The Effects of Ambient Temperature and Atmospheric Humidity on the Diffusion Dynamics of Hydrogen Fluoride Gas Leakage Based on the Computational Fluid Dynamics Method
Abstract
1. Introduction
2. Methodology
2.1. Governing Equation of CFD Model
2.2. Modeling and Verification
2.2.1. Scaling Experiment
2.2.2. Model Validation
3. Simulation Application Background
3.1. Classification of Hazardous Areas
3.2. Actual Environmental Conditions and Boundary Conditions
4. Results and Discussion
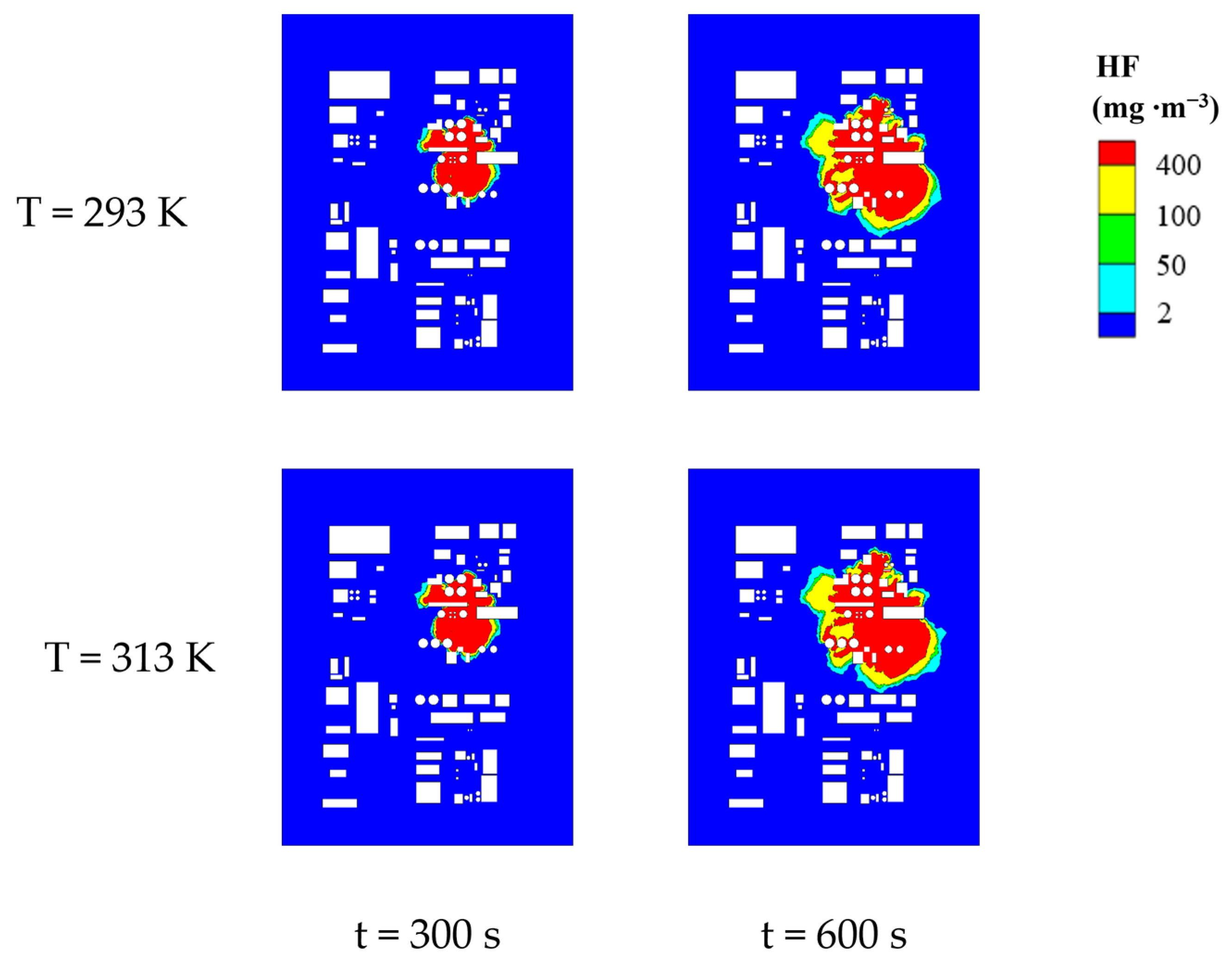
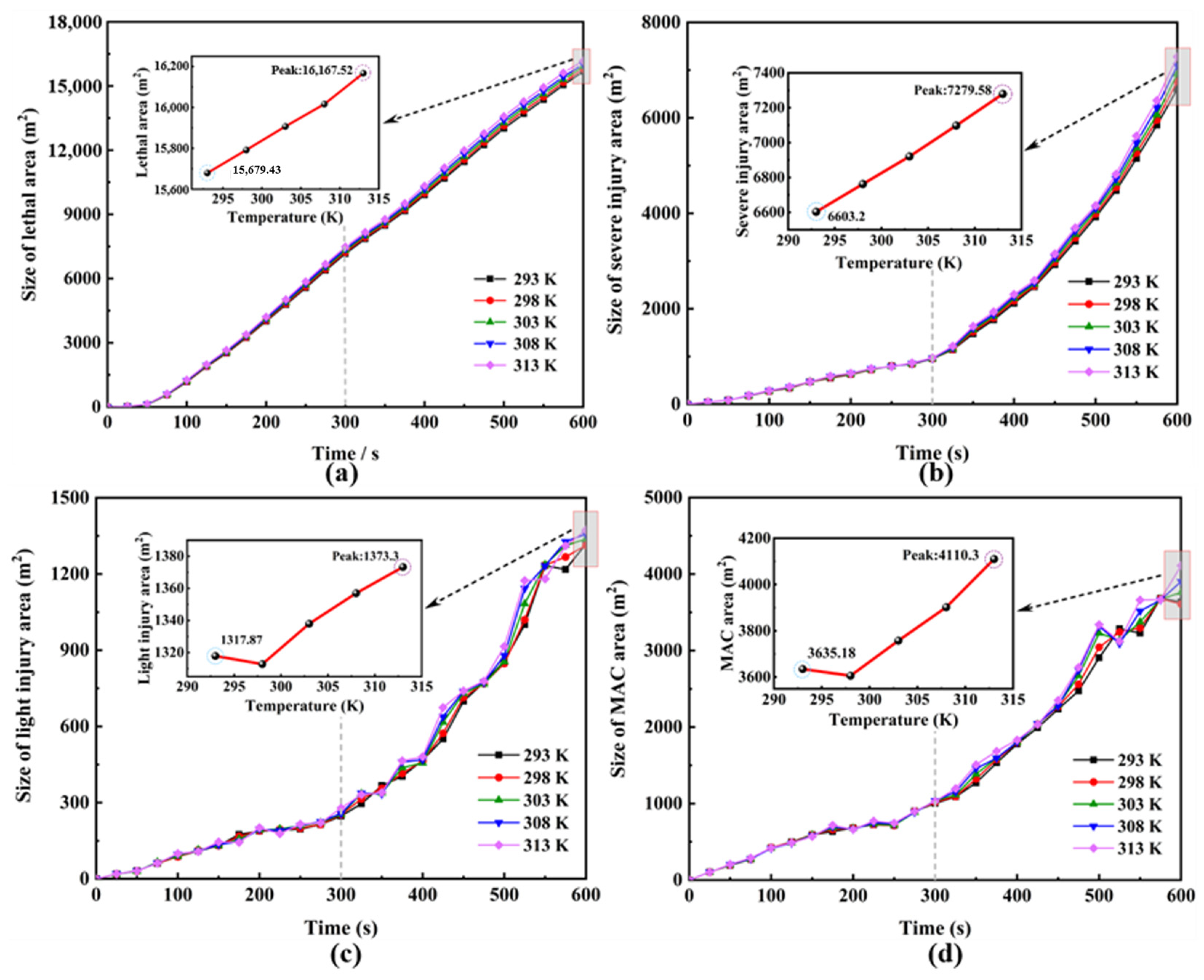
4.1. Patterns of Change in Hazardous Areas at Different Ambient Temperatures
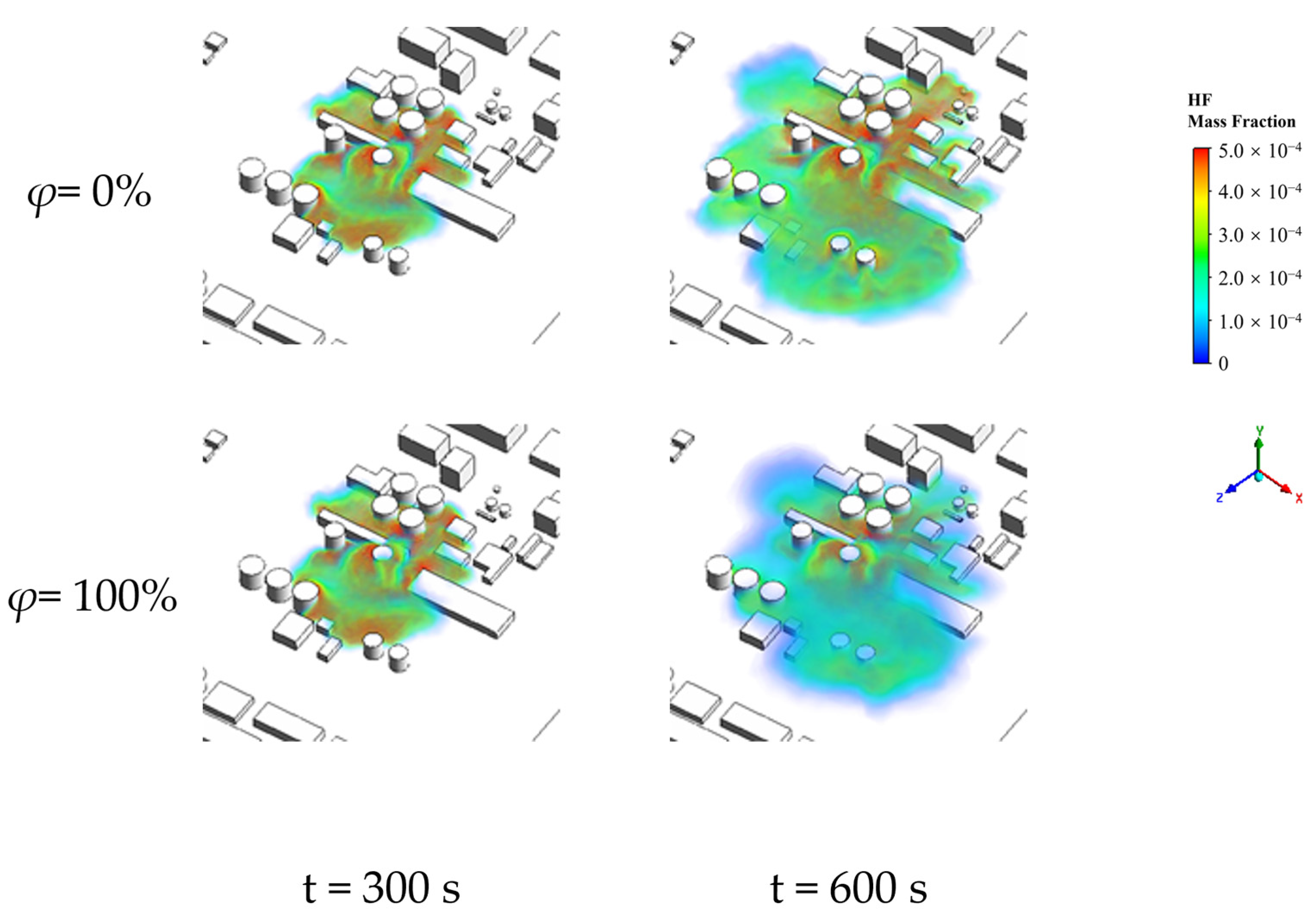
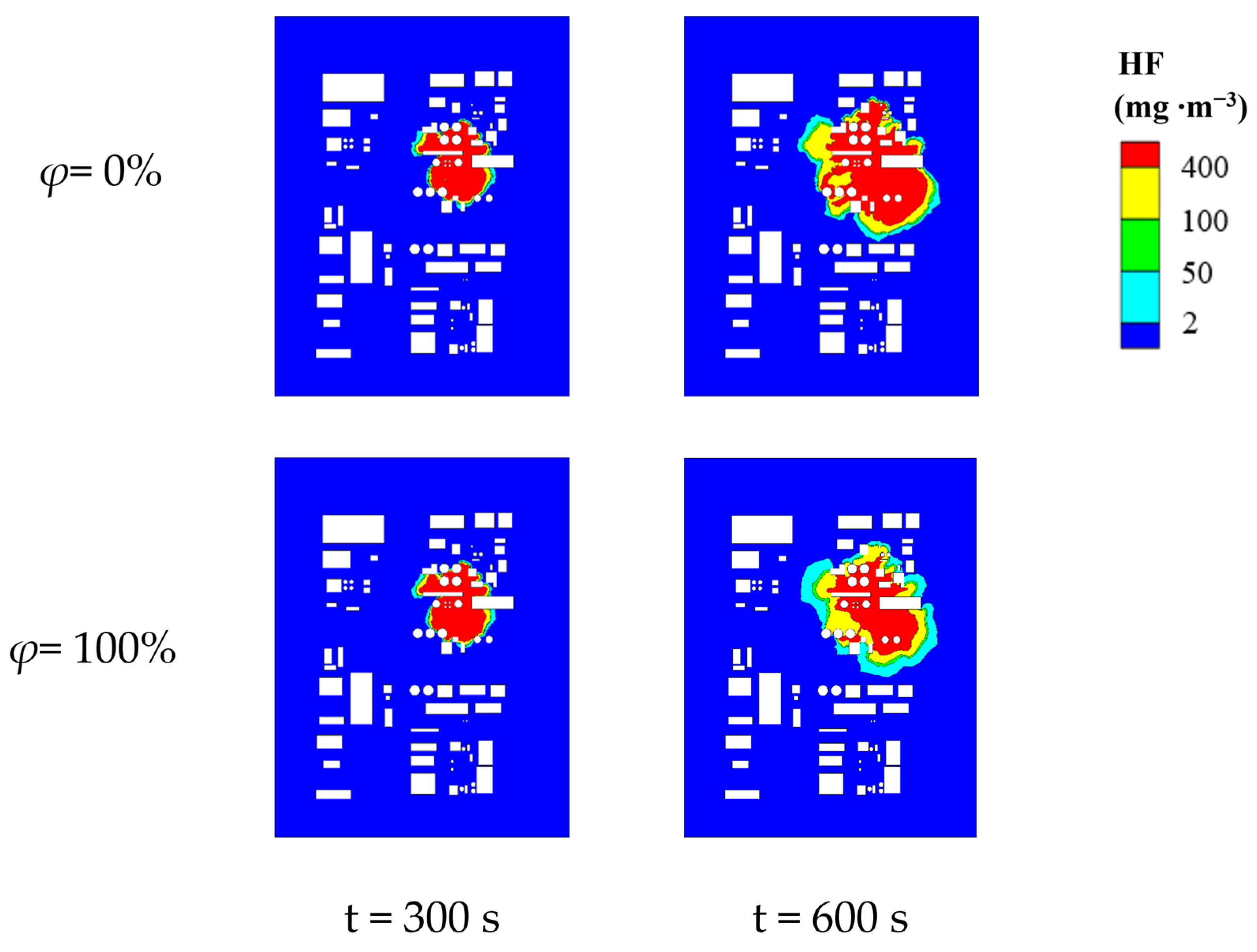
4.2. Patterns of Change in Hazardous Areas at Different Ambient Relative Humidities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jo, J.; Lee, J.; Ahn, Y.; Hwang, Y.S.; Park, J.; Lee, J.; Choi, J. Metabolome and transcriptome analyses of plants grown in naturally attenuated soil after hydrogen fluoride exposure. J. Hazard. Mater. 2022, 437, 129323. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Jeong, I.B. Chemical pneumonitis by prolonged hydrogen fluoride inhalation. Respir. Med. Case Rep. 2021, 32, 101338. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-D. Atmospheric chemistry of hydrogen fluoride. J. Atmos. Chem. 2018, 75, 1–16. [Google Scholar] [CrossRef]
