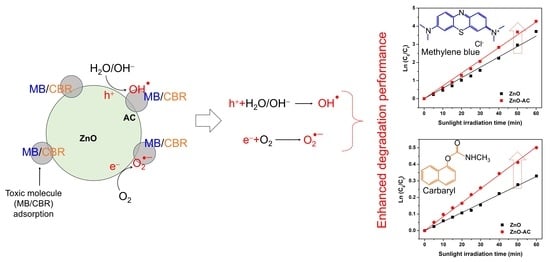
Sustainable Development of ZnO Nanostructure Doping with Water Hyacinth-Derived Activated Carbon for Visible-Light Photocatalysis
Abstract
1. Introduction
2. Materials and Methods
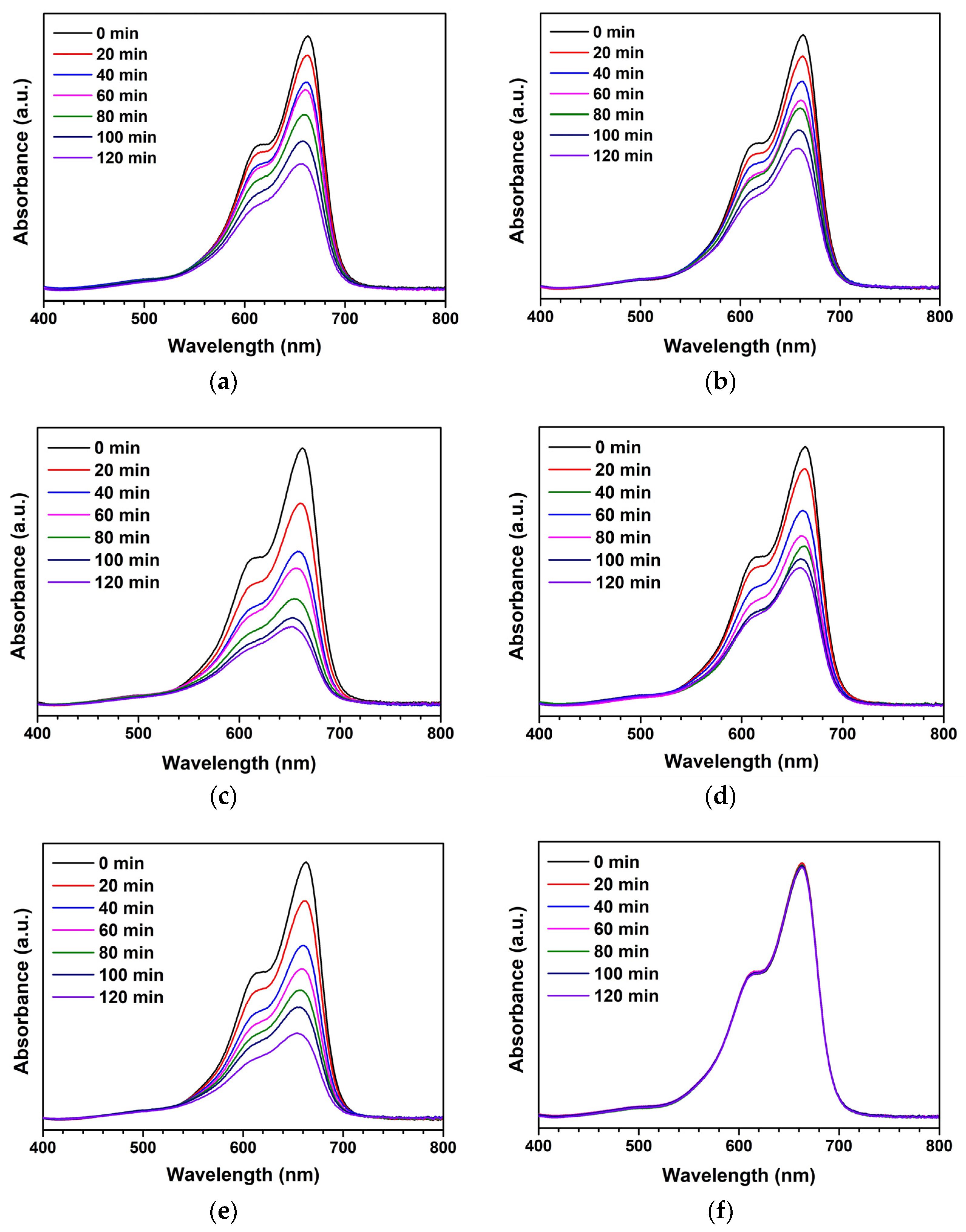
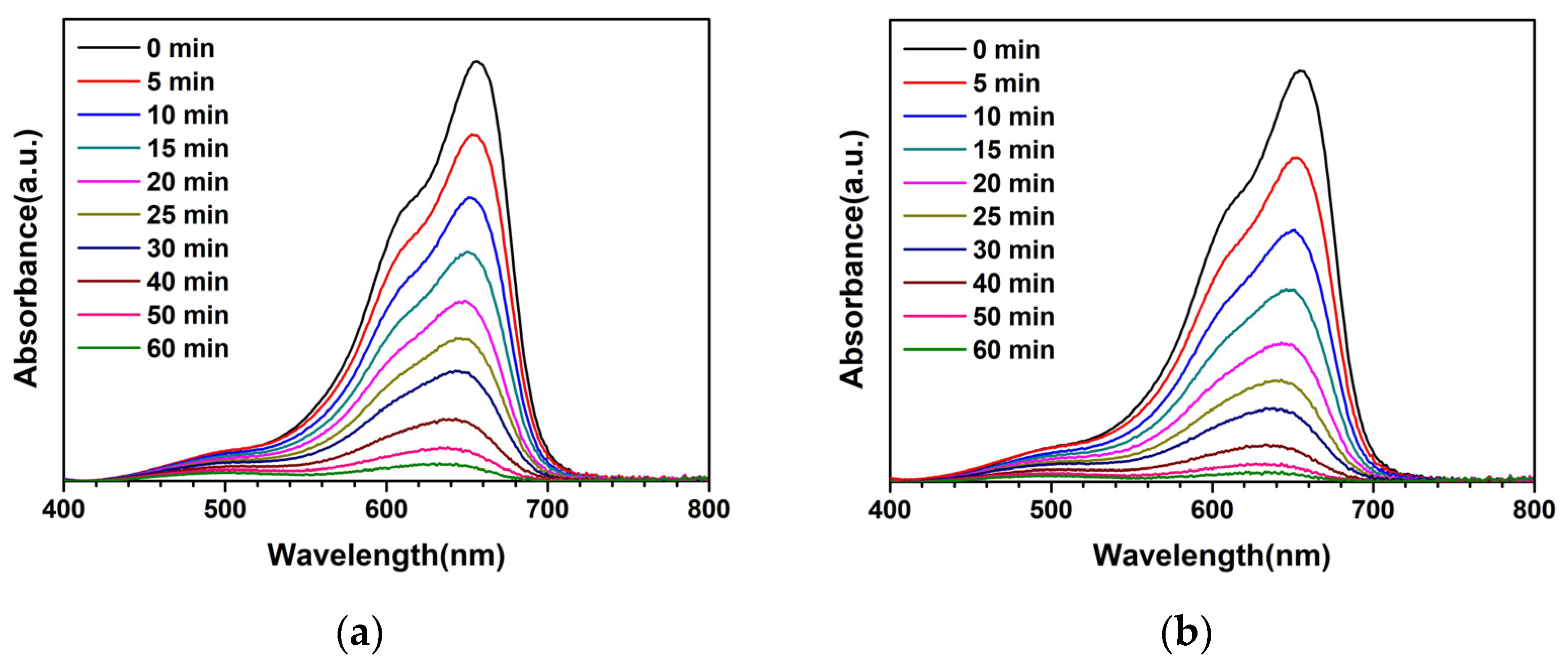
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.W.; Chen, R.A.; Chen, W.Y.; Nguyen, V.H.; Lin, K.Y.A.; Lasek, J. Manipulating and Revealing the Roles of La and Zr Dopants into ZnTiO3 Perovskite toward Heterogeneous Photocatalytic Degradation of Tetracycline Under Visible Light Irradiation. Top. Catal. 2023, 66, 34–40. [Google Scholar] [CrossRef]
- Khalid, H.; Haq, A.; Naqvi, S.A.R.; Usman, M.; Bokhari, T.H. Enhancement of photocatalytic activity of Ba-doped CoO for degradation of Emamectin benzoate in aqueous solution. Environ. Monit. Assess. 2023, 195, 1245. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.Z.; Raza, A.; Qumar, U.; Li, G. Recent advances in engineering strategies of Bi-based photocatalysts for environmental remediation. Sustain. Mater. Technol. 2022, 33, e00478. [Google Scholar] [CrossRef]
- Sultana, K.A.; Hernandez Ortega, J.; Islam, M.T.; Dorado, Z.N.; Alvarado-Tenorio, B.; Galindo-Esquivel, I.R.; Noveron, J.C. Saccharide-Derived Zinc Oxide Nanoparticles with High Photocatalytic Activity for Water Decontamination and Sanitation. Sustain. Chem. 2023, 4, 321–338. [Google Scholar] [CrossRef]
