Absorption and Tissue Distribution of Environmental Pollutant HFPO-DA, and Its Effect on Hepatic Lipid Metabolism Reprogramming in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Stock Solutions, Reference Standards, and Quality Control (QC) Samples
2.3. Animals and Chemical Treatment
2.4. Sample Preparation
2.5. Analytical Instruments and Conditions
2.6. Method Validation
2.6.1. Selectivity
2.6.2. Linearity and Lower Limit of Quantitation (LLOQ)
2.6.3. Precision and Accuracy
2.6.4. Matrix Effect and Extraction Recovery Rate
2.6.5. Stability
2.6.6. Dilution Integrity
2.7. Statistical Analysis
3. Results
3.1. Method Validation
3.1.1. Selectivity
3.1.2. Linearity and LLOQ
3.1.3. Precision and Accuracy
3.1.4. Matrix Effect and Extraction Recovery Rate
3.1.5. Stability
3.1.6. Dilution Integrity
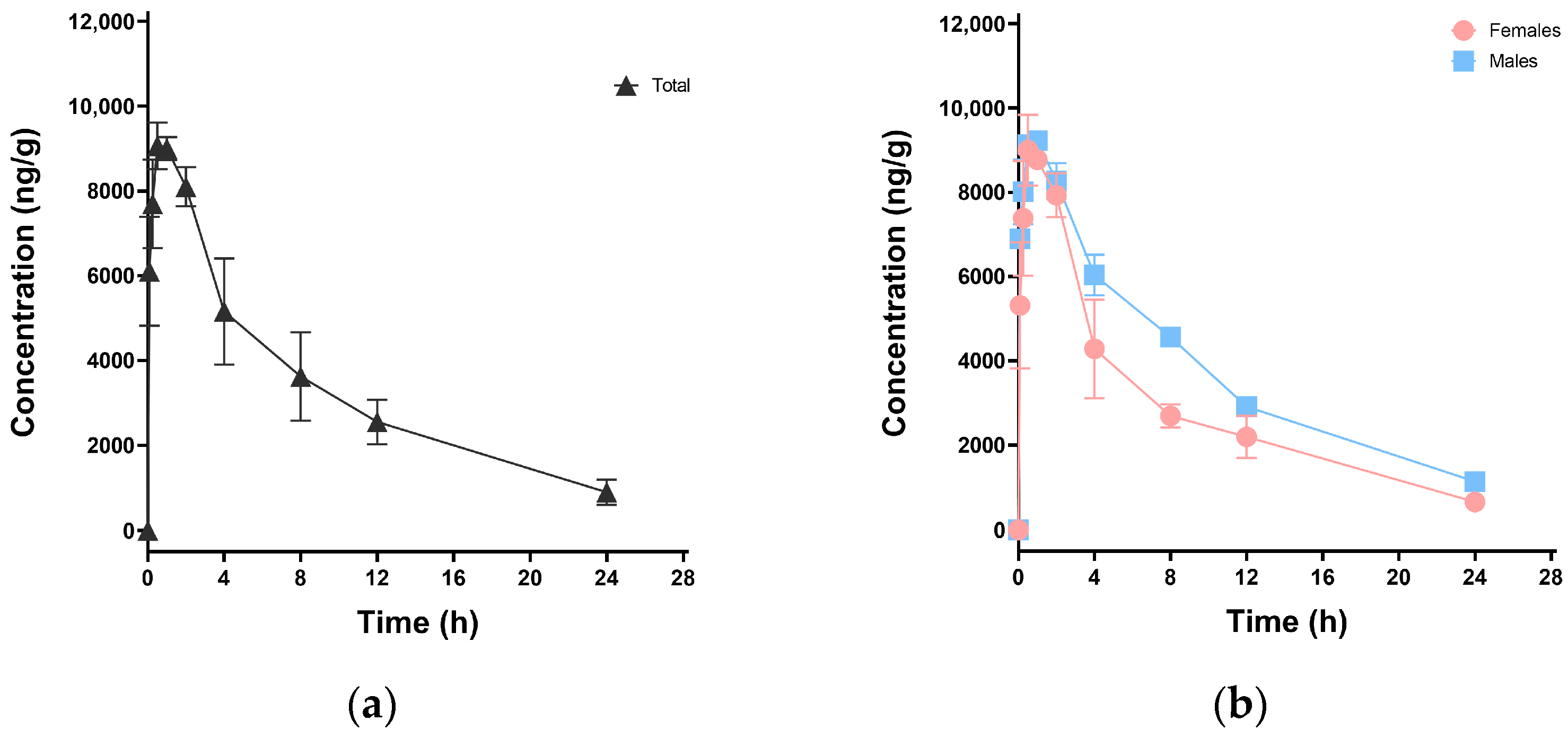
3.2. Pharmacokinetic Study in Mice
3.3. Tissue Distribution in Mice
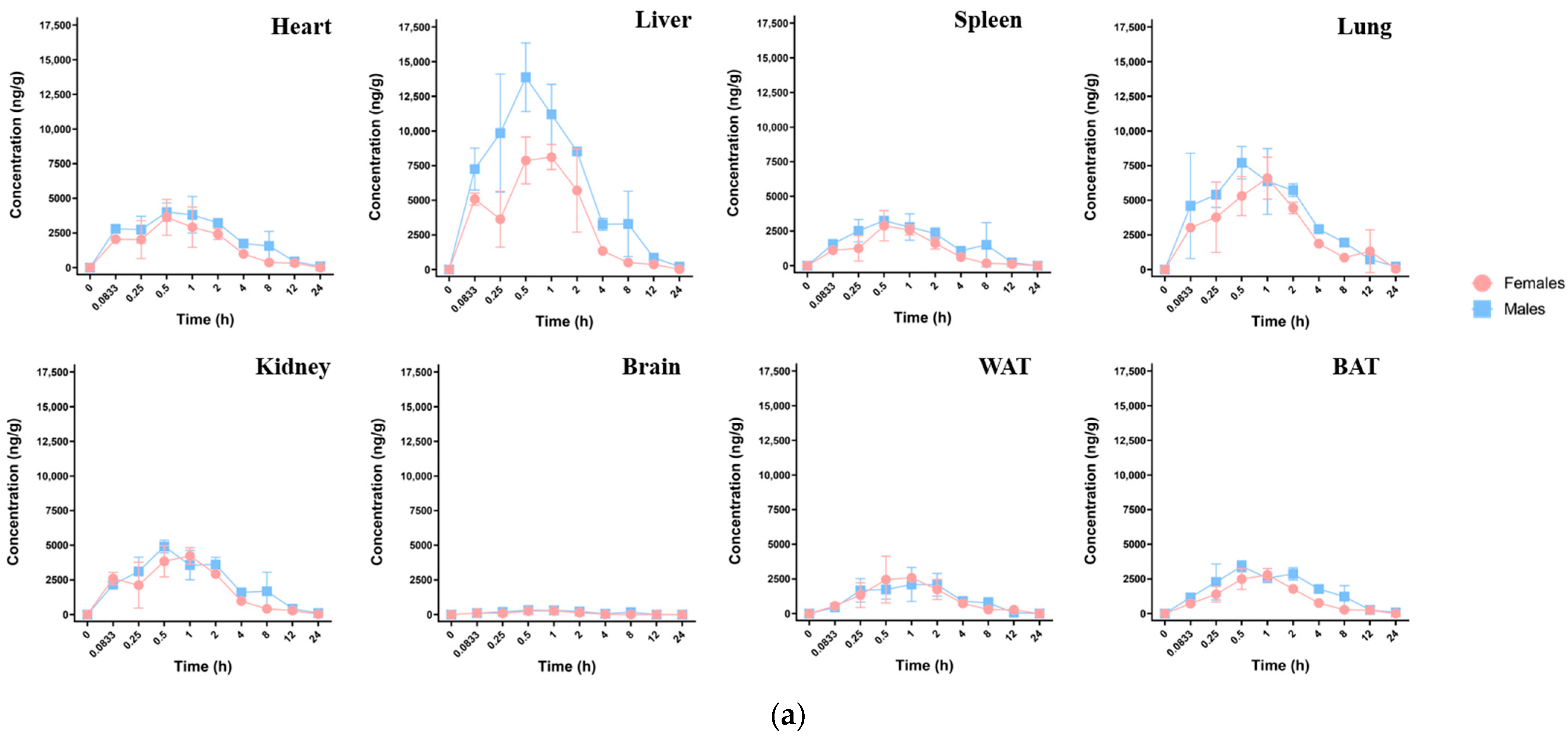
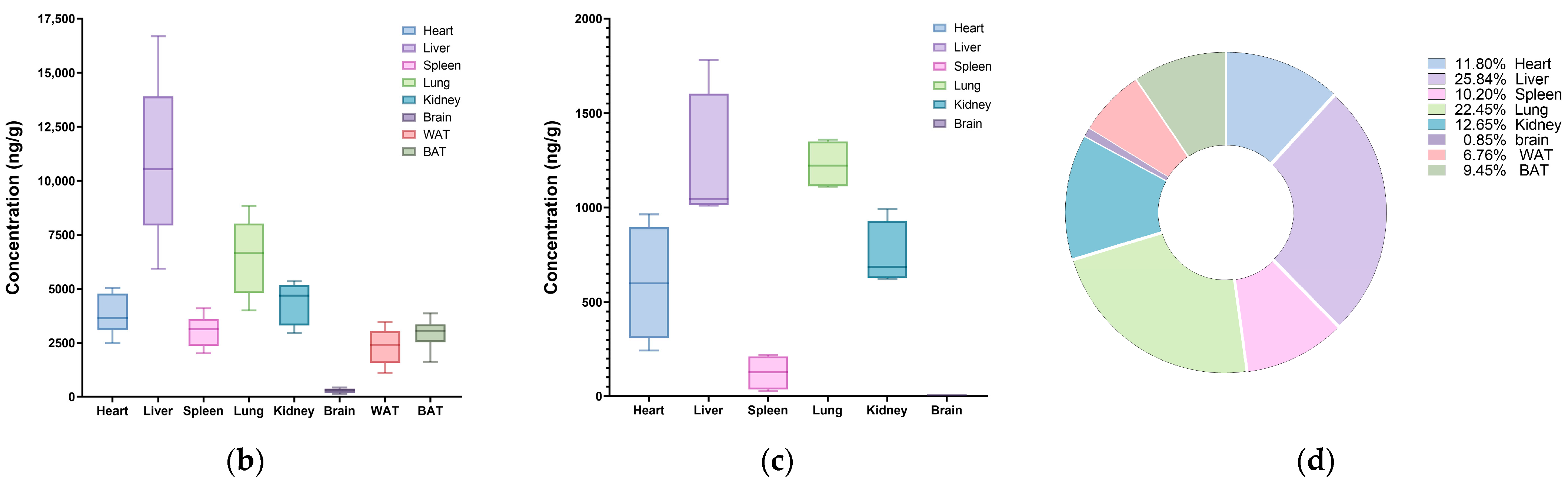
3.3.1. Single-Dose Oral Exposure Tissue Distribution
3.3.2. Multiple-Dose Oral Exposure Tissue Distribution
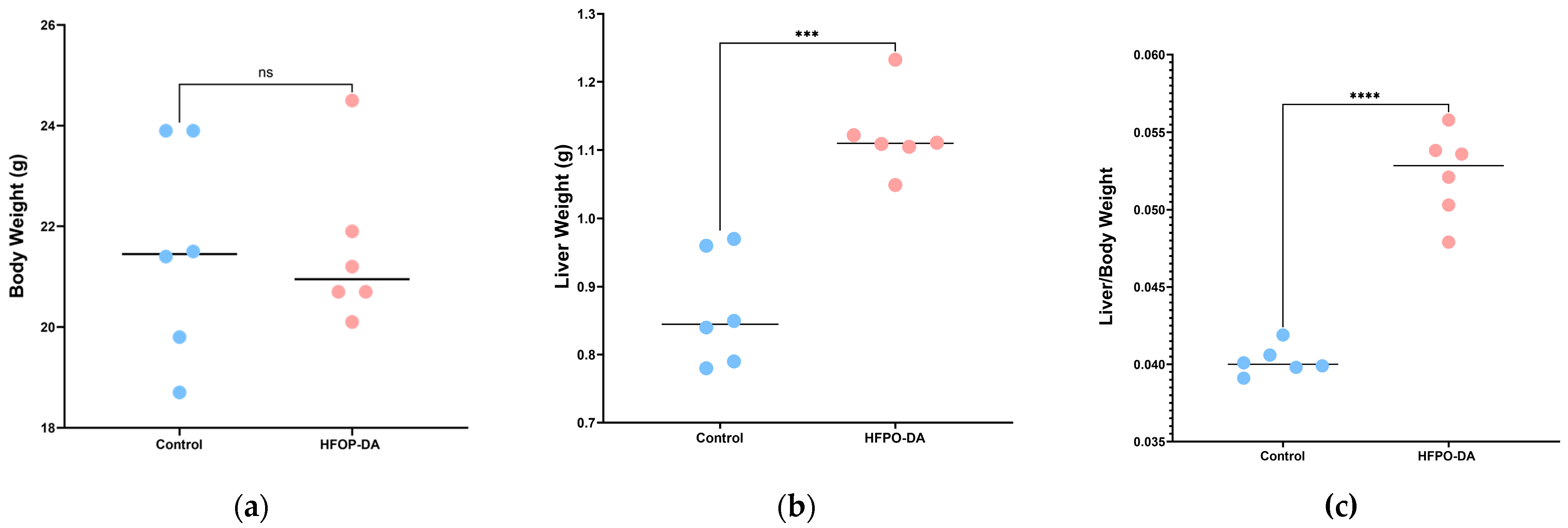
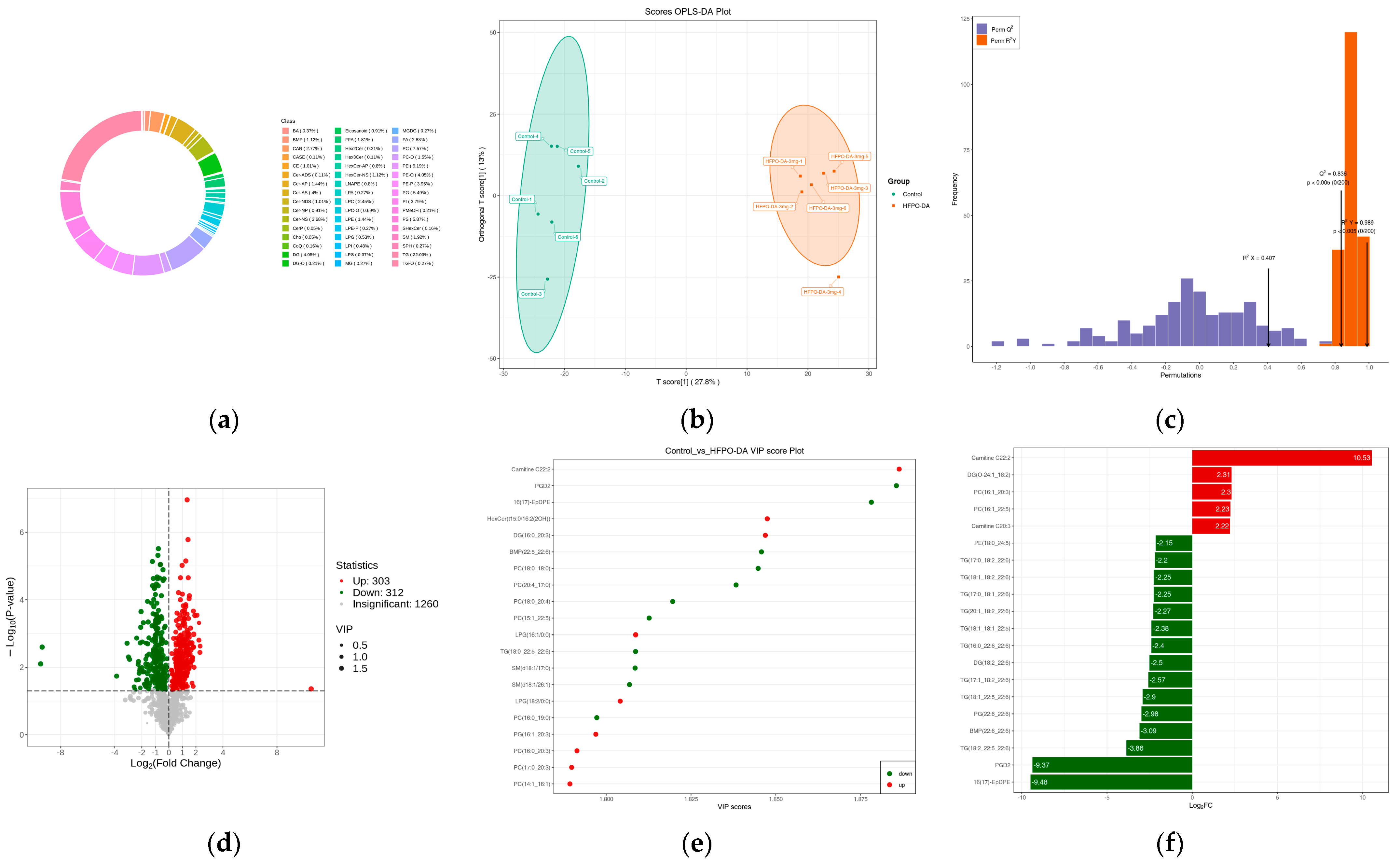
3.3.3. Characteristics of Liver Lipidome Following Multiple-Dose Oral Exposure
4. Discussion
4.1. Method Development
4.2. Pharmacokinetics and Tissue Distribution
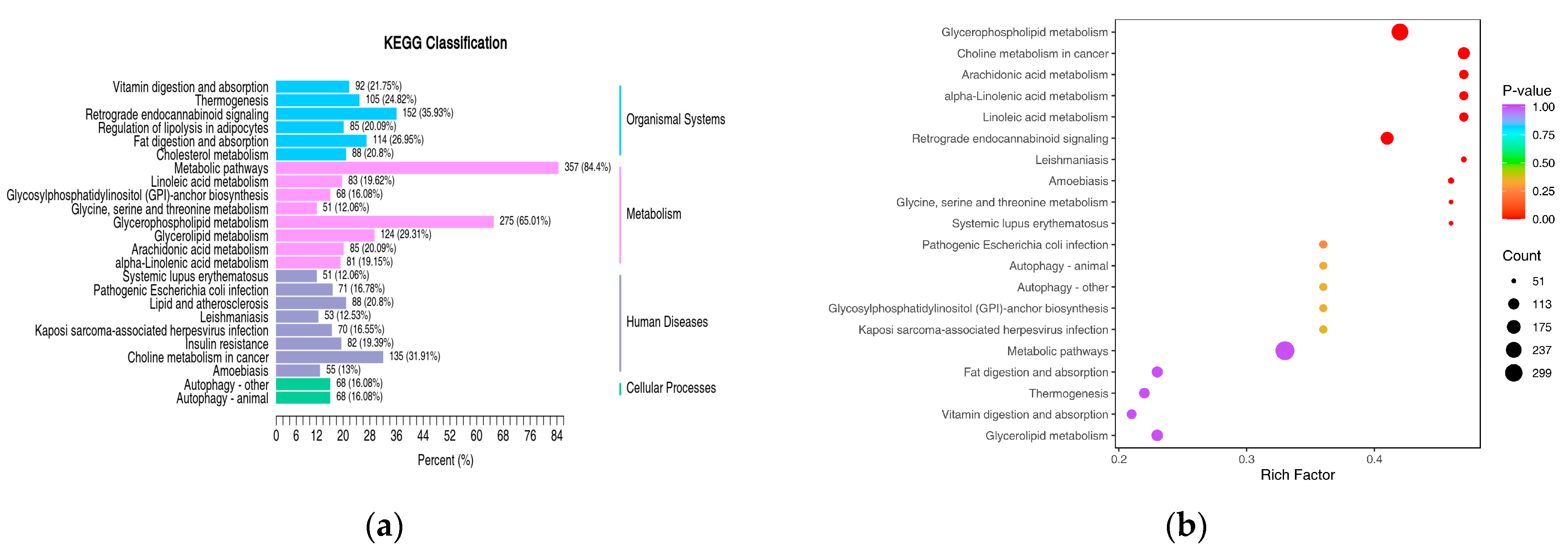
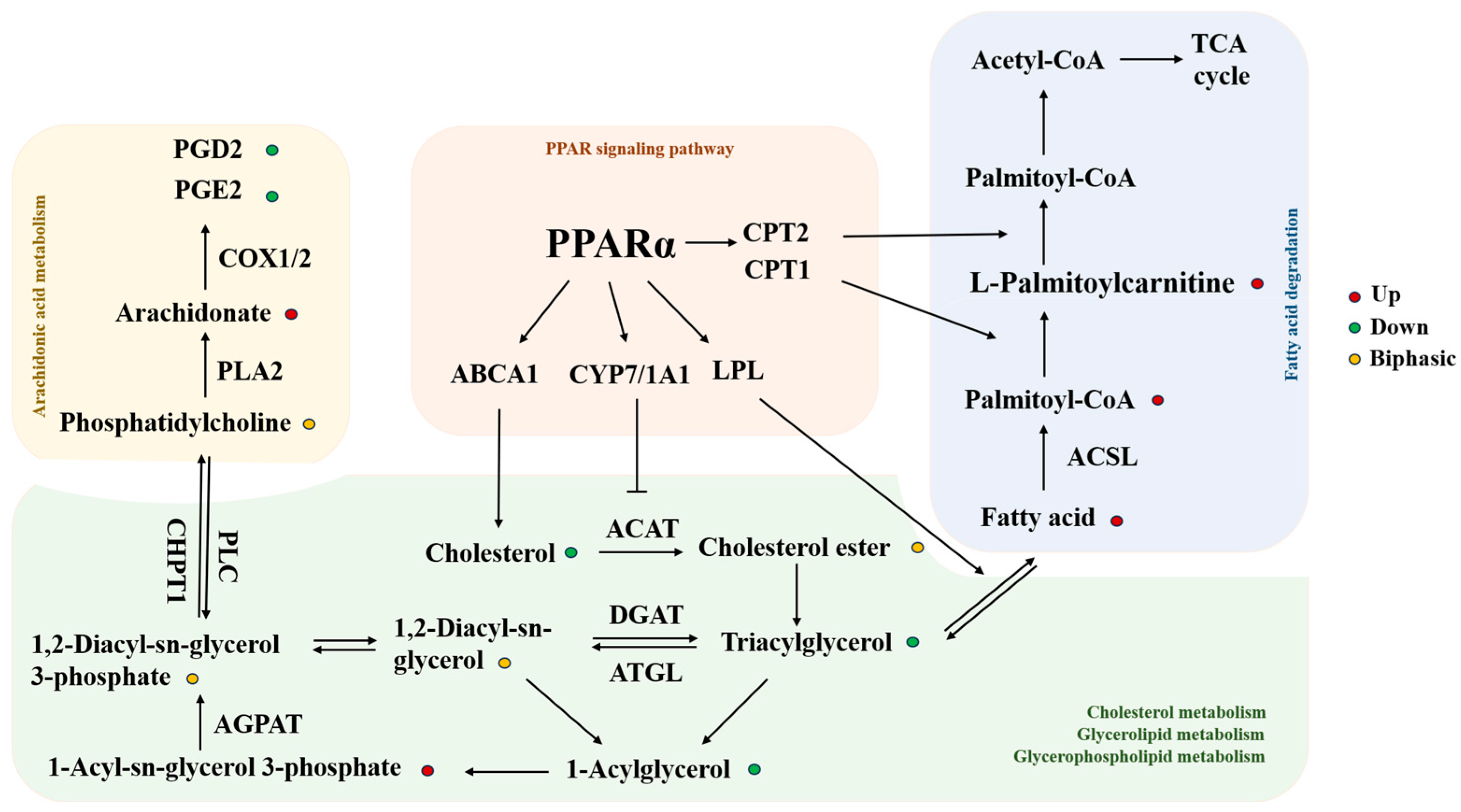
4.3. Lipidomics and KEGG Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stockholm Convention. Alternatives to POPs, Chemicals Listed in Annex A: Perfluorooctanoic Acid (PFOA), Its Salts and PFOA-Related Compounds; Stockholm Convention: Geneva, Switzerland, 2019. [Google Scholar]
- Guo, W.; Hao, W.; Xiao, W. Emerging Perfluorinated Chemical GenX: Environmental and Biological Fates and Risks. Environ. Health 2025, 3, 338–351. [Google Scholar] [CrossRef]
- Feng, X.; Yi, S.; Shan, G.; Chen, X.; Yang, Y.; Yang, L.; Jia, Y.; Zhu, Y.; Zhu, L. Occurrence of perfluoroalkyl substances in the environment compartments near a mega fluorochemical industry: Implication of specific behaviors and emission estimation. J. Hazard. Mater. 2023, 445, 130473. [Google Scholar] [CrossRef]
- Amorim, V.E.; Ferreira, A.S.; Cruzeiro, C.; Cardoso, P.G. Enhancement of per- and Polyfluoroalkyl Substances (PFAS) quantification on surface waters from marinas in the douro river, Portugal. Environ. Res. 2024, 262, 119805. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Lu, X.; Ouyang, K.; Su, G.; Li, Q.; Shi, B.; Meng, J. Environmental occurrence, bioaccumulation and human risks of emerging fluoroalkylether substances: Insight into security of alternatives. Sci. Total Environ. 2024, 922, 171151. [Google Scholar] [CrossRef]
- Petriello, M.C.; Mottaleb, M.A.; Serio, T.C.; Balyan, B.; Cave, M.C.; Pavuk, M.; Birnbaum, L.S.; Morris, A.J. Serum concentrations of legacy and emerging per- and polyfluoroalkyl substances in the Anniston Community Health Surveys (ACHS I and ACHS II). Environ. Int. 2022, 158, 106907. [Google Scholar] [CrossRef]