- Hall, D.; Walker, S. Scaling rules for reduced-scale field releases of hydrogen fluoride. J. Hazard. Mater. 1997, 54, 89–111. [Google Scholar] [CrossRef]
- Krebs, R.; Owens, J.; Luckarift, H. Formation and detection of hydrogen fluoride gas during fire fighting scenarios. Fire Saf. J. 2022, 127, 103489. [Google Scholar] [CrossRef]
- Shang, T.; Wu, D.; Qi, Z. Research on Safety Status and Safety Sealing Transformation of Anhydrous Hydrogen Fluoride Storage Tank Area and Loading and Unloading Area. Zhejiang Chem. Ind. 2022, 53, 37–40. [Google Scholar]
- Yang, S.; Jeon, K.; Kang, D.; Han, C. Accident analysis of the Gumi hydrogen fluoride gas leak using CFD and comparison with post-accidental environmental impacts. J. Loss Prev. Process Ind. 2017, 48, 207–215. [Google Scholar] [CrossRef]
- Ermak, D.; Chan, S.; Morgan, D.; Morris, L. A comparison of dense gas dispersion model simulations with burro series LNG spill test results. J. Hazard. Mater. 1982, 6, 129–160. [Google Scholar] [CrossRef]
- Koopman, R.; Cederwall, R.; Ermak, D.; Goldwire, H.; Hogan, W.; McClure, J.; McRae, T.; Morgan, D.; Rodean, H.; Shinn, J. Analysis of Burro series 40-m3 LNG spill experiments. J. Hazard. Mater. 1982, 6, 43–83. [Google Scholar] [CrossRef]
