The Exposure Peaks of Traffic-Related Ultrafine Particles Associated with Inflammatory Biomarkers and Blood Lipid Profiles
Abstract
1. Background and Introduction
2. Methods
2.1. Study Area and Study Population
2.2. Data Collection and Sample Size
- Basic demographic and behavioral data.
- 2.
- Outcome (health indicator) data.
- 3.
- UFP exposure data.
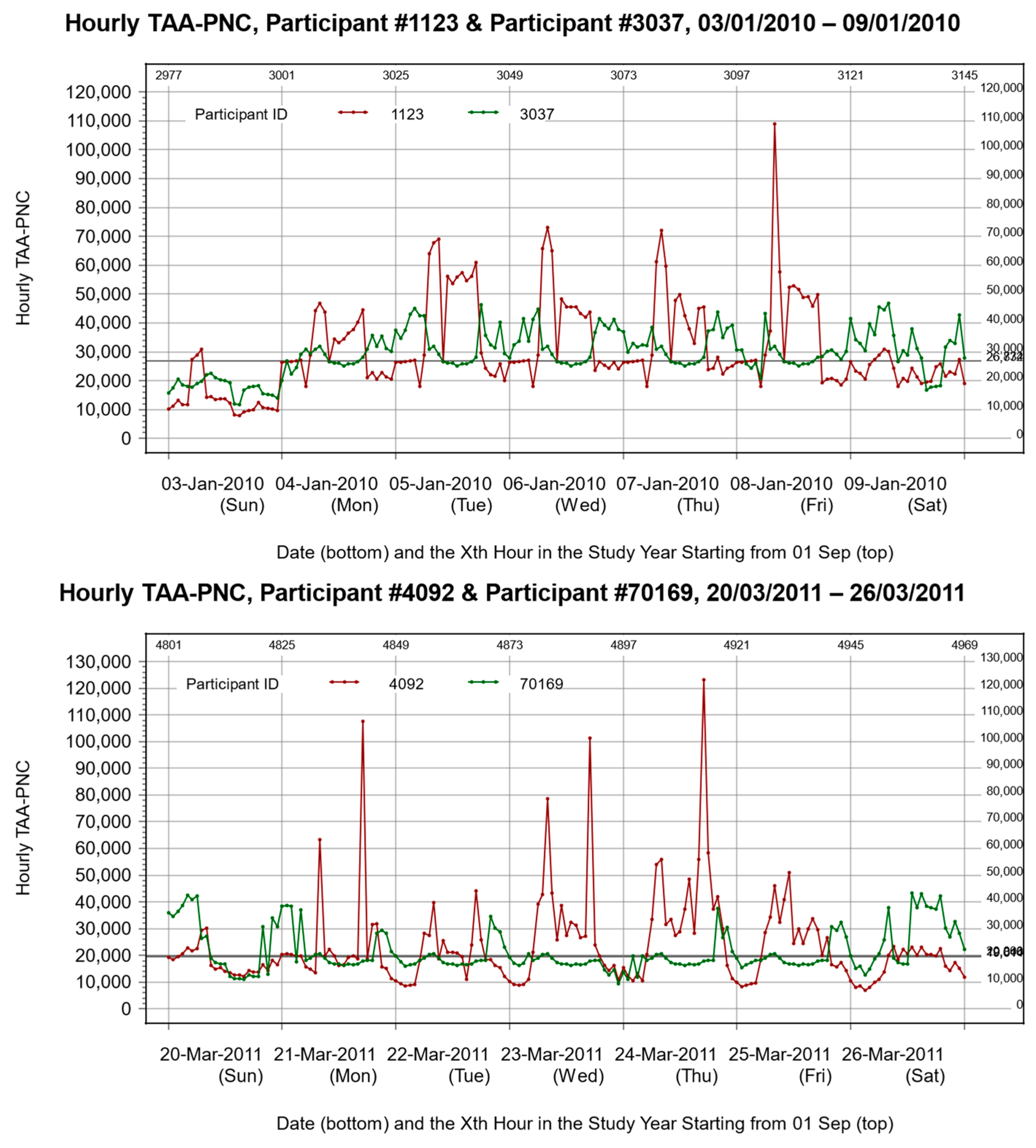
2.3. Processing of UFP Exposure Data
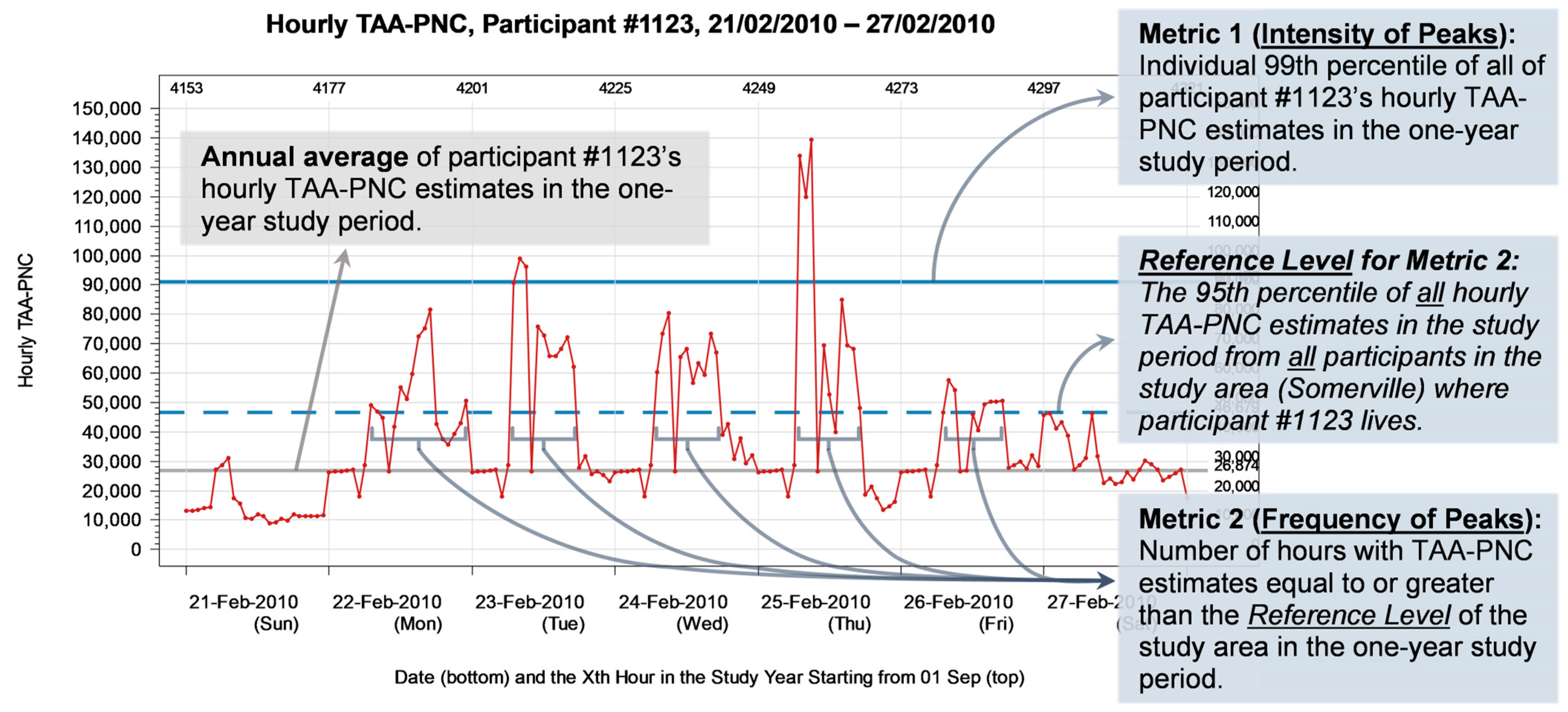
2.4. Definition of Peak Exposure Metrics
- The intensity of peaks.
- 2.
- The frequency of peaks.
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Study Population
3.2. Statistical Characteristics of UFP Exposure Metrics and Health Outcome Indicators
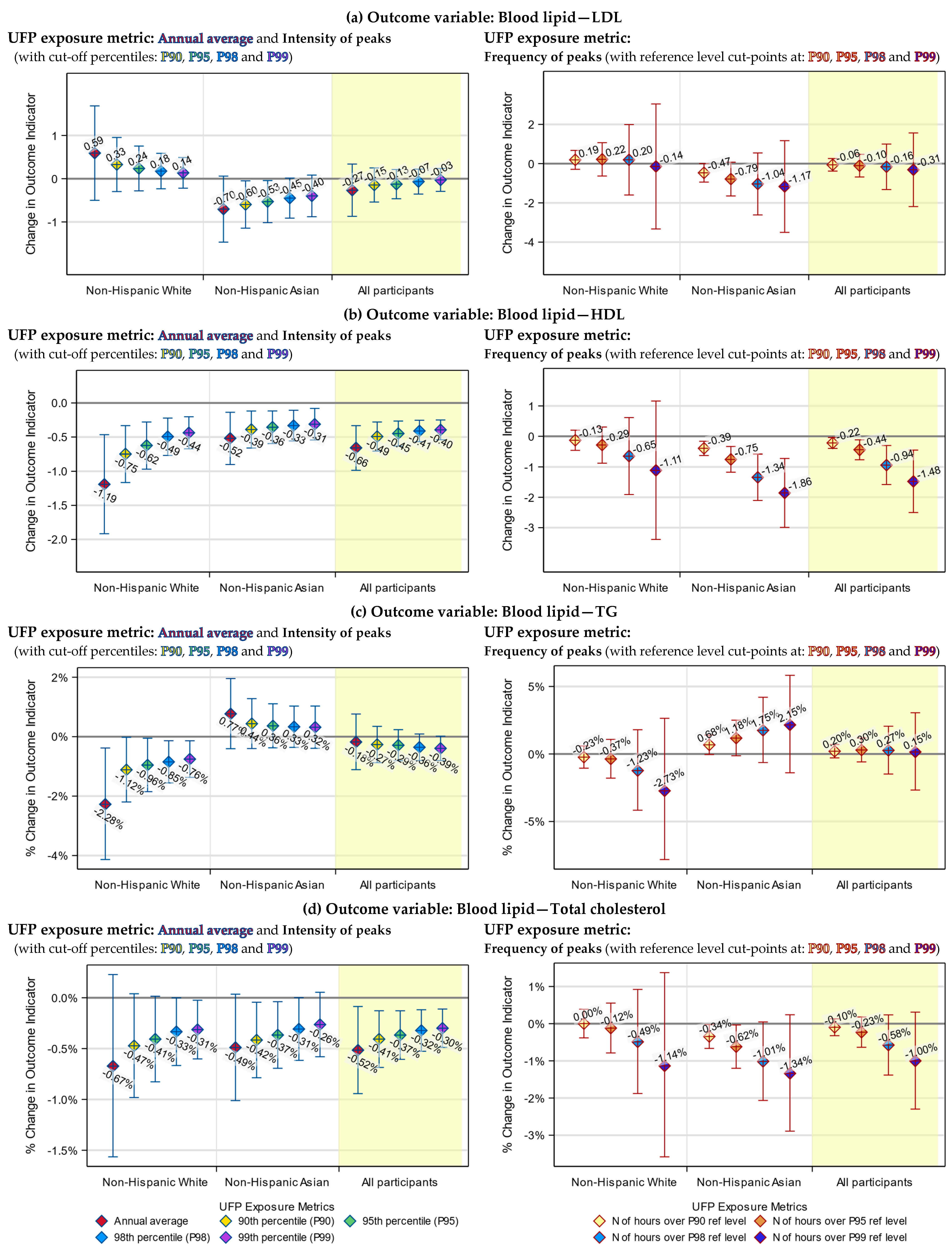
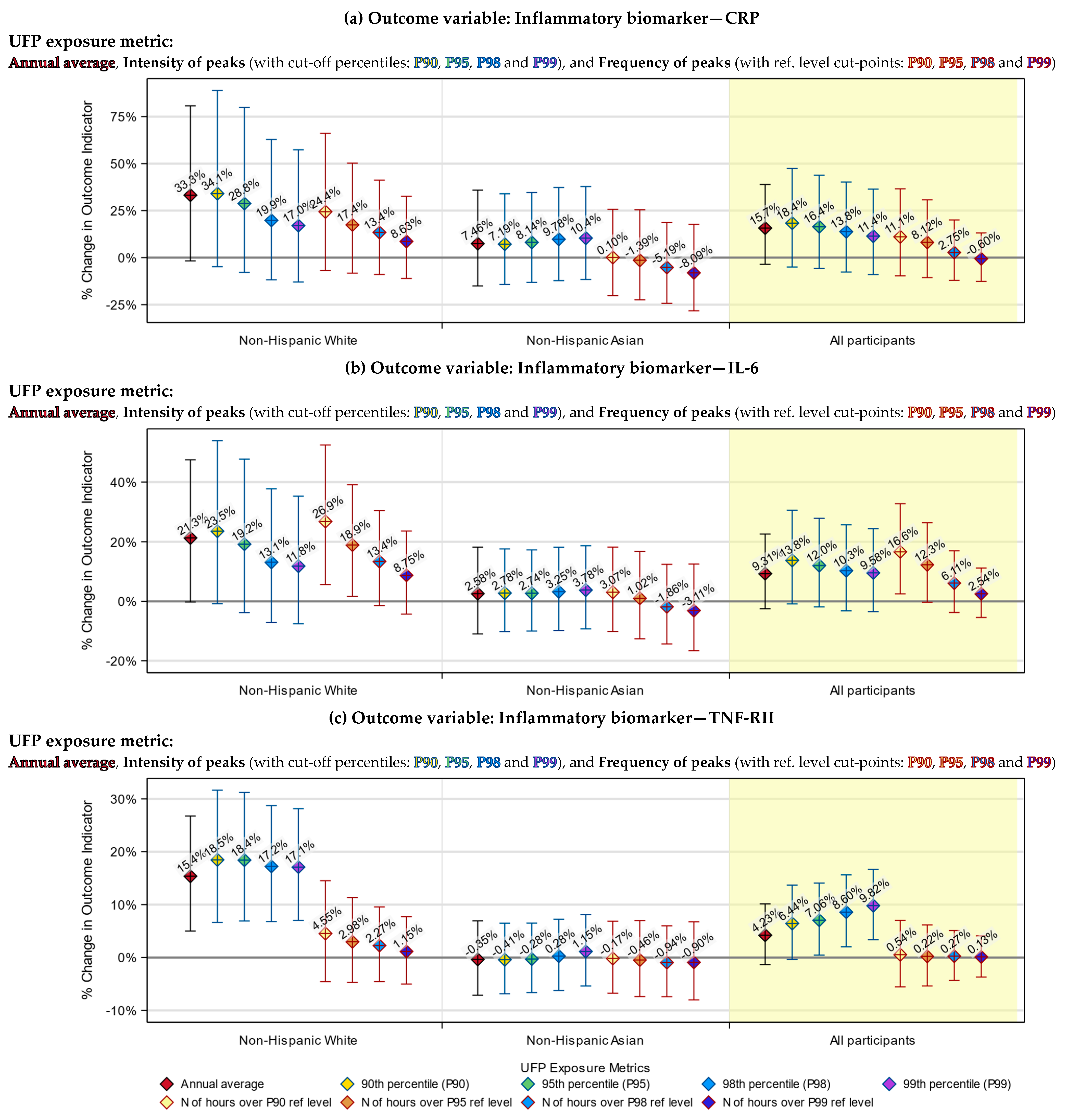
