G, M6G | Plasma | Collect arterial and venous blood samples at specific time intervals → Store plasma at −30 °C until analysis → Thaw plasma before extraction → Add sodium fluoride and potassium oxalate to tubes for stabilization → Centrifuge (2000 g, 5 min) to separate plasma → Solid-phase extraction (SPE) using reversed-phase cartridges → Wash with ammonium formate buffer (pH 3), methanol and acetonitrile → Elute analytes with methanol-acetonitrile gradient → Evaporate under nitrogen → Reconstitute in mobile phase (100 µL methanol–acetonitrile–water–formic acid) → Inject into LC–MS/MS system | [32] | LOD: 5 ng/mL for all analytes | Calibration curves linear over 0.1–50 ng/mL (r > 0.999) | [32] | [32] | Intra-day precision CV < 10%, Inter-day precision CV < 15% | Comparison of intravenous vs. inhalation DIM pharmacokinetics and pharmacodynamics in opioid-dependent patients receiving high-dose maintenance therapy | [20] |
| DIM, 6-MAM Morphine Codeine 6-Acetylcodeine | Sweat | Apply PharmChek sweat patch (PharmChek™, PharmChem Inc., Menlo Park, CA, USA to upper back → Clean skin with 70% isopropanol before application → Wear patch for 7 days before removal → Store absorbent pad at −20 °C in sealed plastic tubes → Elute drugs from sweat patch using 6 mL sodium acetate buffer (pH 4.0) → Perform solid-phase extraction (SPE) using Clean Screen® ZSDAU020 columns (United Chemical Technologies, Bristol, PA, USA) → Wash with distilled water, acetic acid and methanol → Elute analytes with methylene chloride:2-propanol:ammonium hydroxide (78/20/2, v/v/v) → Evaporate eluate under nitrogen at 35 °C → Derivatise with BSTFA + TMCS (60 °C, 20 min, (Supelco, Bellefonte, PA, USA) → Inject into GC–MS system | GC System → Agilent 6890 GC coupled with Agilent 5973 MSD (Agilent Technologies, Santa Clara, CA, USA)→ Column: HP-5MS fused-silica capillary (30 m × 0.32 mm i.d., 0.25 µm film thickness, Agilent Technologies, USA) → Carrier Gas: Helium (1.1 mL/min) → Injection Mode: Splitless (2 µL sample) → Injection Port Temperature: 200 °C Oven Temperature Program → Hold at 100 °C for 0.5 min → Ramp 25 °C/min to 245 °C → Ramp 2 °C/min to 255 °C → Increase at 30 °C/min to 300 °C, hold for 0.7 min → Total run time: 13.5 min Mass Spectrometry Conditions → Ion Source Temperature: 230 °C → Quadrupole Temperature: 150 °C → Ionization Mode: Electron Impact (EI, 70 eV) → Detection Mode: Selected-ion monitoring (SIM) Monitored Ions (SIM Mode) → DIM (m/z 369, 327, 310), HER-d9 (m/z 378) → 6-MAM-TMS (m/z 399, 340, 287), 6-MAM-d3-TMS (m/z 402, 343, 290) → Morphine-TMS (m/z 429, 414, 236), Morphine-d3-TMS (m/z 432, 417, 239) → Cocaine (m/z 182, 303, 272), Cocaine-d3 (m/z 185, 306, 275) → Benzoylecgonine (m/z 240, 361, 346), Benzoylecgonine-d3 (m/z 243, 364, 349) → Methadone (m/z 294, 115, 165) | 2.5 ng/patch | 5–1000 (DIM), 10–1000 (methadone) | 69–78 97–101 102–107 108–112 104–108 | 0.8 to 6.5% | DIM hydrolysis during specimen processing was <11% | forensic toxicology and drug monitoring using sweat patches | [42] |
