Time- and Dose-Dependent Cardiovascular Effects of Nicotine-Containing Electronic Cigarettes in Young Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
2.4. Selection Criteria and Data Extraction
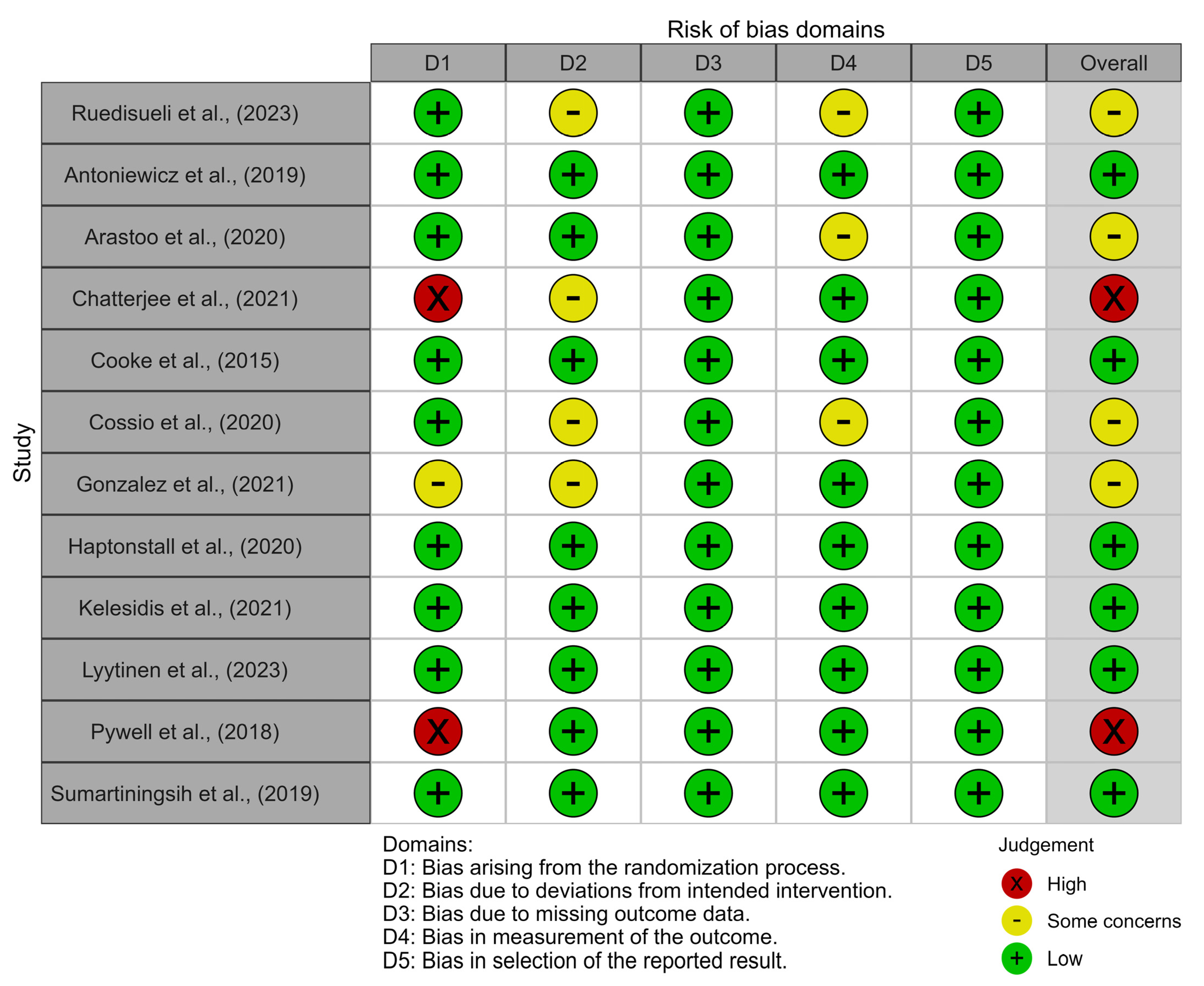
2.5. Quality and Risk of Bias Evaluation
2.6. Statistical Analysis
3. Results
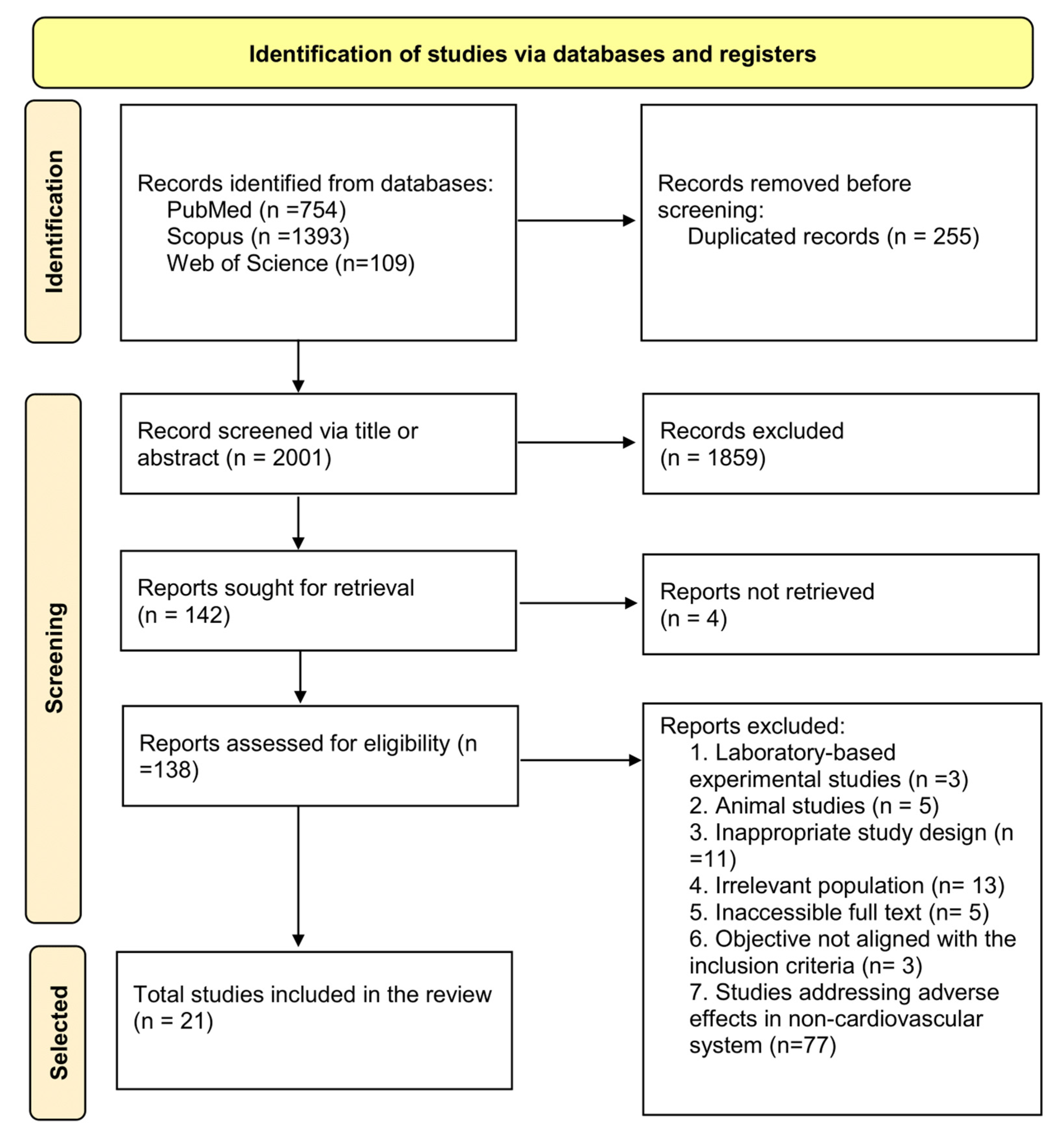
3.1. Studies Selected
3.2. Description and Characteristics of the Studies
3.3. Quality of the Included Studies
3.4. Risk of Bias
3.5. Narrative Synthesis of Individual Study Results
3.5.1. Dysfunction of Endothelial Vasodilatation
3.5.2. Increase in Blood Pressure and Heart Rate
3.5.3. Increase in Cellular Oxidative Stress
3.5.4. Increase in Systemic Inflammatory Activity
3.5.5. Pro-Atherogenic and Pro-Thrombotic Effects
3.5.6. Prolongation of Ventricular Repolarization Time
3.5.7. Heart Rate Variability
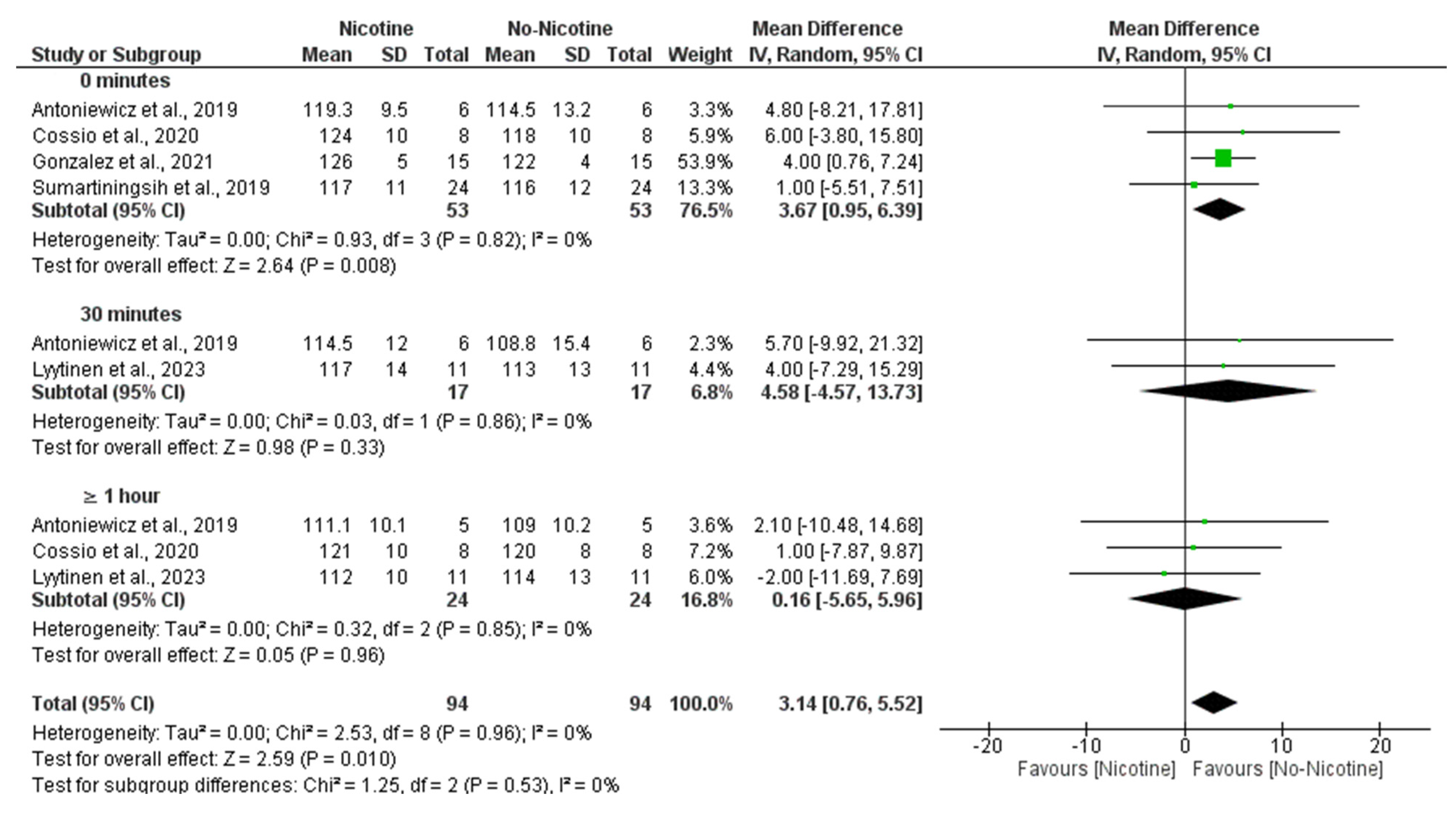
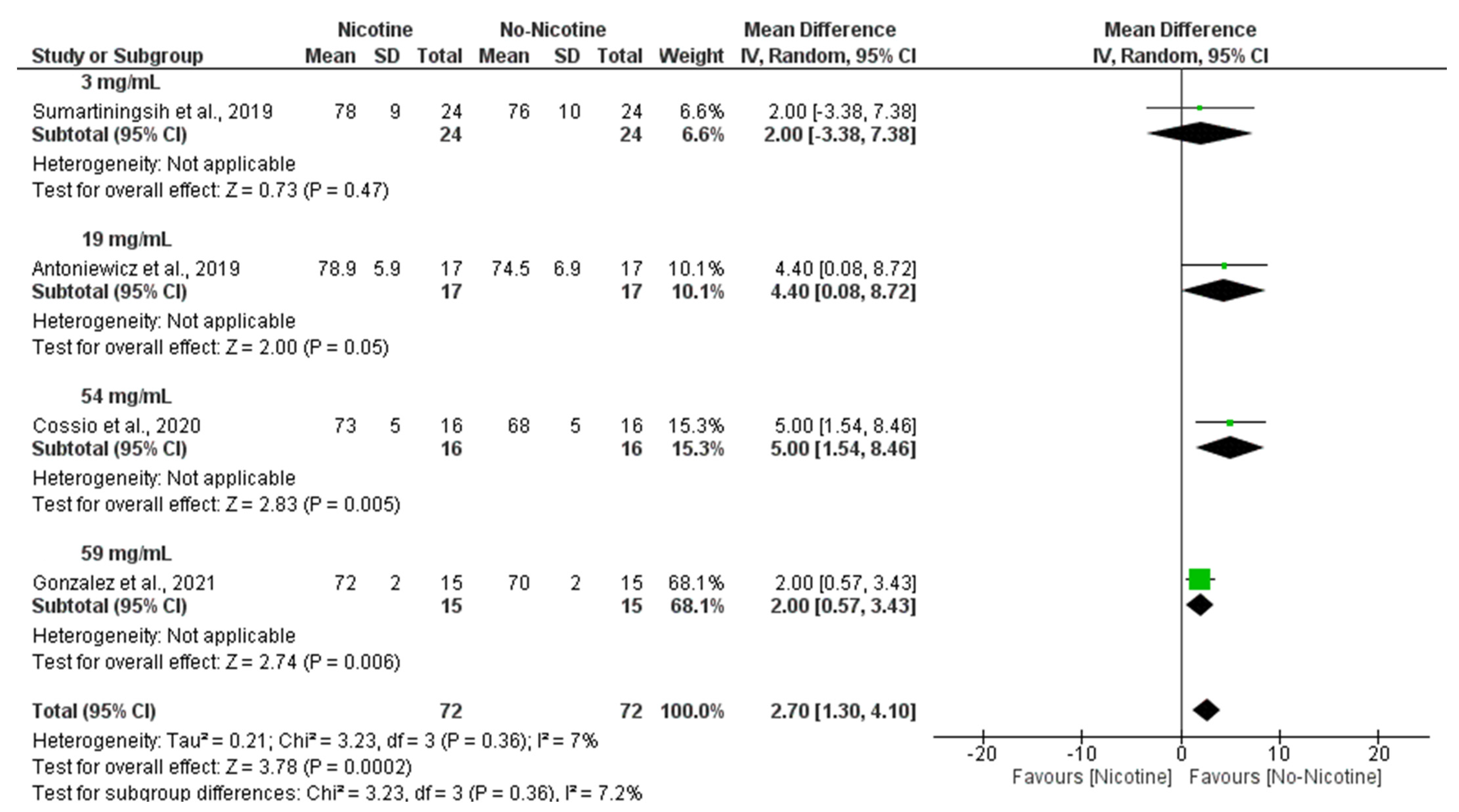
3.6. Meta-Analysis
3.6.1. SBP (Nicotine vs. No Nicotine)
3.6.2. DBP (Nicotine vs. No Nicotine)
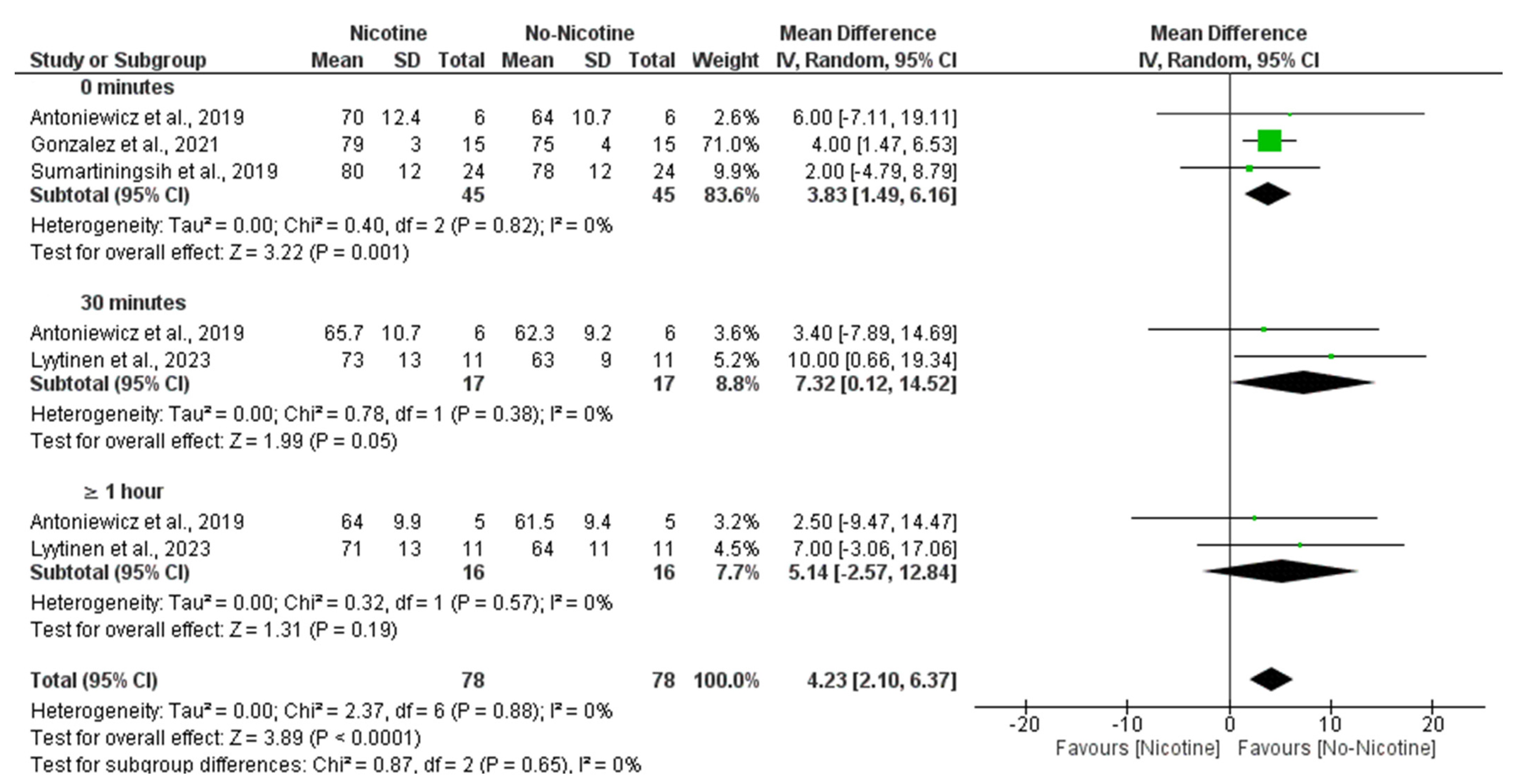
3.6.3. Heart Rate (Nicotine vs. No Nicotine)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| µM | Micromolar |
| ANSM | Autonomic nervous system modulation |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| bpm | Beats per minute |
| CRP | C-reactive protein |
| CT | Conventional tobacco |
| CVC | Cutaneous vascular conductance |
| DBP | Diastolic blood pressure |
| EC | Electronic cigarette |
| ENDS | Electronic nicotine delivery system |
| EVALI | E-cigarette or vaping-associated lung injury |
| FMD | Flow-mediated dilation |
| HMGB1 | High-mobility Group Box 1 Protein |
| HR | Heart rate |
| ICAM | Intercellular adhesion molecule |
| MAP | Mean arterial pressure |
| mmHg | Millimeters of mercury |
| MRI | Magnetic resonance imaging |
| ng/mL | Nanograms per milliliter |
| nmol | Nanomole |
| NO | Nitric oxide |
| PET | Positron emission tomography |
| PU | Perfusion unit |
| PWV | Pulse wave velocity |
| QT | Electrocardiogram QT interval |
| QTc | Corrected QT interval |
| RCT | Randomized controlled trial |
| SBP | Systolic blood pressure |
| SUVmax | Maximum standardized uptake value |
| Tp-e | T-wave peak to end interval |
References
- Orellana-Barrios, M.A.; Payne, D.; Mulkey, Z.; Nugent, K. Electronic Cigarettes—A Narrative Review for Clinicians. Am. J. Med. 2015, 128, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Dirección General de Salud Pública. Informe Sobre Los Cigarrillos Electrónicos: Situación Actual, Evidencia Disponible y Regulación; Ministerio de Sanidad: Madrid, Spain, 2024. [Google Scholar]
- Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated Tobacco Products: Insights into Composition and Toxicity. Toxics 2023, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Hawk, E.T.; Colbert Maresso, K. E-Cigarettes: Unstandardized, Under-Regulated, Understudied, and Unknown Health and Cancer Risks. Cancer Res. 2019, 79, 6079–6083. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Phillips-Waller, A.; Przulj, D.; Pesola, F.; Myers Smith, K.; Bisal, N.; Li, J.; Parrott, S.; Sasieni, P.; Dawkins, L.; et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N. Engl. J. Med. 2019, 380, 629–637. [Google Scholar] [CrossRef]
