Acute Toxicity Assessment of Orally Administered Microplastic Particles in Adult Male Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Microplastic Particle Preparation and Physicochemical Characterization
2.1.1. Microplastic Particle Preparation
2.1.2. Microplastic Particle Physicochemical Characterization
2.2. Acute Oral Toxicity Study
2.2.1. Animals and Treatments
2.2.2. General Health Status
Victual Consumption Estimation
Neurological Testing
Analysis of Clinical Toxicity Signs
2.2.3. Biochemical Parameters in Serum
Common Health Biochemical Marker Assessment
Oxidative Capacity Estimation
2.3. Data Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.E. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. J. Toxicol. Risk Assess. 2019, 5, 21. [Google Scholar] [CrossRef]
- Kannan, K.; Vimalkumar, K. A Review of Human Exposure to Microplastics and Insights into Microplastics as Obesogens. Front. Endocrinol. 2021, 12, 724989. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue Accumulation of Microplastics in Mice and Biomarker Responses Suggest Widespread Health Risks of Exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wang, X.; Ren, T.; Wang, J.; Shan, J. Microplastics Contamination in Food and Beverages: Direct Exposure to Humans. J. Food Sci. 2021, 86, 2816–2837. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Senathirajah, K.; Attwood, S.; Bhagwat, G.; Carbery, M.; Wilson, S.; Palanisami, T. Estimation of the Mass of Microplastics Ingested—A Pivotal First Step towards Human Health Risk Assessment. J. Hazard. Mater. 2021, 404, 124004. [Google Scholar] [CrossRef]
- Weis, J.S.; Palmquist, K.H. Reality Check: Experimental Studies on Microplastics Lack Realism. Appl. Sci. 2021, 11, 8529. [Google Scholar] [CrossRef]
- Walczak, A.P.; Hendriksen, P.J.M.; Woutersen, R.A.; van der Zande, M.; Undas, A.K.; Helsdingen, R.; van den Berg, H.H.J.; Rietjens, I.M.C.M.; Bouwmeester, H. Bioavailability and Biodistribution of Differently Charged Polystyrene Nanoparticles upon Oral Exposure in Rats. J. Nanoparticle Res. 2015, 17, 231. [Google Scholar] [CrossRef]