3.3. Health Indicators Associated with Both the UFP Annual Average and Peak Exposure
3.4. Health Indicators Associated with UFP Peak Exposure Only
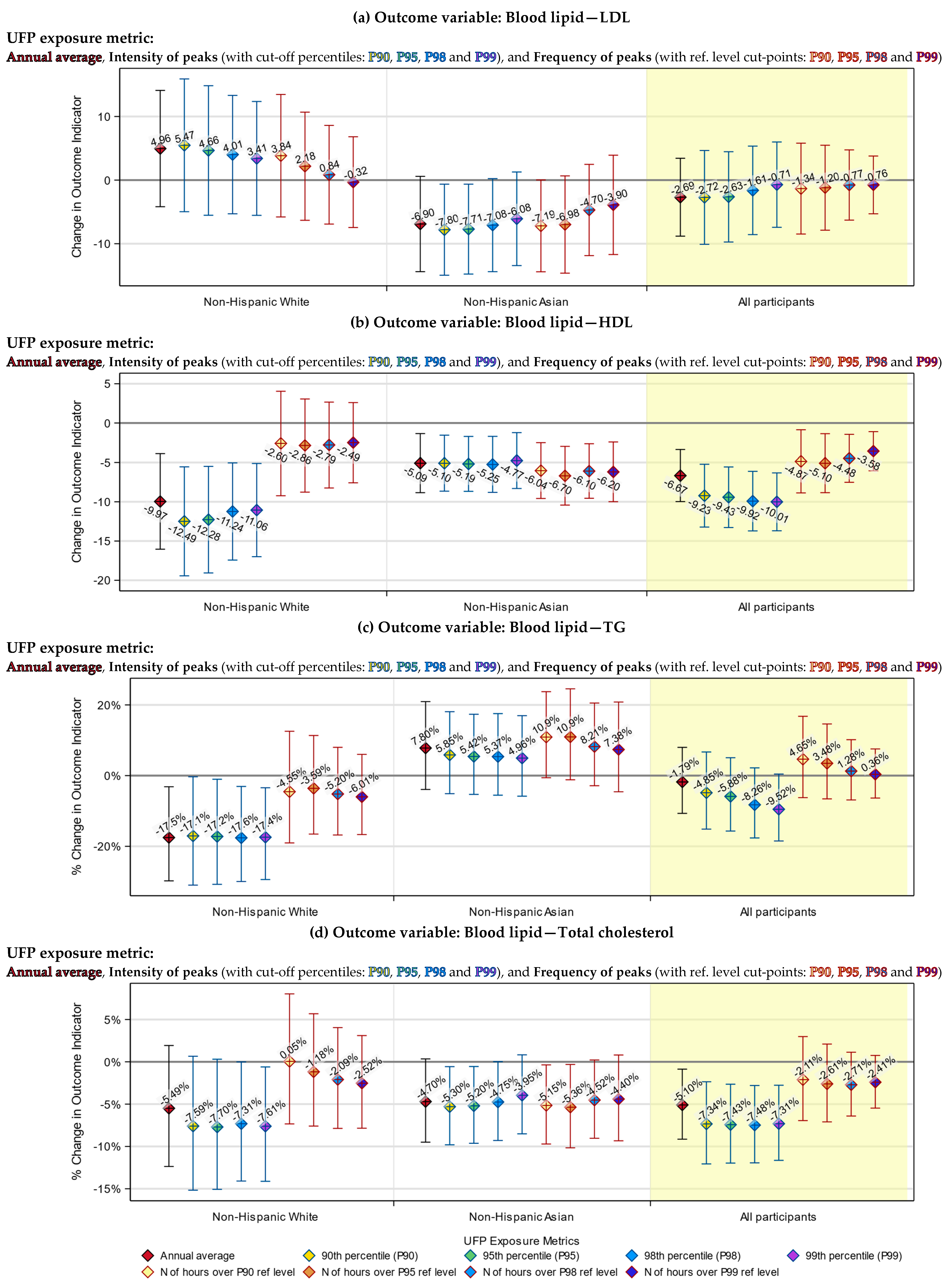
3.5. Racial Differences in the Responses to UFP Exposure
3.6. Differences between the Annual Average and Peak Exposure Metrics
4. Discussion
4.1. Distribution Characteristics of UFP Peak Exposure
4.2. Associations between UFP Peak Exposure and Health Outcomes
4.3. Differences between Racial Groups
4.4. Determination of Cut-off Percentiles for UFP Peak Exposure Metrics
4.5. Comparing UFP Peak Exposure Metrics
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baldauf, R.; Devlin, R.; Gehr, P.; Giannelli, R.; Hassett-Sipple, B.; Jung, H.; Martini, G.; McDonald, J.; Sacks, J.; Walker, K. Ultrafine Particle Metrics and Research Considerations: Review of the 2015 UFP Workshop. Int. J. Environ. Res. Public. Health 2016, 13, 1054. [Google Scholar] [CrossRef]
