1. Introduction
Sedum alfredii is a well-known hyperaccumulator of cadmium (Cd), lead (Pb), and zinc (Zn) that has been extensively used for phytoremediation of heavy metal-contaminated soils [
1,
2,
3]. However, its overall remediation efficiency is constrained by the plant’s low biomass and slow growth rate, which are common characteristics of hyperaccumulator plants. Ref. [
4], constraining the total amount of metal that can be extracted from soils. Therefore, enhancing heavy metal accumulation in the shoots of
S. alfredii is a primary objective for improving its phytoremediation efficiency [
5]. Therefore, achieving this goal requires a better understanding of the regulatory mechanisms governing heavy metal uptake, translocation, and accumulation in
S. alfredii, including the identification of key signal transduction pathways and effective chemical agents to modulate these processes.
To date, multiple biological processes have been implicated in Cd hyperaccumulation by
S. alfredii, including enhanced ion uptake across membranes [
6,
7], efficient xylem loading and translocation of metals [
2,
7], cellular sequestration and compartmentalization of Cd [
8,
9,
10], and detoxification/chelation mechanisms [
6,
11]. These processes operate systematically and cooperatively, ultimately facilitating high Cd accumulation in the plant [
12]. Phytohormones are increasingly recognized as essential regulators that coordinate many of these processes. For instance, the exogenous application of certain plant growth regulators, such as indole-3-acetic acid (IAA), brassinolide (BR), and abscisic acid (ABA), has been shown to enhance Cd phytoextraction by boosting the antioxidant defense system and reducing lipid peroxidation damage [
13]. This evidence suggests that hormonal signaling, often intertwined with redox regulation, plays a crucial role in the accumulation of heavy metals.
Various phytohormones (such as auxins, brassinosteroids, ABA, salicylic acid, jasmonic acid, and ethylene) can modulate heavy metal uptake and tolerance through multiple mechanisms. These hormones regulate the transcription of metal transporter genes [
14,
15]; modulate root system architecture, thereby influencing metal uptake [
16]; enhance antioxidant capacity to mitigate metal-induced oxidative stress [
14,
15]; and engage in crosstalk with one another to fine-tune plant stress responses [
14,
16]. Concurrently, heavy metal stress is closely associated with reactive oxygen species (ROS) signaling in plants. Cd, as a non-essential toxic element, induces oxidative stress that can damage cells and inhibit growth [
17,
18]. Previous studies have shown that S. alfredii displays minimal ROS accumulation at ≤0.1 mM CdCl
2, whereas exposure to ≥0.2 mM CdCl
2 triggers marked oxidative stress responses, including increased H
2O
2 and O
2− production as well as antioxidant enzyme activation [
8]. This threshold-like shift indicates that rising Cd levels rapidly induce ROS signaling, which may interfere with hormone-regulated pathways. However, ROS also function as important secondary messengers in stress signaling and can influence metal uptake processes. For example, elevated ROS levels can affect the expression of metal transporter genes such as
IRT1 and
NRAMP, which mediate the uptake and transport of divalent metal cations, including Cd [
7]. Thus, oxidative signaling is thought to contribute to the regulation of Cd uptake and translocation, potentially playing a role in
S. alfredii’s hyperaccumulation capacity. Moreover, many phytohormones can trigger ROS production and activate downstream defense pathways [
19,
20]. The extensive crosstalk between hormonal signals and ROS enables plants to orchestrate transcriptomic and metabolic adjustments under stress [
19,
20]. This hormone–ROS network is mediated by diverse signaling components, including ROS-generating enzymes like NADPH oxidases [
19], mitogen-activated protein kinase cascades [
21], stress-responsive transcription factors [
19], and secondary signaling molecules such as flavonols [
22].
Despite these insights, the precise regulatory interplay between heavy metal stress, ROS, and phytohormones remains poorly understood. One major challenge is disentangling the direct effects of heavy metals from the signaling interactions among hormones and ROS. In our previous study [
12], we found that exogenous ABA can have a dual effect on Cd accumulation in
S. alfredii depending on the stress intensity. Under moderate Cd exposure—associated with relatively low ROS levels—ABA enhanced Cd uptake and root-to-shoot translocation by upregulating the expression of divalent cation transporter genes. In contrast, under high Cd stress (with elevated ROS), this promotive effect was diminished or even reversed [
12]. These results imply that a basal level of ROS is required for ABA to stimulate Cd accumulation, pointing to a critical ABA–ROS crosstalk in regulating metal transporter activity. However, as these experiments were conducted in the presence of Cd, it was difficult to distinguish responses driven by Cd-induced stress from those activated by hormone-specific signaling.
In the present study, we addressed this knowledge gap by removing external Cd treatment to eliminate direct heavy metal stress signals. This experimental design enabled us to isolate the interactions between phytohormones and ROS in regulating Cd-related transporters, thereby eliminating the confounding influence of concurrent Cd toxicity. We specifically examined how ABA, in the absence of external Cd, influences the expression of Cd transporter genes in S. alfredii via ROS-mediated signaling. By working under Cd-free conditions, we aimed to clarify the role of ABA–ROS crosstalk in the signaling network underlying Cd uptake and accumulation. To our knowledge, this is the first attempt to investigate the regulatory interactions between hormones and ROS in a hyperaccumulator plant without applying heavy metal stress, providing a novel perspective on the mechanisms of Cd hyperaccumulation. The insights gained from this work shed light on how phytohormone-ROS signaling regulates heavy metal transport and may ultimately guide new strategies for enhancing phytoremediation efficiency through targeted manipulation of plant signaling pathways.
3. Results
3.1. The Impact of Exogenous Treatments on Cd Transporter Gene Expression
The expression of genes related to Cd accumulation is shown in
Table 2. Compared with CK, all transporters exhibited significant responses to exogenous phytohormones and H
2O
2, with the strongest regulation observed for
ZIP2 and members of the
Nramp and
HMA families.
Within the Nramp family, low-dose t-Z caused a strong induction of Nramp3 (2.29 vs. 1.00 in CK) and Nramp6 (2.81 vs. 1.00), while high-dose t-Z suppressed both genes (Nramp3 0.47; Nramp6 0.41). A notable dose-dependent divergence was observed under ABA treatment: A high dose of ABA strongly favored Nramp3 (2.22) and Nramp6 (3.44), while a low dose had a milder effect. Similarly, IAA low-dose induced Nramp3 (2.09) and Nramp6 (1.91).