- Rodean, H.C. Effects of a Spill of LNG on Mean Flow and Turbulence under Low Wind Speed, Slightly Stable Atmospheric Conditions. In Atmospheric Dispersion of Heavy Gases and Small Particles; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Koopman, R.P.; Baker, J.; Cederwall, R.T.; Goldwire, H.C., Jr.; Hogan, W.J.; Kamppinen, L.M.; Kiefer, R.D.; McClure, J.W.; McRae, T.G.; Morgan, D.L. LLNL/NWC 1980 LNG Spill Tests; Burro Series Data Report: The Appendices; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1982. [Google Scholar]
- Goldwire, H.C., Jr.; Rodean, H.C.; Cederwall, R.T.; Kansa, E.J.; Koopman, R.P.; McClure, J.W.; McRae, T.G.; Morris, L.K.; Kamppinen, L.; Kiefer, R.D. Coyote Series Data Report LLNL/NWC 1981 LNG Spill Tests Dispersion, Vapor Burn, and Rap-id-Phase-Transition; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1983; Volume 1. [Google Scholar]
- Meroney, R.N. Wind-tunnel experiments on dense gas dispersion. J. Hazard. Mater. 1982, 6, 85–106. [Google Scholar] [CrossRef]
- Heidorn, K.; Murphy, M.; Irwin, P.; Sahota, H.; Misra, P.; Bloxam, R. Effects of obstacles on the spread of a heavy gas—Wind tunnel simulations. J. Hazard. Mater. 1992, 30, 151–194. [Google Scholar] [CrossRef]
- Sweatman, W.L.; Chatwin, P.C. Dosages from instantaneous releases of dense gases in wind tunnels and into a neutrally stable atmosphere. Bound.-Layer Meteorol. 1996, 77, 211–231. [Google Scholar] [CrossRef]