- Morawska, L.; Ristovski, Z.; Jayaratne, E.R.; Keogh, D.U.; Ling, X. Ambient Nano and Ultrafine Particles from Motor Vehicle Emissions: Characteristics, Ambient Processing and Implications on Human Exposure. Atmos. Environ. 2008, 42, 8113–8138. [Google Scholar] [CrossRef]
- Sabaliauskas, K.; Evansa, G.; Jeonga, C.-H. Source Identification of Traffic-Related Ultrafine Particles Data Mining Contest. Procedia Comput. Sci. 2012, 13, 99–107. [Google Scholar] [CrossRef][Green Version]
- U.S. EPA. Integrated Science Assessment (ISA) for Particulate Matter (Final. Report, Dec. 2019); U.S. Environmental Protection Agency: Washington, DC, USA, 2019.
- U.S. EPA. Control of Air Pollution from New Motor Vehicles: Heavy-Duty Engine and Vehicle Standards; U.S. Environmental Protection Agency: Washington, DC, USA, 2022.
- Posner, L.N.; Pandis, S.N. Sources of Ultrafine Particles in the Eastern United States. Atmos. Environ. 2015, 111, 103–112. [Google Scholar] [CrossRef]
- Zhu, Y.; Hinds, W.C.; Kim, S.; Sioutas, C. Concentration and Size Distribution of Ultrafine Particles Near a Major Highway. J. Air Waste Manag. Assoc. 2002, 52, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Karner, A.A.; Eisinger, D.S.; Niemeier, D.A. Near-Roadway Air Quality: Synthesizing the Findings from Real-World Data. Environ. Sci. Technol. 2010, 44, 5334–5344. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Estimation of Population Size and Demographic Characteristics among People Living Near Truck Routes in the Conterminous United States; U.S. Environmental Protection Agency: Washington, DC, USA, 2021.
