Fast Trace Detection of Chlorpyrifos Vapors Using a Handheld Ion Mobility Spectrometer Operated near Ambient Temperature
Highlights
- Detection of Chlorpyrifos vapors at levels below 1 ppmv was accomplished using a ToF IMS operated under near-ambient temperature.
- Both excellent sensitivity and speed in the detection of Chlorpyrifos were achieved using the handheld ToF IMS.
- Ammonia doping of the IMS ensures good selectivity.
- Formation of two product ions represents a strong point for Chlorpyrifos identification.
- Rapid sensing of Chlorpyrifos at trace levels with highly portable IMS devices, demonstrating real-time response (seconds), was shown.
- Potential applications in real-world screening have been highlighted.
Abstract
1. Introduction
2. Materials and Methods
2.1. The IMS Instrument
2.2. Reagents, Sampling and Work Flow Procedure
3. Results
Validation
- Limit of detection LOD = 0.72 ppbv
- Limit of quantification LOQ = 2.41 ppbv
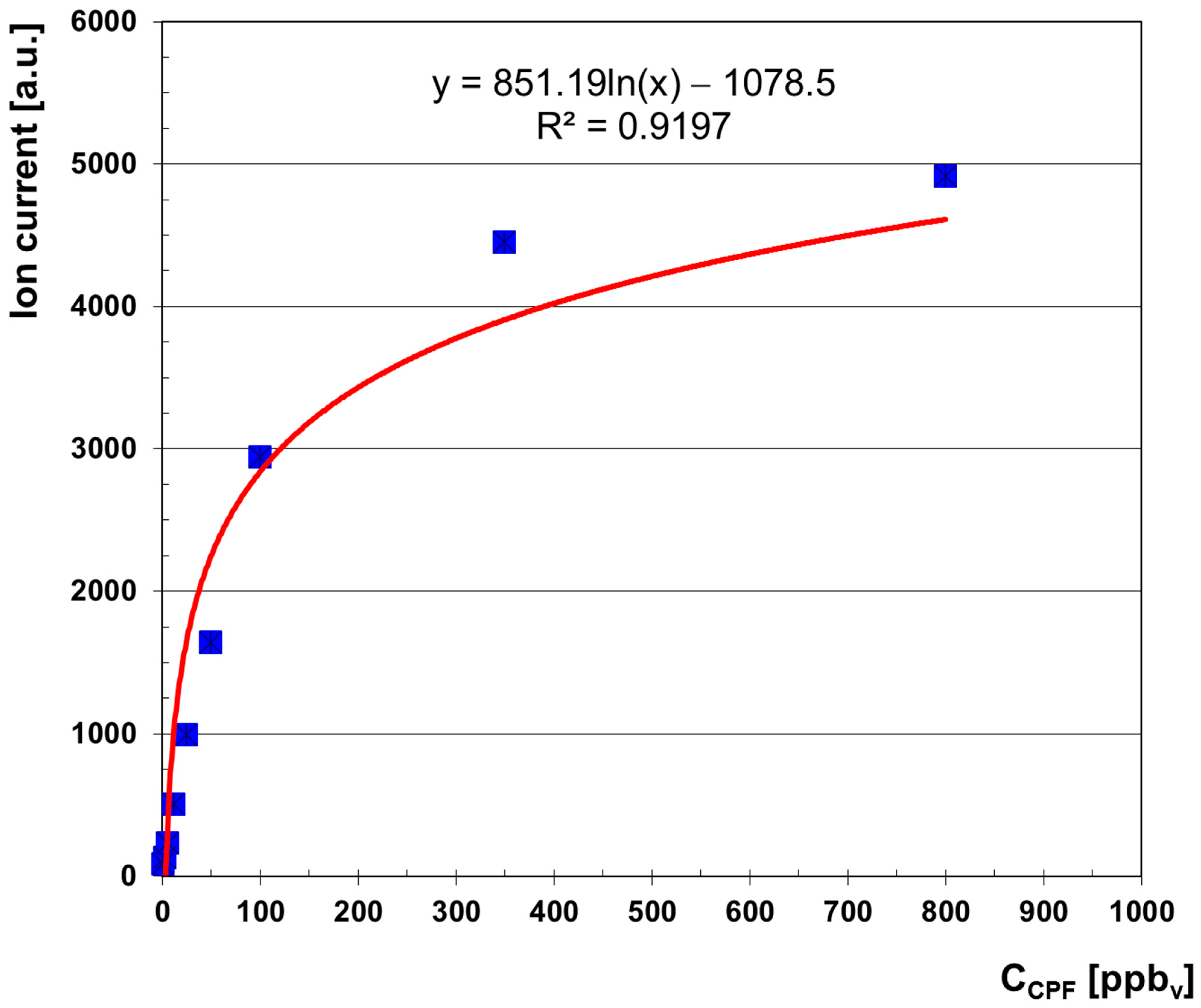
- Linear range: 2.41—50 ppbv
- Equation of linear regression: Y = 33.155·X + 49.622 (R2 = 0.9893)
- Sensitivity S = 32.1 a.u./ppbv.
4. Discussion
- Minimum measured concentration of CPF vapors was 1.5 ppbv (0.022 mg m−3).
- Linear dynamic range is from 2.4 ppbv (0.035 mg m−3) to ca. 100 ppbv (1.46 mg m−3) CPF.