- Nian, M.; Huo, X.; Zhang, J.; Mao, Y.; Jin, F.; Shi, Y.; Zhang, J. Association of emerging and legacy per- and polyfluoroalkyl substances with unexplained recurrent spontaneous abortion. Ecotoxicol. Environ. Saf. 2022, 239, 113691. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Yang, W.J.; Lv, S.H.; Zhu, T.T.; Sharif, H.M.A.; Yang, C.; Du, J.; Lin, H. Is HFPO-DA (GenX) a suitable substitute for PFOA? A comprehensive degradation comparison of PFOA and GenX via electrooxidation. Environ. Res. 2022, 204, 111995. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Lu, C.; Zhang, J.; Li, B.; Du, Z.; Wang, J.; Wang, J.; Juhasz, A.; Yang, Y.; et al. Are HFPO-TA and HFPO-DA safe substitutes for PFOA? A comprehensive toxicity study using zebrafish (Danio rerio) embryos and adults. J. Hazard. Mater. 2025, 484, 136718. [Google Scholar] [CrossRef]
- Wu, S.; Xie, J.; Zhao, H.; Sanchez, O.; Zhao, X.; Freeman, J.L.; Yuan, C. Pre-differentiation GenX exposure induced neurotoxicity in human dopaminergic-like neurons. Chemosphere 2023, 332, 138900. [Google Scholar] [CrossRef]
- Gong, S.; McLamb, F.; Shea, D.; Vu, J.P.; Vasquez, M.F.; Feng, Z.; Bozinovic, K.; Hirata, K.K.; Gersberg, R.M.; Bozinovic, G. Toxicity assessment of hexafluoropropylene oxide-dimer acid on morphology, heart physiology, and gene expression during zebrafish (Danio rerio) development. Environ. Sci. Pollut. Res. 2023, 30, 32320–32336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, J.; Huo, S.; Li, B.; Zhang, J.; Song, M.; Shao, B.; Li, Y. Hexafluoropropylene oxide trimer acid causes fibrosis in mice liver via mitochondrial ROS/cGAS-STING/NLRP3-mediated pyroptosis. Food Chem. Toxicol. 2023, 174, 113706. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xu, J.; Yang, D.; Zhao, X.; Li, X.; Chen, D.; Xing, J.; Shi, Y.; Sun, Y.; Ding, G. Different Life-Stage Exposure to Hexafluoropropylene Oxide Trimer Acid Induces Reproductive Toxicity in Adult Zebrafish (Danio rerio). Environ. Toxicol. Chem. 2023, 42, 2490–2500. [Google Scholar] [CrossRef]
- Thompson, C.M.; Fitch, S.E.; Ring, C.; Rish, W.; Cullen, J.M.; Haws, L.C. Development of an oral reference dose for the perfluorinated compound GenX. J. Appl. Toxicol. 2019, 39, 1267–1282. [Google Scholar] [CrossRef]
- Gannon, S.A.; Fasano, W.J.; Mawn, M.P.; Nabb, D.L.; Buck, R.C.; Buxton, L.W.; Jepson, G.W.; Frame, S.R. Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 2016, 340, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.F.; Guo, Y.F.; Wang, S.K.; Cui, J.S.; Wang, Y.; Shi, Y.L. Distribution, Accumulation, and Amplification of Per- and Polyfluoroalkyl Substances in Organisms in Coastal Waters of Bohai Sea. Huan Jing Ke Xue 2025, 46, 5092–5102. [Google Scholar]
- Barutcu, A.R.; Black, M.B.; Andersen, M.E. Transcriptomic re-analyses of human hepatocyte spheroids treated with PFAS reveals chain length and dose-dependent modes of action. Toxicol. Appl. Pharmacol. 2024, 489, 117013. [Google Scholar] [CrossRef]
- Heintz, M.M.; Klaren, W.D.; East, A.W.; Haws, L.C.; McGreal, S.R.; Campbell, R.R.; Thompson, C.M. Comparison of transcriptomic profiles between HFPO-DA and prototypical PPARα, PPARγ, and cytotoxic agents in mouse, rat, and pooled human hepatocytes. Toxicol. Sci. 2024, 200, 165–182. [Google Scholar] [CrossRef]