- Wongrerkdee, S.; Wongrerkdee, S.; Boonruang, C.; Sujinnapram, S. Enhanced Photocatalytic Degradation of Methylene Blue Using Ti-Doped ZnO Nanoparticles Synthesized by Rapid Combustion. Toxics 2023, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Krobthong, S.; Rungsawang, T.; Wongrerkdee, S. Comparison of ZnO Nanoparticles Prepared by Precipitation and Combustion for UV and Sunlight-Driven Photocatalytic Degradation of Methylene Blue. Toxics 2023, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Nagarajaiah, S.; Nanda, N.; Manjappa, P.; Nagabhushana, B.M.; Gadewar, M.; Rao, S.; Krishna, P.G. Evaluation of apoptosis in human breast cancer cell (MDA-MB-231) induced by ZnO nanoparticles synthesized using Piper betle leaf extract as bio-fuel. Appl. Phys. A 2023, 129, 461. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Selvaraj, V.; Chinnathambi, P.; Hussain, S.; Ali, D.; Kumar, G.; Balaji, P.; Sagadevan, S. Enhanced photocatalytic degradation of methylene blue from aqueous solution using green synthesized ZnO nanoparticles. Biomass Convers. Biorefin. 2023, 13, 17271–17282. [Google Scholar] [CrossRef]
- Yi, Y.; Guan, Q.; Wang, W.; Jian, S.; Li, H.; Wu, L.; Zhang, H.; Jiang, C. Recyclable Carbon Cloth-Supported ZnO@Ag3PO4 Core–Shell Structure for Photocatalytic Degradation of Organic Dye. Toxics 2023, 11, 70. [Google Scholar] [CrossRef]
- Berehe, B.A.; Assen, A.H.; Kumar, A.S.K.; Ulla, H.; Duma, A.D.; Chang, J.Y.; Gedda, G.; Girma, W.M. Highly efficient visible light active ZnO/Cu-DPA composite photocatalysts for the treatment of wastewater contaminated with organic dye. Sci. Rep. 2023, 13, 16454. [Google Scholar] [CrossRef]