- Saturation is estimated to appear at >1000 ppbv (14.57 mg m−3) CPF.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saunders, M.; Magnanti, B.L.; Carreira, C.S.; Yang, A.; Alamo-Hernández, U.; Riojas-Rodriguez, H.; Calamandrei, G.; Koppe, J.G.; Krayer von Krauss, M.; Keune, H.; et al. Chlorpyrifos and neurodevelopmental effects: A literature review and expert elicitation on research and policy. Environ. Health 2012, 11 (Suppl. S1), S5. [Google Scholar] [CrossRef]
- Venerosi, A.; Tait, S.; Stecca, L.; Chiarotti, F.; De Felice, A.; Cometa, M.F.; Volpe, M.T.; Calamandrei, G.; Ricceri, L. Effects of maternal Chlorpyrifos diet on social investigation and brain neuroendocrine markers in the offspring—A mouse study. Environ. Health 2015, 14, 32. [Google Scholar] [CrossRef]
- Lee, I.; Eriksson, P.; Fredriksson, A.; Buratovic, S.; Viberg, H. Developmental neurotoxic effects of two pesticides: Behavior and biomolecular studies on Chlorpyrifos and Carbaryl. Toxicol. Appl. Pharmacol. 2015, 288, 429–438. [Google Scholar] [CrossRef]
- Quirós-Alcalá, L.; Bradman, A.; Nishioka, M.; Harnly, M.E.; Hubbard, A.; McKone, T.E.; Ferber, J.; Eskenazi, B. Pesticides in house dust from urban and farmworker households in California: An observational measurement study. Environ. Health 2011, 10, 19. [Google Scholar] [CrossRef]
- PubChem—National Library of Medicine, National Center of Biotechnology Information. Compound Summary: Chlorpyrifos. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2730 (accessed on 23 July 2025).
- Solomon, K.R.; Williams, W.M.; Mackay, D.; Purdy, J.; Giddings, J.M.; Giesy, J.P. Properties and uses of chlorpyrifos in the United States. Res. Environ. Contam. Toxicol. 2014, 231, 13–34. [Google Scholar] [CrossRef]
- Pacsial-Ong, E.J.; Aguilar, Z.P. Chemical warfare agent detection: A review of current trends and future perspective. Front. Biosci. 2013, 5, 516–543. [Google Scholar] [CrossRef] [PubMed]
- Eiceman, G.; Karpas, Z. Ion Mobility Spectrometry, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2005. [Google Scholar] [CrossRef]
- Mäkinen, M.A.; Anttalainen, O.A.; Sillanpää, M.E.T. Ion Mobility Spectrometry and Its Applications in Detection of Chemical Warfare Agents. Anal. Chem. 2010, 82, 9594–9600. [Google Scholar] [CrossRef]
- Du, Z.; Sun, T.; Zhao, J.; Wang, D.; Zhang, Z.; Yu, W. Development of a plug-type IMS-MS instrument and its applications in resolving problems existing in in-situ detection of illicit drugs and explosives by IMS. Talanta 2018, 184, 65–72. [Google Scholar] [CrossRef]
- Giannoukos, S.; Brkić, B.; Taylor, S.; Marshall, A.; Verbeek, G.F. Chemical Sniffing Instrumentation for Security Applications. Chem. Rev. 2016, 116, 8146–8172. [Google Scholar] [CrossRef] [PubMed]
- Reiss, R.; Hauser, F.; Ehlert, S.; Pütz, M.; Zimmermann, R. Comparison of Different Analytical Methods for the On-Site Analysis of Traces at Clandestine Drug Laboratories. Appl. Sci. 2021, 11, 3754. [Google Scholar] [CrossRef]
- Sisco, E.; Verkouteren, J.; Staymates, J.; Lawrence, J. Rapid detection of fentanyl, fentanyl analogues, and opioids for on-site or laboratory based drug seizure screening using thermal desorption DART-MS and ion mobility spectrometry. Forensic Chem. 2017, 4, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Bocos-Bintintan, V.; Brittain, A.; Thomas, C.L.P. The response of a membrane inlet ion mobility spectrometer to chlorine and the effect of water contamination of the drying media on ion mobility spectrometric responses to chlorine. Analyst 2001, 126, 1539–1544. [Google Scholar] [CrossRef]
- Bocos-Bintintan, V.; Ratiu, I.A. Fast Sensing of Hydrogen Cyanide (HCN) Vapors Using a Hand-Held Ion Mobility Spectrometer with Nonradioactive Ionization Source. Sensors 2021, 21, 5045. [Google Scholar] [CrossRef]