- Kingsley, S.L.; Eliot, M.N.; Carlson, L.; Finn, J.; MacIntosh, D.L.; Suh, H.H.; Wellenius, G.A. Proximity of US Schools to Major Roadways: A Nationwide Assessment. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2.5: The Role of Ultrafine Particles on Adverse Health Effects of Air Pollution. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2844–2855. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of Inhaled Ultrafine Particles to the Brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Cheng, H.; Saffari, A.; Sioutas, C.; Forman, H.J.; Morgan, T.E.; Finch, C.E. Nanoscale Particulate Matter from Urban Traffic Rapidly Induces Oxidative Stress and Inflammation in Olfactory Epithelium with Concomitant Effects on Brain. Environ. Health Perspect. 2016, 124, 1537–1546. [Google Scholar] [CrossRef]
- Tyler, C.R.; Zychowski, K.E.; Sanchez, B.N.; Rivero, V.; Lucas, S.; Herbert, G.; Liu, J.; Irshad, H.; McDonald, J.D.; Bleske, B.E.; et al. Surface Area-Dependence of Gas-Particle Interactions Influences Pulmonary and Neuroinflammatory Outcomes. Part. Fibre Toxicol. 2016, 13, 64. [Google Scholar] [CrossRef]
- Allen, J.L.; Liu, X.; Weston, D.; Prince, L.; Oberdörster, G.; Finkelstein, J.N.; Johnston, C.J.; Cory-Slechta, D.A. Developmental Exposure to Concentrated Ambient Ultrafine Particulate Matter Air Pollution in Mice Results in Persistent and Sex-Dependent Behavioral Neurotoxicity and Glial Activation. Toxicol. Sci. 2014, 140, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, F.; Weichenmeier, I.; van Miert, E.; Takenaka, S.; Karg, E.; Blume, C.; Mempel, M.; Schulz, H.; Bernard, A.; Behrendt, H. Effects of Ultrafine Particles-Induced Oxidative Stress on Clara Cells in Allergic Lung Inflammation. Part. Fibre Toxicol. 2010, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Bengalli, R.; Longhin, E.; Marchetti, S.; Proverbio, M.C.; Battaglia, C.; Camatini, M. The Role of IL-6 Released from Pulmonary Epithelial Cells in Diesel UFP-Induced Endothelial Activation. Environ. Pollut. 2017, 231, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Clifford, S.; Mazaheri, M.; Salimi, F.; Ezz, W.N.; Yeganeh, B.; Low-Choy, S.; Walker, K.; Mengersen, K.; Marks, G.B.; Morawska, L. Effects of Exposure to Ambient Ultrafine Particles on Respiratory Health and Systemic Inflammation in Children. Environ. Int. 2018, 114, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Downward, G.S.; van Nunen, E.J.H.M.; Kerckhoffs, J.; Vineis, P.; Brunekreef, B.; Boer, J.M.A.; Messier, K.P.; Roy, A.; Verschuren, W.M.M.; van der Schouw, Y.T.; et al. Long-Term Exposure to Ultrafine Particles and Incidence of Cardiovascular and Cerebrovascular Disease in a Prospective Study of a Dutch Cohort. Environ. Health Perspect. 2018, 126, 127007. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Weichenthal, S.; Kwong, J.C.; Burnett, R.T.; Hatzopoulou, M.; Jerrett, M.; van Donkelaar, A.; Martin, R.V.; Van Ryswyk, K.; Lu, H.; et al. Associations of Long-Term Exposure to Ultrafine Particles and Nitrogen Dioxide with Increased Incidence of Congestive Heart Failure and Acute Myocardial Infarction. Am. J. Epidemiol. 2019, 188, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.J.; Levy, J.I.; Scammell, M.K.; Peters, J.L.; Patton, A.P.; Reisner, E.; Lowe, L.; Zamore, W.; Durant, J.L.; Brugge, D. Association of Modeled Long-Term Personal Exposure to Ultrafine Particles with Inflammatory and Coagulation Biomarkers. Environ. Int. 2016, 92–93, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Habre, R.; Zhou, H.; Eckel, S.P.; Enebish, T.; Fruin, S.; Bastain, T.; Rappaport, E.; Gilliland, F. Short-Term Effects of Airport-Associated Ultrafine Particle Exposure on Lung Function and Inflammation in Adults with Asthma. Environ. Int. 2018, 118, 48–59. [Google Scholar] [CrossRef]
- Vogli, M.; Peters, A.; Wolf, K.; Thorand, B.; Herder, C.; Koenig, W.; Cyrys, J.; Maestri, E.; Marmiroli, N.; Karrasch, S.; et al. Long-Term Exposure to Ambient Air Pollution and Inflammatory Response in the KORA Study. Sci. Total Environ. 2024, 912, 169416. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Gao, M.; Xiao, X.; Knibbs, L.D.; Morawska, L.; Dharmage, S.C.; Heinrich, J.; Jalaludin, B.; Lin, S.; Guo, Y.; et al. Ultrafine Particles, Blood Pressure and Adult Hypertension: A Population-Based Survey in Northeast China. Environ. Res. Lett. 2021, 16, 094041. [Google Scholar] [CrossRef]