- Robins, A.; Castro, I.; Hayden, P.; Steggel, N.; Contini, D.; Heist, D. A wind tunnel study of dense gas dispersion in a neutral boundary layer over a rough surface. Atmos. Environ. 2001, 35, 2243–2252. [Google Scholar] [CrossRef]
- Tauseef, S.; Rashtchian, D.; Abbasi, S. CFD-based simulation of dense gas dispersion in presence of obstacles. J. Loss Prev. Process Ind. 2011, 24, 371–376. [Google Scholar] [CrossRef]
- Fiates, J.; Santos, R.R.C.; Neto, F.F.; Francesconi, A.Z.; Simoes, V.; Vianna, S.S. An alternative CFD tool for gas dispersion modelling of heavy gas. J. Loss Prev. Process Ind. 2016, 44, 583–593. [Google Scholar] [CrossRef]
- Yoshie, R.; Jiang, G.; Shirasawa, T.; Chung, J. CFD simulations of gas dispersion around high-rise building in non-isothermal boundary layer. J. Wind. Eng. Ind. Aerodyn. 2011, 99, 279–288. [Google Scholar] [CrossRef]
- Ohba, R.; Kouchi, A.; Hara, T.; Vieillard, V.; Nedelka, D. Validation of heavy and light gas dispersion models for the safety analysis of LNG tank. J. Loss Prev. Process Ind. 2004, 17, 325–337. [Google Scholar] [CrossRef]
- Hanna, S.R.; Hansen, O.R.; Ichard, M.; Strimaitis, D. CFD model simulation of dispersion from chlorine railcar releases in industrial and urban areas. Atmos. Environ. 2009, 43, 262–270. [Google Scholar] [CrossRef]