- Heintz, M.M.; Klaren, W.D.; East, A.W.; Haws, L.C.; McGreal, S.R.; Campbell, R.R.; Thompson, C.M. Comparison of transcriptomic profiles between HFPO-DA and prototypical PPARα, PPARγ, and cytotoxic agents in wild-type and PPARα knockout mouse hepatocytes. Toxicol. Sci. 2024, 200, 183–198. [Google Scholar] [CrossRef]
- Murase, W.; Kubota, A.; Hakota, R.; Yasuda, A.; Ikeda, A.; Nakagawa, K.; Shizu, R.; Yoshinari, K.; Kojima, H. Comparative study on gene expression profiles in the liver of male neonatal mice prenatally exposed to PFOA and its alternative HFPO-DA. Toxicology 2025, 511, 154048. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ni, H.; Guo, Y.; Lin, Y.; Ji, J.; Jin, C.; Yuan, F.; Feng, M.; Ji, N.; Zheng, Y.; et al. Hexafluoropropylene oxide dimer acid (HFPO-DA) induced developmental cardiotoxicity and hepatotoxicity in hatchling chickens: Roles of peroxisome proliferator activated receptor alpha. Environ. Pollut. 2021, 290, 118112. [Google Scholar] [CrossRef] [PubMed]
- Chappell, G.A.; Thompson, C.M.; Wolf, J.C.; Cullen, J.M.; Klaunig, J.E.; Haws, L.C. Assessment of the Mode of Action Underlying the Effects of GenX in Mouse Liver and Implications for Assessing Human Health Risks. Toxicol. Pathol. 2020, 48, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, Z.; Guo, H.; Gou, Y.; Dai, J.; Zhou, X.; Sheng, N. GenX analogs exposure induced greater hepatotoxicity than GenX mainly via activation of PPARα pathway while caused hepatomegaly in the absence of PPARα in female mice. Environ. Pollut. 2024, 344, 123314. [Google Scholar] [CrossRef] [PubMed]
- Conley, J.M.; Lambright, C.S.; Evans, N.; McCord, J.; Strynar, M.J.; Hill, D.; Medlock-Kakaley, E.; Wilson, V.S.; Gray, L.J. Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ. Int. 2021, 146, 106204. [Google Scholar] [CrossRef]
- Guo, H.; Sheng, N.; Guo, Y.; Wu, C.; Xie, W.; Dai, J. Exposure to GenX and its novel analogs disrupts fatty acid metabolism in male mice. Environ. Pollut. 2021, 291, 118202. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Liu, Q.J.; Bao, J.X.; Lu, M.T.; Deng, B.Q.; Li, W.W.; Cao, C.C. Counteracting TGM2 by a Fibroin peptide ameliorated Adriamycin-induced nephropathy via regulation of lipid metabolism through PANX1-PPAR α/PANK1 pathway. Transl. Res. 2024, 271, 26–39. [Google Scholar] [CrossRef]
- Comella, F.; Lama, A.; Pirozzi, C.; Annunziata, C.; Piegari, G.; Sodano, F.; Melini, S.; Paciello, O.; Paz, F.L.; Meli, R.; et al. Oleoylethanolamide attenuates acute-to-chronic kidney injury: In vivo and in vitro evidence of PPAR-α involvement. Biomed. Pharmacother. 2024, 171, 116094. [Google Scholar] [CrossRef]
- Zhong, J.; He, X.; Gao, X.; Liu, Q.; Zhao, Y.; Hong, Y.; Zhu, W.; Yan, J.; Li, Y.; Li, Y.; et al. Hyodeoxycholic acid ameliorates nonalcoholic fatty liver disease by inhibiting RAN-mediated PPARα nucleus-cytoplasm shuttling. Nat. Commun. 2023, 14, 5451. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, L.; Liu, J.; Jiang, Y.; Zhang, Y. The emerging role of PPAR-alpha in breast cancer. Biomed. Pharmacother. 2023, 161, 114420. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, C.; Johnson, A.C.; Sun, X.; Ding, X.; Ding, D.; Liu, S.; Liang, X. Source apportionment and crop bioaccumulation of perfluoroalkyl acids and novel alternatives in an industrial-intensive region with fluorochemical production, China: Health implications for human exposure. J. Hazard. Mater. 2022, 423, 127019. [Google Scholar] [CrossRef]