- Baker, R.R. Smoke Generation inside a Burning Cigarette: Modifying Combustion to Develop Cigarettes That May Be Less Hazardous to Health. Prog. Energy Combust. Sci. 2006, 32, 373–385. [Google Scholar] [CrossRef]
- Thirión-Romero, I.; Pérez-Padilla, R.; Zabert, G.; Barrientos-Gutiérrez, I. Respiratory impact of electronic cigarettes and “low-risk” tobacco. Rev. Investig. Clin. Organo Hosp. Enfermedades Nutr. 2019, 71, 17–27. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Encuesta Sobre Uso de Drogas en Enseñanzas Secundarias en España, ESTUDES 2023; Ministerio de Sanidad: Madrid, Spain, 2023. [Google Scholar]
- Salari, N.; Rahimi, S.; Darvishi, N.; Abdolmaleki, A.; Mohammadi, M. The Global Prevalence of E-Cigarettes in Youth: A Comprehensive Systematic Review and Meta-Analysis. Public Health Pract. Oxf. Engl. 2024, 7, 100506. [Google Scholar] [CrossRef]
- Sund, L.J.; Dargan, P.I.; Archer, J.R.H.; Wood, D.M. E-Cigarette or Vaping-Associated Lung Injury (EVALI): A Review of International Case Reports from Outside the United States of America. Clin. Toxicol. Phila. Pa 2023, 61, 91–97. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Stylianou, M.; Agapiou, A. Main and Side Stream Effects of Electronic Cigarettes. J. Environ. Manag. 2019, 238, 10–17. [Google Scholar] [CrossRef]
- Siddiqi, T.J.; Rashid, A.M.; Siddiqi, A.K.; Anwer, A.; Usman, M.S.; Sakhi, H.; Bhatnagar, A.; Hamburg, N.M.; Hirsch, G.A.; Rodriguez, C.J.; et al. Association of Electronic Cigarette Exposure on Cardiovascular Health: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101748. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46, Original work published 1960. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Matheson, C.; Simovic, T.; Heefner, A.; Colon, M.; Tunon, E.; Cobb, K.; Thode, C.; Breland, A.; Cobb, C.O.; Nana-Sinkam, P.; et al. Evidence of Premature Vascular Dysfunction in Young Adults Who Regularly Use E-Cigarettes and the Impact of Usage Length. Angiogenesis 2024, 27, 229–243. [Google Scholar] [CrossRef]
- Sahota, A.; Naidu, S.; Jacobi, A.; Giannarelli, C.; Woodward, M.; Fayad, Z.A.; Mani, V. Atherosclerosis Inflammation and Burden in Young Adult Smokers and Vapers Measured by PET/MR. Atherosclerosis 2021, 325, 110–116. [Google Scholar] [CrossRef]
- Shi, H.; Leventhal, A.M.; Wen, Q.; Ossip, D.J.; Li, D. Sex Differences in the Association of E-Cigarette and Cigarette Use and Dual Use With Self-Reported Hypertension Incidence in US Adults. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2023, 25, 478–485. [Google Scholar] [CrossRef]
- Halstead, K.M.; Wetzel, E.M.; Cho, J.L.; Stanhewicz, A.E. Sex Differences in Oxidative Stress-Mediated Reductions in Microvascular Endothelial Function in Young Adult e-Cigarette Users. Hypertension 2023, 80, 2641–2649. [Google Scholar] [CrossRef]