- Eiceman, G.A.; Nazarov, E.G.; Stone, J.A. Chemical standards in ion mobility spectrometry. Anal. Chim. Acta 2003, 493, 185–194. [Google Scholar] [CrossRef]
- Ghotbadini-Bahraman, N.; Sheibani, A.; Shishehbore, M.R. Off-line coupling of QuEChERS sample preparation to ion mobility spectrometry for the determination of chlorpyrifos residue in pistachio oil. Int. J. Ion Mobil. Spectrom. 2017, 20, 41–45. [Google Scholar] [CrossRef]
- Tabibi, A.; Jafari, M.T. High efficient solid-phase microextraction based on a covalent organic framework for determination of trifluralin and chlorpyrifos in water and food samples by GC-CD-IMS. Food Chem. 2022, 373, 131527. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, L.; Aibaghi, B. Synthesis and characterization of a copper-based metal-organic framework and its application in microextraction and determination of chlorpyrifos by ion mobility spectrometry. Microchem. J. 2024, 207, 111794. [Google Scholar] [CrossRef]
- Amouei, J.; Bazmandegan-Shamili, A.; Ranjbar-Karimi, R.; Moghadam, M.R. Ultrasound-assisted dispersive liquid-liquid microextraction combined with ion mobility spectrometry for the simultaneous preconcentration and determination of dimethoate and chlorpyrifos in fruit, vegetable, and water samples. Anal. Lett. 2024, 57, 58–70. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzadeh, H.; Seidi, S.; Jalilian, N. Preparation of Polyacrylonitrile/Ni-MOF electrospun nanofiber as an efficient fiber coating material for headspace solid-phase microextraction of diazinon and chlorpyrifos followed by CD-IMS analysis. Food Chem. 2021, 350, 129242. [Google Scholar] [CrossRef]
- Rezayat, M.R.; Jafari, M.T.; Rahmanian, F. Thin film nanofibers containing ZnTiO3 nanoparticles for rapid evaporation of extraction solvent: Application to the preconcentration of chlorpyrifos prior to its quantification by ion mobility spectrometry. Microchim. Acta 2019, 186, 35. [Google Scholar] [CrossRef]
- Karami, K.; Mardaniboldaji, A.; Rezayat, M.R.; Bayat, P.; Jafari, M.T. Novel UiO-66-NH2/Gly/GO Nanocomposite Adsorbent for Ultra-trace Analyzing of Chlorpyrifos Pesticide by Ion Mobility Spectrometry. ChemistrySelect 2021, 6, 3370–3377. [Google Scholar] [CrossRef]
- Kalhor, H.; Hashemipour, S.; Yaftian, M.R. Ultrasound-Assisted Emulsification-Microextraction/Ion Mobility Spectrometry Combination: Application for Analysis of Organophosphorus Pesticide Residues in Rice Samples. Food Anal. Methods 2016, 9, 3006–3014. [Google Scholar] [CrossRef]
- Heydari, M.; Jafari, M.T.; Saraji, M.; Soltani, R.; Dinari, M. Covalent triazine-based framework-grafted functionalized fibrous silica sphere as a solid-phase microextraction coating for simultaneous determination of fenthion and chlorpyrifos by ion mobility spectrometry. Microchim. Acta 2021, 188, 4. [Google Scholar] [CrossRef]
- Mehrani, Z.; Ebrahimzadeh, H.; Aliakbar, A.R.; Asgharinezhad, A.A. A poly(4-nitroaniline)/poly(vinyl alcohol) electrospun nanofiber as an efficient nanosorbent for solid phase microextraction of diazinon and chlorpyrifos from water and juice samples. Microchim. Acta 2018, 185, 384. [Google Scholar] [CrossRef]
- Kermani, M.; Jafari, M.T.; Saraji, M. Porous magnetized carbon sheet nanocomposites for dispersive solid-phase microextraction of organophosphorus pesticides prior to analysis by gas chromatography-ion mobility spectrometry. Microchim. Acta 2019, 186, 88. [Google Scholar] [CrossRef]
- Saraji, M.; Jafari, M.T.; Mossaddegh, M. Carbon nanotubes@silicon dioxide nanohybrids coating for solid-phase microextraction of organophosphorus pesticides followed by gas chromatography–corona discharge ion mobility spectrometric detection. J. Chrom. A 2016, 1429, 30–39. [Google Scholar] [CrossRef]