- Tang, J.; Ye, L.; Wang, B. Absorption, distribution, metabolism, and excretion (ADME) of R,S-Goitrin in Radix Isatidis in rats by LC-MS/MS determination. Fitoterapia 2025, 183, 106514. [Google Scholar] [CrossRef]
- De Girolamo, A.; Lippolis, V.; Pascale, M. Overview of Recent Liquid Chromatography Mass Spectrometry-Based Methods for Natural Toxins Detection in Food Products. Toxins 2022, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zeng, R.; Pan, M.; Zhou, Y.; Tang, H.; Shen, W.; Tang, Y.; Lei, P. Pharmacokinetics, bioavailability, and tissue distribution of MRTX1133 in rats using UHPLC-MS/MS. Front. Pharmacol. 2024, 15, 1509319. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Kong, Y.; Peng, Y.; Cui, X. Uptake, distribution, and depuration of emerging per- and polyfluoroalkyl substances in mice: Role of gut microbiota. Sci. Total Environ. 2022, 853, 158372. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Chaturvedi, P.; Gupta, P.C.; Awasthi, S.K.; Kalani, A. Molecular epigenetics in the transition of white to brown fat. Pathol. Res. Pract. 2025, 272, 156073. [Google Scholar] [CrossRef]
- Zurlinden, T.J.; Dzierlenga, M.W.; Kapraun, D.F.; Ring, C.; Bernstein, A.S.; Schlosser, P.M.; Morozov, V. Estimation of species- and sex-specific PFAS pharmacokinetics in mice, rats, and non-human primates using a Bayesian hierarchical methodology. Toxicol. Appl. Pharmacol. 2025, 499, 117336. [Google Scholar] [CrossRef]
- Hari, A.; AbdulHameed, M.; Balik-Meisner, M.R.; Mav, D.; Phadke, D.P.; Scholl, E.H.; Shah, R.R.; Casey, W.; Auerbach, S.S.; Wallqvist, A.; et al. Exposure to PFAS chemicals induces sex-dependent alterations in key rate-limiting steps of lipid metabolism in liver steatosis. Front. Toxicol. 2024, 6, 1390196. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, Y.; Wu, Y.; Li, H.; Yang, H.; Deng, Q.; He, B.; Yan, F.; Li, Y.; Chen, F. Association of per- and polyfluoroalkyl substances (PFAS) with periodontitis: The mediating role of sex hormones. BMC Oral Health 2024, 24, 243. [Google Scholar] [CrossRef]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef]
- Schmitt, C.; Koller, M.; Köhler, A.; Worek, F. Determination of tissue distribution of VX and its metabolites EMPA and EA-2192 in various rat tissues by LC-ESI-MS/MS after phosphotriesterase treatment. Toxicol. Lett. 2024, 398, 13–18. [Google Scholar] [CrossRef]
- Abu-Salah, A.; Cesur, M.F.; Anchan, A.; Ay, M.; Langley, M.R.; Shah, A.; Reina-Gonzalez, P.; Strazdins, R.; Çakır, T.; Sarkar, S. Comparative Proteomics Highlights that GenX Exposure Leads to Metabolic Defects and Inflammation in Astrocytes. Environ. Sci. Technol. 2024, 58, 20525–20539. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ng, C. Absorption, distribution, and toxicity of per- and polyfluoroalkyl substances (PFAS) in the brain: A review. Environ. Sci.-Process Impacts 2021, 23, 1623–1640. [Google Scholar] [CrossRef]