- Li, R.; Navab, M.; Pakbin, P.; Ning, Z.; Navab, K.; Hough, G.; Morgan, T.E.; Finch, C.E.; Araujo, J.A.; Fogelman, A.M.; et al. Ambient Ultrafine Particles Alter Lipid Metabolism and HDL Anti-Oxidant Capacity in LDLR-Null Mice. J. Lipid Res. 2013, 54, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Roswall, N.; Poulsen, A.H.; Hvidtfeldt, U.A.; Hendriksen, P.F.; Boll, K.; Halkjær, J.; Ketzel, M.; Brandt, J.; Frohn, L.M.; Christensen, J.H.; et al. Exposure to Ambient Air Pollution and Lipid Levels and Blood Pressure in an Adult, Danish Cohort. Environ. Res. 2023, 220, 115179. [Google Scholar] [CrossRef] [PubMed]
- Ossoli, A.; Cetti, F.; Gomaraschi, M. Air Pollution: Another Threat to HDL Function. Int. J. Mol. Sci. 2023, 24, 317. [Google Scholar] [CrossRef]
- Jordakieva, G.; Grabovac, I.; Valic, E.; Schmidt, K.E.; Graff, A.; Schuster, A.; Hoffmann-Sommergruber, K.; Oberhuber, C.; Scheiner, O.; Goll, A.; et al. Occupational Exposure to Ultrafine Particles in Police Officers: No Evidence for Adverse Respiratory Effects. J. Occup. Med. Toxicol. 2018, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Tobías, A.; Rivas, I.; Reche, C.; Alastuey, A.; Rodríguez, S.; Fernández-Camacho, R.; Sánchez de la Campa, A.M.; de la Rosa, J.; Sunyer, J.; Querol, X. Short-Term Effects of Ultrafine Particles on Daily Mortality by Primary Vehicle Exhaust versus Secondary Origin in Three Spanish Cities. Environ. Int. 2018, 111, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J. Studying Peak Exposure—Toxicology and Exposure Statistics. In X2001—Exposure Assessment in Epidemiology and Practice; National Institute for Working Life: Stockholm, Sweden, 2001; pp. 207–209. ISBN 91-7045-607-0. [Google Scholar]
- Holz, O.; Mücke, M.; Paasch, K.; Böhme, S.; Timm, P.; Richter, K.; Magnussen, H.; Jörres, R.A. Repeated Ozone Exposures Enhance Bronchial Allergen Responses in Subjects with Rhinitis or Asthma: Repeated Ozone Exposure and Allergen. Clin. Exp. Allergy 2002, 32, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Pathmanathan, S. Repeated Daily Exposure to 2 Ppm Nitrogen Dioxide Upregulates the Expression of IL-5, IL-10, IL-13, and ICAM-1 in the Bronchial Epithelium of Healthy Human Airways. Occup. Environ. Med. 2003, 60, 892–896. [Google Scholar] [CrossRef]
- Sundblad, B.-M.; von Scheele, I.; Palmberg, L.; Olsson, M.; Larsson, K. Repeated Exposure to Organic Material Alters Inflammatory and Physiological Airway Responses. Eur. Respir. J. 2009, 34, 80–88. [Google Scholar] [CrossRef]
- Lin, C.; Lane, K.J.; Griffiths, J.K.; Brugge, D. A New Exposure Metric for the Cumulative Effect of Short-Term Exposure Peaks of Traffic-Related Ultrafine Particles. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 615–628. [Google Scholar] [CrossRef]
- Joint CCl/CLIVAR/JCOMM Expert Team on Climate Change Detection and Indices (ETCCDI). Definitions of the 27 Core Indices. Available online: http://etccdi.pacificclimate.org/list_27_indices.shtml (accessed on 21 January 2023).
- Perkins, S.E.; Alexander, L.V. On the Measurement of Heat Waves. J. Clim. 2013, 26, 4500–4517. [Google Scholar] [CrossRef]
- Fuller, C.H.; Patton, A.P.; Lane, K.; Laws, M.B.; Marden, A.; Carrasco, E.; Spengler, J.; Mwamburi, M.; Zamore, W.; Durant, J.L.; et al. A Community Participatory Study of Cardiovascular Health and Exposure to Near-Highway Air Pollution: Study Design and Methods. Rev. Environ. Health 2013, 28, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.P.; Collins, C.; Naumova, E.N.; Zamore, W.; Brugge, D.; Durant, J.L. An Hourly Regression Model for Ultrafine Particles in a Near-Highway Urban Area. Environ. Sci. Technol. 2014, 48, 3272–3280. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.P.; Zamore, W.; Naumova, E.N.; Levy, J.I.; Brugge, D.; Durant, J.L. Transferability and Generalizability of Regression Models of Ultrafine Particles in Urban Neighborhoods in the Boston Area. Environ. Sci. Technol. 2015, 49, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Padró-Martínez, L.T.; Patton, A.P.; Trull, J.B.; Zamore, W.; Brugge, D.; Durant, J.L. Mobile Monitoring of Particle Number Concentration and Other Traffic-Related Air Pollutants in a near-Highway Neighborhood over the Course of a Year. Atmos. Environ. 