Formaldehyde Fumigation: Antibacterial Profile and Toxic Effects on Hatching Eggs
Abstract
Highlights
- Formaldehyde (FA) fumigation reduced bacterial load on eggshells.
- Increasing FA concentration intensified eggshell microstructural damage and embryonic tracheal lesions.
- Reduced chick weight and impaired chick quality are among the toxic effects of FA fumigation.
- Fumigation with FA as an antibacterial treatment for hatching eggs poses risks to poultry survival, and consequently, to the efficiency and quality of poultry production.
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halgrain, M.; Georgeault, S.; Bernardet, N.; Hincke, M.T.; Réhault-Godbert, S. Concomitant Morphological Modifications of the Avian Eggshell, Eggshell Membranes and the Chorioallantoic Membrane During Embryonic Development. Front. Physiol. 2022, 13, 838013. [Google Scholar] [CrossRef]
- Oliveira, G.d.S.; McManus, C.; dos Santos, V.M. Multivariate Analysis of Microbiological and Incubation Parameters in Hatching Eggs Sanitized with or Without Essential Oils. Vet. Sci. 2025, 12, 600. [Google Scholar] [CrossRef]
- Cadirci, S. Disinfection of Hatching Eggs by Formaldehyde Fumigation-a Review. Eur. Poult. Sci. 2009, 73, 116–123. [Google Scholar] [CrossRef]
- Oliveira, G.D.S.; McManus, C.; Santos, P.H.; de Sousa, D.E.; Jivago, J.L.; de Castro, M.B.; Dos Santos, V.M. Hatching egg sanitizers based on essential oils: Microbiological parameters, hatchability, and poultry health. Antibiotics 2024, 13, 1066. [Google Scholar] [CrossRef]
- Oliveira, G.S.; Nascimento, S.T.; dos Santos, V.M.; Silva, M.G. Clove Essential Oil in the Sanitation of Fertile Eggs. Poult. Sci. 2020, 99, 5509–5516. [Google Scholar] [CrossRef]
- Cony, H.C.; Vieira, S.L.; Berres, J.; Gomes, H.A.; Coneglian, J.L.B.; Freitas, D.M.D. Técnicas de pulverização e imersão com distintos desinfetantes sobre ovos incubáveis. Ciênc. Rural 2008, 38, 1407–1412. [Google Scholar] [CrossRef]
- Clímaco, W.L.D.S.; Melo, É.D.F.; Vaz, D.P.; Saldanha, M.M.; Pinto, M.F.V.D.S.; Fernandes, L.C.C.; Baião, N.C.; Oliveira, L.G.D.; Sant’Anna, F.M.D.; Souza, M.R.D.; et al. Eggshell Microbiology and Quality of Hatching Eggs Subjected to Different Sanitizing Procedures. Pesq. Agrop. Bras. 2018, 53, 1177–1183. [Google Scholar] [CrossRef]
- Melo, E.F.; Clímaco, W.L.S.; Triginelli, M.V.; Vaz, D.P.; de Souza, M.R.; Baião, N.C.; Pompeu, M.A.; Lara, L.J.C. An Evaluation of Alternative Methods for Sanitizing Hatching Eggs. Poult. Sci. 2019, 98, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, I.P.; Rieger, G.; Garcia, R.G.; Valentim, J.K.; Caldara, F.R.; de Oliveira Troguilho, A.; Sgavioli, S. Optimal paraformaldehyde levels for disinfection of eggs used in vaccine production. Poult. Sci. 2025, 104, 104614. [Google Scholar] [CrossRef]
- USDA. United States Departament of Agriculture. 2000. Available online: https://www.ams.usda.gov/sites/default/files/media/Egg%20Grading%20Manual.pdf (accessed on 13 July 2025).