- Eusébio, S.; Soares, A.R.; Fiúza, P.; Garcia, T. Amiodarone-Induced Interstitial Pneumonia: A Cause of Respiratory Failure. Cureus J. Med. Sci. 2024, 16, e74253. [Google Scholar] [CrossRef]
- Harb, I.; Ashour, H.; Rashed, L.A.; Mostafa, A.; Samir, M.; Aboulhoda, B.E.; El-Hanbuli, H.; Rashwan, E.; Mahmoud, H. Nicorandil mitigates amiodarone-induced pulmonary toxicity and fibrosis in association with the inhibition of lung TGF-β1/PI3K/Akt1-p/mTOR axis in rats. Clin. Exp. Pharmacol. Physiol. 2023, 50, 96–106. [Google Scholar] [CrossRef]
- Ibrahim, F.G.; Mousa, M.R. The protective potential of alpha lipoic acid on amiodarone-induced pulmonary fibrosis and hepatic injury in rats. Mol. Cell. Biochem. 2021, 476, 3433–3448. [Google Scholar] [CrossRef]
- Dragon, J.; Hoaglund, M.; Badireddy, A.R.; Nielsen, G.; Schlezinger, J.; Shukla, A. Perfluoroalkyl Substances (PFAS) Affect Inflammation in Lung Cells and Tissues. Int. J. Mol. Sci. 2023, 24, 8539. [Google Scholar] [CrossRef]
- Sassano, M.; Seyyedsalehi, M.S.; Kappil, E.M.; Zhang, S.; Zheng, T.; Boffetta, P. Exposure to per- and poly-fluoroalkyl substances and lung, head and neck, and thyroid cancer: A systematic review and meta-analysis. Environ. Res. 2025, 266, 120606. [Google Scholar] [CrossRef]
- Mei, J.; Jiang, J.; Li, Z.; Pan, Y.; Xu, K.; Gao, X.; Yuan, J.; Li, L.; Wang, Y.; Wang, L.; et al. Increased perfluorooctanoic acid accumulation facilitates the migration and invasion of lung cancer cells via remodeling cell mechanics. Proc. Natl. Acad. Sci. USA 2024, 121, e1886392175. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; You, D.J.; Taylor-Just, A.J.; Linder, K.E.; Atkins, H.M.; Ralph, L.M.; De la Cruz, G.; Bonner, J.C. Pulmonary exposure of mice to ammonium perfluoro(2-methyl-3-oxahexanoate) (GenX) suppresses the innate immune response to carbon black nanoparticles and stimulates lung cell proliferation. Inhal. Toxicol. 2022, 34, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Wozniak, A.K.; Burkard, N.J.; Freaney, M.L.; Costamagna-Soto, A.; O’Conor, K.; Bakhoda, A.; Eisenberg, S.M.; Zhao, W.; Liow, J.S.; et al. [11C] Fentanyl: Radiosynthesis and Preclinical PET Imaging for Its Pharmacokinetics. Res. Sq. 2025, rs.3.rs-7367969. [Google Scholar]
- Mastropasqua, F.; Luurtsema, G.; Filosa, C.; Colabufo, N.A. Designing a Small Molecule for PET Radiotracing: [18F]MC225 in Human Trials for Early Diagnosis in CNS Pathologies. Molecules 2025, 30, 3696. [Google Scholar] [CrossRef]
- Xiang, E.; Guo, Q.; Dai, Y.G.; Sun, X.X.; Liu, J.; Fan, C.P.; Wang, Y.Q.; Qiu, S.K.; Wang, H.; Guo, Y. Female-specific activation of pregnane X receptor mediates sex difference in fetal hepatotoxicity by prenatal monocrotaline exposure. Toxicol. Appl. Pharmacol. 2020, 406, 115137. [Google Scholar] [CrossRef]
- Domingo, J.L. A review of the occurrence and distribution of Per- and polyfluoroalkyl substances (PFAS) in human organs and fetal tissues. Environ. Res. 2025, 272, 121181. [Google Scholar] [CrossRef]