- Boas, Z.; Gupta, P.; Moheimani, R.S.; Bhetraratana, M.; Yin, F.; Peters, K.M.; Gornbein, J.; Araujo, J.A.; Czernin, J.; Middlekauff, H.R. Activation of the “Splenocardiac Axis” by Electronic and Tobacco Cigarettes in Otherwise Healthy Young Adults. Physiol. Rep. 2017, 5, e13393. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Sharma, M.; Sharma, E.; Ruedisueli, I.; Tran, E.; Middlekauff, H.R. Chronic Electronic Cigarette Use and Atherosclerosis Risk in Young People: A Cross-Sectional Study-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Moheimani, R.S.; Bhetraratana, M.; Yin, F.; Peters, K.M.; Gornbein, J.; Araujo, J.A.; Middlekauff, H.R. Increased Cardiac Sympathetic Activity and Oxidative Stress in Habitual Electronic Cigarette Users: Implications for Cardiovascular Risk. JAMA Cardiol. 2017, 2, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Ruedisueli, I.; Lakhani, K.; Nguyen, R.; Gornbein, J.; Middlekauff, H.R. Electronic Cigarettes Prolong Ventricular Repolarization in People Who Smoke Tobacco Cigarettes: Implications for Harm Reduction. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H821–H832. [Google Scholar] [CrossRef]
- Youn, J.Y.; Middlekauff, H.R.; Reudiseuli, I.; Huang, K.; Cai, H. Endothelial Damage in Young Adult E-Cigarette Users. Redox Biol. 2023, 62, 102688. [Google Scholar] [CrossRef]
- Antoniewicz, L.; Brynedal, A.; Hedman, L.; Lundbäck, M.; Bosson, J.A. Acute Effects of Electronic Cigarette Inhalation on the Vasculature and the Conducting Airways. Cardiovasc. Toxicol. 2019, 19, 441–450. [Google Scholar] [CrossRef]
- Arastoo, S.; Haptonstall, K.P.; Choroomi, Y.; Moheimani, R.; Nguyen, K.; Tran, E.; Gornbein, J.; Middlekauff, H.R. Acute and Chronic Sympathomimetic Effects of E-Cigarette and Tobacco Cigarette Smoking: Role of Nicotine and Non-Nicotine Constituents. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H262–H270. [Google Scholar] [CrossRef]
- Chatterjee, S.; Caporale, A.; Tao, J.Q.; Guo, W.; Johncola, A.; Strasser, A.A.; Leone, F.T.; Langham, M.C.; Wehrli, F.W. Acute E-Cig Inhalation Impacts Vascular Health: A Study in Smoking Naïve Subjects. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H144–H158. [Google Scholar] [CrossRef]
- Cooke, W.H.; Pokhrel, A.; Dowling, C.; Fogt, D.L.; Rickards, C.A. Acute Inhalation of Vaporized Nicotine Increases Arterial Pressure in Young Non-Smokers: A Pilot Study. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2015, 25, 267–270. [Google Scholar] [CrossRef]
- Cossio, R.; Cerra, Z.A.; Tanaka, H. Vascular Effects of a Single Bout of Electronic Cigarette Use. Clin. Exp. Pharmacol. Physiol. 2020, 47, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.E.; Cooke, W.H. Acute Effects of Electronic Cigarettes on Arterial Pressure and Peripheral Sympathetic Activity in Young Nonsmokers. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H248–H255. [Google Scholar] [CrossRef] [PubMed]
- Haptonstall, K.P.; Choroomi, Y.; Moheimani, R.; Nguyen, K.; Tran, E.; Lakhani, K.; Ruedisueli, I.; Gornbein, J.; Middlekauff, H.R. Differential Effects of Tobacco Cigarettes and Electronic Cigarettes on Endothelial Function in Healthy Young People. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H547–H556. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Tran, E.; Nguyen, R.; Zhang, Y.; Sosa, G.; Middlekauff, H.R. Association of 1 Vaping Session With Cellular Oxidative Stress in Otherwise Healthy Young People With No History of Smoking or Vaping: A Randomized Clinical Crossover Trial. JAMA Pediatr. 2021, 175, 1174–1176. [Google Scholar] [CrossRef]