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The Weight of Nations: An Estimation of Adult Human Biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Zaric, M.; Drakulic, D.; Dragic, M.; Gusevac Stojanovic, I.; Mitrovic, N.; Grkovic, I.; Martinovic, J. Molecular Alterations and Effects of Acute Dehydroepiandrosterone Treatment Following Brief Bilateral Common Carotid Artery Occlusion: Relevance to Transient Ischemic Attack. Neuroscience 2019, 410, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Dekanski, D.; Spremo-Potparević, B.; Bajić, V.; Živković, L.; Topalović, D.; Sredojević, D.N.; Lazić, V.; Nedeljković, J.M. Acute Toxicity Study in Mice of Orally Administrated TiO2 Nanoparticles Functionalized with Caffeic Acid. Food Chem. Toxicol. 2018, 115, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Miletić, J.; Drakulić, D.; Pejić, S.; Petković, M.; Ilić, T.V.; Miljković, M.; Stefanović, A.; Prostran, M.; Stojanov, M. Prooxidant–Antioxidant Balance, Advanced Oxidation Protein Products and Lipid Peroxidation in Serbian Patients with Parkinson’s Disease. Int. J. Neurosci. 2018, 128, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in House Dust from 12 Countries and Associated Human Exposure. Environ. Int. 2020, 134, 105314. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Bhattacharya, A.; Khare, S.K. Enzymatic Remediation of Polyethylene Terephthalate (PET)–Based Polymers for Effective Management of Plastic Wastes: An Overview. Front. Bioeng. Biotechnol. 2020, 8, 602325. [Google Scholar] [CrossRef]
- Sobhani, Z.; Lei, Y.; Tang, Y.; Wu, L.; Zhang, X.; Naidu, R.; Megharaj, M.; Fang, C. Microplastics Generated When Opening Plastic Packaging. Sci. Rep. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Kiran, B.R.; Kopperi, H.; Venkata Mohan, S. Micro/Nano-Plastics Occurrence, Identification, Risk Analysis and Mitigation: Challenges and Perspectives. Rev. Environ. Sci. Biotechnol. 2022, 21, 169–203. [Google Scholar] [CrossRef]
- Chércoles Asensio, R.; San Andrés Moya, M.; de la Roja, J.M.; Gómez, M. Analytical Characterization of Polymers Used in Conservation and Restoration by ATR-FTIR Spectroscopy. Anal. Bioanal. Chem. 2009, 395, 2081–2096. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Huang, Y.-Z.; Lin, J.-N.; Li, J. Microplastic Constituent Identification from Admixtures by Fourier-Transform Infrared (FTIR) Spectroscopy: The Use of Polyethylene Terephthalate (PET), Polyethylene (PE), Polypropylene (PP), Polyvinyl Chloride (PVC) and Nylon (NY) as the Model Constituents. Environ. Technol. Innov. 2021, 23, 101798. [Google Scholar] [CrossRef]