The HMA family was highly sensitive to exogenous stimuli but showed distinct patterns among members. High-dose treatments of t-Z, ABA, GA3, and H2O2 consistently enhanced HMA2 and HMA4. For example, ABA low-dose enhanced HMA2 (2.75) and HMA4 (2.45), and GA3 low-dose strongly promoted HMA2 (3.88) and HMA4 (4.26). The most substantial increases were observed under high-dose H2O2 treatment, with HMA2 and HMA4 expression levels reaching 5.41-fold and 6.15-fold, respectively, relative to the control (CK). In contrast, while all treatments suppressed HMA3 expression, the effect was least pronounced under low-dose IAA and low-dose t-Z, with relative expression levels of 0.90 and 0.72, respectively.
Among all genes tested, the
ZIP2 transporter exhibited the most dramatic expression changes, with its transcript levels increasing by orders of magnitude under specific treatments (
Table 2). From a baseline expression of nearly 1.0 in CK, it increased to 39.9 under t-Z low-dose, 158.7 under ABA high-dose, 87.6 under GA
3 low-dose, and 71.1 under GA
3 high-dose. Even under milder treatments such as IAA low-dose,
ZIP2 was strongly upregulated (29.6), highlighting it as the most hormone-responsive transporter in this dataset. By contrast,
ZIP3 was generally suppressed, with values falling below CK under most treatments (e.g., 0.26 under ABA high-dose). For
IRT1, expression was usually reduced relative to CK; however, IAA at high doses significantly upregulated
IRT1 (1.73 vs. 1.00), suggesting a dose-specific redirection of Cd uptake routes under auxin influence.
In summary, the response to hormone treatment was highly gene-specific. Low-dose treatments often preferentially induced Nramp transporters (Nramp3/6), whereas high-dose treatments, particularly high-dose H2O2, strongly promoted HMA2 and HMA4. Across all genes, ZIP2 was the most significantly upregulated, showing increases of two to three orders of magnitude.
3.2. Effects of Exogenous Hormone Application on Endogenous Phytohormones
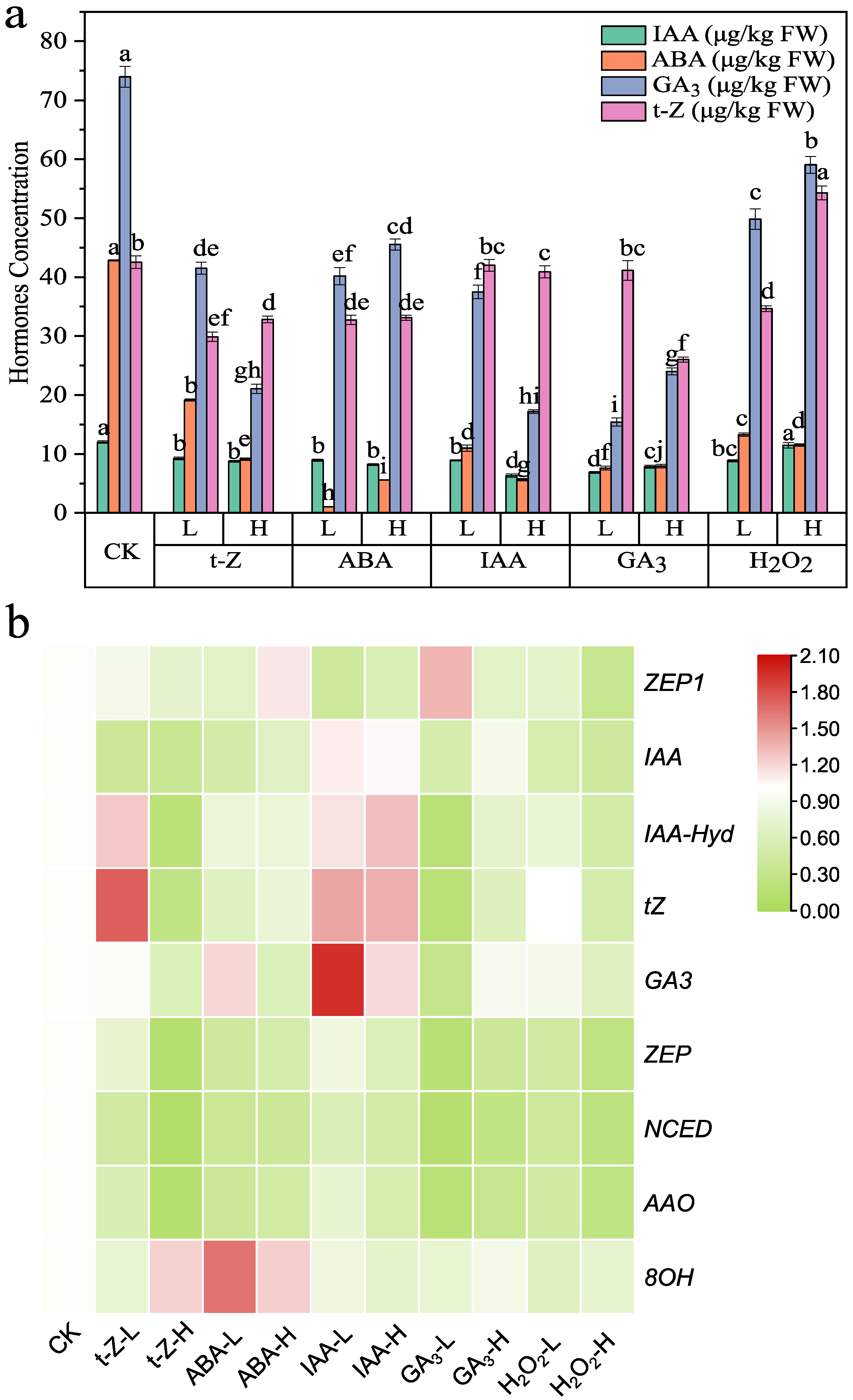
The effects of endogenous phytohormone contents in Shoots are shown in
Figure 1a,
Table S2. Raw values for
Figure 1a. Most treatments resulted in a general decline in endogenous phytohormone levels, although the extent varied among hormones. The most potent suppression was observed for endogenous ABA, the accumulation of which was nearly eliminated by high-dose applications of either ABA or GA
3 (5.6 μg/kg and 7.9 μg/kg, respectively, vs. 42.9 μg/kg in CK). GA
3 levels were likewise highly sensitive, dropping from 74 μg/kg in CK to 21.1 μg/kg under high-dose t-Z and 17.2 μg/kg under high-dose IAA, while H
2O
2 treatments also reduced GA
3 to 49.8 μg/kg (low-dose) and 59.0 μg/kg (High-dose). IAA showed a more moderate decline, decreasing from 12.0 μg/kg in CK to ~9.3 μg/kg under low-dose t-Z and 7.9 under high-dose GA
3, with the most potent inhibition under high-dose IAA (6.3 μg/kg). In contrast, t-Z was less consistently suppressed; although most treatments lowered its content to ~30–33 μg/kg, high-dose H
2O
2 significantly promoted it to 54.3 μg/kg, ~28% above CK. Collectively, these results indicate that exogenous hormone applications generally downregulated endogenous phytohormone pools. Among them, ABA and GA
3 levels were the most significantly reduced. The magnitude of inhibition was often dose dependent, while specific treatments, notably high-dose H
2O
2, deviated from this trend by enhancing t-Z despite reducing ABA and GA
3. In general, inhibitory effects were dose-dependent, with higher concentrations producing more substantial reductions. However, exceptions existed, such as high-dose H
2O
2, which significantly elevated t-Z despite decreasing ABA and GA
3.