- Lyytinen, G.; Brynedal, A.; Anesäter, E.; Antoniewicz, L.; Blomberg, A.; Wallén, H.; Bosson, J.A.; Hedman, L.; Mobarrez, F.; Tehrani, S.; et al. Electronic Cigarette Vaping with Nicotine Causes Increased Thrombogenicity and Impaired Microvascular Function in Healthy Volunteers: A Randomised Clinical Trial. Cardiovasc. Toxicol. 2023, 23, 255–264. [Google Scholar] [CrossRef]
- Pywell, M.J.; Wordsworth, M.; Kwasnicki, R.M.; Chadha, P.; Hettiaratchy, S.; Halsey, T. The Effect of Electronic Cigarettes on Hand Microcirculation. J. Hand Surg. 2018, 43, 432–438. [Google Scholar] [CrossRef]
- Ruedisueli, I.; Arastoo, S.; Gupta, P.K.; Gornbein, J.; Middlekauff, H.R. Neural-Hematopoietic-Inflammatory Axis in Nonsmokers, Electronic Cigarette Users, and Tobacco Smokers. Physiol. Rep. 2022, 10, e15412. [Google Scholar] [CrossRef]
- Sumartiningsih, S.; Lin, H.-F.; Lin, J.-C. Cigarette Smoking Blunts Exercise-Induced Heart Rate Response among Young Adult Male Smokers. Int. J. Environ. Res. Public. Health 2019, 16, 1032. [Google Scholar] [CrossRef]
- Evans, M.E. Update: Interim Guidance for Health Care Professionals Evaluating and Caring for Patients with Suspected E-Cigarette, or Vaping, Product Use–Associated Lung Injury and for Reducing the Risk for Rehospitalization and Death Following Hospital Discharge—United States, December 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 68, 1189–1194. [Google Scholar] [CrossRef]
- Adkins, S.H.; Anderson, K.N.; Goodman, A.B.; Twentyman, E.; Danielson, M.L.; Kimball, A.; Click, E.S.; Ko, J.Y.; Evans, M.E.; Weissman, D.N.; et al. Demographics, Substance Use Behaviors, and Clinical Characteristics of Adolescents With e-Cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) in the United States in 2019. JAMA Pediatr. 2020, 174, e200756. [Google Scholar] [CrossRef]
- Campus, B.; Fafard, P.; St Pierre, J.; Hoffman, S.J. Comparing the Regulation and Incentivization of E-Cigarettes across 97 Countries. Soc. Sci. Med. 2021, 291, 114187. [Google Scholar] [CrossRef]
- Pearson, J.L.; Sharma, E.; Rui, N.; Halenar, M.J.; Johnson, A.L.; Cummings, K.M.; Hammad, H.T.; Kaufman, A.R.; Tworek, C.; Goniewicz, M.L.; et al. Association of Electronic Nicotine Delivery System Use With Cigarette Smoking Progression or Reduction Among Young Adults. JAMA Netw. Open 2020, 3, e2015893. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Norcross, J.C. Stages of Change. Psychother. Theory Res. Pract. Train. 2001, 38, 443–448. [Google Scholar] [CrossRef]
- Ministerio de la Presidencia y Para las Administraciones Territoriales Real Decreto 579/2017, de 9 de Junio, por el que se Regulan Determinados Aspectos Relativos a la Fabricación, Presentación y Comercialización de los Productos del Tabaco y los Productos Relacionados. 2017. Available online: https://www.boe.es/eli/es/rd/2017/06/09/579/con (accessed on 29 July 2025).
- El Hajj Moussa, F.; Hayeck, N.; Hajir, S.; El Hage, R.; Salman, R.; Karaoghlanian, N.; Saliba, N.A. Enhancement of Benzene Emissions in Special Combinations of Electronic Nicotine Delivery System Liquid Mixtures. Chem. Res. Toxicol. 2024, 37, 227–233. [Google Scholar] [CrossRef]
- Komura, M.; Sato, T.; Yoshikawa, H.; Nitta, N.A.; Suzuki, Y.; Koike, K.; Kodama, Y.; Seyama, K.; Takahashi, K. Propylene Glycol, a Component of Electronic Cigarette Liquid, Damages Epithelial Cells in Human Small Airways. Respir. Res. 2022, 23, 216. [Google Scholar] [CrossRef]