- Hanif, M.A.; Akter, J.; Kim, Y.S.; Kim, H.G.; Hahn, J.R.; Kwac, L.K. Highly Efficient and Sustainable ZnO/CuO/g-C3N4 Photocatalyst for Wastewater Treatment under Visible Light through Heterojunction Development. Catalysts 2022, 12, 151. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Li, G. Recent Advances in Aerobic Photo-Oxidation over Small-Sized IB Metal Nanoparticles. Photochem 2022, 2, 528–538. [Google Scholar] [CrossRef]
- Mirikaram, N.; Pérez-Molina, Á.; Morales-Torres, S.; Salemi, A.; Maldonado-Hódar, F.J.; Pastrana-Martínez, L.M. Photocatalytic Perfomance of ZnO-Graphene Oxide Composites towards the Degradation of Vanillic Acid under Solar Radiation and Visible-LED. Nanomaterials 2021, 11, 1576. [Google Scholar] [CrossRef]
- Kaewtrakulchai, N.; Faungnawakij, K.; Eiad-Ua, A. Parametric Study on Microwave-Assisted Pyrolysis Combined KOH Activation of Oil Palm Male Flowers Derived Nanoporous Carbons. Materials 2020, 13, 2876. [Google Scholar] [CrossRef]
- Krobthong, S.; Wongrerkdee, S.; Pimpang, P.; Moungsrijun, S.; Sujinnapram, S.; Nilphai, S.; Rungsawang, T.; Wongrerkdee, S. ZnO Nanoparticles Coprecipitation with Aluminum and Copper Ions for Efficient Photocatalytic Degradation of Commercial Glyphosate. Integr. Ferroelectr. 2022, 222, 69–83. [Google Scholar] [CrossRef]
- Abou Zeid, S.; Perez, A.; Bastide, S.; Le Pivert, M.; Rossano, S.; Remita, H.; Hautière, N.; Leprince-Wang, Y. Antibacterial and Photocatalytic Properties of ZnO Nanostructure Decorated Coatings. Coatings 2024, 14, 41. [Google Scholar] [CrossRef]