- Olarewaju, E.; Obeng-Gyasi, E. Association of Per- and Polyfluoroalkyl Substances with Pan-Cancers Associated with Sex Hormones. Toxics 2025, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Ingelido, A.M.; Fuscoletti, V.; Abballe, A.; Cerroni, M.; Dellatte, E.; Iacovella, N.; Marra, V.; Valentini, S.; Lucentini, L.; De Felip, E.; et al. Human exposure to PFASs in a contaminated area: Associations between water and serum levels. Chemosphere 2025, 385, 144575. [Google Scholar] [CrossRef]
- Abinaya, B.; Waseem, M.; Kashif, M.; Srinivasan, H. Lipidomics: An excellent tool for chronic disease detection. Curr. Res. Transl. Med. 2022, 70, 103346. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Z.; Li, X.; Chen, J.; Liang, X.; Li, J. Genx disturbs the indicators of hepatic lipid metabolism even at environmental concentration in drinking water via pparα signaling pathways. Chem. Res. Toxicol. 2024, 37, 98–108. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, J.; Tan, B.; Chi, S. Effects of α-lipoic acid (LA) supplementation in high-fat diet on the growth, glycolipid metabolism and liver health of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2025, 157, 110072. [Google Scholar] [CrossRef]
- Huang, X.X.; Li, L.; Jiang, R.H.; Yu, J.B.; Sun, Y.Q.; Shan, J.; Yang, J.; Ji, J.; Cheng, S.Q.; Dong, Y.F.; et al. Lipidomic analysis identifies long-chain acylcarnitine as a target for ischemic stroke. J. Adv. Res. 2024, 61, 133–149. [Google Scholar] [CrossRef]
- McCann, M.R.; de la Rosa, M.V.G.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yang, H.; Li, M. Emerging Roles of Lysophosphatidic Acid in Macrophages and Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 12524. [Google Scholar] [CrossRef]
- He, L.; She, X.; Guo, L.; Gao, M.; Wang, S.; Lu, Z.; Guo, H.; Li, R.; Nie, Y.; Xing, J.; et al. Hepatic AKAP1 deficiency exacerbates diet-induced MASLD by enhancing GPAT1-mediated lysophosphatidic acid synthesis. Nat. Commun. 2025, 16, 4286. [Google Scholar] [CrossRef]
- Kirkwood-Donelson, K.I.; Chappel, J.; Tobin, E.; Dodds, J.N.; Reif, D.M.; DeWitt, J.C.; Baker, E.S. Investigating mouse hepatic lipidome dysregulation following exposure to emerging per- and polyfluoroalkyl substances (PFAS). Chemosphere 2024, 354, 141654. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Xu, Y.; Lu, G.; Hu, Y.; Mao, W.; Ke, L.; Tong, Z.; Xia, Y.; Ma, S.; Dong, X.; et al. AAV-mediated hepatic LPL expression ameliorates severe hypertriglyceridemia and acute pancreatitis in Gpihbp1 deficient mice and rats. Mol. Ther. 2024, 32, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Tang, C.K. ABCA1, ABCG1, and Cholesterol Homeostasis. Adv. Exp. Med. Biol. 2022, 1377, 95–107. [Google Scholar]
- Chen, L.; Zhao, Z.W.; Zeng, P.H.; Zhou, Y.J.; Yin, W.J. Molecular mechanisms for ABCA1-mediated cholesterol efflux. Cell Cycle 2022, 21, 1121–1139. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.R.; Liu, X.W.; Li, S.H.; Qin, Z.; Bai, L.X.; Ge, W.B.; Li, J.Y.; Yang, Y.J. Untargeted lipidomics and metagenomics reveal the mechanism of aspirin eugenol ester relieving hyperlipidemia in ApoE-/- mice. Front. Nutr. 2022, 9, 1030528. [Google Scholar] [CrossRef]