| DIM, 6-MAM Morphine M3G M6G | Blood–Brain | Collect whole blood and brain tissue samples from mice → Immediately add ice-cold acidic buffer containing sodium fluoride (4 mg/mL, Sigma-Aldrich, USA) → Homogenize brain tissue in ammonium formate buffer (pH 3.1) → Perform protein precipitation using ice-cold acetonitrile/methanol (85:15) → Centrifuge at 4500 rpm (3900× g) at 4 °C for 10 min (Eppendorf 5810R, Hamburg, Germany) → Evaporate supernatant to dryness at 40 °C under nitrogen (TurboVap LV, Zymark/Caliper, Hopkinton, MA, USA) → Reconstitute residue in mobile phase (3% acetonitrile/97% ammonium formate buffer, pH 3.1) → Inject into LC–MS/MS system | LC System → Waters 2695 Separations Module (Waters Corporation, Milford, MA, USA) → Column: Xterra MS C18 (2.1 × 150 mm, 3.5 µm particle size, Waters Corporation, Milford, MA, USA) → Column Temperature: 35 °C → Mobile Phase: Gradient elution with ammonium formate buffer (pH 3.1) and acetonitrile → Flow Rate: 0.2 mL/min → Run Time: 16 min Mass Spectrometry Conditions → Ionization Source → Electrospray ionization (ESI) in positive-ion mode → Mass Spectrometer: Quattro Premier XE Triple Quadrupole → Detection Mode: Multiple Reaction Monitoring (MRM) → Monitored Analytes → DIM, 6-MAM, MorphineM3G, M6G. | 1 (3) 0.3 (1) 0.5 (1) 7 (20) 0.6 (1) | 2–890 1–160 3–1430 20–11,500 2–1150 | n.a. (n.a) 110 (+44) 104 (+61) 87 (−8) 87 (−12) | 4.7–13 4–15 2.1–11 3–10 2.4–7 | Heroin: Stabile for 1 week. DIM is more stable in brain tissue than in blood. | forensic toxicology and clinical drug monitoring | [43] |
| DIM, 6-MAM, Morphine, Codeine, 6-Acetylcodeine | Hair | Collect hair samples from subjects in DIM-maintenance and methadone substituted groups → Segment hair into 1 cm sections for analysis → Wash hair samples with deionized water, petroleum benzine and dichloromethane → Dry and cut into small pieces (~50 mg) → Add methanol and internal standards (DIM-d9, 6-MAM-d3, morphine-d3, cocaine-d3, benzoylecgonine-d3, cocaethylene-d8, acetylcodeine-d3, Cerilliant, Round Rock, TX, USA / Lipomed, Arlesheim, Switzerland) → Ultrasonication extraction (5 h at 50 °C, Branson Ultrasonics, Danbury, CT, USA) → Evaporate methanol extract under nitrogen at 50 °C (TurboVap LV, Zymark/Caliper, Hopkinton, MA, USA)→ Reconstitute in phosphate buffer (pH 6) → Perform solid-phase extraction (SPE) → Elute analytes with dichloromethane/propanol-2/ammonia mixture → Derivatise with MSTFA + pyridine + isooctane (90 °C, 15 min, (Sigma-Aldrich, St. Louis, MO, USA) → Inject into GC–MS system | GC System → Agilent 6890 Plus GC coupled with Agilent 5973N MSD (Chromtech, Idstein, Germany) → Column: HP-5MS fused-silica capillary (30 m × 0.25 mm i.d., 0.25 µm film thickness, Santa Clara, CA, USA) → Carrier Gas: Helium (1.0 mL/min) → Injection Mode: Splitless (1 µL sample) → Injection Port Temperature: 280 °C Oven Temperature Program → Hold at 180 °C for 1 min → Ramp 15 °C/min to 190 °C, hold for 10 min → Ramp 5 °C/min to 250 °C → Increase at 30 °C/min to 290 °C, hold for 2 min → Total run time: 21.28 min Mass Spectrometry Conditions → Ion Source Temperature: 230 °C → Quadrupole Temperature: 150 °C → Transfer Line Temperature: 290 °C → Ionization Mode: Electron Impact (EI, 70 eV) → Detection Mode: Selected-ion monitoring (SIM) | 0.04 (0.21) 0.02 (0.15) 0.03 (0.11) 0.02 (0.04) | 0.5–25 | n.a. | n.a. | n.a. | Comparison of DIM maintenance program and methadone substituted group | [44] |