- Irfan, M.; Hussain, H.; Saleem, B.; Saleem, M.; Shukrullah, S.; Legutko, S.; Petrů, J.; Naz, M.Y.; Pagáč, M.; Rahman, S.; et al. Evaluation of Ultrasonically ZnO Loading Effect on Photocatalytic Self-Cleaning, UV Protection and Antibacterial Activity of Plasma/Citric Acid-Activated Cotton Fabric. Nanomaterials 2022, 12, 2122. [Google Scholar] [CrossRef]
- Sujinnapram, S.; Wongrerkdee, S. Synergistic effects of structural, crystalline, and chemical defects on the photocatalytic performance of Y-doped ZnO for carbaryl degradation. J. Environ. Sci. 2023, 124, 667–677. [Google Scholar] [CrossRef]
- Wongrerkdee, S.; Krobthong, S. Synthesis, Characterization, and Photocatalytic Property of Ba-Doped ZnO Nanoparticles Synthesized Using Facile Precipitation. Integr. Ferroelectr. 2022, 224, 192–204. [Google Scholar] [CrossRef]
- Sheik Mydeen, S.; Raj Kumar, R.; Sambathkumar, S.; Kottaisamy, M.; Vasantha, V.S. Facile Synthesis of ZnO/AC Nanocomposites using Prosopis juliflora for Enhanced Photocatalytic Degradation of Methylene Blue and Antibacterial Activity. Optik 2020, 224, 165426. [Google Scholar] [CrossRef]
- Phophayu, S.; Pimpang, P.; Wongrerkdee, S.; Sujinnapram, S.; Wongrerkdee, S. Modified graphene quantum dots-zinc oxide nanocomposites for photocatalytic degradation of organic dyes and commercial herbicide. J. Reinf. Plast. Compos. 2020, 39, 81–94. [Google Scholar] [CrossRef]