- Mineki, M.; Kobayashi, M. Microstructural changes in stored hen egg yolk. Jpn. Poult. Sci. 1998, 35, 285–294. [Google Scholar] [CrossRef]
- Vale, I.R.R.; Oliveira, G.d.S.; de Jesus, L.M.; de Castro, M.B.; McManus, C.; dos Santos, V.M. Sustainable Bacterial Control of Hatching Eggshells Using Essential Oils. Antibiotics 2024, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Mahato, P.L.; Weatherby, T.; Ewell, K.; Jha, R.; Mishra, B. Scanning Electron Microscope-Based Evaluation of Eggshell Quality. Poult. Sci. 2024, 103, 103428. [Google Scholar] [CrossRef]
- Boerjan, M. Chick Vitality and Uniformity. Int. Hatch. Pract. 2006, 20, 7–8. [Google Scholar]
- Upadhyaya, I.; Yin, H.B.; Nair, M.S.; Chen, C.H.; Upadhyay, A.; Darre, M.J.; Venkitanarayanan, K. Efficacy of Fumigation with Trans-Cinnamaldehyde and Eugenol in Reducing Salmonella enterica serovar Enteritidis on Embryonated Egg Shells. Poult. Sci. 2015, 94, 1685–1690. [Google Scholar] [CrossRef]
- Hayretdaǧ, S.; Kolankaya, D. Investigation of the Effects of Pre-Incubation Formaldehyde Fumigation on the Tracheal Epithelium of Chicken Embryos and Chicks. Turk. J. Vet. Anim. Sci. 2008, 32, 263–267. [Google Scholar]
- Souto, H.N.; de Campos Júnior, E.O.; Campos, C.F.; Rodrigues, T.S.; Pereira, B.B.; Morelli, S. Biomonitoring birds: The use of a micronuclei test as a tool to assess environmental pollutants on coffee farms in southeast Brazil. Environ. Sci. Pollut. Res. 2018, 25, 24084–24092. [Google Scholar] [CrossRef]
- Benvindo-Souza, M.; Oliveira, E.A.S.; Assis, R.A.; Santos, C.G.A.; Borges, R.E.; e Silva, D.D.M.; de Souza Santos, L.R. Micronucleus test in tadpole erythrocytes: Trends in studies and new paths. Chemosphere 2020, 240, 124910. [Google Scholar] [CrossRef] [PubMed]