3.3. Effects of Exogenous Treatments on Phytohormone-Related Metabolic Enzymes
The expression patterns of phytohormone-related metabolic enzymes in shoots are shown in
Figure 1b. In the ABA biosynthetic branch (
ZEP/NCED/AAO), most treatments suppressed transcript levels relative to CK. Consistent with this overall trend,
ZEP1 was reduced under several conditions (e.g., t-Z low-dose ≈ 0.87; t-Z high-dose ≈ 0.71; low-dose ABA ≈ 0.66; H
2O
2 high-dose ≈ 0.33 vs. CK ≈ 1.00), whereas GA
3 low-dose produced an opposite effect with a significant increase of
ZEP1 to ~1.35. This dose-dependent divergence highlights that GA
3 at low levels can relieve the repression of ABA biosynthesis, while oxidative or cytokinin inputs tend to down-modulate it.
In the ABA catabolic branch, ABA 8′-hydroxylase (ABA8′OH) was generally repressed across treatments, although notable exceptions occurred under ABA (both low- and high-dose) and high-dose t-Z, which induced its expression (e.g., ABA low-dose ≈ 1.65; ABA high-dose ≈ 1.24; t-Z high-dose ≈ 1.23). This suggests that ABA turnover is generally reduced under most conditions, but can be enhanced by specific hormonal cues.
Within the IAA metabolic module, most exogenous treatments downregulated the IAA biosynthetic marker (e.g., t-Z low-dose ≈ 0.40; t-Z high-dose ≈ 0.35; ABA low-dose ≈ 0.49; GA3 low-dose ≈ 0.51; H2O2 high-dose ≈ 0.42), whereas IAA low-dose (≈1.10) and IAA high-dose (≈1.06) promoted its expression, consistent with an auxin-mediated positive feedback on its own synthesis. In the IAA catabolic arm, IAA-Hyd was selectively upregulated by t-Z low-dose and both IAA doses, but remained repressed or unchanged under most other treatments; notably, ABA, H2O2, and t-Z high-dose preferentially suppressed IAA synthesis more than IAA-Hyd, while both GA3 doses showed the opposite tendency (i.e., relatively more potent effects on the catabolic side).
For t-Z metabolism, t-Z synthetase was induced under IAA treatments (both doses), but was suppressed by t-Z high-dose and most other treatments. Increasing the dose of t-Z or H2O2 generally intensified the inhibition of t-Z synthetase, whereas GA3 displayed a reverse dose–response trend, with stronger promotion emerging at higher concentrations.
Finally, GA3 synthetase showed hormone-specific and dose-dependent regulation: IAA treatments and ABA low-dose enhanced expression, while t-Z (especially high-dose), ABA high-dose, H2O2, and GA3 high-dose led to significant suppression. Overall, low-dose applications tended to exert mild promotion or partial maintenance of GA3 biosynthesis, whereas high-dose inputs more frequently drove repression.
Together, these data indicate that exogenous cues reshape hormone biosynthesis and catabolism in a dose- and hormone-specific manner. ABA biosynthesis is broadly repressed (with GA3 low-dose as a key exception), while ABA8′OH-mediated catabolism is context-dependent—generally reduced but inducible by ABA and t-Z. Auxin imposes positive feedback on its own synthesis.