- Critchell, K.; Hoogenboom, M.O. Effects of Microplastic Exposure on the Body Condition and Behaviour of Planktivorous Reef Fish (Acanthochromis Polyacanthus). PLoS ONE 2018, 13, e0193308. [Google Scholar] [CrossRef]
- Rafiee, M.; Dargahi, L.; Eslami, A.; Beirami, E.; Jahangiri-rad, M.; Sabour, S.; Amereh, F. Neurobehavioral Assessment of Rats Exposed to Pristine Polystyrene Nanoplastics upon Oral Exposure. Chemosphere 2018, 193, 745–753. [Google Scholar] [CrossRef]
- Hussain, N. Recent Advances in the Understanding of Uptake of Microparticulates across the Gastrointestinal Lymphatics. Adv. Drug Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef]
- Kashiwada, S. Distribution of Nanoparticles in the See-through Medaka (Oryzias Latipes). Environ. Health Perspect. 2006, 114, 1697–1702. [Google Scholar] [CrossRef]
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health Impacts of Environmental Contamination of Micro- and Nanoplastics: A Review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of Nanomaterial Physicochemical Properties on in Vivo Toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef]
- Mirmiran, P.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Elevated Serum Levels of Aminotransferases in Relation to Unhealthy Foods Intake: Tehran Lipid and Glucose Study. BMC Endocr. Disord. 2019, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Berk, P.; Korenblat, K. Approach to the Patient with Jaundice or Abnormal Liver Tests. In Goldman’s Cecil Medicine; Elsevier: Amsterdam, The Netherlands, 2012; pp. 956–966. [Google Scholar] [CrossRef]
- Giannini, E.G. Liver Enzyme Alteration: A Guide for Clinicians. Can. Med. Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.K. Abnormal Liver Function Tests Associated with Severe Rhabdomyolysis. World J. Gastroenterol. 2020, 26, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Ulvik, A.; Rios-Avila, L.; Midttun, Ø.; Gregory, J.F. Direct and Functional Biomarkers of Vitamin B6 Status. Annu. Rev. Nutr. 2015, 35, 33–70. [Google Scholar] [CrossRef]
- Diehl, A.M.; Potter, J.; Boitnott, J.; Van Duyn, M.A.; Herlong, H.F.; Mezey, E. Relationship between Pyridoxal 5′-Phosphate Deficiency and Aminotransferase Levels in Alcoholic Hepatitis. Gastroenterology 1984, 86, 632–636. [Google Scholar] [CrossRef]