- Sassano, M.F.; Davis, E.S.; Keating, J.E.; Zorn, B.T.; Kochar, T.K.; Wolfgang, M.C.; Glish, G.L.; Tarran, R. Evaluation of E-Liquid Toxicity Using an Open-Source High-Throughput Screening Assay. PLoS Biol. 2018, 16, e2003904. [Google Scholar] [CrossRef] [PubMed]




| Author, Year | Study Type | Groups | Sample Size | Mean Age | Sex (F/M) | EC Nicotine (mg/mL) * | Measured Effects | Assessed Variable | Results |
|---|---|---|---|---|---|---|---|---|---|
| Antoniewicz L et al. (2019) [29] | Randomized, double-blinded, crossover study | Non- smokers | 15 | 26 ± 3 | 9/6 | 19 | Vascular and pulmonary function following exposure to EC inhalation with or without nicotine | PWV (m/s) | Inhalation of ECs with and without nicotine led to an increase in BP. However, HR and arterial stiffness increased only with nicotine-containing ECs. |
| SBP (mmHg) | |||||||||
| DBP (mmHg) | |||||||||
| HR (bpm) | |||||||||
| Arastoo S et al. (2020) [30] | Open-label randomized crossover study | EC | 58 | 28 ± 5 | 19/39 | 50 | Sympathetic activation following exposure to inhalation sessions with nicotine-containing ECs, ECs without nicotine, nicotine inhalers, and CTs | SBP (mmHg) | EC and CT users experienced increases in BP and HR following inhalation of ECs and CT, respectively. These changes were more pronounced among participants who inhaled CT and were primarily attributed to the effects of nicotine. |
| CT | 42 | 27 ± 6 | 15/27 | DBP (mmHg) | |||||
| HR (bpm) | |||||||||
| Boas et al. (2017) [24] | Cross-sectional study based on an experimental protocol | EC | 9 | 29 ± 1.5 | 2/7 | NE | Metabolic activity of the splenocardiac axis assessed by PET-FDG | SUVmax | Both CT and EC showed increased FDG uptake in the spleen and aorta, with a greater increase observed in CT smokers. |
| CT | 9 | 27 ± 1.5 | 2/7 | Paraoxonase 1 enzyme activity (nmol p-nitrophenol/min/mL) | |||||
| Non- smokers | 9 | 28 ± 1.5 | 3/6 | ||||||
| Chatterjee S et al. (2021) [31] | Experimental repeated-measures study | Non- smokers | 31 | 24 ± 4 | 14/17 | 0 | Changes in inflammation and oxidative stress assessed by blood biomarkers and MRI following acute inhalation of ECs | CRP (ng/mL) | Subjects exposed to ECs exhibited increases in CRP, ICAM, ASC, and HMGB1 levels, along with a decrease in venous oxygen saturation and alterations in vascular diameter and resistance. |
| ICAM (ng/mL) | |||||||||
| HMGB1 (ng/mL) | |||||||||
| ASC containing a CARD (ng/mL) | |||||||||
| NO (μmol/L) | |||||||||
| FMD (%) | |||||||||
| PWV (m/s) | |||||||||
| Pulsatility index (dimensionless) | |||||||||
| Cooke WH et al. (2015) [32] | Randomized, counterbalanced, and double-blinded pilot study | Non- smokers | 20 | 23 ± 1 | 10/10 | 18 | Changes in BP, HR, and autonomic cardiovascular control following EC inhalation | HR (ECG R–R interval) | Acute nicotine inhalation increased SBP and DBP, as well as HR, but did not affect autonomic cardiovascular control under conditions of orthostatic stress. |
| Autonomic nervous system modulation (bursts/min) | |||||||||
| SBP (mmHg) | |||||||||
| DBP (mmHg) | |||||||||