2012, 61, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.J.; Levy, J.I.; Scammell, M.K.; Patton, A.P.; Durant, J.L.; Mwamburi, M.; Zamore, W.; Brugge, D. Effect of Time-Activity Adjustment on Exposure Assessment for Traffic-Related Ultrafine Particles. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Patton, A.P.; Perkins, J.; Zamore, W.; Levy, J.I.; Brugge, D.; Durant, J.L. Spatial and Temporal Differences in Traffic-Related Air Pollution in Three Urban Neighborhoods near an Interstate Highway. Atmos. Environ. 2014, 99, 309–321. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Dhaniyala, S. Vertical and Horizontal Concentration Distributions of Ultrafine Particles near a Highway. Atmos. Environ. 2012, 46, 225–236. [Google Scholar] [CrossRef]
- Chih-Da, W.; Piers, M.; Melly, S.; Lane, K.; Adamkiewicz, G.; Durant, J.L.; Brugge, D.; Spengler, J.D. Mapping the Vertical Distribution of Population and Particulate Air Pollution in a Near-Highway Urban Neighborhood: Implications for Exposure Assessment. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 297–304. [Google Scholar] [CrossRef]
- Mao, S.; Li, S.; Wang, C.; Liu, Y.; Li, N.; Liu, F.; Huang, S.; Liu, S.; Lu, Y.; Mao, Z.; et al. Is Long-Term PM1 Exposure Associated with Blood Lipids and Dyslipidemias in a Chinese Rural Population? Environ. Int. 2020, 138, 105637. [Google Scholar] [CrossRef]
- Bell, G.; Mora, S.; Greenland, P.; Tsai, M.; Gill, E.; Kaufman, J.D. Association of Air Pollution Exposures with High-Density Lipoprotein Cholesterol and Particle Number: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Brugge, D.; Lee, A.C.; Woodin, M.; Rioux, C. Native and Foreign Born as Predictors of Pediatric Asthma in an Asian Immigrant Population: A Cross Sectional Survey. Environ. Health 2007, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Corlin, L.; Woodin, M.; Thanikachalam, M.; Lowe, L.; Brugge, D. Evidence for the Healthy Immigrant Effect in Older Chinese Immigrants: A Cross-Sectional Study. BMC Public Health 2014, 14, 603. [Google Scholar] [CrossRef] [PubMed]
- Newbold, K.B. Chronic Conditions and the Healthy Immigrant Effect: Evidence from Canadian Immigrants. J. Ethn. Migr. Stud. 2006, 32, 765–784. [Google Scholar] [CrossRef]
- Choi, W.; He, M.; Barbesant, V.; Kozawa, K.H.; Mara, S.; Winer, A.M.; Paulson, S.E. Prevalence of Wide Area Impacts Downwind of Freeways under Pre-Sunrise Stable Atmospheric Conditions. Atmos. Environ. 2012, 62, 318–327. [Google Scholar] [CrossRef]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Arhami, M.; Polidori, A.; Gillen, D.L.; Kleinman, M.T.; Schauer, J.J.; Sioutas, C. Association of Biomarkers of Systemic Inflammation with Organic Components and Source Tracers in Quasi-Ultrafine Particles. Environ. Health Perspect. 2010, 118, 756–762. [Google Scholar] [CrossRef]
- Simon, M.C.; Patton, A.P.; Naumova, E.N.; Levy, J.I.; Kumar, P.; Brugge, D.; Durant, J.L. Combining Measurements from Mobile Monitoring and a Reference Site to Develop Models of Ambient Ultrafine Particle Number Concentration at Residences. Environ. Sci. Technol. 2018, 52, 6985–6995. [Google Scholar] [CrossRef]

| Health Outcome Indicator | N * | Mean | Standard Deviation | Median | Minimum | Lower Quartile | Upper Quartile | Maximum |
|---|---|---|---|---|---|---|---|---|
| Inflammatory Biomarkers: | ||||||||
| CRP (mg/L) | ||||||||
| Near-highway areas | 323 | 3.26 | 6.17 | 1.29 | 0.05 | 0.50 | 3.35 | 58.52 |
| Urban background areas | 88 | 2.52 | 4.25 | 1.11 | 0.06 | 0.51 | 2.33 | 30.99 |
| All study areas | 411 | 3.10 | 5.81 | 1.27 | 0.05 | 0.50 | 3.27 | 58.52 |
| IL-6 (pg/mL) | ||||||||
| Near-highway areas | 323 | 2.21 | 2.57 | 1.36 | 0.34 | 0.83 | 2.45 | 21.55 |
| Urban background areas | 88 | 1.55 | 1.46 | 1.15 | 0.31 | 0.75 | 1.75 | 8.77 |
| All study areas | 411 | 2.07 | 2.39 | 1.29 | 0.31 | 0.81 | 2.24 | 21.55 |
| TNF-RII (pg/mL) | ||||||||
| Near-highway areas | 323 | 2633.21 | 1394.61 | 2242.41 | 995.86 | 1812.80 | 2981.22 | 13,455.50 |
| Urban background areas | 88 | 2539.83 | 1151.53 | 2249.52 | 1091.80 | 1890.92 | 2797.49 | 9150.00 |
| All study areas | 411 | 2613.21 | 1345.49 | 2244.44 | 995.86 | 1838.81 | 2954.23 | 13,455.50 |
| Blood Lipid Indicators: | ||||||||
| LDL (mg/dL) | ||||||||
| Near-highway areas | 284 | 91.98 | 35.29 | 88.50 | 1.00 | 70.00 | 108.00 | 281.00 |
| Urban background areas | 80 | 93.45 | 29.14 | 88.00 | 38.00 | 72.00 | 106.50 | 196.00 |
| All study areas | 364 | 92.31 | 34.00 | 88.00 | 1.00 | 70.50 | 108.00 | 281.00 |