3.4. Effects of Exogenous Treatments on Reactive Oxygen Species (ROS) and Related Metabolic Enzymes
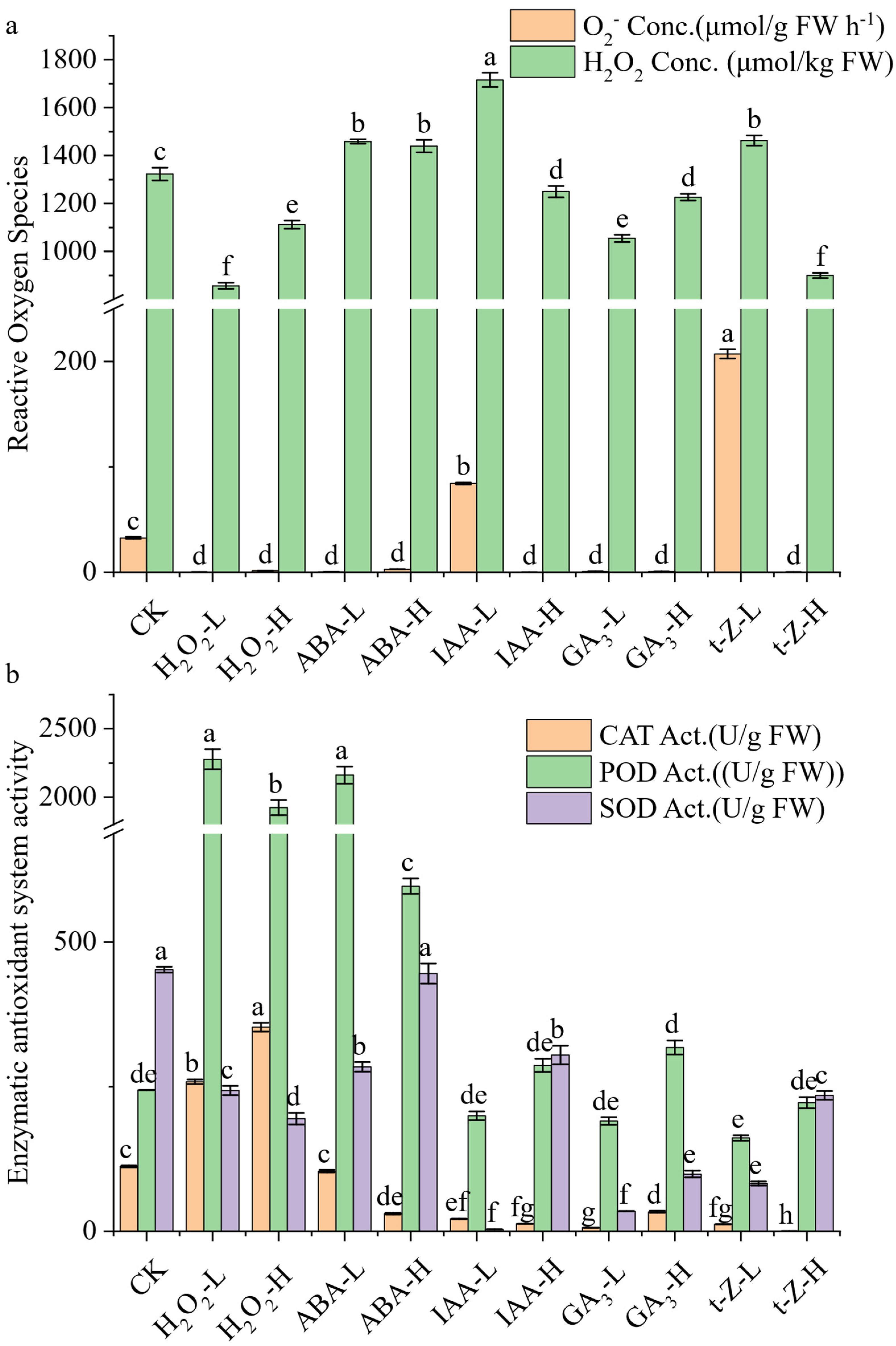
The responses of endogenous ROS and associated antioxidant enzymes to exogenous treatments are shown in
Figure 2.
The basal production rate of O2− in the control (CK) group was 32.6 μmol g−1 FW h−1. Several treatments significantly lowered this rate; notably, low-dose H2O2 and high-dose t-Z decreased the production to nearly undetectable levels (0.24 μmol g−1 FW h−1 and 0.27 μmol g−1 FW h−1, respectively). In stark contrast, other treatments markedly elevated the O2− production rate. Low-dose IAA markedly elevated O2− to 84.3 μmol g−1 FW h−1, while low-dose t-Z induced the strongest promotion, reaching 207.2 μmol g−1 FW h−1 over six times the CK value.
Regarding H2O2 accumulation, CK shoots contained 1323 μmol kg−1 FW. Low-dose IAA significantly increased H2O2 to 1716 μmol kg−1 FW, while low-dose ABA (1459 μmol kg−1 FW) also caused a slight but significant increase. Conversely, high-dose t-Z reduced H2O2 to 900 μmol kg−1 FW, and both GA3 treatments lowered it to 1055 μmol kg−1 FW (low-dose) and 1226 μmol kg−1 FW (high-dose). Exogenous H2O2 treatments also decreased endogenous H2O2, with low-dose H2O2 at 857 μmol kg−1 FW and high-dose H2O2 at 1112 μmol kg−1 FW.
Antioxidant enzyme activities showed substantial divergence among treatments. SOD activity in CK was 452 U g−1 FW, but most treatments suppressed it drastically, with low-dose IAA showing the most severe inhibition (3.6 U g−1 FW). High-dose IAA partially restored SOD (305 U g−1 FW), whereas high-dose ABA (446 U g−1 FW) remained nearly unchanged relative to the control. POD activity in CK was 244 U g−1 FW, but treatments with low-dose H2O2 (2276 U g−1 FW) and low-dose ABA (2161 U g−1 FW) caused nearly tenfold increases. High-dose H2O2 (1922 U g−1 FW) and high-dose ABA (597 U g−1 FW) also enhanced POD relative to CK, though to a lesser extent. IAA and GA3 treatments remained below 320 U g−1 FW, while t-Z showed intermediate effects (161–222 U g−1 FW). CAT activity was 112 U g−1 FW in CK. Low-dose and high-dose H2O2 treatments elevated CAT to 258 U g−1 FW and 353 U g−1 FW, respectively, more than doubling the control. Conversely, CAT activity was strongly inhibited by most hormonal treatments. Specifically, activity levels were reduced to 31 U g−1 FW under high-dose ABA, 21 U g−1 FW with low-dose IAA, 6 U g−1 FW with low-dose GA3, and a mere 5.3 U g−1 FW under high-dose t-Z, indicating profound enzymatic suppression.
Collectively, these results reveal a dual regulatory pattern: specific treatments (e.g., low-dose IAA, t-Z) promoted ROS accumulation, whereas others (e.g., H2O2, GA3, high-dose t-Z) enhanced ROS scavenging capacity by stimulating POD and CAT activities.