| DIM, 6-MAM, Morphine, M3G, M6G, Normorphine | Pediatric Plasma | Collect pediatric plasma sample (≤250 µL) → Add 300 µL of 0.01 M ammonium carbonate buffer (pH 9.3) → Add 25 µL of internal standard solution (25 ng/mL; containing DIM-d9, 6-MAM-d3, MOR-d3, M3G-d3, M6G-d3; Cerilliant, Round Rock, TX, USA) → Vortex mix → Load onto Bond Elut C18 SPE cartridge (Varian, Palo Alto, CA, USA ) (preconditioned with methanol, water and buffer) → Wash twice with 1 mL of 0.01 M ammonium carbonate (BDH, Poole, UK) (pH 9.3) → Dry cartridge for 10 min → Elute analytes with 2 mL methanol → Evaporate eluate under nitrogen at 50 °C → Reconstitute residue in 80 µL of initial mobile phase → Inject 20 µL into LC–MS/MS system | LC System → Thermo Finnigan LCQ Deca XP Plus ion-trap mass spectrometer with Surveyor LC interface (Thermo Finnigan, San Jose, CA, USA) → Column → Synergy Polar-RP (150 × 2.0 mm, 4 µm, Phenomenex, Torrance, CA, USA) with guard column Mobile Phase → 10 mM ammonium formate (Acros Organics, Morris Plains, NJ, USA) (pH 3) (A) and acetonitrile (B) (BDH, Poole, UK) Gradient Elution → Start at 97% A → Decrease to 84.5% at 8 min → 74% at 13 min → 20% at 26 min → Hold 3 min → Return to 97% A Flow Rate → 0.3 mL/min (first 8 min) → 0.2 mL/min (13–26 min) → Return to 0.3 mL/min Injection Volume → 20 µL Column Temperature → 30 °C Autosampler Temperature → 4 °C. Diamorphine (DIM): m/z 310 → 268, 328 6-MAM: m/z 211 → 229, 268 Morphine (MOR): m/z 201 → 229, 268 M3G & M6G: m/z 286 → 462 Normorphine (NMOR): m/z 254 → 229, 272 | 0.08 (0.2) 0.1 (0.2) 0.1 (0.3) 0.1 (0.3) 0.1 (0.3) 0.1 (O.26) | 0.1–250 | 94 (−2) 95 (−2) 98 (−2) 96 (−3) 96 (−4) 97 (+1) | 4–9 | In Room temperature: 50% with 4 h, Stable in autosampler, up to 8% flowing 3 cycle freeze and thaw, less than 5% under deep freeze up to months | Plasma samples from children under treatment for acute-to-severe pain | [1] |
| DIM, 6-MAM, Morphine, M3G | Blood, Vitreous Humor | Vitreous Humor (VH): 100 µL sample spiked with 50 µL internal standard (IS; 0.5 µM in water, Lipomed GmbH, Arlesheim, Switzerland). → Liquid–liquid extraction (LLE) with 500 µL acetonitrile/methanol (85:15, v/v, Labscan Ltd., Gliwice, Poland). Blood: 50 µL IS added to whole blood. Protein precipitation followed by LLE using 500 µL acetonitrile/methanol (85:15). | LC System → Waters Quattro Premier XE Triple Quadrupole (Waters Corporation, Milford, MA, USA) → Column → XTerra MS C18 (150 × 2.1 mm, 3.5 µm, Waters Corporation, Milford, MA, USA) Mobile Phase → Ammonium formate buffer (A) and acetonitrile (B); pH adjusted via formic acid (exact gradient referenced from urine method [42].Gradient Elution → Time-resolved step gradient adapted from prior validated assay; maintained reproducible retention and peak shape for DIM, 6-MAM, MOR and M3G Flow Rate → 0.3 mL/min Injection Volume → Not explicitly stated, but consistent with microdialysis assays (typically 10–20 µL) Column Temperature → Ambient laboratory conditions assumed (~25 °C) Autosampler Temperature → Cooling system maintained at 6 °C during dialysate collection | LOD = 0.5 ng/mL | Not specified | Not specified | Not specified | Validated method for DIM metabolite detection | Comparison of DIM and metabolite distribution in blood vs. VH using a pig model to assess forensic toxicology applications | [7] |
| DIM, 6-MAM, Morphine, | Human Plasma | Collect 100 µL human plasma sample → Add 200 µL internal standard solution (O-methylcodeine, 100 ng/mL in methanol, Council of Europe, France) → Vortex for 30 s → Centrifuge at 14,000 rpm, 4 °C, for 5 min → Filter supernatant into Eppendorf tube → For DAM, 6-MAM, MOR, (Barcelona, Spain) → Add 50 µL of 0.1% formic acid (Merck, Darmstadt, Germany) to 50 µL supernatant (Method A) → For M3G, M6G → Evaporate 150 µL supernatant to dryness at 25 °C under nitrogen → Reconstitute in 50 µL of 0.1% formic acid → Add 50 µL from Method A to same vial (Method B) → Inject into LC–MS/MS system | LC System → Waters Alliance 2795 HPLC with Quattro Premier MS (Waters Corp., Milford, MA, USA) → Column: X-Bridge Phenyl (150 × 4.6 mm, 5 µm, Waters Corp., Milford, MA, USA), 35 °C Mobile Phase → A: 5 mM ammonium formate (pH 3.0, Sigma-Aldrich, St. Louis, MO, USA) → B: 0.1% formic acid in acetonitrile (Merck, Darmstadt, Germany) Gradient Elution → Start at 5% B (1 min) → Ramp to 90% B (3 min) → Hold at 90% B (2 min) → Return to initial Flow Rate → 1.0 mL/min Injection Volume → 30 µL Run Time → 8 min | 10 ng/mL | 10–2000 (M3G), 10–1000 (others) | above 93% | 2.4–12.5% | above 93% | Used in clinical trial with 12 healthy volunteers DAM and 6-MAM were not detected in plasma after oral administration | [45] |
| DIM, 6-MAM, Morphine | Hair | Collect 10–30 scalp hair strands (apex region) → Wash with distilled water (5 min) → Acetone (Scharlau, Barcelona, Spain) rinse (1 min) → Dichloromethane rinse (2 min) → Air dry and segment hair into 3–4 mm fragments → Weigh 50 mg hair sample → Add 500 µL of 1 M NaOH → Incubate at 50 °C for 3 h (alkaline digestion, Memmert oven, Germany) → Add 2 mL MTBE → Rotary mix for 30 min → Centrifuge for 10 min (Eppendorf 5810R, Hamburg, Germany) → Transfer organic layer → Add 150 µL of 1% formic acid (Panreac, Barcelona, Spain) → Rotary mix for 20 min (IKA, Germany) → Centrifuge for 10 min (Eppendorf 5810R, Germany) → Aspirate and discard upper layer → Inject 1 µL aqueous phase into GC–MS system | GC System → Shimadzu GC–MS QP-2010 (Shimadzu Corporation, Kyoto, Japan) → Column → HP-5 crosslinked 5% phenylmethyl polysiloxane fused-silica capillary (25 m × 0.32 mm i.d., 0.17 µm film thickness, Agilent Technologies, Palo Alto, CA, USA) → Carrier Gas → Helium, 99.9% purity, flow rate: 1.0 mL/min → Injection Mode → Splitless, sample volume: 1 µL → Injection Port Temperature → 280 °C → Oven Temperature Program → Hold at 110 °C for 3 min → Ramp 10 °C/min to 210 °C, hold for 2 min → Ramp 20 °C/min to 300 °C, hold for 5 min → Total run time: 18 min Mass Spectrometry Conditions → Ionization Mode → Electron Impact (EI, 70 eV) → Detection Mode → Selected-ion monitoring (SIM) for m/z < 300 → Ion Source Temperature → 230 °C → Transfer Line Temperature and Quadrupole Temperature ( Not specified) | LOD: 6-MAM, 0.03–0.07 LOQ: 6-MAM: 0.2 and 0.1 ng/mg for addict and Rehab subjects | 7.80 ng/mg (regular addicts), 2.34 ng/mg (rehabilitation subjects) | 87–94% | 1–8% | n.a. | Hair samples from 20 regular DIM addicts and 20 undergoing rehabilitation | [46] |
3.1.2. GC–MS Methods
Hair Analysis by GC–MS
Sweat Analysis by GC–MS
Biological Fluid Analysis by GC–MS
3.1.3. HPLC–UV/FLD/DAD Methods
3.1.4. LC–MS(/MS) Methods
APCI–MS Methods
ESI–MS(/MS) with On-Line and Mixed-Mode SPE
3.2. Sample Matrices and Sampling Considerations
3.3. Stability of DIM
4. Discussion
4.1. Sensitivity vs. Throughput
4.2. Hydrolysis Control
4.3. Matrix Compatibility
4.4. Metabolite Coverage
4.5. Automation and Emerging Technologies
4.6. Limitations and Future Work
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APCI | Atmospheric pressure chemical ionisation |
| AUC | Area under the curve |
| DAD | Diode-array detection |
| EI | Electron Impact |
| HPLC | High-performance liquid chromatography |
| MRM | Multiple Reaction Monitoring |
| SIM | Selected ion monitoring |
| SPE | Solid-phase extraction |
| SRM | Selected Reaction Monitoring |
| VH | Vitreous Humor |
References