- Dadashzadeh, M.; Khan, F.; Abbassi, R.; Hawboldt, K. Combustion products toxicity risk assessment in an offshore installation. Process Saf. Environ. Prot. 2014, 92, 616–624. [Google Scholar] [CrossRef]
- Souza, A.O.; Luiz, A.M.; Neto, A.T.P.; Araujo, A.C.B.; Silva, H.B.; Silva, S.K.; Alves, J.J.N. A new correlation for hazardous area classification based on experiments and CFD predictions. Process Saf. Prog. 2019, 38, 21–26. [Google Scholar] [CrossRef]
- Liang, Q.; Miao, Y.; Zhang, G.; Liu, S. Simulating Microscale Urban Airflow and Pollutant Distributions Based on Computational Fluid Dynamics Model: A Review. Toxics 2023, 11, 927. [Google Scholar] [CrossRef] [PubMed]
- Galeev, A.; Starovoytova, E.; Ponikarov, S. Numerical simulation of the consequences of liquefied ammonia instantaneous release using FLUENT software. Process Saf. Environ. Prot. 2013, 91, 191–201. [Google Scholar] [CrossRef]
- Barros, P.L.; Luiz, A.M.; Nascimento, C.A.; Neto, A.T.; Alves, J.J. On the non-monotonic wind influence on flammable gas cloud from CFD simulations for hazardous area classification. J. Loss Prev. Process Ind. 2020, 68, 104278. [Google Scholar] [CrossRef]
- Shao, X.; Zhao, Z.; Liu, Z.; Yang, H.; Hu, C. Exposure risk and emergency evacuation modeling of toxic gas leakage in urban areas under the influence of multiple meteorological factors. Environ. Pollut. 2023, 333, 122044. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y. Numerical simulation of toxic gas diffusion in confined space. IOP Conf. Ser. Earth Environ. Sci. 2020, 450, 012096. [Google Scholar] [CrossRef]