3.5. Correlation Analysis Between Endogenous Phytohormones, Antioxidant Enzymes, and Metal Transporter Genes
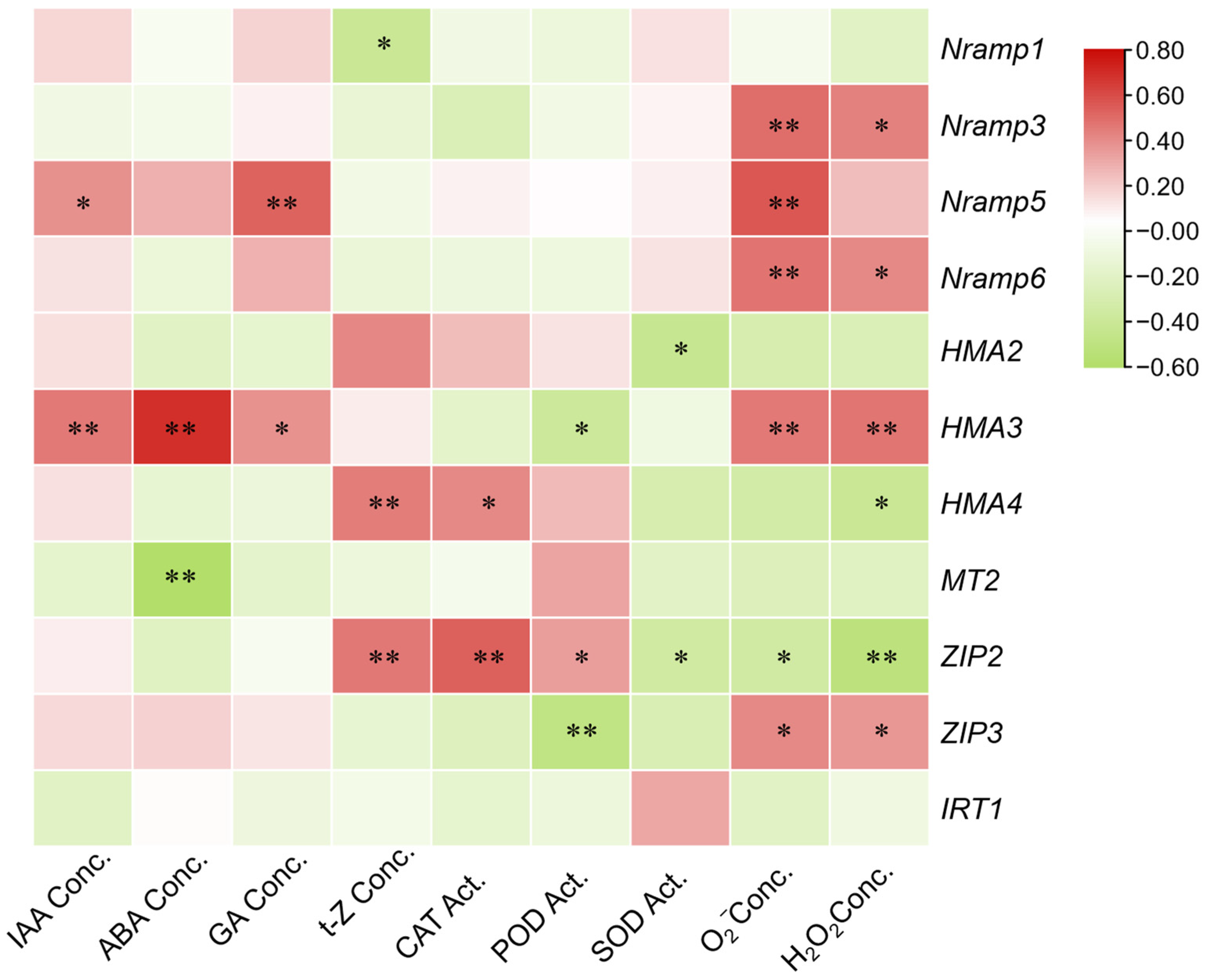
To further elucidate the regulatory network among endogenous phytohormones, antioxidant enzymes, and metal transporter genes, correlation analyses were conducted (
Figure 3). In the first correlation matrix, phytohormones exhibited strong internal coherence. IAA and GA displayed a highly significant positive correlation (r = 0.895,
p < 0.01), while ABA was also positively correlated with IAA (r = 0.696,
p < 0.01) and GA (r = 0.622,
p < 0.01). Moreover, t-Z showed significant positive correlations with IAA, ABA, and GA (r = 0.355–0.419,
p < 0.05). By contrast, antagonistic patterns were observed between hormones and antioxidant enzymes. ABA was negatively correlated with SOD (r = −0.442,
p < 0.01), and GA was negatively correlated with POD (r = −0.569,
p < 0.01). In addition, antioxidant enzymes displayed strong synergy, as evidenced by positive correlations between POD and SOD (r = 0.405,
p < 0.05) and between POD and CAT (r = 0.777,
p < 0.01). These findings suggest that phytohormones tend to accumulate in a coordinated manner, whereas their increase often coincides with a reduction in antioxidant enzyme activities.
In the second correlation matrix, diverse associations were observed between phytohormones and genes encoding metal transporters. IAA was negatively correlated with Nramp1 (r = −0.439, p < 0.05), Nramp5 (r = −0.399, p < 0.05), and HMA3 (r = −0.407, p < 0.05), but positively correlated with MT2 (r = 0.374, p < 0.05). ABA exhibited the strongest influence, showing a highly significant negative correlation with HMA3 (r = −0.648, p < 0.01), while positively correlating with MT2 (r = 0.774, p < 0.01) and ZIP2 (r = 0.567, p < 0.01). Similarly, GA was negatively correlated with Nramp1 (r = −0.432, p < 0.05), Nramp5 (r = −0.596, p < 0.01), and Nramp6 (r = −0.448, p < 0.05). By contrast, t-Z exhibited only weak or non-significant correlations with most transporters. Collectively, these results highlight that ABA plays a central role in shaping transporter expression, particularly by suppressing HMA3 while enhancing ZIP2, whereas IAA and GA predominantly act to inhibit Nramp-mediated metal uptake.