| Cossio R et al. (2020) [33] | Randomized crossover study | Non- smokers | 16 | 24 ± 3 | 7/9 | 54 | Vascular function: HR and BP following inhalation sessions with nicotine-containing and nicotine-free ECs | FMD (%) | EC with and without nicotine did not produce any significant changes in HR, SBP, DBP, or endothelial function. |
| SBP (mmHg) | |||||||||
| DBP (mmHg) | |||||||||
| Gonzalez JE et al. (2021) [34] | Randomized crossover study | Non- smokers | 15 | 21 ± 1 | 6/9 | 59 | Changes in BP and ANSM following exposure to a single inhalation session of EC with and without nicotine | SBP (mmHg) | Inhalation of nicotine-containing EC increased BP and HR, along with a reduction in ANSM modulation. Inhalation of the placebo EC had no effect on sympathetic activity. |
| DBP (mmHg) | |||||||||
| HR (bpm) | |||||||||
| ANSM (bursts/min) | |||||||||
| Cardiovagal reflex sensitivity (ms/mmHg) | |||||||||
| Halstead KM et al. (2023) [23] | Cross-sectional study | EC | 20 | 21 ± 2 | 10/10 | NE | Endothelium-dependent VD assessed by measuring CVC responses to local heating with tempol | CVC (%) | EC users exhibited a reduction in NO-dependent VD compared to non-smokers, attributed to an excess of reactive oxygen species in plasma. Differences were more pronounced in women. |
| Non- smokers | 20 | 21 ± 2 | 10/10 | Percentage of NO-dependent vasodilatation (%) | |||||
| Haptonstall KP et al. (2020) [35] | Open-label, randomized crossover study | EC | 49 | 27 ± 5 | 13/36 | 50 | Endothelial VD function following exposure to EC, CT, and a placebo | FMD (%) | EC use did not alter FMD, unlike CT inhalation, which induced endothelial dysfunction, consistent with the notion that non-nicotine constituents in CT smoke mediate the impairment. |
| HR (bpm) | |||||||||
| SBP (mmHg) | |||||||||
| CT | 40 | 27 ± 5 | 14/26 | DBP (mmHg) | |||||
| MAP (mmHg) | |||||||||
| Non- smokers | 47 | 26 ± 5 | 25/22 | Shear stress (velocity/diameter) | |||||
| SSIRH (dynes/cm2) | |||||||||
| Reactive hyperemia velocity (cm/s) | |||||||||
| Kelesidis T et al. (2021) [36] | Randomized clinical crossover trial | EC | 12 | 24 ± 4 | 13/19 | 50 | Changes in oxidative stress cell counts in blood following a single inhalation session of EC | Counts of blood CD45, CD16, CD14 monocytes, T cells, and natural killer (NK) cells | Both EC and CT users exhibited increased levels of oxidative stress compared to non-smokers. However, CT users showed a greater increase than EC users. |
| CT | 9 | 25 ± 4 | |||||||
| Non- smokers | 11 | 24 ± 2 | |||||||
| Kelesidis T et al. (2023) [25] | Cross-sectional study | EC | 21 | 23 ± 1 | 12/9 | NE | Pro-atherogenic events assessed by plasma measurement of parameters related to transendothelial monocyte migration and foam cell formation | Percentage of transendothelial migration of macrophages and monocytes | Transendothelial migration of monocytes and macrophages, as well as foam cell formation, were significantly increased in EC users compared to non-smokers. However, these increases were significantly lower than those observed in CT users. |
| CT | 18 | 24 ± 1 | 9/9 | ||||||
| Non- smokers | 21 | 23 ± 2 | 10/11 | ||||||