- Ray, L.; Sarangi, R.; Chatterjee, A.; Ganguly, S.; Nanda, S. A Comparative Study of Serum Aminotransferases in Chronic Kidney Disease with and without End-Stage Renal Disease: Need for New Reference Ranges. Int. J. Appl. Basic Med. Res. 2015, 5, 31. [Google Scholar] [CrossRef]
- Sette, L.; Lopes, E. The Reduction of Serum Aminotransferase Levels Is Proportional to the Decline of the Glomerular Filtration Rate in Patients with Chronic Kidney Disease. Clinics 2015, 70, 346–349. [Google Scholar] [CrossRef]
- Olaniyan, J.M.; Muhammad, H.L.; Makun, H.A.; Busari, M.B.; Abdullah, A.S. Acute and Sub-Acute Toxicity Studies of Aqueous and Methanol Extracts of Nelsonia Campestris in Rats. J. Acute Dis. 2016, 5, 62–70. [Google Scholar] [CrossRef]
- Banaee, M.; Gholamhosseini, A.; Sureda, A.; Soltanian, S.; Fereidouni, M.S.; Ibrahim, A.T.A. Effects of microplastic exposure on the blood biochemical parameters in the pond turtle (Emys orbicularis). Environ. Sci. Pollut. Res. 2021, 28, 9221–9234. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.-D.H. Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. blood biomarkers. Chemosphere 2019, 228, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Oyakhire, F.; Emokpae, M.A.; Ogie, E.; Valentine, E.E. Effect of Diabetes Mellitus on the Excretory Function of the Liver. Med. Lab. Technol. J. 2021, 7, 155. [Google Scholar] [CrossRef]
- Anadón, A.; Castellano, V.; Rosa Martínez-Larrañaga, M. Biomarkers of Drug Toxicity. In Biomarkers in Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 593–607. [Google Scholar] [CrossRef]
- Limdi, J.K. Evaluation of Abnormal Liver Function Tests. Postgrad. Med. J. 2003, 79, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lv, W.; Jiao, Y.; Liu, Z.; Li, Y.; Cai, M.; Wu, D.; Zhou, W.; Zhao, Y. Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile red claw crayfish, Cherax quadricarinatus. Aquat. Toxicol. 2020, 224, 105497. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, V.; Salazar, J.; Calvo, M.; Martínez, M.; Añez, R.; Rivas-Ríos, J.; Chacín, M.; Hernández, J.; Graterol, M.; Rojas, J. Importance of High Triglycerides Levels between Novel Coronary Risk Factors. Rev. Colomb. Cardiol. 2017, 24, 583–591. [Google Scholar] [CrossRef]