- Pontiggia, M.; Landucci, G.; Busini, V.; Derudi, M.; Alba, M.; Scaioni, M.; Bonvicini, S.; Cozzani, V.; Rota, R. CFD model simulation of LPG dispersion in urban areas. Atmos. Environ. 2011, 45, 3913–3923. [Google Scholar] [CrossRef]
- Shih, T.H.; Liou, W.W.; Shabbir, A.; Yang, Z.; Zhu, J. A New k-(Eddy Viscosity Model for High Reynolds Number Turbulent Flows-Model Development and Validation. Comput. Fluids 1994, 24, 227–238. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Z.; Ma, Y.; Zhang, L.; Wang, T.; Wang, S.; Zhang, Y. Study on Hazardous Areas of Hydrogen Fluoride Diffusion Based on CFD Simulation. Processes 2021, 9, 1545. [Google Scholar] [CrossRef]
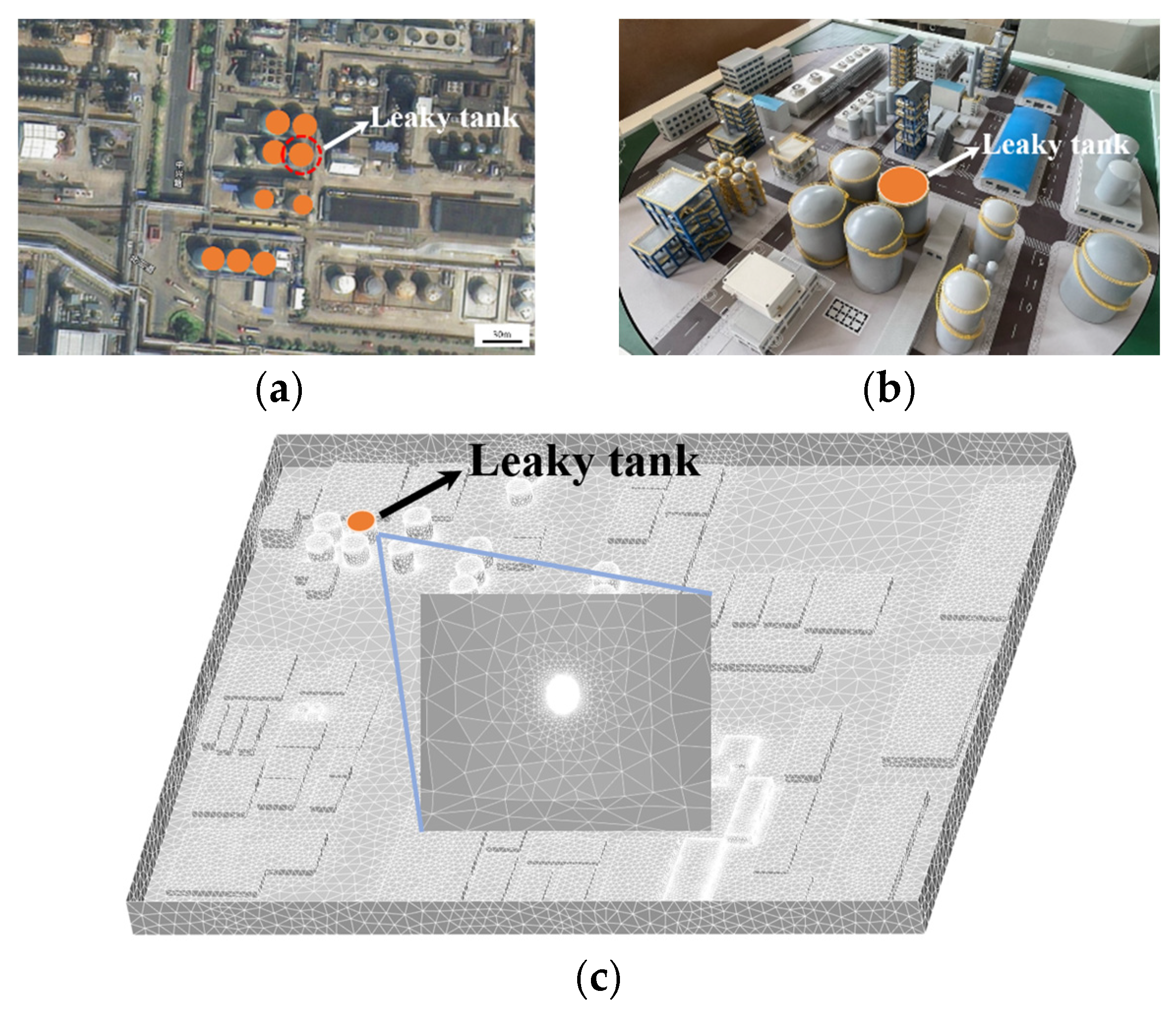
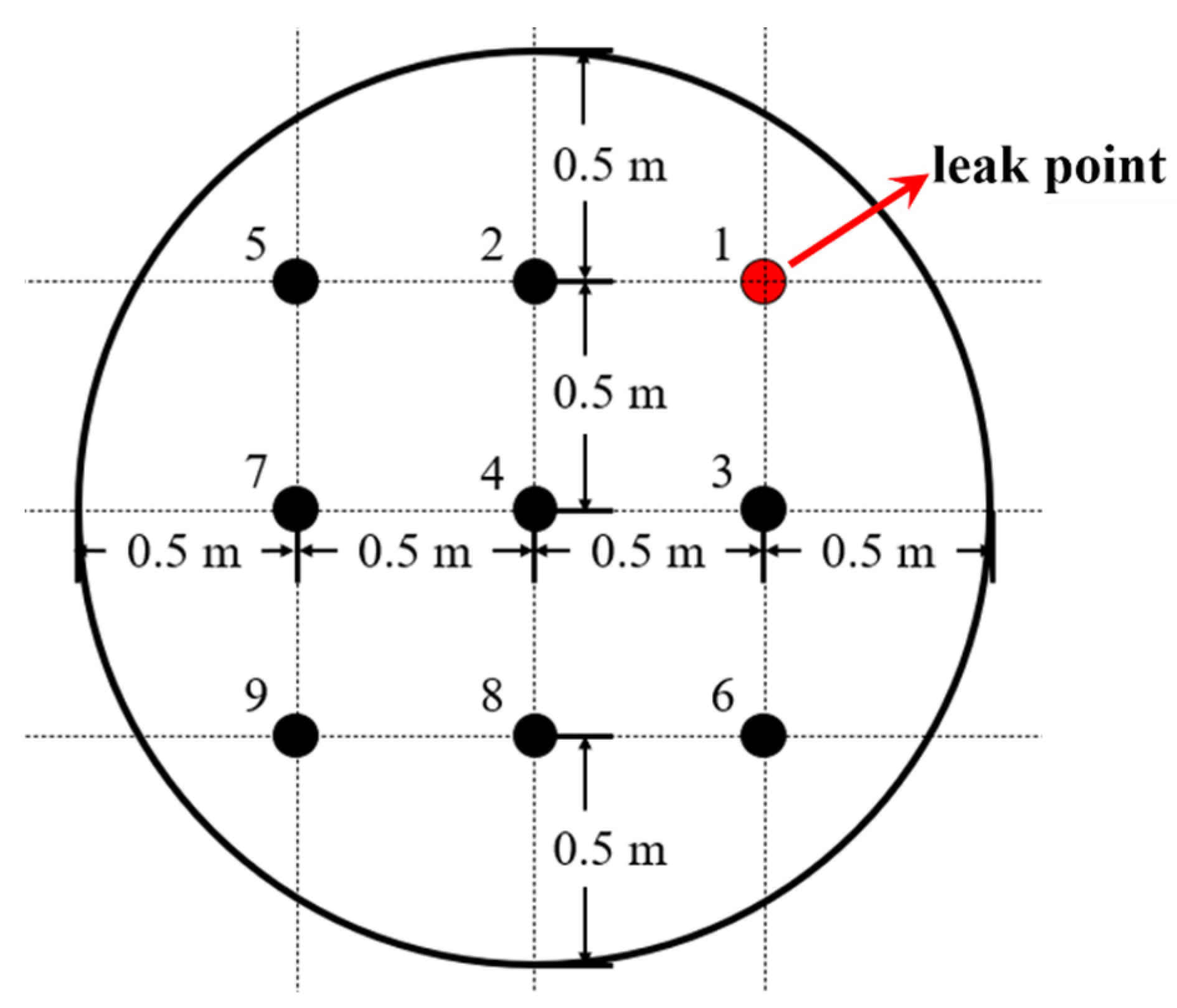
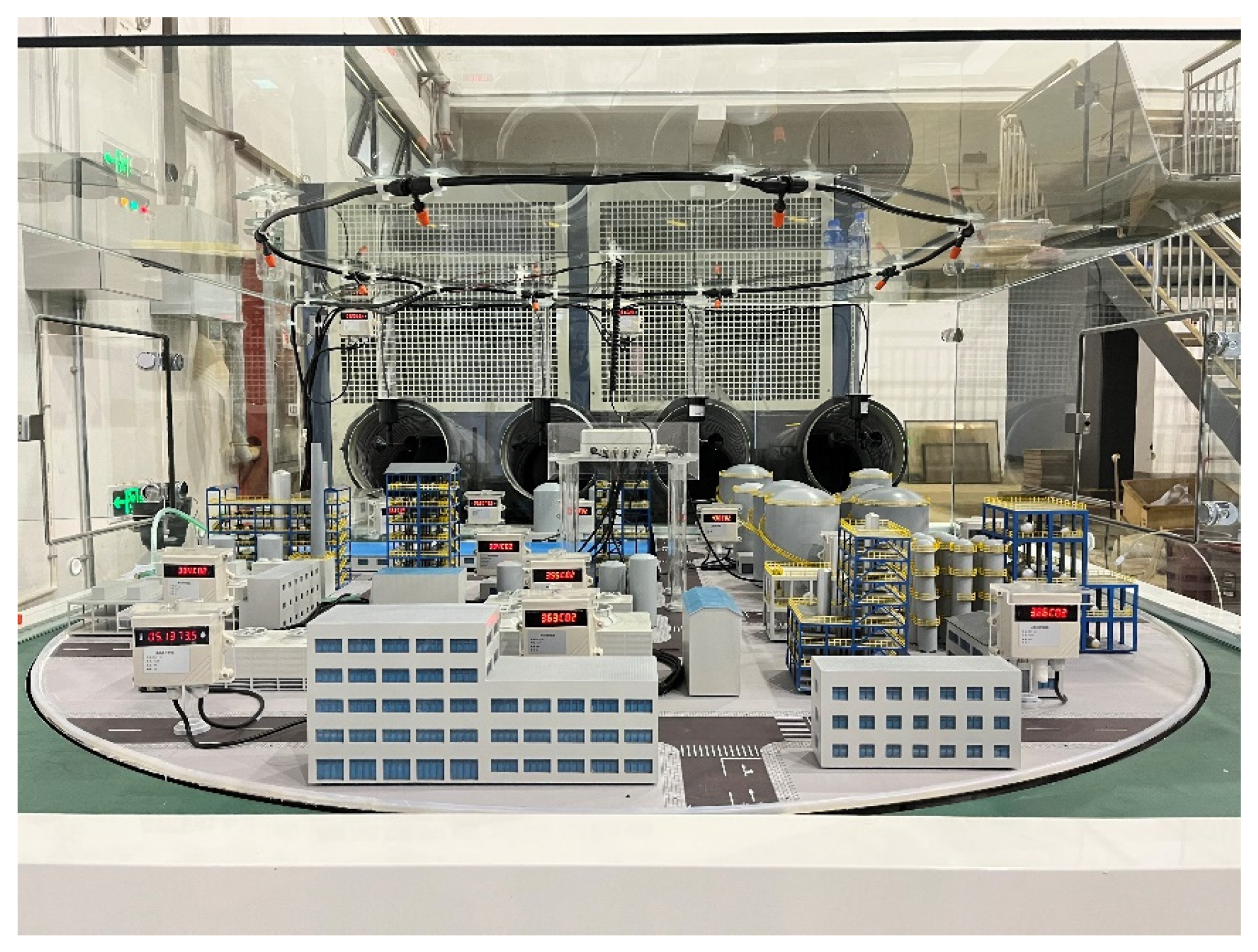
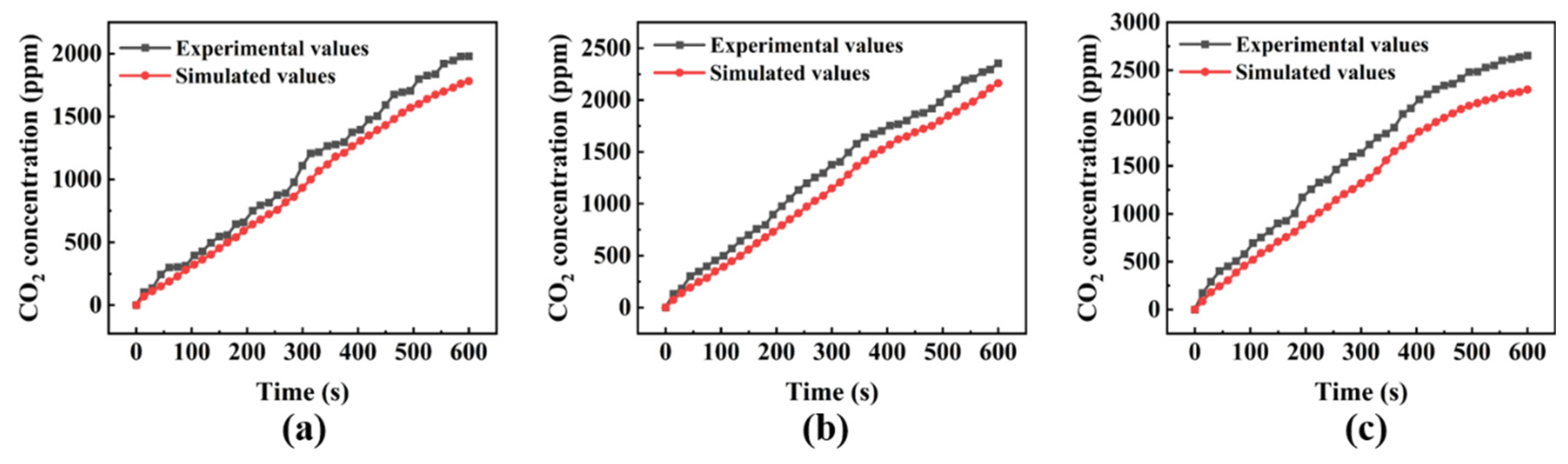


| Level | Wind Speed (m/s) | Model Wind Speed (m/s) |
|---|---|---|
| 0 | 0 ≤ Ur ≤ 0.2 | 0 ≤ Um ≤ 0.043 |
| 1 | 0.2 < Ur ≤ 1.5 | 0.043 < Um ≤ 0.32 |
| 2 | 1.5 < Ur ≤ 3.3 | 0.32 < Um ≤ 0.71 |
| 3 | 3.3 < Ur ≤ 5.4 | 0.71 < Um ≤ 1.2 |
| Name of Substance | HF | CO2 |
|---|---|---|
| Boiling/K | 292.66 | 216.15 |
| Melting point/K | 189.78 | 194.65 |
| Relative Vapor Density(ρair = 1, 299.65 K) | 1.7 | 1.5 |