3.6. Integrated Multivariate Analysis (PLS-SEM) of Transporters, Hormones, and ROS
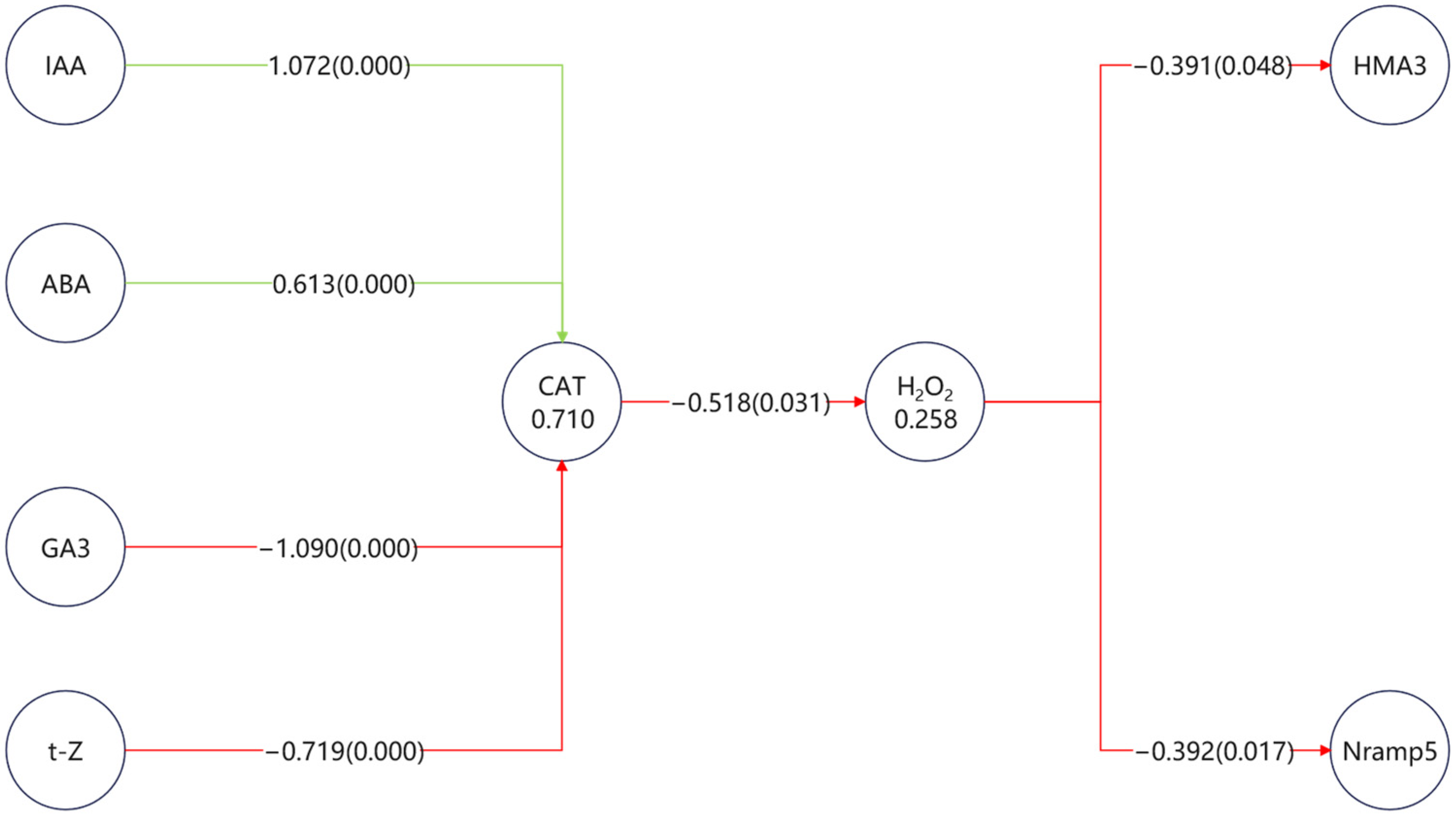
All measured parameters, including hormone metabolic enzymes, hormone levels, antioxidant enzyme activities, ROS, and transporter expression, were incorporated into a PLS-SEM framework to reconstruct potential regulatory pathways. The complete model is provided in the
Supplementary Materials (Figure S1), while
Figure 4. highlights only the significant paths that directly connect hormones (via ROS metabolism) to transporters.
The results revealed a CAT–H2O2 axis as the central regulatory hub, through which multiple hormones indirectly shaped transporter expression. Hormones exerted contrasting effects on CAT activity: IAA (+1.07) and ABA (+0.61) acted as positive regulators, while GA3 (−1.09) and t-Z (−0.72) served as negative regulators. These opposing inputs determined the ROS balance, with elevated CAT activity suppressing H2O2 accumulation (−0.52).
Downstream, ROS exerted a consistent negative effect on transporter expression, significantly reducing Nramp5 (−0.39, p = 0.017) and HMA3 (−0.39, p = 0.048). Thus, positive hormonal inputs (IAA, ABA) alleviated ROS pressure and indirectly favored transporter regulation, whereas negative inputs (GA3, t-Z) promoted ROS accumulation and ultimately repressed transporter activity.
In terms of relative magnitude, GA3 showed the strongest suppression, while IAA had the strongest stimulation, indicating that auxin and gibberellin represent the dominant but antagonistic forces within this network.
3.7. Moderation Analysis of Hormone–Transporter Relationships by H2O2 Inhibition
To validate the SEM-inferred hormone–ROS–transporter pathways, we performed a moderation analysis using H
2O
2 inhibition (
Table 3). The manipulation check confirmed that the inhibitor effectively reduced H
2O
2 accumulation in a Cd-dependent manner. Although the overall inhibitor main effect was not significant (β = −0.031,
p = 0.128), a potent inhibitor × Cd Level interaction (β = −0.371,
p < 0.001) indicated that H
2O
2 accumulation was significantly suppressed under high Cd conditions. These patterns are consistent with the SEM structure, in which CAT lowers H
2O
2 and H
2O
2 negatively regulates
HMA3 and
Nramp5.
Simple slope analyses revealed distinct patterns of hormone–transporter regulation. For HMA3, ABA (p = 0.048) and t-Z (p = 0.02) showed significant negative effects in the absence of inhibitor, which became non-significant under inhibition, suggesting that both hormones act through an H2O2-dependent pathway. In contrast, GA3 and IAA had no significant effect on HMA3 in either condition. For Nramp5, ABA exhibited a striking reversal: a significant negative effect without inhibitor (p = 0.01) shifted to a significant positive effect under inhibition (p < 0.01). t-Z again showed a significant negative association without the inhibitor (p < 0.001), which disappeared with inhibition. IAA displayed a robust positive effect on Nramp5 across both groups (p < 0.001), with nearly identical slopes, indicating an H2O2-independent mechanism. GA3 had no significant influence on Nramp5.