- Al-Asmari, A.; Anderson, R.A.; Kidd, S.; Thomson, A.H. Method for the quantification of diamorphine and its metabolites in pediatric plasma samples by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2010, 34, 177–195. [Google Scholar] [CrossRef]
- Maas, A.; Madea, B.; Hess, C. Confirmation of recent heroin abuse: Accepting the challenge. Drug Test. Anal. 2018, 10, 54–71. [Google Scholar] [CrossRef]
- Goldberger, B.A.; Cone, E.J.; Grant, T.M.; Caplan, Y.H.; Levine, B.S.; Smialek, J.E. Disposition of heroin and its metabolites in heroin-related deaths. J. Anal. Toxicol. 1994, 18, 22–28. [Google Scholar] [CrossRef]
- United Nation Office on Drugs and Crime. Available online: https://www.unodc.org/unodc/data-and-analysis/world-drug-report-2024.html (accessed on 1 January 2020).
- Zughaibi, T.A.; Assiri, Z.; Mirza, A.; Alharbi, H.; Alzahrani, A.E.; Alahmadi, S.A.; Alsolami, F.; Al-Saadi, A.; Almoustady, M.; Al-Zahrani, S.; et al. A quantitative and comparative study of heroin-related metabolites in different postmortem fluids and tissues. Toxics 2025, 13, 229. [Google Scholar] [CrossRef]
- Al-Asmari, A.I.; Alharbi, H.; Al-Zahrani, A.E.; Zughaibi, T.A. Heroin-related fatalities in Jeddah, Saudi Arabia, between 2008 and 2018. Toxics 2023, 11, 248. [Google Scholar] [CrossRef]
- Gottås, A.; Arnestad, M.; Halvorsen, P.S.; Bachs, L.C.; Høiseth, G. Pharmacokinetics of heroin and its metabolites in vitreous humor and blood in a living pig model. Forensic Toxicol. 2016, 34, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, K.M.; Kullak-Ublick, G.A.; Reichel, C.; Meier, P.J.; Fattinger, K. Arterial and venous pharmacokinetics of intravenous heroin in subjects who are addicted to narcotics. Clin. Pharmacol. Ther. 2001, 70, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Dams, R.; Murphy, C.M.; Lambert, W.E.; Huestis, M.A. Urine drug testing for opioids, cocaine, and metabolites by direct injection liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 1665–1670. [Google Scholar] [CrossRef]
- Baselt, R.C. Heroin. In Disposition of Toxic Drugs and Chemicals in Man, 11th ed.; Biomedical Publications: Foster City, CA, USA, 2017; pp. 1031–1036. [Google Scholar]
- Cone, E.J.; Holicky, B.A.; Grant, T.M.; Darwin, W.D.; Goldberger, B.A. Pharmacokinetics and pharmacodynamics of intranasal “snorted” heroin. J. Anal. Toxicol. 1993, 17, 327–337. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Keenan, R.M.; Henningfield, J.E.; Cone, E.J. Pharmacokinetics and pharmacodynamics of smoked heroin. J. Anal. Toxicol. 1994, 18, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Umans, J.G.; Chiu, T.S.K.; Lipman, R.A.; Schultz, M.F.; Shin, S.-U.; Inturrisi, C.E. Determination of heroin and its metabolites by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1982, 233, 213–225. [Google Scholar] [CrossRef]
- Inturrisi, C.E.; Max, M.B.; Foley, K.M.; Schultz, M.; Shin, S.-U.; Houde, R.W. The pharmacokinetics of heroin in patients with chronic pain. N. Engl. J. Med. 1984, 310, 1213–1217. [Google Scholar] [CrossRef]
- Goldberger, B.A.; Caplan, Y.H.; Maguire, T.; Cone, E.J. Testing human hair for drugs of abuse. III. Identification of heroin and 6-acetylmorphine as indicators of heroin use. J. Anal. Toxicol. 1991, 15, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Cone, E.J.; Hillsgrove, M.J.; Jenkins, A.J.; Keenan, R.M.; Darwin, W.D. Sweat testing for heroin, cocaine, and metabolites. J. Anal. Toxicol. 1994, 18, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P.; Brenneisen, R.; Bundeli, P.; Mangin, P. Sweat testing for heroin and metabolites in a heroin maintenance program. Clin. Chem. 1997, 43, 736–739. [Google Scholar] [CrossRef]