- Negash, A.; Mohammed, S.; Weldekirstos, H.d.; Ambaye, A.D.; Gashu, M. Enhanced photocatalytic degradation of methylene blue dye using eco-friendly synthesized rGO@ZnO nanocomposites. Sci. Rep. 2023, 13, 22234. [Google Scholar] [CrossRef]
- Zeljković, S.; Balaban, M.; Gajić, D.; Vračević, S.; Ivas, T.; Vranković, D.; Jelić, D. Mechanochemically induced synthesis of N-ion doped ZnO: Solar photocatalytic degradation of methylene blue. Green Chem. Lett. Rev. 2022, 15, 869–880. [Google Scholar] [CrossRef]
- Karthik, K.V.; Raghu, A.V.; Reddy, K.R.; Ravishankar, R.; Sangeeta, M.; Shetti, N.P.; Reddy, C.V. Green synthesis of Cu-doped ZnO nanoparticles and its application for the photocatalytic degradation of hazardous organic pollutants. Chemosphere 2022, 287, 132081. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kumar, V.; Singh, J.; Datt, J.; Sharma, R. Effect of Cu-N co-doping on the dielectric properties of ZnO nanoparticles. Mater. Technol. 2022, 37, 2644–2658. [Google Scholar] [CrossRef]
- Ščajev, P.; Durena, R.; Onufrijevs, P.; Miasojedovas, S.; Malinauskas, T.; Stanionyte, S.; Zarkov, A.; Zukuls, A.; Bite, I.; Smits, K. Morphological and optical property study of Li doped ZnO produced by microwave-assisted solvothermal synthesis. Mater. Sci. Semicond. Process. 2021, 135, 106069. [Google Scholar] [CrossRef]
- Russo, V.; Ghidelli, M.; Gondoni, P.; Casari, C.S.; Bassi, A.L. Multi-wavelength Raman scattering of nanostructured Al-doped zinc oxide. J. Appl. Phys. 2014, 115, 073508. [Google Scholar] [CrossRef]