- Bekhet, G.; Khalifa, A.Y.Z. Essential Oil Sanitizers to Sanitize Hatching Eggs. J. Appl. Anim. Res. 2022, 50, 695–701. [Google Scholar] [CrossRef]
- Gopar, M.d.C.F. Efecto de la Fumigación con Gas Formaldehído Sobre la Viabilidad del Embrión de Pollo y Sobre la Integridad de la Cutícula del Huevo. Bachelor’s Thesis, Trabajo Final (Medicina Veterinaria y Zootecnia)—Universidad Nacional Autónoma de México, Facultad de Medicina Veterinaria y Zootecnia, Ciudad de México, Mexico, 1992. [Google Scholar]
- Baylan, M.; Akpınar, G.C.; Canogullari, S.D.; Ayasan, T. The Effects of Using Garlic Extract for Quail Hatching Egg Disinfection on Hatching Results and Performance. Rev. Bras. Cienc. Avic. 2018, 20, 343–350. [Google Scholar] [CrossRef]
- Amoah, I.B.; Asiedu, P.; Arthur, C.T.; Aboagye, I.F. Effect of Formaldehyde Treatment on Bacteria-Infected Hatching Eggs of Gallus gallus domesticus Linnaeus, 1758. Ghana J. Sci. 2020, 61, 82–90. [Google Scholar] [CrossRef]
- Zeweil, H.S.; Rizk, R.E.; Bekhet, G.M.; Ahmed, M.R. Comparing the Effectiveness of Egg Disinfectants against Bacte-ria and Mitotic Indices of Developing Chick Embryos. J. Basic Appl. Zool. 2015, 70, 1–15. [Google Scholar] [CrossRef]
- Badran, A.M.M.; Osman, A.M.R.; Yassein, D.M.M. Comparative Study of the Effect of Some Disinfectants on Em-bryonic Mortality, Hatchability, and Some Blood Components. Egypt. Poult. Sci. J. 2018, 38, 1069–1081. [Google Scholar] [CrossRef]