- Katagi, M.; Nishikawa, M.; Tatsuno, M.; Miki, A.; Tsuchihashi, H. Column-switching high-performance liquid chromatography–electrospray ionization mass spectrometry for identification of heroin metabolites in human urine. J. Chromatogr. B Biomed. Sci. Appl. 2001, 751, 177–185. [Google Scholar] [CrossRef]
- Klous, M.G.; Huitema, A.D.R.; Rook, E.J.; Hillebrand, M.J.X.; Hendriks, V.M.; Van den Brink, W.; Beijnen, J.H.; Van Ree, J.M. Pharmacokinetic comparison of two methods of heroin smoking: ‘Chasing the dragon’ versus the use of a heating device. Eur. Neuropsychopharmacol. 2005, 15, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Rook, E.J.; Van Ree, J.M.; Van Den Brink, W.; Hillebrand, M.J.X.; Huitema, A.D.R.; Hendriks, V.M.; Beijnen, J.H. Pharmacokinetics and pharmacodynamics of high doses of pharmaceutically prepared heroin, by intravenous or by inhalation route in opioid-dependent patients. Basic Clin. Pharmacol. Toxicol. 2006, 98, 86–96. [Google Scholar] [CrossRef]
- Maurer, H.H. Hyphenated high-resolution mass spectrometry—The “all-in-one” device in analytical toxicology? Anal. Bioanal. Chem. 2021, 413, 2303–2309. [Google Scholar] [CrossRef]
- Gaugler, S.; Al-Mazroua, M.K.; Issa, S.Y.; Rykl, J.; Grill, M.; Qanair, A.; Cebolla, V.L. Fully automated forensic routine dried blood spot screening for workplace testing. J. Anal. Toxicol. 2019, 43, 212–220. [Google Scholar] [CrossRef]
- Mardal, M.; Andreasen, M.F.; Mollerup, C.B.; Stockham, P.; Telving, R.; Thomaidis, N.S.; Diamanti, K.S.; Linnet, K.; Dalsgaard, P.W. Highresnps.com: An online crowd-sourced HR-MS database for suspect and non-targeted screening of new psychoactive substances. J. Anal. Toxicol. 2019, 43, 520–527. [Google Scholar] [CrossRef]
- Kumari Sharma, P. Heroin drug: Production, chemistry, effects and analysis. Acad. J. Forensic Sci. 2020, 3, 2581–4273. [Google Scholar]
- Bosch, M.E.; Sánchez, A.R.; Rojas, F.S.; Ojeda, C.B. Morphine and its metabolites: Analytical methodologies for its determination. J. Pharm. Biomed. Anal. 2007, 43, 799–815. [Google Scholar] [CrossRef]
- Amini, R.; Rahimpour, E.; Jouyban, A. Determination of morphine and its metabolites in the biological samples: An updated review. Bioanalysis 2020, 12, 1161–1194. [Google Scholar] [CrossRef]
- Musshoff, F.; Lachenmeier, K.; Wollersen, H.; Lichtermann, D.; Madea, B. Opiate concentrations in hair from subjects in a controlled heroin-maintenance program and from opiate-associated fatalities. J. Anal. Toxicol. 2005, 29, 345–352. [Google Scholar] [CrossRef]
- Al-Asmari, A.I.; Anderson, R.A. Method for quantification of opioids and their metabolites in autopsy blood by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2007, 31, 394–408. [Google Scholar] [CrossRef]
- Svensson, J.-O. Determination of morphine, morphine-6-glucuronide and normorphine in plasma and urine with high-performance liquid chromatography and electrochemical detection. J. Chromatogr. B Biomed. Sci. Appl. 1986, 375, 174–178. [Google Scholar] [CrossRef]
- Skoppl, G.; Ganssmann, B.; Cone, E.J.; Aderjan, R. Plasma concentrations of heroin and morphine-related metabolites after intranasal and intramuscular administration. J. Anal. Toxicol. 1997, 21, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, D.; Lehmann, T.; Hämmig, R.; Bührer, M.; Brenneisen, R. High-performance liquid chromatographic monitoring of intravenously administered diacetylmorphine and morphine and their metabolites in human plasma. J. Chromatogr. B Biomed. Appl. 1997, 694, 233–238. [Google Scholar] [CrossRef]