- Bairos, J.A.; Njoku, U.; Zafar, M.; Akl, M.G.; Li, L.; Parlakgul, G.; Arruda, A.P.; Widenmaier, S.B. Sterol O-acyltransferase (SOAT/ACAT) activity is required to form cholesterol crystals in hepatocyte lipid droplets. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159512. [Google Scholar] [CrossRef]
- Hai, Q.; Smith, J.D. Acyl-Coenzyme A: Cholesterol Acyltransferase (ACAT) in Cholesterol Metabolism: From Its Discovery to Clinical Trials and the Genomics Era. Metabolites 2021, 11, 543. [Google Scholar] [CrossRef]
- Szántó, M.; Gupte, R.; Kraus, W.L.; Pacher, P.; Bai, P. PARPs in lipid metabolism and related diseases. Prog. Lipid Res. 2021, 84, 101117. [Google Scholar] [CrossRef]
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Chen, Y.; Wu, J.; Lin, Y.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Mechanism of liver x receptor alpha in intestine, liver and adipose tissues in metabolic associated fatty liver disease. Int. J. Biol. Macromol. 2025, 307, 142275. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, T.A.; Zalzala, M.H. FXR crosstalk with other nuclear receptors. J. Physiol. Biochem. 2025, 307, 142275. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple Polyphenol Extract Improves High-Fat Diet-Induced Hepatic Steatosis by Regulating Bile Acid Synthesis and Gut Microbiota in C57BL/6 Male Mice. J. Agric. Food Chem. 2021, 69, 6829–6841. [Google Scholar] [CrossRef]
- Beuers, U.; Banales, J.M.; Karpen, S.J.; Keitel, V.; Williamson, C.; Trauner, M. The history and future of bile acid therapies. J. Hepatol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
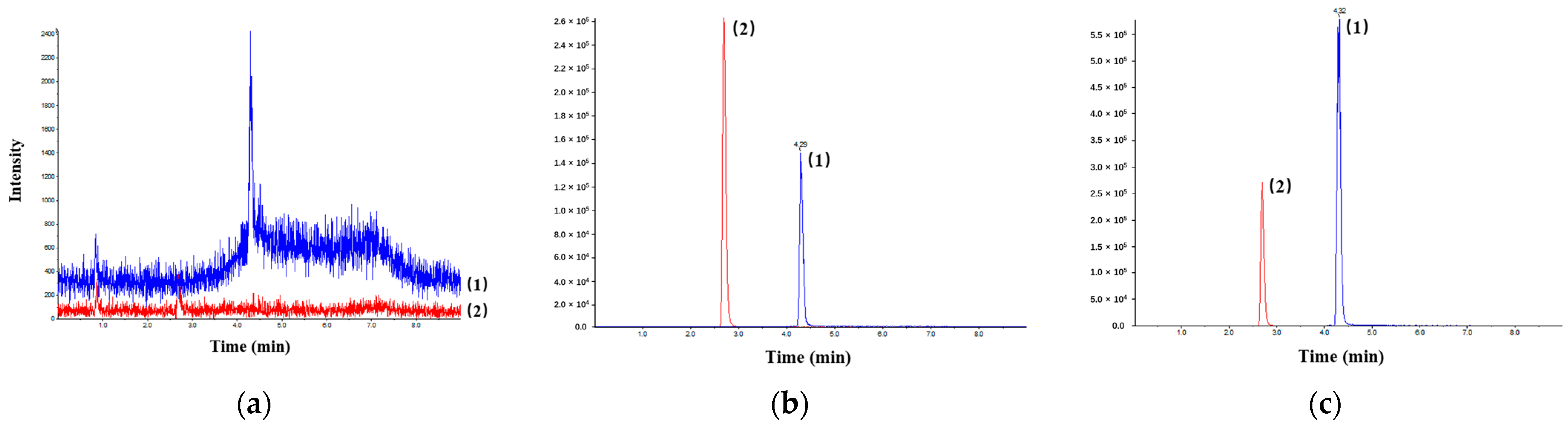
| Analyte | Linear Range (ng·mL−1) | Linear Regression Equation | Correlation Coefficient (r) | LLOQ (ng·mL−1) | RSD of LLOQ (%) | RE of LLOQ (%) |
|---|---|---|---|---|---|---|
| serum | 2–1000 | y = 1.52 × 104x + 1.62 × 104 | 0.9976 | 2 | 12.65 | 0.01 |
| HFPO-DA | Concentration (ng·mL−1) | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (ng·mL−1) | RSD (%) | RE (%) | Mean ± SD (ng·mL−1) | RSD (%) | RE (%) | ||
| Low concentration | 6 | 6.85 ± 0.34 | 4.97 | 14.10 | 6.84 ± 0.18 | 2.67 | −2.27 |
| Medium concentration | 400 | 394.27 ± 5.76 | 1.46 | −1.43 | 395.43 ± 7.62 | 1.93 | −1.14 |
| High concentration | 800 | 802.36 ± 9.49 | 1.18 | 0.30 | 800.07 ± 6.92 | 0.86 | 0.01 |
| HFPO-DA | Concentration (ng·mL−1) | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | ||
| Low concentration | 6 | 96.92 ± 3.21 | 3.31 | 96.97 ± 2.18 | 2.24 |
| medium concentration | 400 | 95.00 ± 1.28 | 1.35 | 96.81 ± 1.98 | 2.05 |
| High concentration | 800 | 97.76 ± 2.35 | 2.40 | 98.03 ± 1.27 | 1.29 |
| Condition | Concentration (ng·mL−1) | Mean ± SD (ng·mL−1) | RSD (%) | RE (%) |
|---|---|---|---|---|
| Storage at room temperature for five hours | 6 | 6.82 ± 0.26 | 3.81 | 13.71 |
| 400 | 393.25 ± 5.21 | 1.33 | −1.69 | |
| 800 | 797.22 ± 13.10 | 1.64 | −0.35 | |
| Storage at −80 °C for 15 days | 6 | 6.88 ± 0.29 | 4.20 | 14.71 |
| 400 | 396.80 ± 6.87 | 1.73 | −0.80 | |
| 800 | 806.89 ± 10.03 | 1.24 | 0.86 | |
| Three freeze–thaw cycles | 6 | 6.73 ± 0.22 | 3.32 | 12.19 |
| 400 | 389.53 ± 13.49 | 3.46 | −2.62 | |
| 800 | 802.12 ± 11.35 | 1.41 | 0.26 |