- Batterjee, M.G.; Nabi, A.; Kamli, M.R.; Alzahrani, K.A.; Danish, E.Y.; Malik, M.A. Green Hydrothermal Synthesis of Zinc Oxide Nanoparticles for UV-Light-Induced Photocatalytic Degradation of Ciprofloxacin Antibiotic in an Aqueous Environment. Catalysts 2022, 12, 1347. [Google Scholar] [CrossRef]
- Gaitán-Alvarez, J.; Berrocal, A.; Mantanis, G.I.; Moya, R.; Araya, F. Acetylation of tropical hardwood species from forest plantations in Costa Rica: An FTIR spectroscopic analysis. J. Wood Sci. 2020, 66, 49. [Google Scholar] [CrossRef]
- Algarni, T.S.; Al-Mohaimeed, A.M.; Al-Odayni, A.-B.; Abduh, N.A.Y. Activated Carbon/ZnFe2O4 Nanocomposite Adsorbent for Efficient Removal of Crystal Violet Cationic Dye from Aqueous Solutions. Nanomaterials 2022, 12, 3224. [Google Scholar] [CrossRef]
- Tien, T.-M.; Chen, E.L. A Novel ZnO/Co3O4 Nanoparticle for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2023, 13, 852. [Google Scholar] [CrossRef]
- Lemraski, E.G.; Sharafinia, S. Kinetics, equilibrium and thermodynamics studies of Pb2+ adsorption onto new activated carbon prepared from Persian mesquite grain. J. Mol. Liq. 2016, 219, 482–492. [Google Scholar] [CrossRef]
- Soliman, A.I.A.; Abdel-Wahab, A.M.A.; Abdelhamid, H.N. Hierarchical porous zeolitic imidazolate frameworks (ZIF-8) and ZnO@N-doped carbon for selective adsorption and photocatalytic degradation of organic pollutants. RSC Adv. 2022, 12, 7075–7084. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, Q.; Li, J.; Li, G. Hollow sphere manganese–ceria solid solution enhances photocatalytic activity in tetracycline degradation. New J. Chem. 2023, 47, 21264–21269. [Google Scholar] [CrossRef]