| Solubility in water | soluble | soluble |
| Vapor pressure (298.15 K)/mmHg | 25 | 4.19 × 10−5 |
| Stickiness(1–100 kPa, 273.15 K)/mPa·S | 1.14 × 10−2 | 6.4 × 10−2 |
| Toxicity level | 4 | / |
| Tank Pressure (MPa) | Leakage Source Diameter (m) | HF Density (kg/m3) | Air Density (kg/m3) | Leakage Source Intensity (kg/s) |
|---|---|---|---|---|
| 3 | 0.02 | 2.2 | 1.29 | 0.18 |
| Environmental Temperature /K | Size of Lethal Area/m2 | Size of Severe Injury Area/m2 | Size of Light Injury Area/m2 | Size of MAC Area/m2 | Size of Total Hazardous Area/m2 |
|---|---|---|---|---|---|
| 293 | 15,679.43 | 6603.2 | 1317.87 | 3635.18 | 27,235.68 |
| 298 | 15,792.01 | 6762.57 | 1312.78 | 3606.17 | 27,473.53 |
| 303 | 15,907 | 6920.88 | 1337.95 | 3757.85 | 27,923.68 |
| 308 | 16,016.22 | 7098.02 | 1356.97 | 3902.23 | 28,373.44 |
| 313 | 16,167.52 | 7279.58 | 1373.3 | 4110.3 | 28,930.70 |
| Atmospheric Humidity/% | Size of Lethal Area/m2 | Size of Severe Injury Area/m2 | Size of Light Injury Area/m2 | Size of MAC Area/m2 | Size of Total Hazardous Area/m2 |
|---|---|---|---|---|---|
| 0 | 15,792.01 | 6762.57 | 1312.78 | 3606.17 | 27,473.53 |
| 20 | 15,590.19 | 6731.74 | 1376.47 | 3889.93 | 27,588.33 |
| 40 | 14,682.11 | 6409.93 | 1554.76 | 4467.84 | 27,114.64 |
| 60 | 13,288.35 | 6470.93 | 1505.1 | 5759.3 | 27,023.68 |
| 80 | 11,565.67 | 6392.83 | 1448.52 | 6510.89 | 25,917.91 |