| Lyytinen G et al. (2023) [37] | Randomized double-blind crossover study | Occasional smokers or snus users | 22 | 27 ± 7 | 15/7 | 19 | Fibrin and platelet thrombus formation following exposure to EC with and without nicotine, with subsequent measurement of plasma markers | Time to reach 10 kPa (s) | Inhalation of nicotine-containing EC, compared to nicotine-free EC, resulted in increased platelet and fibrin thrombus formation, and a reduction in microcirculatory VD capacity. |
| Time to reach occlusion pressure (s) | |||||||||
| Baseline blood flow and after 30 min infusion of acetylcholine or SNP | |||||||||
| Matheson C et al. (2024) [20] | Cross-sectional study | EC | 21 | 23 ± 3 | 11/10 | NE | Vascular function of subjects assessed by vascular reactivity tests | Red blood cell flow (UP) | EC users exhibited lower levels of hyperemia following vascular occlusion, reduced VD in response to acetylcholine infusion, and decreased heat-induced VD compared to controls. No differences were observed in endothelium-independent VD. |
| CVC (UP/mmHg) | |||||||||
| Non- smokers | 21 | 25 ± 5 | 12/9 | FMD (%) | |||||
| Moheimani R et al. (2017) [26] | Cross-sectional case–control study | EC | 16 | 29 ± 1 | 3/13 | NE | Variations in HR and oxidative stress assessed through plasma parameters | HR (bpm) | EC use was associated with an increase in HR and elevated oxidative stress levels compared to the non-smoking group Habitual EC use was associated with a shift in cardiac autonomic balance toward sympathetic predominance and increased oxidative stress, both associated with increased cardiovascular risk. |
| LDL oxidative capacity (dimensionless) | |||||||||
| HDL antioxidant index (dimensionless) | |||||||||
| Non- smokers | 18 | 27 ± 1 | 11/7 | Paraoxonase-1 activity (nmol p-nitrophenol/min/mL) | |||||
| Plasma fibrinogen (mg/dL) | |||||||||
| Pywell MJ et al. (2018) [38] | Pilot experimental repeated-measures study | CT | 7 | 26 ± 1 | NE | 24 | Hand microvascular blood flow assessed by Doppler following inhalation of EC with and without nicotine | Superficial and deep blood flow (arbitrary units, equivalent to ml/min) | CT smokers exhibited a decrease in hand microvascular blood flow during inhalation of nicotine-containing ECs. |
| Non- smokers | 8 | 25 ± 3 | |||||||
| Ruedisueli I et al. (2023) [27] | Randomized crossover experimental study with a cross-sectional baseline comparison | EC | 34 | 23 ± 3 | 17/17 | 50 | Changes in ventricular repolarization assessed by ECG in subjects following inhalation of CT and EC | Tp-e interval (ms) | Inhalation of ECs, unlike CT inhalation, resulted in prolongation of ventricular repolarization indices. This effect was observed only in men and not in women. |
| Tp-e/QT ratio | |||||||||
| CT | 35 | 24 ± 3 | 21/14 | Tp-e/QTc ratio | |||||
| QT (ms) | |||||||||
| Non- smokers | 41 | 24 ± 4 | 21/20 | QTc (ms) | |||||
| HR (bpm) | |||||||||
| Ruedisueli S et al. (2022) [39] | Cross-sectional comparative | EC | 16 | 25 ± 4 | 6/10 | NE | Metabolic activity of the splenocardiac axis, associated with sympathetic activity and atherosclerosis development, assessed by PET-FDG | SUVmax | Underscored the role of the sympathetic nervous system in provoking inflammatory monocyte proliferation, instigating atherosclerotic development. No differences were observed in metabolic activity among the subjects studied. |
| CT | 14 | 27 ± 6 | 7/7 | ||||||
| Non- smokers | 15 | 25 ± 4 | 6/9 | ||||||