- Jafari, M.T.; Saraji, M.; Kermani, M. Sol-gel electrospinning preparation of hybrid carbon silica nanofibers for extracting organophosphorus pesticides prior to analyzing them by gas chromatography-ion mobility spectrometry. J. Chrom. A 2018, 1558, 1–13. [Google Scholar] [CrossRef]
- Mohammadi, V.; Jafari, M.T.; Saraji, M. Solvent holder-assisted liquid-phase microextraction using nano-structure biomass-derived carbonaceous aerogel combined with ion mobility spectrometry for simultaneous determination of ethion and chlorpyrifos. Microchim. Acta 2020, 187, 232. [Google Scholar] [CrossRef]
- Bahrami, H.; Rezaei, B.; Jafari, M.T. Coupling of a novel electrospun polyacrylonitrile/amino-Zr-MOF nanofiber as a thin film for microextraction-corona discharge-ion mobility spectrometry for the analysis of chlorpyrifos in water samples. Anal. Meth. 2019, 11, 1073–1079. [Google Scholar] [CrossRef]
- Saraji, M.; Jafari, M.T.; Sherafatmand, H. Sol–gel/nanoclay composite as a solid-phase microextraction fiber coating for the determination of organophosphorus pesticides in water samples. Anal. Bioanal. Chem. 2015, 407, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Aladaghlo, Z.; Fakhari, A.R. Development of a new solvent-assisted dispersive solid-phase extraction followed by ion mobility spectrometry for trace determination of organophosphorus pesticides in environmental water samples. Sep. Sci. Plus 2019, 2, 291–299. [Google Scholar] [CrossRef]
- Amini, S.; Amiri, M.; Ebrahimzadeh, H.; Seidi, S.; Hejabri Kandeh, S. Synthesis of magnetic Cu/CuFe2O4@MIL-88A(Fe) nanocomposite and application to dispersive solid-phase extraction of chlorpyrifos and phosalone in water and food samples. J. Food Comp. Anal. 2021, 104, 104128. [Google Scholar] [CrossRef]
- Rezayat, M.R.; Jafari, M.T.; Mohammadipour, L. A configuration for cooling assisted organic solvent coated thin film microextraction after dispersive liquid-liquid microextraction method: A microextraction method for ultra-trace analyzing of volatile sample. Heliyon 2024, 10, e33230. [Google Scholar] [CrossRef]
- Kermani, M.; Jafari, M.T.; Saraji, M. Self-rotating stir mesh screen sorptive extraction for analyzing chlorpyrifos by ion mobility spectrometry. Anal. Meth. 2021, 13, 2631–2644. [Google Scholar] [CrossRef]
- Heidarbeigi, M.; Saraji, M.; Jafari, M.T. Silica aerogel modified electrospun polyacrylonitrile as a sorbent for thin-film microextraction of chlorpyrifos from real samples coupled with corona discharge ion mobility spectrometry detection. Anal. Meth. 2022, 14, 4106–4112. [Google Scholar] [CrossRef]
- Gallart-Mateu, D.; Armenta, S.; de la Guardia, M. Indoor and outdoor determination of pesticides in air by ion mobility spectrometry. Talanta 2016, 161, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Hauck, B.C.; Davis, E.J.; Clark, A.E.; Siems, W.F.; Harden, C.S.; McHugh, V.M.; Hill, H.H. Determining the water content of a drift gas using reduced ion mobility measurements. Int. J. Mass Spectrom. 2014, 368, 37–44. [Google Scholar] [CrossRef]
- Bocoș-Bințințan, V.; Bocoș-Bințințan, P.-F.; Rozsypal, T.; Beldean-Galea, M.S. Trace Detection of Di-Isopropyl Methyl Phosphonate DIMP, a By-Product, Precursor, and Simulant of Sarin, Using Either Ion Mobility Spectrometry or GC-MS. Toxics 2025, 13, 102. [Google Scholar] [CrossRef] [PubMed]
| Substance Name and Formula | Properties |
|---|---|