- Mustafa, A.A.; Mirza, R.A.; Aziz, H.I. Lavender Essential Oil in Sanitation on Fertile Egg. Passer J. Basic Appl. Sci. 2023, 5, 377–381. [Google Scholar] [CrossRef]
- Maharjan, P.; Cox, S.; Gadde, U.; Clark, F.D.; Bramwell, K.; Watkins, S.E. Evaluation of chlorine dioxide based product as a hatchery sanitizer. Poult. Sci. 2017, 96, 560–565. [Google Scholar] [CrossRef]
- Sommer, S.; Buraczewska, I.; Kruszewski, M. Micronucleus Assay: The State of Art, and Future Directions. Int. J. Mol. Sci. 2020, 21, 1534. [Google Scholar] [CrossRef] [PubMed]
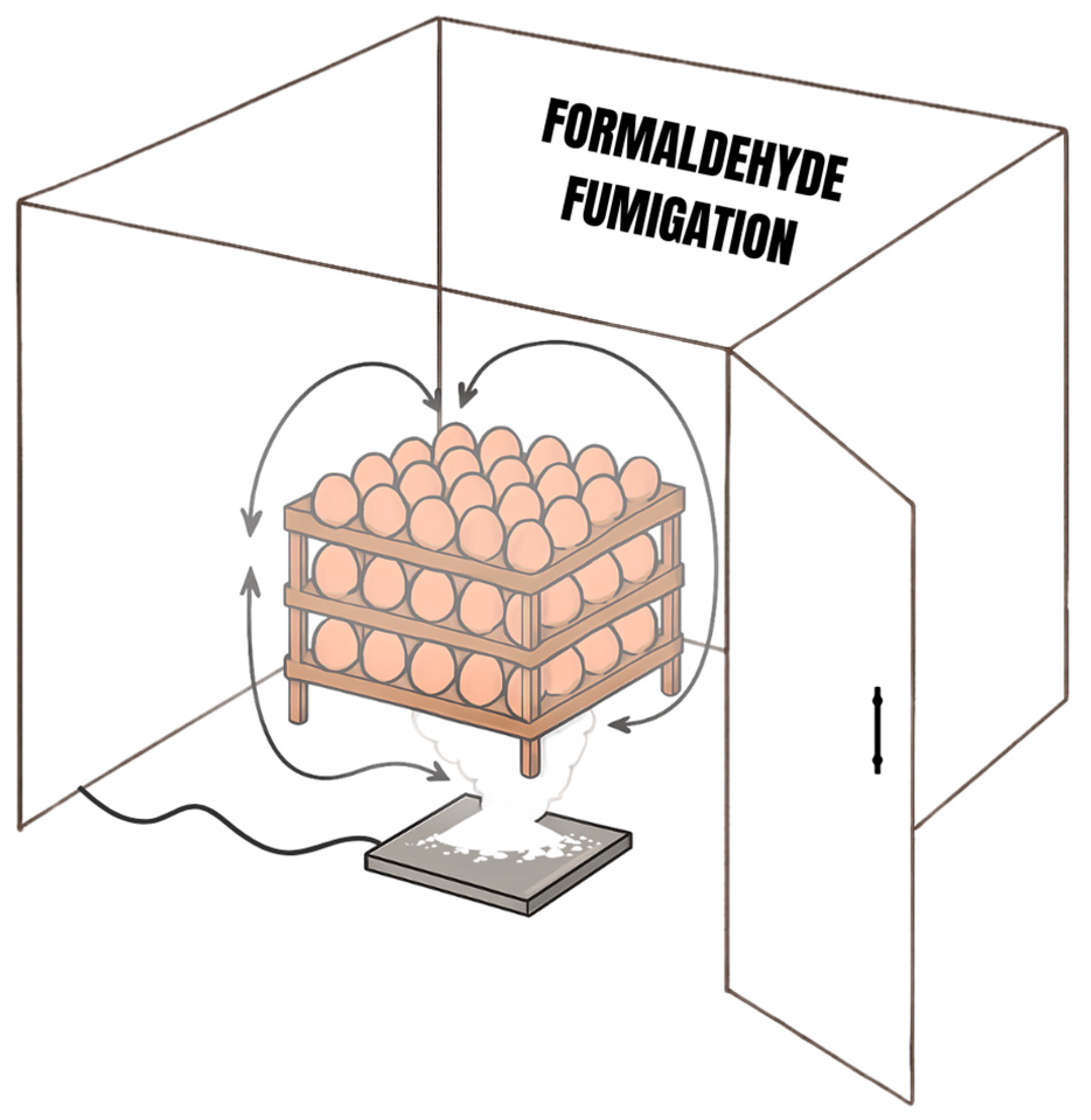
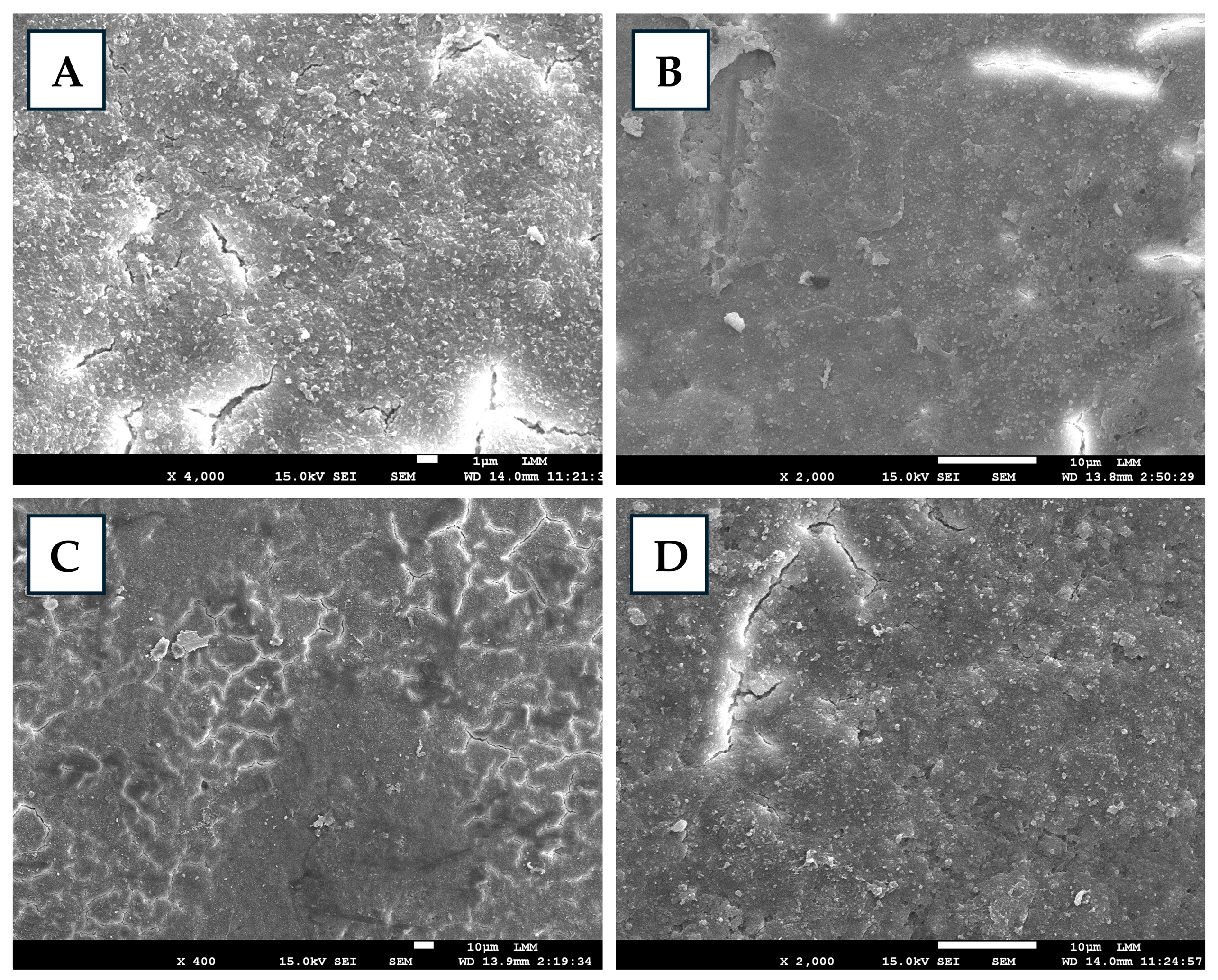



| Treatment | Concentration | Fumigation Time | Fumigation Temperature | Fumigation Humidity | Number of Eggs |
|---|---|---|---|---|---|
| Control | . | . | 24–26 °C | 73–79% | 350 |
| FA-I | 2.5 g/m3 | 15 min | 24–26 °C | 73–79% | 350 |
| FA-II | 5 g/m3 | 15 min | 24–26 °C | 73–79% | 350 |
| FA-III | 10 g/m3 | 15 min | 24–26 °C | 73–79% | 350 |
| Treatment | Eggshells | |
|---|---|---|
| TAMB (log10 CFU/mL) | ENT | |
| Control | 1.57 ± 0.49 a | <10 CFU/mL |
| FA-I | <10 CFU/mL b | <10 CFU/mL |
| FA-II | <10 CFU/mL b | <10 CFU/mL |
| FA-III | <10 CFU/mL b | <10 CFU/mL |
| p value | <0.0009 | |
| Treatment | Yolk sacs | |
| TAMB (log10 CFU/mL) | ENT | |
| Control | 2.40 ± 0.22 a | <10 CFU/mL |
| FA-I | 1.85 ± 0.33 a | <10 CFU/mL |
| FA-II | 1.78 ± 0.16 a | <10 CFU/mL |
| FA-III | 1.77 ± 0.43 a | <10 CFU/mL |
| p value | 0.0964 | |
| YTAMB | HI | EED | IED | LED | CE | |
|---|---|---|---|---|---|---|
| ETAMB | 0.52 ns | 0.10 ns | 0.25 ns | −0.16 ns | 0.08 ns | 0.41 ns |
| YTAMB | 0.01 ns | −0.13 ns | −0.17 ns | −0.06 ns | 0.67 * | |