Fisher’s r-to-z tests further confirmed these interaction patterns. Significant group differences in correlations were detected for ABA–
HMA3, ABA–
Nramp5, t-Z–
HMA3, and t-Z–
Nramp5, while IAA–
Nramp5 remained consistently positive across groups, and GA
3–transporter pairs showed no significant differences. Finally, leaf Cd concentrations varied among treatments (
Supplementary Table S3). Across Cd doses (0.1–0.5 mM), application of the H
2O
2 inhibitor generally resulted in higher Cd accumulation compared to the corresponding non-inhibitor controls (e.g., from 15.2 to 33.9 at 0.1 mM Cd). Although Cd accumulation reflects multiple pathways beyond those captured by the SEM, the consistent increase under inhibition supports the functional relevance of H
2O
2-mediated regulation of Cd handling in leaves. Detailed measurements of endogenous hormone contents, H
2O
2 concentrations, and transporter expression levels are provided in
Supplementary Tables S4–S6 for reference.
4. Discussion
The regulation of metal transporter expression involves intricate interactions among heavy metals, ROS, and phytohormones, and the precise signaling pathways remain largely unresolved [
29,
30]. Previous studies have demonstrated that Cd levels strongly affect how phytohormones influence Cd uptake and translocation [
12,
13], making it difficult to decouple direct hormonal or ROS effects from Cd-induced responses. In this study, Cd itself was excluded from the treatments to minimize this interference and to investigate the extent to which phytohormones and ROS alone influence the expression of Cd-related transporters in
S. alfredii. Our findings provide correlative evidence suggesting that hormone–ROS interactions could be important modulators of transporter expression, thereby offering candidate pathways for further functional investigation.
PLS-SEM analysis identified phytohormones as central regulators of Cd uptake and accumulation, acting either positively or negatively depending on the treatment. Among them, H
2O
2 and CAT emerged as pivotal mediators that bridge hormonal signals with Cd transporter responses, particularly
HMA3 and
Nramp5. ABA, IAA, GA
3, and t-Z displayed contrasting roles in shaping Cd transporter expression. SEM highlighted that ABA and IAA exerted a consistent positive impact on leaf catalase (CAT) activity, whereas t-Z and GA
3 suppressed CAT. This hormone-specific regulation underscores CAT as an early functional node in the hormonal control of Cd handling in leaves. Previous studies support such trends, showing that ABA can enhance CAT activity under stress conditions [
31,
32], and that auxin–antioxidant crosstalk contributes to stress adaptation [
31]. In contrast, the observed suppression of CAT by t-Z and GA
3 is consistent with their roles in balancing growth and stress responses [
33]. Together, these observations indicate that ABA and IAA function as positive regulators of CAT in leaves, whereas t-Z and GA
3 act as suppressors, reflecting a hormone-driven modulation of antioxidant readiness.
Within this framework, leaf CAT acts as a proximal regulatory switch linking hormone inputs to Cd-transporter transcription. Treatments that increased CAT (ABA, IAA) were associated with maintained or elevated expression of
HMA3 and
Nramp5, while reduced CAT (t-Z, GA
3) coincided with transporter repression. This observation aligns with our SEM model, in which CAT-mediated detoxification of H
2O
2 alleviates its inhibitory pressure on both
HMA3 and
Nramp5, thereby allowing for sustained transporter expression. Thus, hormone-driven CAT elevation relieves ROS-mediated repression of transporter genes, while CAT suppression reinforces it. This interpretation aligns with established roles of catalase in maintaining intracellular H
2O
2 homeostasis and the broader recognition of H
2O
2 as a transcription-modulating signal in plant stress responses, including heavy metal exposure [
34,
35].
At the transporter level, the SEM highlighted that H
2O
2 negatively regulates both
HMA3 and
Nramp5, but their functions in leaves are complementary rather than antagonistic. Upregulation of
HMA3 reflects enhanced vacuolar sequestration of Cd in mesophyll or storage cells—a hallmark strategy of hyperaccumulators that enables high shoot Cd accumulation while buffering cytosolic toxicity [
36]. In contrast, while
Nramp5 is classically considered a root uptake transporter, emerging evidence suggests it can contribute to metal partitioning in aerial tissues; for example,
OsNramp5 in rice leaf sheaths mediates Mn unloading from the xylem [
37]. By analogy, elevated
Nramp5 expression in
S. alfredii leaves may facilitate Cd distribution into safe compartments, synergizing with
HMA3-mediated sequestration. Together, these two transporters define a leaf-centric Cd accumulation module, in which
HMA3 enhances vacuolar storage and
Nramp5 supports controlled cellular distribution.
Taken together, these interlinked mechanisms establish a coherent framework: hormones modulate CAT, CAT modulates transporter expression, and transporters cooperate to compartmentalize Cd in leaves. This layered model explains how S. alfredii achieves effective shoot-level Cd accumulation through a hormone–CAT–transporter cascade, highlighting the leaf as a central site for coordinated regulation of Cd buildup.
To test the robustness of the SEM-derived relationships, we employed an intervention-based moderation design that perturbed the ROS node. These tests partially validated the relationships inferred from the SEM, though some discrepancies were observed. Specifically, hormone effects on
HMA3/
Nramp5 that were significant in the SEM emerged as H
2O
2-dependent only for ABA and t-Z (their effects vanished under H
2O
2 inhibition), whereas IAA’s positive association with
Nramp5 persisted under inhibition, and GA
3 showed no robust impact. By contrast, the negative influence of H
2O
2 on both transporters remained consistent with the SEM. Taken together, the intervention results narrow the SEM: they support a core pathway in which ABA and t-Z stimulate H
2O
2 accumulation that subsequently suppresses
HMA3 and
Nramp5, while revealing that IAA–
Nramp5 regulation can occur independently of H
2O
2. This pattern is mechanistically plausible because H
2O
2 is a central stress signal that integrates with multiple hormone pathways [
38], and ABA can upregulate catalase isoforms to buffer H
2O
2, whereas cytokinins can promote ROS production in specific tissues and contexts [
39].