- Li, Z.; Xu, L.; Babar, Z.U.D.; Raza, A.; Zhang, Y.; Gu, X.; Miao, Y.X.; Zhao, Z.; Li, G. Fabrication of MXene-Bi2WO6 heterojunction by Bi2Ti2O7 hinge for extraordinary LED-light-driven photocatalytic performance. Nano Res. 2023, in press. [CrossRef]
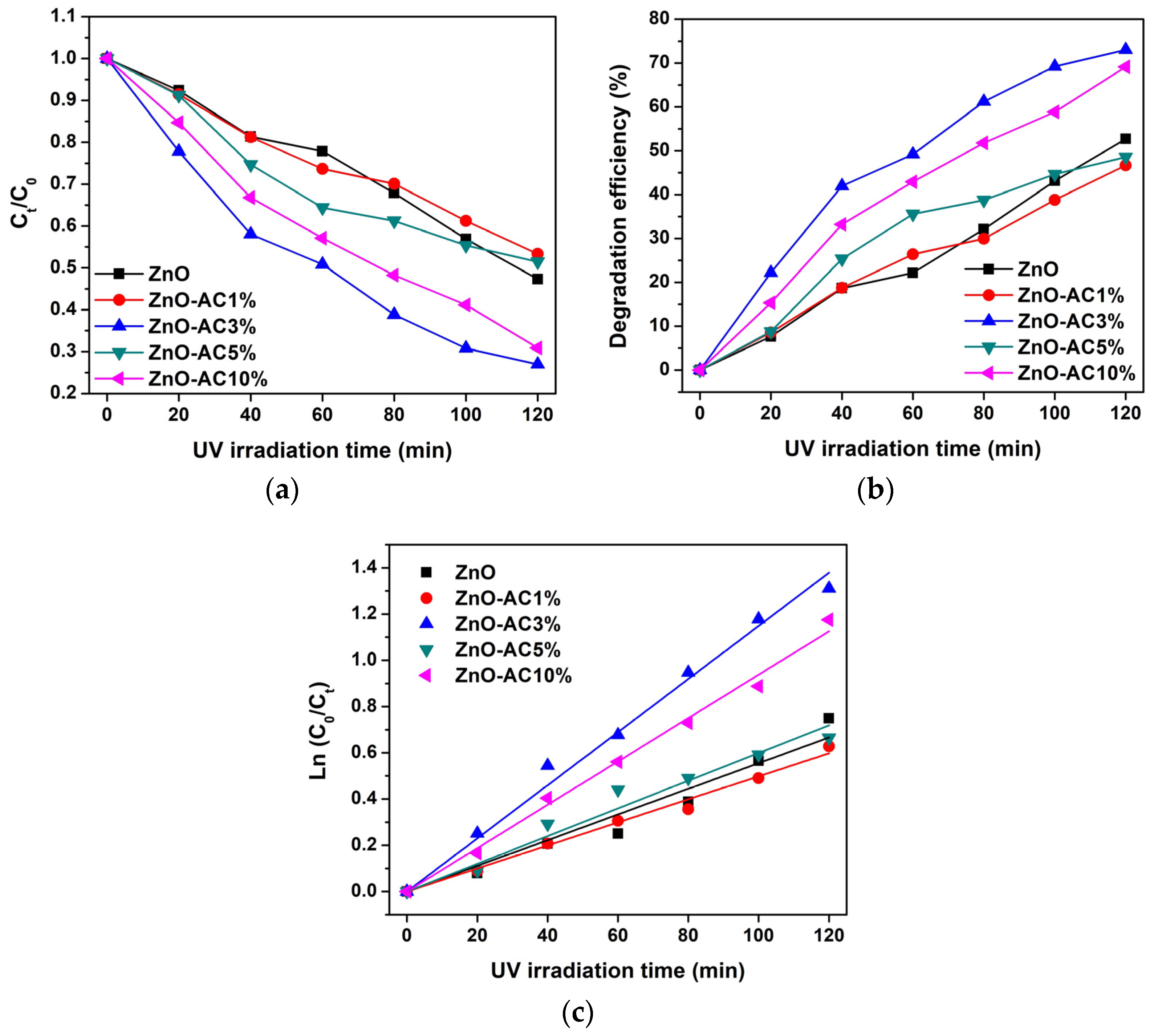
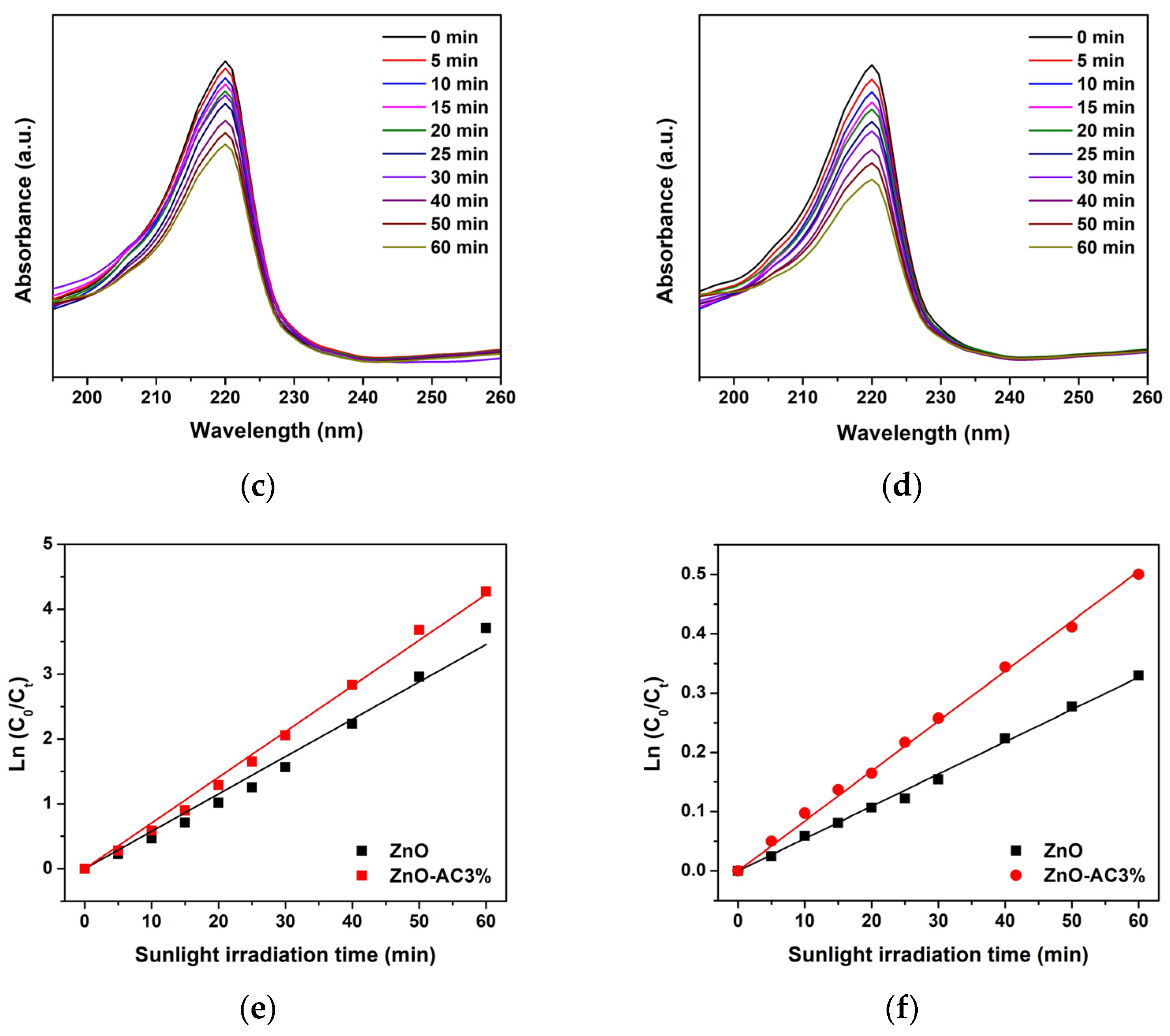

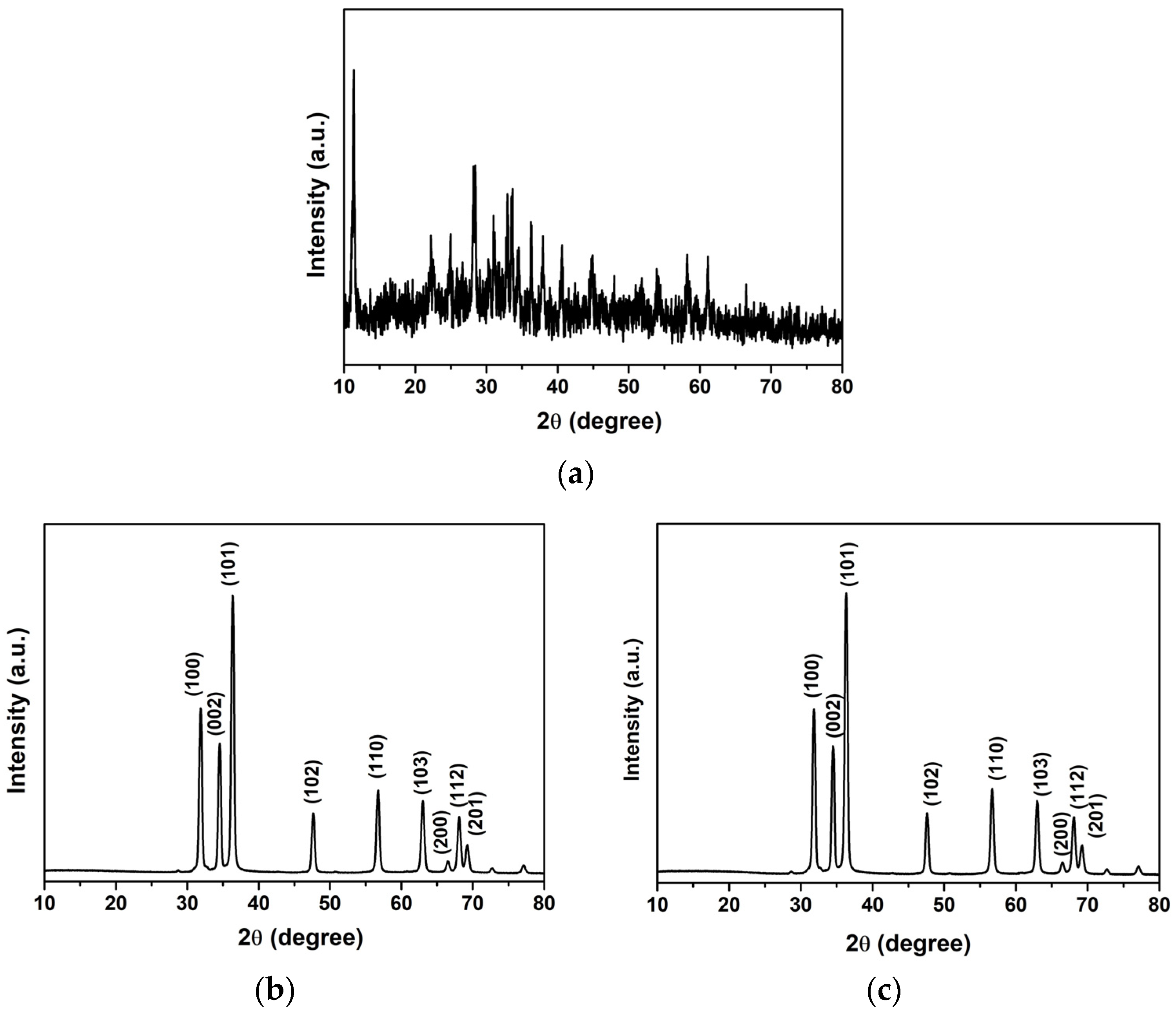
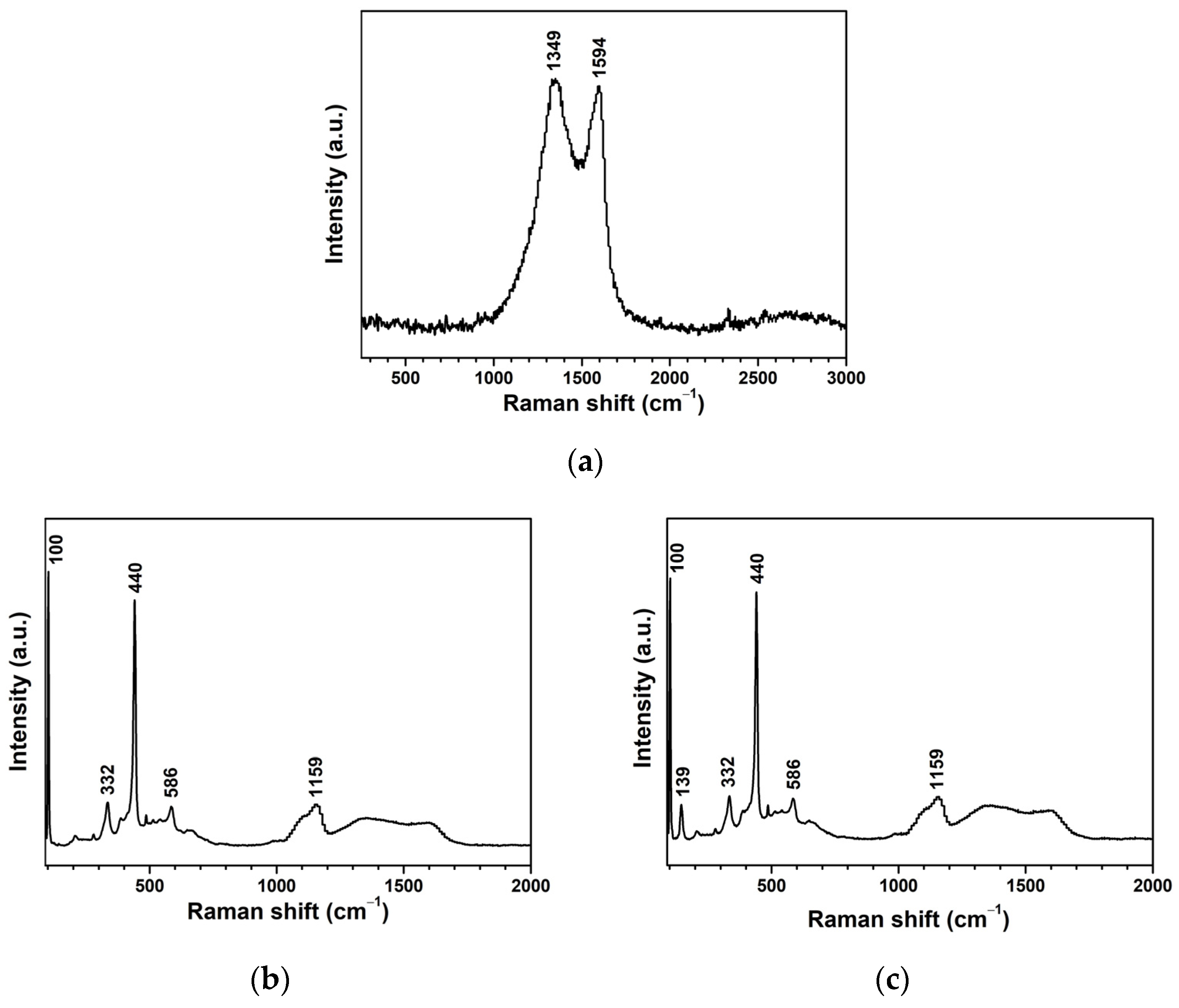

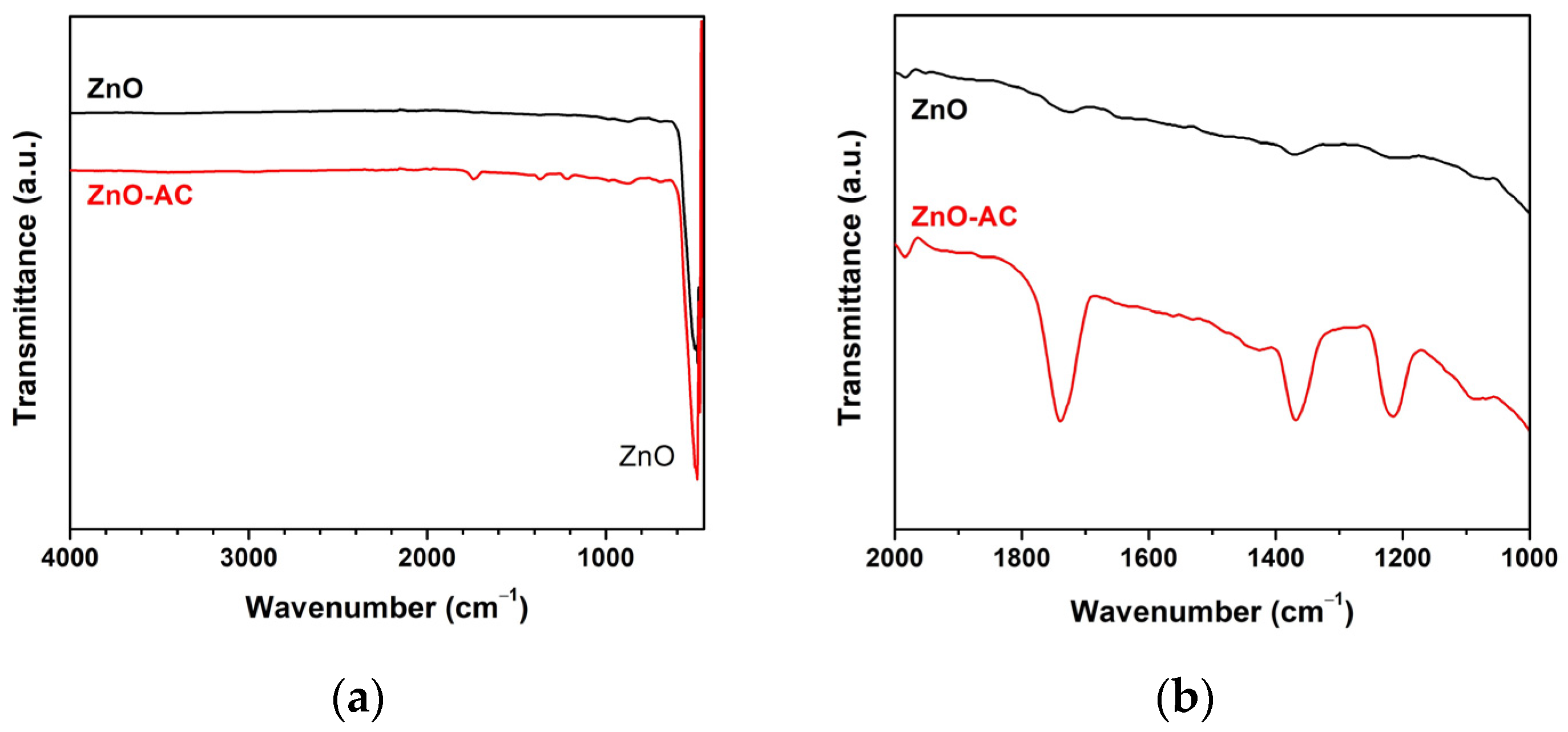


| Sample | kr (10−3 min−1) | τ (min) | R2 |
|---|---|---|---|
| ZnO | 5.55 | 125 | 0.9814 |
| ZnO-AC1% | 4.98 | 139 | 0.9960 |
| ZnO-AC3% | 11.49 | 60 | 0.9966 |
| ZnO-AC5% | 5.99 | 116 | 0.9884 |
| ZnO-AC10% | 9.38 | 74 | 0.9975 |
| Sample | MB Concentration | Photocatalyst Dosage in MB Solution | Light Source | kr (10−3 min−1) | Ref. |
|---|---|---|---|---|---|
| Ti-ZnO | 5 mg/L | 0.1 g/100 mL | UV | 2.54 | [5] |
| GQDs-ZnO | 10 mg/L | 0.2 g/100 mL | UV | 3.79 | [21] |
| rGO@ZnO | 15 mg/L | 20 mg/100 mL | Sunlight | 5.03 | [22] |
| N-ZnO | 10 mg/L | 0.1 g/100 mL | Sunlight | 19.6 | [23] |
| Cu-ZnO | 10 mg/L | 25 mg/100 mL | UV | 25.4 | [24] |
| ZnO-AC3% | 5 mg/L | 0.1 g/100 mL | UV | 11.49 | This work |
| Sample | Pore Diameter (nm) | Pore Volume (cm3/g) | SBET (m2/g) |
|---|---|---|---|
| ZnO | 6.55 | 3.44 × 10−2 | 27.08 |