| 100 | 10,154.04 | 6648.83 | 1670.52 | 7630.59 | 26,103.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Liu, Y.; Jiang, H.; Bai, Z.; Sun, L.; Liu, J.; Zhao, W. The Effects of Ambient Temperature and Atmospheric Humidity on the Diffusion Dynamics of Hydrogen Fluoride Gas Leakage Based on the Computational Fluid Dynamics Method. Toxics 2024, 12, 184. https://doi.org/10.3390/toxics12030184
Zhou Z, Liu Y, Jiang H, Bai Z, Sun L, Liu J, Zhao W. The Effects of Ambient Temperature and Atmospheric Humidity on the Diffusion Dynamics of Hydrogen Fluoride Gas Leakage Based on the Computational Fluid Dynamics Method. Toxics. 2024; 12(3):184. https://doi.org/10.3390/toxics12030184
Chicago/Turabian StyleZhou, Zhengqing, Yuzhe Liu, Huiling Jiang, Zhiming Bai, Lingxia Sun, Jia Liu, and Wenwen Zhao. 2024. "The Effects of Ambient Temperature and Atmospheric Humidity on the Diffusion Dynamics of Hydrogen Fluoride Gas Leakage Based on the Computational Fluid Dynamics Method" Toxics 12, no. 3: 184. https://doi.org/10.3390/toxics12030184
APA StyleZhou, Z., Liu, Y., Jiang, H., Bai, Z., Sun, L., Liu, J., & Zhao, W. (2024). The Effects of Ambient Temperature and Atmospheric Humidity on the Diffusion Dynamics of Hydrogen Fluoride Gas Leakage Based on the Computational Fluid Dynamics Method. Toxics, 12(3), 184. https://doi.org/10.3390/toxics12030184