| Sahota A et al. (2021) [21] | Cross sectional study | EC | 20 | 26 ± 4 | 7/13 | NE | Presence of atherosclerosis parameters in the carotid artery assessed by PET-MRI | SUVmax | CT and EC smokers exhibited atherosclerotic plaques in carotid MRI images, unlike the non-smoker group. This damage was more pronounced in EC smokers. |
| CT | 20 | 27 ± 3 | 7/13 | ||||||
| Non- smokers | 20 | 25 ± 2 | 8/12 | ||||||
| Shi H et al. (2023) [22] | Prospective longitudinal cohort study | EC | 372 | 18 to 24 | 177/196 | NE | Development of HTN following exclusive use of EC, CT, or dual consumption of both, based on a retrospective analysis of survey data | HTN yes/no | Exclusive use of CT was associated with an increased risk of HTN in women. Neither EC use nor combined EC and CT consumption were associated with HTN risk in either men or women. |
| CT | 4933 | 2573/2360 | |||||||
| EC + CT | 573 | 291/283 | |||||||
| Non- smokers | 10486 | 5725/ 4761 | |||||||
| Sumartiningsih S et al. (2019) [40] | Randomized crossover experimental study with repeated measures | EC CT | 24 | 23 ± 2 | 0/24 | 3 | Cardiac response to exercise following exposure to nicotine-free EC, nicotine-containing EC, and CT | HR (bpm) | Inhalation of CT resulted in an increase in HR and a decrease in DBP. Significant acute autonomic cardiac modulation during exercise was induced by acute episodes of EC and CT smoking. |
| SBP (mmHg) | |||||||||
| DBP (mmHg) | |||||||||
| Maximal oxygen consumption (ml/kg/min) | |||||||||
| Time to exhaustion (sec) | |||||||||
| Youn JY et al. (2023) [28] | Cross sectional study | EC | 13 | 22 ± 1 | 6/7 | NE | Endothelial damage function assessed through plasma nitrite levels | Circulating plasma nitrite levels (µM) | Nitrite levels were lower in EC users compared to both the control and CT groups. |
| CT | 11 | 23 ± 1 | 6/5 | ||||||
| Non- smokers | 9 | 22 ± 1 | 3/6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranchal-Lavela, C.; Casanova-Rodríguez, D.; Ranchal-Sanchez, A.; Torre-Aguilar, M.J.D.L.; Jurado-Castro, J.M. Time- and Dose-Dependent Cardiovascular Effects of Nicotine-Containing Electronic Cigarettes in Young Adults: A Systematic Review and Meta-Analysis. Toxics 2025, 13, 831. https://doi.org/10.3390/toxics13100831
Ranchal-Lavela C, Casanova-Rodríguez D, Ranchal-Sanchez A, Torre-Aguilar MJDL, Jurado-Castro JM. Time- and Dose-Dependent Cardiovascular Effects of Nicotine-Containing Electronic Cigarettes in Young Adults: A Systematic Review and Meta-Analysis. Toxics. 2025; 13(10):831. https://doi.org/10.3390/toxics13100831
Chicago/Turabian StyleRanchal-Lavela, Carmen, David Casanova-Rodríguez, Antonio Ranchal-Sanchez, María José De La Torre-Aguilar, and Jose Manuel Jurado-Castro. 2025. "Time- and Dose-Dependent Cardiovascular Effects of Nicotine-Containing Electronic Cigarettes in Young Adults: A Systematic Review and Meta-Analysis" Toxics 13, no. 10: 831. https://doi.org/10.3390/toxics13100831
APA StyleRanchal-Lavela, C., Casanova-Rodríguez, D., Ranchal-Sanchez, A., Torre-Aguilar, M. J. D. L., & Jurado-Castro, J. M. (2025). Time- and Dose-Dependent Cardiovascular Effects of Nicotine-Containing Electronic Cigarettes in Young Adults: A Systematic Review and Meta-Analysis. Toxics, 13(10), 831. https://doi.org/10.3390/toxics13100831