Chlorpyrifos O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate C9H11Cl3NO3PS CAS#: 2921-88-2 EC#: 220-864-4 | Molecular mass: 350.57 g mol−1 Melting point: ca. 41…42 °C Boiling point: decomposes at approx. 160 °C Physical state: solid, white/colorless crystals Density: 1.4 g cm−3 (@ 20 °C) Relative density of vapors: 12.1 (air = 1) Vapor pressure: ca. 2·10−5 Torr @ 25 °C Volatility: 0.38 mg m−3 @ 25 °C Octanol–water partition coefficient (log Kow): 4.9…5.2 Solubility in water: low—ca. 1.4 mg L−1 (@ 25 °C) Soluble in organic solvents (acetone, benzene, carbon disulfide, etc.) Acute toxicity: medium to high, with LD50 of ca. 150 mg kg−1 (rat, oral), ca. 500…1000 mg kg−1 (goats, oral), ca. 1200 mg kg−1 (rabbit, dermal) and LC50 of ca. 550 mg m−3 (rat, inhalation, 4 h) Minimum risk level: 0.003 mg kg−1 day−1 (acute, oral) and 0.001 mg kg−1 day−1 (chronic, oral) Reported fatal dose: 300 mg kg−1 (human, adult) Occupational air level (OSHA): 0.04 mg m−3 (8 h) TWA: 0.2 mg m−3 (UK); STEL: 0.6 mg m−3 (UK) NIOSH recommended exposure limit (REL): 0.2 mg m−3 (10 h, skin); 0.6 mg m−3 (15 min, skin) TLV (threshold limit value)–TWA: 0.1 mg m−3 (8 h; inhalable fraction and vapor), skin EPA limit in air: 1 μg m−3 (adults) and 0.5 μg m−3 (children) Estimated AVDI (average daily food intake) in the U.S.: 0.04·10−3 mg day−1 in 1980; 0.8·10−3 mg day−1 in 1990. Conversion: 1 ppmv = 14.57 mg m−3 (20 °C) |
| Vstandard sol.−SRM [μL] | CCPF [μg m−3] | CCPF [ppbv] |
|---|---|---|
| 8 | 22 | 1.5 |
| 16 | 44 | 3 |
| 32 | 89 | 6 |
| 63 | 175 | 12 |
| 132 | 366 | 25 |
| 265 | 736 | 50 |
| Vstandard sol.−Nurelle D [μL] | CCPF [μg m−3] | CCPF [ppbv] |
| 2.1 | 1458 | 100 |
| 7.4 | 5140 | 350 |
| 16.8 | 11,667 | 800 |
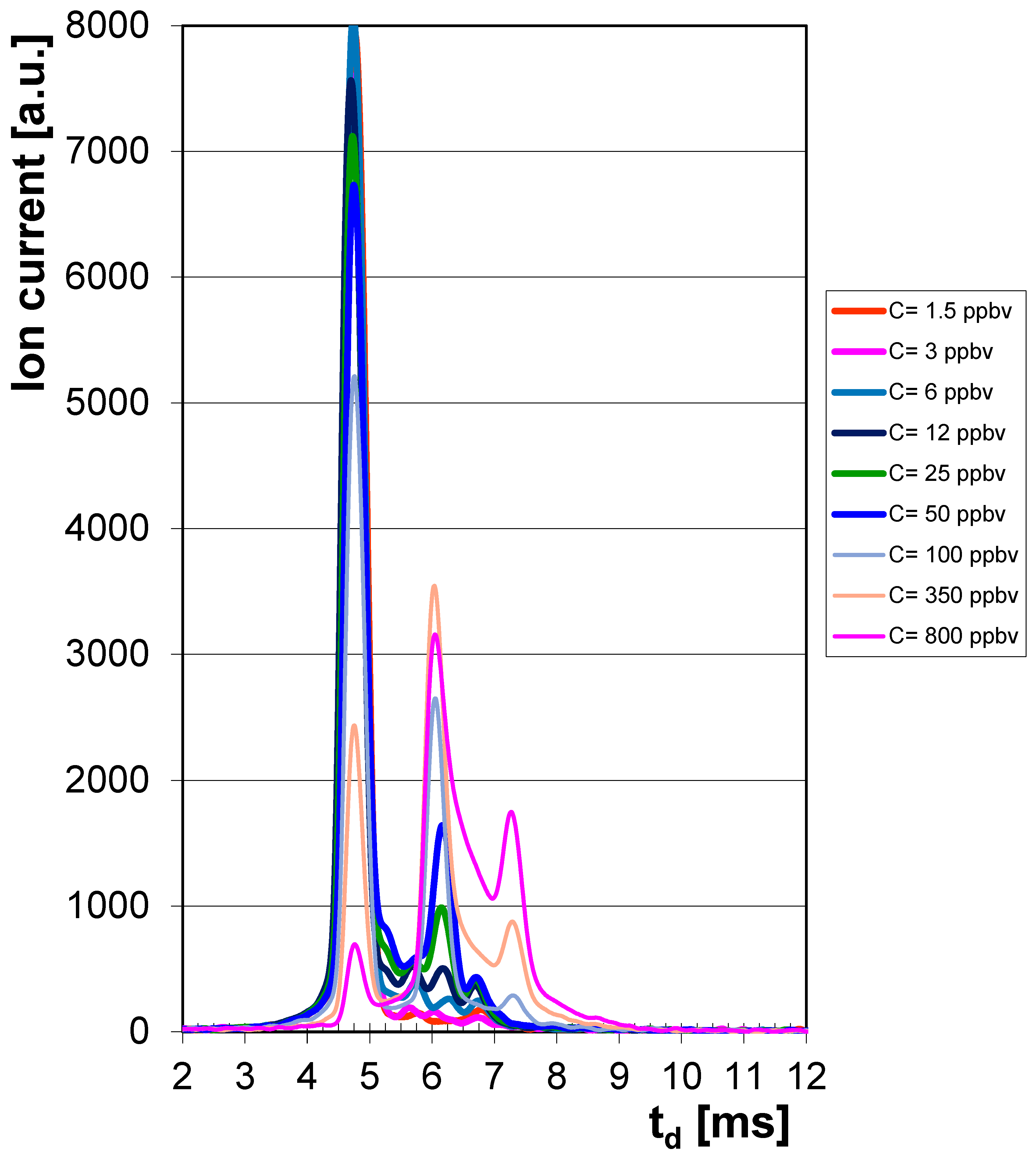
| CCPF [ppbv] | Drift Time Monomer td [ms] | Peak Height Monomer hmax [a.u.] | Drift Time Dimer td [ms] | Peak Height Dimer hmax [a.u.] |
|---|---|---|---|---|
| 1.5 | 6.14 | 85 ± 6 | - | - |
| 3 | 6.14 | 130 ± 9 | - | - |
| 6 | 6.14 | 230 ± 14 | - | - |
| 12 | 6.16 | 500 ± 26 | - | - |
| 25 | 6.14 | 990 ± 58 | - | - |
| 50 | 6.16 | 1640 ± 84 | - | - |
| 100 | 6.06 | 2650 ± 152 | 7.28 | 290 ± 16 |
| 350 | 6.06 | 3540 ± 194 | 7.28 | 890 ± 48 |
| 800 | 6.06 | 3160 ± 168 | 7.26 | 1750 ± 92 |
| Operation Mode | Ion Drift Time, td [ms] | Reduced Ion Mobility 1, K0 [cm2 V−1 s−1] | Reduced Ion Mobility 2, K0 [cm2 V−1 s−1] | |
|---|---|---|---|---|
| Pos RIP: | 4.74 | 2.278 | 2.251 | |
| Positive | PIP 1 (monomer): | 6.06 | 1.783 | 1.760 |
| PIP 2 (dimer): | 7.28 | 1.484 | 1.465 | |
| Ion Drift Time, td [ms] | Peak Width at Half Maximum, Δtd [ms] | Resolving Power, RIMS | |
|---|---|---|---|
| Pos RIP: | 4.74 | 0.35 | 13.5 |
| PIP 1 (monomer): | 6.06 | 0.36 | 16.8 |
| PIP 2 (dimer): | 7.28 | 0.42 | 17.3 |
| Application/Instrument Used | K0 [cm2 V−1 s−1] | Quant. | Ref. |
|---|---|---|---|
| Determination of CPF residue in pistachio oil after liquid extraction by IMS: Home-built IMS (Isfahan Univ. of Technology) with corona discharge ionization (NRS). Drift length ld = 11 cm. E = 500 V cm−1. TIMS cell = 190 °C. Drift gas: N2. Extraction of CPF from pistachio oil was performed with hexane. Liquid extract was injected into the IMS at 220 °C. Linear dynamic range LDR: 0.4 to 20 μg g−1. | Only one product ion peak was noticed, at td = ca. 11 ms (POS mode). K0 not reported! | LOD 0.1 μg g−1 LOQ 0.3 μg g−1 | [17] |
| Determination of CPF in water and food samples by tandem GC-IMS: CPF was extracted by SPME from various matrices (fruits, vegetables, water), and then, analysis by GC—IMS was performed. NRS IMS (corona discharge) was used. TIMS cell = 200 °C. Drift length ld = 11 cm (?). E = 500 V cm−1. Drift gas: N2. Linear dynamic range LDR: 0.50 to 25 μg L−1. | Only one product ion peak was observed (td = ca. 11.2 ms), overlapping with that produced by Trifluralin, at td ca. 11.4 ms (POS mode). K0 not reported! | LOD 0.15 μg L−1 LOQ 0.50 μg L−1 | [18] |