| HI | −0.87 ** | −0.03 ns | −0.48 ns | −0.54 * | ||
| EED | 0.21 ns | 0.10 ns | 0.34 ns | |||
| IED | −0.47 ns | −0.24 ns | ||||
| LED | 0.11 ns |
| Group | EWBS (g) | EWDT (g) | EWL (%) | HI (%) | EED (%) | IED (%) | LED (%) |
|---|---|---|---|---|---|---|---|
| Control | 65.83 ± 0.35 a | 57.82 ± 0.29 a | 12.17 ± 0.66 a | 90.40 ± 3.14 ab | 3.27 ± 1.14 ab | 0.30 ± 0.60 a | 3.57 ± 1.68 a |
| FA-I | 65.57 ± 0.67 a | 57.35 ± 1.15 a | 12.54 ± 0.93 a | 96.05 ± 0.98 a | 0.89 ± 1.14 b | 0.00 ± 0.00 a | 2.68 ± 0.60 a |
| FA-II | 65.32 ± 0.29 a | 57.29 ± 0.31 a | 12.30 ± 0.45 a | 91.74 ± 2.60 ab | 4.12 ± 2.15 ab | 0.60 ± 1.19 a | 2.38 ± 2.57 a |
| FA-III | 64.92 ± 0.51 a | 56.53 ± 0.91 a | 12.93 ± 0.75 a | 88.64 ± 4.11 b | 5.95 ± 3.22 a | 0.30 ± 0.60 a | 3.57 ± 2.38 a |
| p value | 0.0980 | 0.1736 | 0.4863 | 0.0223 | 0.0345 | 0.7256 | 0.7628 |
| Treatment | CW (g) | CQ |
|---|---|---|
| Control | 45.69 ± 0.78 a | 9.23 ± 0.95 ab |
| FA-I | 44.63 ± 0.31 ab | 9.68 ± 0.62 a |
| FA-II | 44.93 ± 0.56 ab | 9.35 ± 0.98 ab |
| FA-III | 43.96 ± 0.81 b | 9.10 ± 1.03 b |
| p value | 0.0187 | 0.0339 |
| Treatment | SN | ECN | GCH | LI | ECD |
|---|---|---|---|---|---|
| Control | 1 | − | − | − | − |
| Control | 2 | − | − | − | − |
| Control | 3 | − | − | − | − |
| Control | 4 | − | − | − | − |
| Control | 5 | − | − | − | − |
| Control | 6 | − | − | − | − |
| FA−I | 1 | − | + | + | ++ |
| FA−I | 2 | − | + | − | ++ |
| FA−I | 3 | − | + | − | ++ |
| FA−I | 4 | − | + | − | + |
| FA−I | 5 | − | + | − | ++ |
| FA−I | 6 | − | + | + | ++ |
| FA−II | 1 | − | + | − | + |
| FA−II | 2 | − | + | − | +++ |
| FA−II | 3 | − | ++ | − | ++ |