- Rook, E.J.; Hillebrand, M.J.X.; Rosing, H.; Van Ree, J.M.; Beijnen, J.H. The quantitative analysis of heroin, methadone and their metabolites and the simultaneous detection of cocaine, acetylcodeine and their metabolites in human plasma by high-performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2005, 824, 213–221. [Google Scholar] [CrossRef]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of new methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef]
- Goldberger, B.A.; Darwin, W.D.; Grant, T.M.; Allen, A.C.; Caplan, Y.H.; Cone, E.J. Measurement of Heroin and Its Metabolites by Isotope-Dilution Electron-Impact Mass Spectrometry. Clin. Chem. 1993, 39, 670–675. [Google Scholar] [CrossRef]
- Polettini, A.; Groppi, A.; Montagna, M. Rapid and highly selective GC/MS/MS detection of heroin and its metabolites in hair. Forensic Sci. Int. 1993, 63, 217–225. [Google Scholar] [CrossRef]
- Wang, W.-L.; Darwin, W.D.; Cone, E.J. Simultaneous assay of cocaine, heroin and metabolites in hair, plasma, saliva and urine by gas chromatography—Mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 1994, 660, 279–290. [Google Scholar] [CrossRef]
- Guillot, J.G.; Lefebvre, M.; Weber, J.P. Determination of heroin, 6-acetylmorphine, and morphine in biological fluids using their propionyl derivatives with ion trap GC–MS. J. Anal. Toxicol. 1997, 21, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zuccaro, P.; Ricciarello, R.; Pichini, S.; Pacifici, R.; Altieri, I.; Pellegrini, M.; D’Ascenzo, G. Simultaneous determination of heroin, 6-monoacetylmorphine, morphine, and its glucuronides by liquid chromatography-atmospheric pressure ionspray-mass spectrometry. J. Anal. Toxicol. 1997, 21, 268–277. [Google Scholar] [CrossRef]
- Gyr, E.; Brenneisen, R.; Bourquin, D.; Lehmann, T.; Vonlanthen, D.; Hug, I. Pharmacodynamics and pharmacokinetics of intravenously, orally and rectally administered diacetylmorphine in opioid dependents, a two-patient pilot study within a heroin-assisted treatment program. Int. J. Clin. Pharmacol. Ther. 2000, 38, 486–491. [Google Scholar] [CrossRef]
- Girardin, F.; Rentsch, K.M.; Schwab, M.A.; Maggiorini, M.; Pauli-Magnus, C.; Kullak-Ublick, G.A.; Meier, P.J.; Fattinger, K. Pharmacokinetics of high doses of intramuscular and oral heroin in narcotic addicts. Clin. Pharmacol. Ther. 2003, 74, 341–352. [Google Scholar] [CrossRef]
- Capella-Peiró, M.E.; Bose, D.; Gil-Agustí, M.; Esteve-Romero, J.; Carda-Broch, S. Direct injection determination of benzoylecgonine, heroin, 6-monoacetylmorphine and morphine in serum by MLC. J. Chromatogr. A 2005, 1073, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Brunet, B.R.; Barnes, A.J.; Scheidweiler, K.B.; Mura, P.; Huestis, M.A. Development and Validation of a solid-phase extraction gas chromatography–mass spectrometry method for the simultaneous quantification of methadone, heroin, cocaine and metabolites in sweat. Anal. Bioanal. Chem. 2008, 392, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Karinen, R.; Andersen, J.M.; Ripel, Å.; Hasvold, I.; Hopen, A.B.; Mørland, J.; Christophersen, A.S. Determination of heroin and its main metabolites in small sample volumes of whole blood and brain tissue by reversed-phase liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2009, 33, 345–350. [Google Scholar] [CrossRef]