| ZnO-AC | 3.83 | 4.40 × 10−2 | 28.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krobthong, S.; Rungsawang, T.; Khaodara, N.; Kaewtrakulchai, N.; Manatura, K.; Sukiam, K.; Wathinputthiporn, D.; Wongrerkdee, S.; Boonruang, C.; Wongrerkdee, S. Sustainable Development of ZnO Nanostructure Doping with Water Hyacinth-Derived Activated Carbon for Visible-Light Photocatalysis. Toxics 2024, 12, 165. https://doi.org/10.3390/toxics12030165
Krobthong S, Rungsawang T, Khaodara N, Kaewtrakulchai N, Manatura K, Sukiam K, Wathinputthiporn D, Wongrerkdee S, Boonruang C, Wongrerkdee S. Sustainable Development of ZnO Nanostructure Doping with Water Hyacinth-Derived Activated Carbon for Visible-Light Photocatalysis. Toxics. 2024; 12(3):165. https://doi.org/10.3390/toxics12030165
Chicago/Turabian StyleKrobthong, Sucheewan, Tipawan Rungsawang, Naphatson Khaodara, Napat Kaewtrakulchai, Kanit Manatura, Khewika Sukiam, Donchida Wathinputthiporn, Sawitree Wongrerkdee, Chatdanai Boonruang, and Sutthipoj Wongrerkdee. 2024. "Sustainable Development of ZnO Nanostructure Doping with Water Hyacinth-Derived Activated Carbon for Visible-Light Photocatalysis" Toxics 12, no. 3: 165. https://doi.org/10.3390/toxics12030165
APA StyleKrobthong, S., Rungsawang, T., Khaodara, N., Kaewtrakulchai, N., Manatura, K., Sukiam, K., Wathinputthiporn, D., Wongrerkdee, S., Boonruang, C., & Wongrerkdee, S. (2024). Sustainable Development of ZnO Nanostructure Doping with Water Hyacinth-Derived Activated Carbon for Visible-Light Photocatalysis. Toxics, 12(3), 165. https://doi.org/10.3390/toxics12030165