Methodologically, these discrepancies are expected. PLS-SEM is a variance-based, prediction-oriented composite approach; it estimates conditional associations under a prespecified model but does not, by itself, establish causal effects or guarantee that an association will survive perturbation of an internal node (here, H
2O
2). Endogeneity, omitted variables, and unmodeled interactions (e.g., the strong Cd dose × inhibitor effect we observed) can yield significant paths that do not generalize under intervention [
40]. Moderation analyses—by directly testing slope changes across perturbed vs. unperturbed conditions—probe when a relation holds and therefore often constrain SEM-based hypotheses [
41]. Our findings thus support the use of SEM as a hypothesis-generating tool, with intervention-based tests serving to subsequently refine the model by identifying and removing non-robust pathways (retain ABA/t-Z ROS-dependent links), flag IAA–
Nramp5 as ROS-independent, and deem GA
3 effects negligible under the tested regime.
Biologically, the hormone-specific divergence we uncovered is also consistent with the literature: (i) H
2O
2 sits at the hub of metal stress signaling (MAPKs, redox-sensitive TFs), so blocking it should attenuate hormone effects that route through ROS [
38]; (ii) ABA can elevate catalase expression/activity and thereby prevent excessive H
2O
2 accumulation, aligning with its H
2O
2-dependent footprint here [
39]; (iii) cytokinin (t-Z) can induce ROS (e.g., in guard cells) and thus reasonably shows H
2O
2-dependent transport regulation [
38]; and (iv) some auxin outputs can be routed via parallel (ROS-independent) modules, explaining the IAA–
Nramp5 persistence under inhibition.
While PLS–SEM provides valuable conditional insights by isolating direct effects under controlled model architecture, it remains inherently limited in capturing the full richness of hormone–hormone interactions, especially when feedback loops, dose sensitivity, and metabolic conversions are involved.
Our expression profiles reveal that IAA is not significantly associated with the expression of hormone-metabolizing enzymes (e.g., IAA synthases or hydrolases) under non-IAA treatments. However, IAA treatments themselves selectively induced IAA-hydrolase, suggesting a self-regulatory turnover mechanism to prevent overaccumulation. This aligns with the notion that auxin homeostasis is maintained by tight metabolic feedback loops rather than broad cross-hormonal control.
By contrast, t-Z (a cytokinin) consistently suppressed ABA biosynthetic enzymes, reflecting potential antagonism, which is consistent with patterns observed by O’Brien et al. [
42], where cytokinin–ABA crosstalk modulates development and stress adaptation in a context-dependent manner. Meanwhile, ABA appears to suppress GA
3 biosynthesis, and vice versa, showcasing their classical antagonism in balancing growth and stress responses. This relationship aligns with findings reviewed in Emamverdian et al., where the interplay between heavy metal stress-associated ABA and GA directs protective versus growth-promoting outcomes [
31]. Furthermore, GA
3 displayed dose-dependent metabolic control: low-dose GA
3 partly enhanced its biosynthesis, whereas high-dose treatment led to suppression—highlighting a concentration-sensitive regulation fitting recent reviews on GA
3 crosstalk dynamics under stress [
43].
Together, these patterns support a model of hormone crosstalk operating via metabolic modulation, including mutual antagonism and self-regulation, rather than through broad direct activation or suppression. This observation underscores that complex crosstalk is more clearly revealed at the level of enzyme expression, even in the absence of significant pairwise correlations. It also suggests that hormones self-regulate within a layered network, which aligns with their distinct roles in stress adaptation.
A striking finding of this study was the dramatic upregulation of
ZIP2, which far exceeded that of any gene within the core CAT–H
2O
2 pathway. Its exclusion from the PLS-SEM framework is therefore highly informative, strongly suggesting the existence of a parallel, ROS-independent regulatory network that is highly sensitive to hormonal cues. The discovery of this distinct pathway thus opens a novel avenue for future research. While correlation analysis revealed notable associations between several phytohormones (e.g., IAA, ABA, GA
3, t-Z) and transporter genes, such as
ZIP family members (e.g.,
ZIP2,
ZIP6) and
NRAMPs (e.g.,
Nramp1), these hormone–transporter pairs did not emerge as significant in the PLS-SEM framework. This discrepancy suggests that their regulation is likely mediated by alternative molecular pathways rather than the CAT–H
2O
2 signaling module. For example,
ZIP transporters—responsible for the uptake and redistribution of essential and non-essential metals, such as Zn, Fe, Mn, and Cd—are often regulated by metal-responsive transcription factors and hormonal cues, independent of ROS signaling. Structural control of these genes is typically mediated by
bZIP-related transcriptional networks responsive to metal homeostasis rather than oxidative stress signals [
44]. Similarly,
NRAMP family proteins, although classically associated with root uptake, include members such as tobacco
NtNRAMP3, which is preferentially expressed in the leaf xylem and likely functions in local metal unloading to maintain homeostasis. This expression pattern underscores its potential regulation by developmental or metal-status signals rather than ROS fluctuations [
45]. Moreover, evolving evidence supports the involvement of hormone-responsive transcription factors (e.g.,
NAC,
WRKY,
MYB families) in directly influencing transporter gene expression. These TFs often respond to hormonal stimuli or metal stress through regulatory cascades distinct from ROS-mediated pathways [
46]. Lastly, hormonal effects on transporters may be context- or dose-specific, only evident under particular physiological conditions. Such nuanced regulatory trends may yield detectable correlations but may not be strong or consistent enough to emerge as direct pathways in the PLS-SEM model, which prioritizes robust and dominant interactions.
This study offers new insights into the hormone-ROS signaling network in S. alfredii; however, the precise molecular mechanisms require further investigation. Future research can be extended on both the mechanistic and application fronts. At the mechanistic level, the priority will be to identify the key molecular components of the H2O2 signaling pathway, such as its upstream sensor proteins and downstream protein kinase cascades, to construct a complete signal transduction chain. At the application level, our findings offer potential targets for enhancing phytoremediation efficiency. We propose that genetic engineering or chemical priming approaches could be used to strategically bolster the antioxidant system and signaling responsivity of S. alfredii. Such efforts could optimize its bioaccumulation capacity and environmental resilience, thus advancing its potential in the remediation of contaminated soils.