- Musshoff, F.; Lachenmeier, K.; Lichtermann, D.; Madea, B. Cocaine and opiate concentrations in hair from subjects in a heroin maintenance program in comparison to a methadone substituted group. Int. J. Leg. Med. 2009, 123, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vicente, R.; Fernández-Nieva, Z.; Navarro, A.; Gascón-Crespí, I.; Farré-Albaladejo, M.; Igartua, M.; Hernández, R.M.; Pedraz, J.L. Development and validation of a bioanalytical method for the simultaneous determination of heroin, its main metabolites, naloxone and naltrexone by LC–MS/MS in human plasma samples: Application to a clinical trial of oral administration of a heroin/naloxone formulation. J. Pharm. Biomed. Anal. 2015, 114, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ghauri, M.A.; Hassan, F.; Hassan, Y.; Atif, N.; Adnan, A. Detection of 6-monoacetylemorphine in hair sample of heroin addicts using gas chromatography–mass spectrometry and significance of rehabilitation program. Future J. Pharm. Sci. 2021, 7, 98. [Google Scholar] [CrossRef]
- Bogusz, M.J.; Maier, R.-D.; Driessen, S. Morphine, morphine-3-glucuronide, morphine-6-glucuronide, and 6-monoacetylmorphine determined by means of atmospheric pressure chemical ionization-mass spectrometry-liquid chromatography in body fluids of heroin victims. J. Anal. Toxicol. 1997, 21, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Madea, B. Review of Biologic Matrices (Urine, Blood, Hair) as Indicators of Recent or Ongoing Cannabis Use. Ther. Drug Monit. 2006, 28, 155–163. [Google Scholar] [CrossRef]
- Wijesekera, A.R.L.; Abeysinghe, D.M.U.J.; Pathirana, K.C. Studies on the degradation of heroin. Forensic Sci. Int. 1994, 67, 147–154. [Google Scholar] [CrossRef]
- Gottås, A.; Øiestad, E.L.; Boix, F.; Vindenes, V.; Ripel, Å.; Thaulow, C.H.; Mørland, J. Levels of heroin and its metabolites in blood and brain extracellular fluid after I.v. heroin administration to freely moving rats. Br. J. Pharmacol. 2013, 170, 546–556. [Google Scholar] [CrossRef]
| Matrix | Key Considerations | Typical Sensitivity (LOD/LOQ) |
|---|---|---|
| Hair | Requires extensive washing and decontamination; keratin binding complicates extraction | Ultra-trace (pg–ng/mg) |
| Sweat | Patch collection; low analyte abundance; risk of environmental contamination | ng/patch |
| Blood/Plasma/Urine | Matrix effects from proteins and salts; need for SPE cleanup | Sub-ng/mL achievable |
| Oral Fluid (Saliva) | Pre-collection rinsing needed; risk of oral cavity contamination | Low ng/mL |
| Tissues/Vitreous Humor | Postmortem redistribution; heterogeneous composition | ng/g or ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asmari, A.I. Analytical Methods for the Determination of Diamorphine (Heroin) in Biological Matrices: A Review. Toxics 2025, 13, 867. https://doi.org/10.3390/toxics13100867
Al-Asmari AI. Analytical Methods for the Determination of Diamorphine (Heroin) in Biological Matrices: A Review. Toxics. 2025; 13(10):867. https://doi.org/10.3390/toxics13100867
Chicago/Turabian StyleAl-Asmari, Ahmed Ibrahim. 2025. "Analytical Methods for the Determination of Diamorphine (Heroin) in Biological Matrices: A Review" Toxics 13, no. 10: 867. https://doi.org/10.3390/toxics13100867
APA StyleAl-Asmari, A. I. (2025). Analytical Methods for the Determination of Diamorphine (Heroin) in Biological Matrices: A Review. Toxics, 13(10), 867. https://doi.org/10.3390/toxics13100867