| Dilution | Concentration After Dilution (ng·mL−1) | Mean ± SD (ng·mL−1) | RSD (%) | RE (%) |
|---|---|---|---|---|
| 1/25 | 160 | 153.45 ± 5.02 | 0.75 | 0.24 |
| 1/10 | 400 | 394.22 ± 6.20 | 3.27 | −4.09 |
| 1/5 | 800 | 801.94 ± 5.98 | 1.57 | −1.45 |
| Mice | Cmax (ng·mL−1) | Tmax (h) | AUC(0-t) (h·ng·mL−1) | AUC(0-∞) (h·ng·mL−1) | t1/2 (h) | CLz/F (mL·h−1·kg−1) | Vz/F (mL·kg−1) |
|---|---|---|---|---|---|---|---|
| Total | 9066.67 | 0.5 | 80,566.05 | 90,893.73 | 7.95 | 330.06 | 3783.25 |
| Female | 9000.00 | 0.5 | 69,272.50 | 76,438.65 | 7.54 | 392.47 | 4265.69 |
| Male | 9226.67 | 1.0 | 91,859.61 | 105,429.62 | 8.23 | 284.55 | 3379.24 |
| Analyte | Mouse | Cmax (ng·g−1) | Tmax (h) | AUC(0-t) (h·ng·g−1) | t1/2 (h) |
|---|---|---|---|---|---|
| Heart | Total | 3815.96 | 0.50 | 20,350.10 | 3.67 |
| Female | 3627.05 | 0.50 | 13,012.38 | 3.29 | |
| Male | 4004.87 | 0.50 | 25,686.48 | 4.26 | |
| Liver | Total | 10,877.13 | 0.50 | 44,324.97 | 4.43 |
| Female | 8113.31 | 1.00 | 28,178.67 | 3.37 | |
| Male | 13,879.58 | 0.50 | 60,471.27 | 4.12 | |
| Spleen | Total | 3542.87 | 0.50 | 17,731.19 | 2.76 |
| Female | 3243.90 | 0.50 | 18,624.10 | 1.75 | |
| Male | 4237.26 | 1.00 | 16,838.28 | 4.03 | |
| Lung | Total | 6508.74 | 0.50 | 38,229.16 | 4.80 |
| Female | 6598.41 | 1.00 | 34,744.64 | 3.72 | |
| Male | 7710.97 | 0.50 | 41,713.68 | 4.69 | |
| Kidney | Total | 4379.75 | 0.50 | 21,633.41 | 4.47 |
| Female | 4237.26 | 1.00 | 16,838.28 | 4.03 | |
| Male | 4917.66 | 0.50 | 26,428.54 | 4.41 | |
| Brain | Total | 287.10 | 0.50 | 934.90 | 4.28 |
| Female | 264.56 | 1.00 | 548.26 | - * | |
| Male | 321.85 | 0.50 | 1276.86 | 9.00 | |
| WAT | Total | 2345.75 | 1.00 | 11,786.75 | 1.90 |
| Female | 2587.39 | 1.00 | 9771.23 | 3.87 | |
| Male | 2104.11 | 1.00 | 12,120.13 | 2.36 | |
| BAT | Total | 2952.58 | 0.50 | 16,224.88 | 3.90 |
| Female | 2796.12 | 1.00 | 11,440.93 | 2.96 | |
| Male | 3412.95 | 0.50 | 21,008.84 | 4.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Jiang, W.; Long, Z.; Cui, Y.; Zhu, G.; Liu, R.; Kong, D.; Yu, W.; Li, Y.; Hai, C. Absorption and Tissue Distribution of Environmental Pollutant HFPO-DA, and Its Effect on Hepatic Lipid Metabolism Reprogramming in Mice. Toxics 2025, 13, 850. https://doi.org/10.3390/toxics13100850
Peng J, Jiang W, Long Z, Cui Y, Zhu G, Liu R, Kong D, Yu W, Li Y, Hai C. Absorption and Tissue Distribution of Environmental Pollutant HFPO-DA, and Its Effect on Hepatic Lipid Metabolism Reprogramming in Mice. Toxics. 2025; 13(10):850. https://doi.org/10.3390/toxics13100850
Chicago/Turabian StylePeng, Jie, Wei Jiang, Zi Long, Yueying Cui, Guizhen Zhu, Rui Liu, Deqin Kong, Weihua Yu, Yuliang Li, and Chunxu Hai. 2025. "Absorption and Tissue Distribution of Environmental Pollutant HFPO-DA, and Its Effect on Hepatic Lipid Metabolism Reprogramming in Mice" Toxics 13, no. 10: 850. https://doi.org/10.3390/toxics13100850
APA StylePeng, J., Jiang, W., Long, Z., Cui, Y., Zhu, G., Liu, R., Kong, D., Yu, W., Li, Y., & Hai, C. (2025). Absorption and Tissue Distribution of Environmental Pollutant HFPO-DA, and Its Effect on Hepatic Lipid Metabolism Reprogramming in Mice. Toxics, 13(10), 850. https://doi.org/10.3390/toxics13100850