| Determination of CPF after microextraction using Cu COF by IMS: Samples: water, soil, pear fruits. IMS model IMS 300 (TOF Tech., Iran) with NRS (corona discharge). TIMS cell = 200 °C. Drift length ld = 11 cm (?). E = 500 V cm−1 (?). Linear dynamic range LDR: 1.0 to 400.0 ng mL−1. | Only one product ion peak was noticed, at td = ca. 11.7 ms (POS mode). K0 not reported! | LOD 0.65 ng mL−1 | [19] |
| Determination of CPF after ultrasound-assisted microextraction using IMS: Samples: water, food (vegetables, fruits). IMS model IMS 400 (TOF Tech. Pars Co., Iran) with NRS (corona discharge). Drift length ld = 11 cm; drift gas: dry air. TIMS cell = 200 °C. E = 500 V cm−1. Linear dynamic range LDR: 5.0 to 200.0 μg L−1. | Only one product ion peak was noticed (POS mode), at td = ca. 10.3 ms. K0 not reported! | LOD 1.3 μg L−1 | [20] |
| Determination of CPF after headspace SPME by IMS: Various aqueous samples (river, farm, groundwater) and fruit juices. IMS model CD-1400 (TOF Tech. Pars Co., Isfahan, Iran) with NRS (corona discharge). Drift length ld = 11 cm; drift gas: nitrogen. TIMS cell = 190 °C. E = 500 V cm−1 (?). Linear dynamic range LDR: 0.5 to 300.0 ng mL−1. | Only one product ion peak was noticed, at td = ca. 9.3 ms (POS mode). K0 not reported! | LOD 0.2 ng mL−1 | [21] |
| Determination of CPF after DLLME by IMS: Samples of water, fruits (apples), vegetables (tomatoes). IMS (Teif Azmon Espadana Co., Isfahan, Iran) with NRS (corona discharge). Drift length ld = 11 cm; drift gas: nitrogen. TIMS cell = 160 °C. E = 450 V cm−1. Linear dynamic range LDR: 0.1 to 3.0 μg L−1. | Only one product ion peak was reported, at td = ca. 10.9 ms (and at 12.2 ms in spiked samples!) (POS mode). K0 not reported! | LOD 0.04 μg L−1 | [22] |
| Determination of CPF after SPME by IMS: Sample: standard solution of CPF. IMS (Teif Azmon Espadana Co., Isfahan, Iran) with NRS (corona discharge). Drift length ld = 11 cm; drift gas: nitrogen. TIMS cell = 160 °C. E= 420 V cm−1. Linear dynamic range LDR: 0.5 to 20.0 μg L−1. | Only one product ion peak was noticed, at td = ca. 12.4 ms (POS mode). K0 not reported! | LOD 0.15 μg L−1 LOQ 0.5 μg L−1 | [23] |
| Determination of CPF after ultrasound-assisted emulsification microextraction using IMS: Samples: rice paddy water, rice. Home-built IMS with RS (63Ni). Drift length ld = 10 cm; drift gas: nitrogen. TIMS cell = 200 °C. E = 550 V cm−1. Linear dynamic range LDR: 8.9 to 750.0 μg L−1. | Only one product ion peak was reported, at td = ca. 11.5 ms (POS mode). K0 not reported! | LOD 3.2 μg L−1 | [24] |
| Determination of CPF after SPME by IMS: Samples: water, fruits (grape, tangerine). IMS (Teif Azmon Espadana Co., Isfahan Univ. of Technology, Iran) with NRS (corona discharge). Drift length ld = 11 cm; drift gas: nitrogen. TIMS cell = 160 °C. E = 400 V cm−1. Linear dynamic range LDR: 0.1 to 10.0 μg L−1. | Only one product ion peak was noticed, at td = ca. 11.4 ms (POS mode). K0 not reported! | LOD 0.05 μg L−1 LOQ 0.10 μg L−1 | [25] |
| Determination of CPF after SPME by IMS: Samples: water, fruit juices. IMS model CD-1400 (Theif Azmoon Espadana Co., Isfahan, Iran) with NRS (corona discharge). Drift length ld = 11 cm; drift gas: nitrogen. TIMS cell = 200 °C. E = 500 V cm−1 (?). Linear dynamic range LDR: 2 to 250.0 ng mL−1. | Only one product ion peak was noticed, at td = ca. 9.2 ms (POS mode). K0 not reported! | LOD 0.6 ng mL−1 | [26] |
| Determination of CPF by tandem GC-IMS after SPME: CPF was extracted by dispersive SPME from various matrices (water, fruits, vegetables), and then, analysis by GC—IMS was performed. IMS manufactured by Teif Azmon Espadana Co., Isfahan, Iran, with NRS (corona discharge) was used. Drift length ld = 11 cm. TIMS cell = 200 °C. Drift gas: N2. E = 500 V cm−1. Linear dynamic range LDR: 2 to 1000 μg L−1. | Only one product ion peak was observed (td = ca. 11.8 ms), overlapped with peak produced by Malathion (ca. 12.0 ms).—POS mode. K0 not reported! | LOD 0.85 μg L−1 LOQ 2 μg L−1 | [27] |