- Pranata, R.; Huang, I.; Lim, M.A.; Yonas, E.; Vania, R.; Lukito, A.A.; Nasution, S.A.; Siswanto, B.B.; Kuswardhani, R.A.T. Elevated De Ritis Ratio Is Associated with Poor Prognosis in COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 676581. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, Y.; Long, J.; Yang, X.; Bao, L.; Yang, Z.; Wu, B.; Si, R.; Zhao, W.; Peng, C.; et al. Polystyrene Microplastic Exposure Induces Insulin Resistance in Mice via Dysbacteriosis and Pro-Inflammation. Sci. Total Environ. 2022, 838, 155937. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Liu, L.; Mo, H.; Wei, S.; Raftery, D. Quantitative Analysis of Urea in Human Urine and Serum by 1H Nuclear Magnetic Resonance. Analyst 2012, 137, 595–600. [Google Scholar] [CrossRef]
- Banaee, M.; Nemadoost Haghi, B.; Tahery, S.; Shahafve, S.; Vaziriyan, M. Effects of Sub-Lethal Toxicity of Paraquat on Blood Biochemical Parameters of Common Carp, Cyprinus Carpio (Linnaeus, 1758). Iran. J. Toxicol. 2016, 10, 1–5. [Google Scholar] [CrossRef]
- Babaei, A.A.; Rafiee, M.; Khodagholi, F.; Ahmadpour, E.; Amereh, F. Physiological Stress Response of the Wistar Albino Rats Orally Exposed to Polystyrene Nanoparticles. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Mohamed, F.; Endre, Z.; Jayamanne, S.; Pianta, T.; Peake, P.; Palangasinghe, C.; Chathuranga, U.; Jayasekera, K.; Wunnapuk, K.; Shihana, F.; et al. Mechanisms Underlying Early Rapid Increases in Creatinine in Paraquat Poisoning. PLoS ONE 2015, 10, e0122357. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]

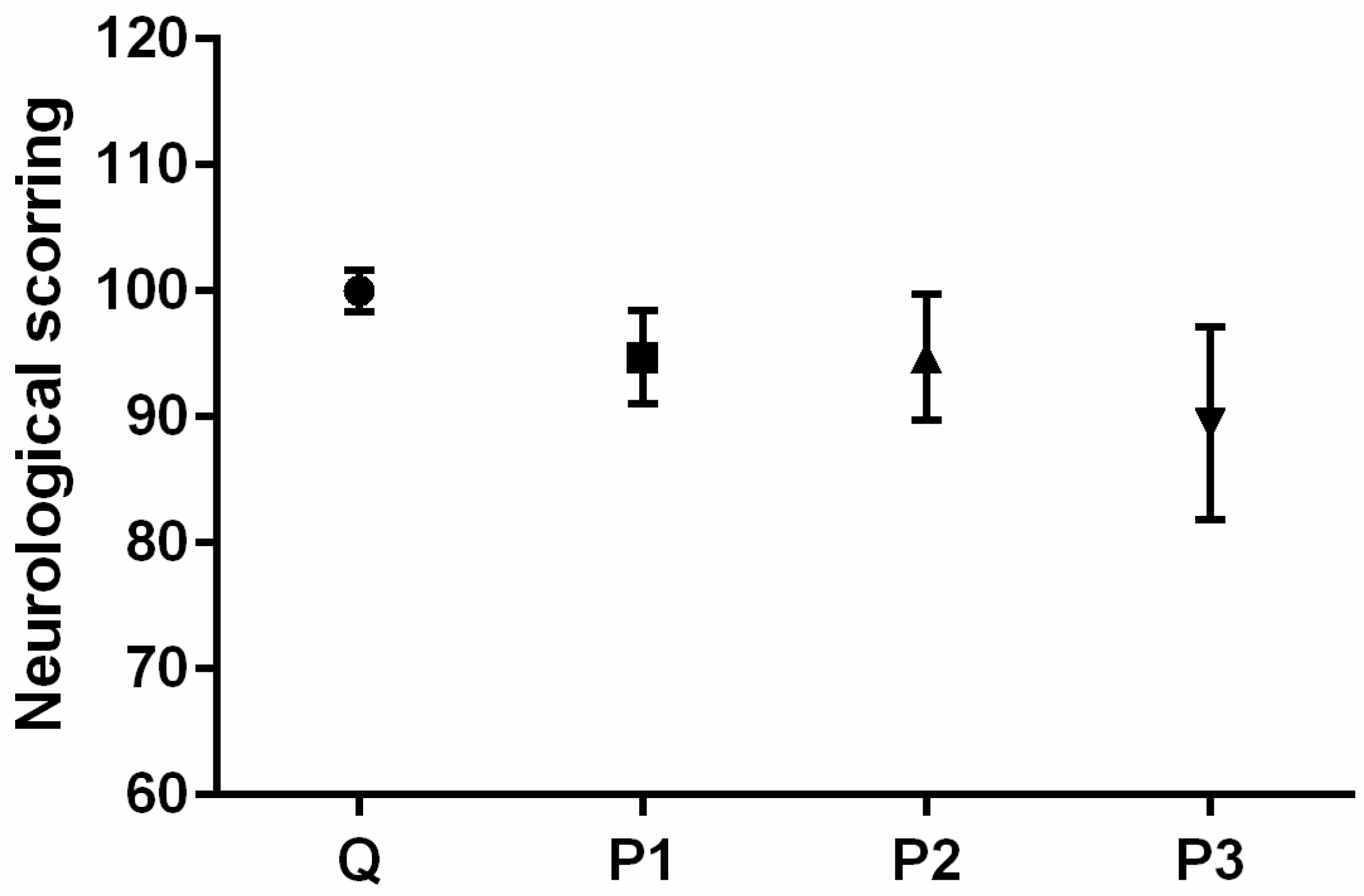
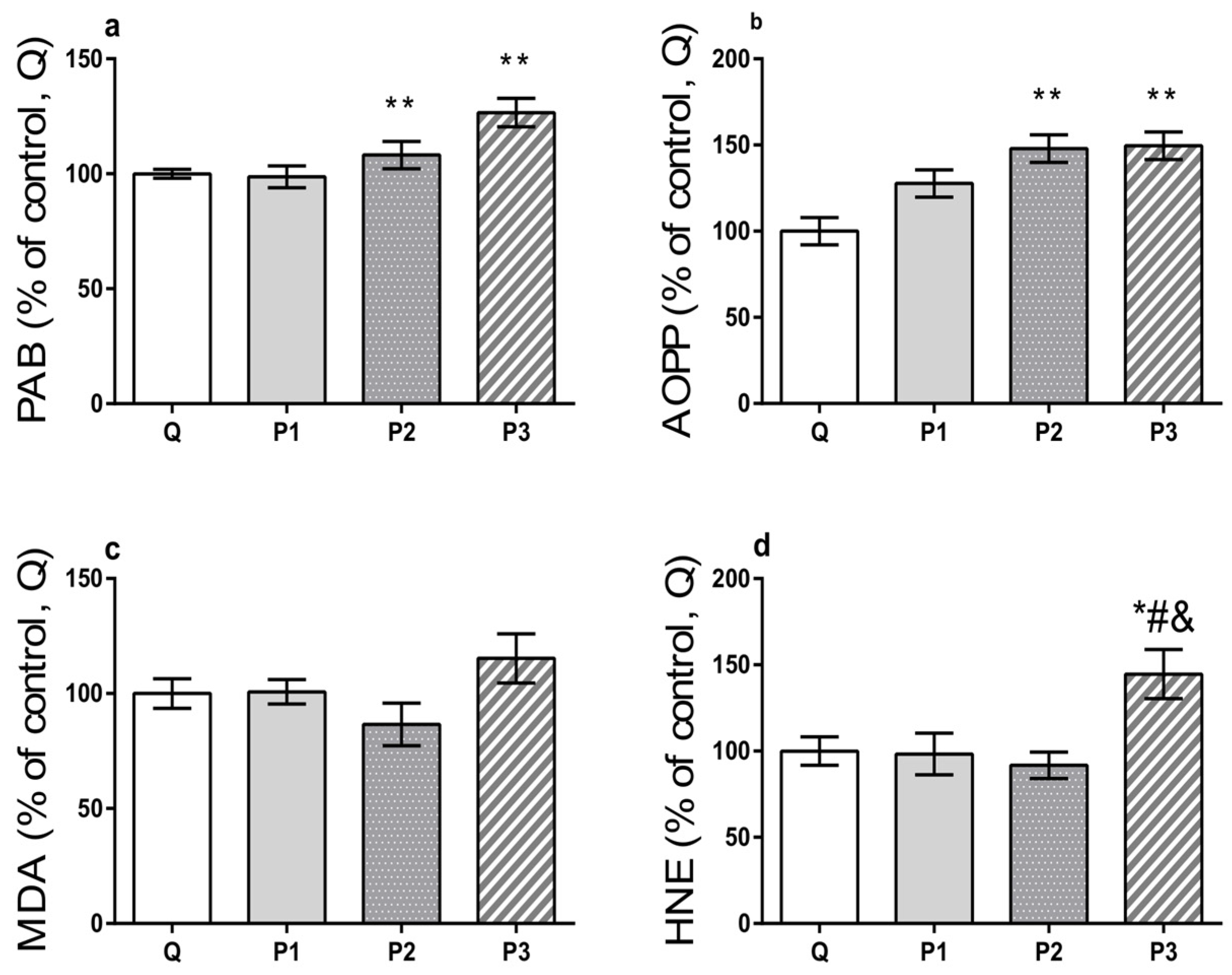
| Group | Q | P1 | P2 | P3 | |
|---|---|---|---|---|---|
| Parameter | |||||
| Food intake (g) | 25.20 ± 0.49 | 23.80 ± 0.73 | 20.40 ± 1.47 * | 20.60 ± 1.47 * | |
| Water intake (mL) | 51.80 ± 0.49 | 50.60 ± 0.98 | 45.80 ± 1.71 * | 46.00 ± 1.22 * | |
| Group | Q | P1 | P2 | P3 | |
|---|---|---|---|---|---|
| Parameter | |||||
| Agitation | - | - | - | - | |
| Convulsion | - | - | - | - | |
| Ataxia | - | - | - | - | |
| Touch response | - | - | - | - | |
| Piloerection | - | - | - | - | |
| Sleepiness | - | - | - | - | |
| Lethargy | - | - | - | - | |