| FA−II | 4 | − | ++ | − | ++ |
| FA−II | 5 | − | + | + | ++ |
| FA−II | 6 | − | + | + | ++ |
| FA−III | 1 | − | ++ | − | ++ |
| FA−III | 2 | − | ++ | + | ++ |
| FA−III | 3 | − | ++ | + | +++ |
| FA−III | 4 | − | ++ | + | +++ |
| FA−III | 5 | − | ++ | + | + |
| FA−III | 6 | + | + | − | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, P.H.G.d.S.; Oliveira, G.d.S.; Cerqueira, L.d.A.; Jivago, J.L.d.P.R.; Paixão, S.S.R.M.; de Castro, M.B.; McManus, C.; Santos, V.M.d. Formaldehyde Fumigation: Antibacterial Profile and Toxic Effects on Hatching Eggs. Toxics 2025, 13, 851. https://doi.org/10.3390/toxics13100851
Santos PHGdS, Oliveira GdS, Cerqueira LdA, Jivago JLdPR, Paixão SSRM, de Castro MB, McManus C, Santos VMd. Formaldehyde Fumigation: Antibacterial Profile and Toxic Effects on Hatching Eggs. Toxics. 2025; 13(10):851. https://doi.org/10.3390/toxics13100851
Chicago/Turabian StyleSantos, Pedro Henrique Gomes de Sá, Gabriel da Silva Oliveira, Liz de Albuquerque Cerqueira, José Luiz de Paula Rôlo Jivago, Susana Suely Rodrigues Milhomem Paixão, Márcio Botelho de Castro, Concepta McManus, and Vinícius Machado dos Santos. 2025. "Formaldehyde Fumigation: Antibacterial Profile and Toxic Effects on Hatching Eggs" Toxics 13, no. 10: 851. https://doi.org/10.3390/toxics13100851
APA StyleSantos, P. H. G. d. S., Oliveira, G. d. S., Cerqueira, L. d. A., Jivago, J. L. d. P. R., Paixão, S. S. R. M., de Castro, M. B., McManus, C., & Santos, V. M. d. (2025). Formaldehyde Fumigation: Antibacterial Profile and Toxic Effects on Hatching Eggs. Toxics, 13(10), 851. https://doi.org/10.3390/toxics13100851