| Determination of CPF after SPME by tandem GC-IMS: CPF was extracted by SPME from various matrices (water, fruits, vegetables), and then, analysis by GC—IMS was performed. Home-built IMS (Isfahan Univ., Iran), with NRS (corona discharge) used. Drift length ld = 11 cm; drift gas: nitrogen. TIMS cell = 230 °C. E = 500 V cm−1. Linear dynamic range LDR: 0.025 to 2.0 μg L−1 (river water, wastewater); 0.75 to 20.0 μg kg−1 (pears, grapes); 0.50 to 15.0 μg kg−1 (eggplants). | Only one product ion peak was observed, with td = ca. 14.1 ms (POS mode). K0 not reported! | LOD 0.010 μg L−1 LOQ 0.025 μg L−1 (water) LOD 0.30 μg L−1 LOQ 0.75 μg L−1 (pears, grapes) | [28] |
| Determination of CPF after SPME by tandem GC-IMS: CPF was extracted by SPME from various matrices (water, milk, serum, fruits), and then, analysis by GC—IMS was performed. Home-built IMS (Isfahan Univ. of Technology, Iran), with NRS (corona discharge). Drift length ld = 11 cm; drift gas: N2. TIMS cell = 200 °C. E = 500 V cm−1. Linear dynamic range LDR: 0.05 to 20.0 μg L−1 (water). | No IMS spectrum was provided. K0 not reported! | LOD 0.019 μg L−1 (water) LOQ 0.050 μg L−1 (water) | [29] |
| Determination of CPF after liquid phase micro-extraction by IMS: Samples: water, vegetables. IMS (Teif Azmon Espadana Co., Isfahan, Iran) with NRS (secondary electrospray ionization SESI). Drift length ld = 11 cm; drift gas: N2. TIMS cell = 150 °C. E = 567 V cm−1. Linear dynamic range LDR: 1 to 70.0 μg L−1. | Only one product ion peak was noticed, at td = 12.28 ms (POS mode). K0 = 1.37 cm2 V−1 s−1 (with nicotinamide as mobility standard) | LOD 0.21 μg L−1 LOQ 0.70 μg L−1 | [30] |
| Determination of CPF after micro-extraction by IMS: Sample: water. Home-built IMS (Isfahan Univ., Iran), with NRS (corona discharge) was used. Drift length ld = 11 cm; drift gas: N2. TIMS cell = 150 °C. E = 400 V cm−1. Linear dynamic range LDR: 2 to 200 μg L−1. | Only one product ion peak was noticed, at td = ca. 10.6 ms (POS mode). K0 not reported! | LOD 0.6 μg L−1 LOQ 2.0 μg L−1 | [31] |
| Determination of CPF after SPME by tandem GC-IMS: Sample: water. Home-built GC-IMS (Isfahan Univ., Iran), with NRS (corona discharge) was used. Drift gas: N2. TIMS cell = 235 °C. E = 500 V cm−1. Linear dynamic range LDR: 0.02 to 5 μg L−1. | Only one product ion peak was noticed, at td = ca. 12.1 ms (POS mode). K0 = 1.27 cm2 V−1 s−1 (with nicotinamide as mobility standard). | LOD 0.012 μg L−1 | [32] |
| Determination of CPF after dispersive solid-phase extraction by IMS: Samples: water, vegetables. Home-built IMS model 1000 (Isfahan Univ. of Technology, Iran), with NRS (corona discharge) was used. Drift length ld = 16 cm; drift gas: N2. TIMS cell = 200 °C. E = 500 V cm−1. Reactant ion: NH4+. Linear dynamic range LDR: 1 to 500 ng mL−1. | Only one product ion peak was noticed, at td = ca. 8.8 ms. K0 not reported! | LOD 0.3 ng mL−1 LOQ 1 ng mL−1 | [33] |
| Determination of CPF after dispersive solid-phase extraction by IMS: Samples: water, fruit juice, vegetables. IMS model CD-1400 (TOF Tech, Isfahan, Iran), with NRS (corona discharge), was used. Drift length ld = 11 cm; drift gas: N2. TIMS cell = 200 °C. E = 636 V cm−1. Linear dynamic range LDR: 0.6 to 300.0 ng mL−1. | Only one product ion peak was noticed, at td = ca. 11 ms (POS mode). K0 not reported! | LOD 0.2 ng mL−1 | [34] |