| Respiratory distress | - | - | - | - | |
| Mortality | 0/5 | 0/5 | 0/5 | 0/5 | |
| Group | Q | P1 | P2 | P3 | |
|---|---|---|---|---|---|
| Parameter | |||||
| AST (U/L) | 118.00 ± 7.23 | 131.00 ± 10.25 | 163.20 ± 9.61 * | 167.80 ± 13.84 * | |
| ALT (U/L) | 46.00 ± 5.33 | 24.80 ± 4.68 * | 37.6 ± 2.44 | 40.20 ± 4.33 | |
| Bilirubin µmol/L) | 0.94 ± 0.07 | 1.04 ± 0.07 | 1.08 ± 0.13 | 1.06 ± 0.07 | |
| GGT (U/L) | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.22 ± 0.02 | 0.20 ± 0.05 | |
| ALP (U/L) | 114.40 ± 6.29 | 119.60 ± 6.81 | 123.80 ± 6.95 | 129.20 ± 7.04 | |
| Cholesterol (mmol/L) | 1.88 ± 0.16 | 2.08 ± 0.17 | 2.14 ± 0.13 | 2.14 ± 0.08 | |
| HDL (mmol/L) | 1.40 ± 0.11 | 1.42 ± 0.11 | 1.44 ± 0.15 | 1.32 ± 0.13 | |
| LDL (mmol/L) | 1.30 ± 0.07 | 1.28 ± 0.09 | 1.46 ± 0.09 | 1.48 ± 0.10 | |
| Triglycerides (mmol/L) | 1.92 ± 0.16 | 2.36 ± 0.16 | 2.56 ± 0.12 * | 2.58 ± 0.18 * | |
| Glucose (mmol/L) | 7.80 ± 0.50 | 7.94 ± 0.45 | 8.32 ± 0.53 | 8.16 ± 0.54 | |
| Urea (mmol/L) | 8.02 ± 0.57 | 8.66 ± 0.50 | 10.66 ± 0.83 * | 11.22 ± 0.63 * | |
| Creatinine (µmol/L) | 43.80 ± 4.50 | 47.20 ± 2.96 | 60.20 ± 4.37 * | 63.40 ± 3.01 *# | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guševac Stojanović, I.; Drakulić, D.; Todorović, A.; Martinović, J.; Filipović, N.; Stojanović, Z. Acute Toxicity Assessment of Orally Administered Microplastic Particles in Adult Male Wistar Rats. Toxics 2024, 12, 167. https://doi.org/10.3390/toxics12030167
Guševac Stojanović I, Drakulić D, Todorović A, Martinović J, Filipović N, Stojanović Z. Acute Toxicity Assessment of Orally Administered Microplastic Particles in Adult Male Wistar Rats. Toxics. 2024; 12(3):167. https://doi.org/10.3390/toxics12030167
Chicago/Turabian StyleGuševac Stojanović, Ivana, Dunja Drakulić, Ana Todorović, Jelena Martinović, Nenad Filipović, and Zoran Stojanović. 2024. "Acute Toxicity Assessment of Orally Administered Microplastic Particles in Adult Male Wistar Rats" Toxics 12, no. 3: 167. https://doi.org/10.3390/toxics12030167
APA StyleGuševac Stojanović, I., Drakulić, D., Todorović, A., Martinović, J., Filipović, N., & Stojanović, Z. (2024). Acute Toxicity Assessment of Orally Administered Microplastic Particles in Adult Male Wistar Rats. Toxics, 12(3), 167. https://doi.org/10.3390/toxics12030167