| Determination of CPF after dispersive liquid–liquid microextraction by IMS: Samples: water, vegetables (potato). IMS model CD-1400 (Teif Azmon Espadana Co., Isfahan, Iran), with NRS (corona discharge) was used. Drift length ld = 11 cm; electric field E = 420 V cm−1; drift gas: N2. TIMS cell = 160 °C. Linear dynamic range LDR: 0.1 to 7.0 μg L−1. | Only one product ion peak was noticed, at td = ca. 13.2 ms (POS mode). K0 not reported! | LOD 0.03 μg L−1 LOQ 0.1 μg L−1 | [35] |
| Determination of CPF after stir mesh screen sorptive extraction (SMSE) by IMS: Samples: water (river, well, agricultural wastewater); fruits (apple). IMS with thermal desorption unit (Teif Azmon Espadana Co., Isfahan Univ. of Technology, Iran), with NRS (corona discharge) was used. Drift length ld = 11 cm; electric field E = 500 V cm−1; drift gas: nitrogen. TIMS cell = 150 °C. Linear dynamic range LDR: 0.1 to 20.0 μg L−1. | Only one product ion peak was noticed, at td = ca. 11.3 ms (POS mode). K0 not reported! | LOD 0.035 μg L−1 LOQ 0.100 μg L−1 | [36] |
| Determination of CPF after thin film micro-extraction (SMSE) by IMS: Samples: water (well, river, agricultural wastewater); fruits (tangerine). IMS with thermal desorption unit (Isfahan Univ. of Technology, Iran), with NRS (corona discharge) was used. Drift length ld = 11 cm; electric field E = 450 V cm−1; drift gas: N2. TIMS cell = 160 °C. Linear dynamic range LDR: 1 to 100 μg L−1. | Only one product ion peak was noticed, at td = ca. 12.8 ms (POS mode). K0 not reported! | LOD 0.3 μg L−1 | [37] |
| Indoor and outdoor determination of pesticides in air after active sampling on Teflon membranes by IMS: Samples: air. Commercial IMS with RS (63Ni) with thermal desorption, model Ionscan-LS (Smiths Detection, Morristown, NJ, USA). Drift Tube length: 7 cm; electric field E = 252 V cm−1; drift gas: dry air. TIMS cell = 237 °C. Linear dynamic range LDR—over 2 to 10 ng. | One product ion peak in the Negative ion mode, at td = 11.93 ms. K0 = 1.561 cm2 V−1 s−1 (using 4-nitrobenzonitrile as mobility standard, with K0 = 1.655 cm2 V−1 s−1). | LOD 600 pg LOQ 1960 pg | [38] |
| Determination of CPF vapors by IMS: ToF IMS, model LCD-3.2E (NRS—corona discharge), operated near ambient T (ca. 28 °C); ammonia doped. Calibration: from 1.5 to 800 ppbv Linear range: from 2.5 to 100 ppbv Saturation: >1000 ppbv | 1.727 (monomer) 1.461 (dimer) | LOD 0.72 ppbv LOQ 2.41 ppbv | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocoș-Bințințan, V.; Dodea, A.-M.; Rozsypal, T.; Pătruț, A.; Roșian, G.; Martiniuc, A.-V.; Moraru, A.-G.; Vasc, S.; Bocoș-Bințințan, M.-P. Fast Trace Detection of Chlorpyrifos Vapors Using a Handheld Ion Mobility Spectrometer Operated near Ambient Temperature. Toxics 2025, 13, 843. https://doi.org/10.3390/toxics13100843
Bocoș-Bințințan V, Dodea A-M, Rozsypal T, Pătruț A, Roșian G, Martiniuc A-V, Moraru A-G, Vasc S, Bocoș-Bințințan M-P. Fast Trace Detection of Chlorpyrifos Vapors Using a Handheld Ion Mobility Spectrometer Operated near Ambient Temperature. Toxics. 2025; 13(10):843. https://doi.org/10.3390/toxics13100843
Chicago/Turabian StyleBocoș-Bințințan, Victor, Ancuța-Maria Dodea, Tomáš Rozsypal, Adrian Pătruț, Gheorghe Roșian, Aurel-Vasile Martiniuc, Alin-Gabriel Moraru, Simina Vasc, and Maria-Paula Bocoș-Bințințan. 2025. "Fast Trace Detection of Chlorpyrifos Vapors Using a Handheld Ion Mobility Spectrometer Operated near Ambient Temperature" Toxics 13, no. 10: 843. https://doi.org/10.3390/toxics13100843
APA StyleBocoș-Bințințan, V., Dodea, A.-M., Rozsypal, T., Pătruț, A., Roșian, G., Martiniuc, A.-V., Moraru, A.-G., Vasc, S., & Bocoș-Bințințan, M.-P. (2025). Fast Trace Detection of Chlorpyrifos Vapors Using a Handheld Ion Mobility Spectrometer Operated near Ambient Temperature. Toxics, 13(10), 843. https://doi.org/10.3390/toxics13100843