| HDL (mg/dL) | ||||||||
| Near-highway areas | 298 | 49.57 | 20.93 | 47.00 | 15.00 | 36.00 | 60.00 | 100.00 |
| Urban background areas | 85 | 52.48 | 20.93 | 51.00 | 15.00 | 39.00 | 67.00 | 100.00 |
| All study areas | 383 | 50.22 | 20.94 | 47.00 | 15.00 | 37.00 | 62.00 | 100.00 |
| Triglycerides (mg/dL) | ||||||||
| Near-highway areas | 318 | 129.95 | 86.12 | 110.50 | 11.00 | 63.00 | 166.00 | 500.00 |
| Urban background areas | 89 | 105.47 | 62.49 | 91.00 | 50.00 | 62.00 | 139.00 | 444.00 |
| All study areas | 407 | 124.59 | 82.10 | 100.00 | 11.00 | 63.00 | 160.00 | 500.00 |
| Total cholesterol (mg/dL) | ||||||||
| Near-highway areas | 319 | 167.86 | 48.50 | 165.00 | 100.00 | 134.00 | 193.00 | 400.00 |
| Urban background areas | 89 | 163.17 | 40.22 | 158.00 | 100.00 | 140.00 | 186.00 | 268.00 |
| All study areas | 408 | 166.84 | 46.81 | 162.50 | 100.00 | 135.00 | 190.00 | 400.00 |
| Near-Highway Neighborhoods | Urban Background Neighborhoods | All Study Areas | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 61.01 (12.56) | 60.75 (13.17) | 60.95 (12.68) |
| Body Mass Index | 27.64 (6.83) | 27.24 (6.86) | 27.55 (6.83) |
| N (%) | N (%) | N (%) | |
| Gender | |||
| Female | 193 (58.31%) | 56 (61.54%) | 249 (59.00%) |
| Male | 138 (41.69%) | 35 (38.46%) | 173 (41.00%) |
| Race/Ethnicity | |||
| Non-Hispanic White | 138 (41.69%) | 43 (47.25%) | 181 (42.89%) |
| Non-Hispanic Black | 23 (6.95%) | 7 (7.69%) | 30 (7.11%) |
| Non-Hispanic Asian | 132 (39.88%) | 36 (39.56%) | 168 (39.81%) |
| Other | 38 (11.48%) | 5 (5.49%) | 43 (10.19%) |
| Education level | |||
| Lower than 12th Grade | 125 (37.76%) | 16 (17.58%) | 141 (33.41%) |
| 12th Grade (High school diploma) | 100 (30.21%) | 24 (26.37%) | 124 (29.38%) |
| Some undergraduate college | 74 (22.36%) | 30 (32.97%) | 104 (24.64%) |
| Some postgraduate | 32 (9.67%) | 21 (23.08%) | 53 (12.56%) |
| Smoking status | |||
| Non-smoker | 157 (47.43%) | 49 (53.85%) | 206 (48.82%) |
| Former smoker | 104 (31.42%) | 24 (26.37%) | 128 (30.33%) |
| Current smoker | 70 (21.15%) | 18 (19.78%) | 88 (20.85%) |
| Employment status | |||
| Not employed | 205 (62.50%) | 51 (57.30%) | 256 (61.39%) |
| Employed or full-time student | 123 (37.50%) | 38 (42.70%) | 161 (38.61%) |
| (Missing) 2 | 3 | 2 | 5 |
| Birthplace | |||
| Outside the United States | 193 (58.48%) | 41 (46.07%) | 234 (55.85%) |
| In the United States | 137 (41.52%) | 48 (53.93%) | 185 (44.15%) |
| (Missing) 2 | 1 | 2 | 3 |
| Total 1 | 331 | 91 | 422 |
| N 2 | Mean | Standard Deviation | Median | Minimum | Lower Quartile | Upper Quartile | Maximum | |
|---|---|---|---|---|---|---|---|---|
| Annual average of TAA-PNC exposure (unit: cm−3) | ||||||||
| Near-highway areas | 513 | 2.33 × 104 | 0.48 × 104 | 2.35 × 104 | 1.08 × 104 | 2.02 × 104 | 2.71 × 104 | 3.47 × 104 |
| Urban background areas | 188 | 1.30 × 104 | 0.31 × 104 | 1.20 × 104 | 0.88 × 104 | 1.03 × 104 | 1.57 × 104 | 2.37 × 104 |
| All study areas | 701 | 2.06 × 104 | 0.64 × 104 | 2.14 × 104 | 0.88 × 104 | 1.54 × 104 | 2.56 × 104 | 3.47 × 104 |
| Intensity of TAA-PNC exposure peaks (unit: cm−3) 3 | ||||||||
| Near-highway areas | 513 | 6.09 × 104 | 1.19 × 104 | 6.39 × 104 | 2.53 × 104 | 5.48 × 104 | 6.98 × 104 | 8.19 × 104 |
| Urban background areas | 188 | 3.82 × 104 | 0.87 × 104 | 3.96 × 104 | 2.19 × 104 | 3.16 × 104 | 4.34 × 104 | 9.12 × 104 |
| All study areas | 701 | 5.48 × 104 | 1.50 × 104 | 5.90 × 104 | 2.19 × 104 | 4.21 × 104 | 6.75 × 104 | 9.12 × 104 |
| Frequency of TAA-PNC exposure peaks (unit: hours) 4 | ||||||||
| Near-highway areas | 513 | 577.39 | 337.73 | 526 | 1 | 392 | 735 | 2196 |
| Urban background areas | 188 | 42.59 | 77.64 | 27 | 0 | 12 | 58 | 958 |
| All study areas | 701 | 433.96 | 375.84 | 427 | 0 | 67 | 645 | 2196 |
| Cut-off Percentile 1 | Near-Highway Neighborhoods | Urban Background Neighborhoods | All Study Areas | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 2 (%) | N2 3 (%) | Ntotal 4 | N1 2 (%) | N2 3 (%) | Ntotal 4 | N1 2 (%) | N2 3 (%) | Ntotal 4 | |||
| 90th percentile | 513 (100.00%) | 0 (0.00%) | 513 | 188 (100.00%) | 0 (0.00%) | 188 | 701 (100.00%) | 0 (0.00%) | 701 | ||
| 95th percentile | 513 (100.00%) | 0 (0.00%) | 513 | 180 (95.74%) | 8 (4.26%) | 188 | 693 (98.86%) | 8 (1.14%) | 701 | ||
| 98th percentile | 509 (99.22%) | 4 (0.78%) | 513 | 145 (77.13%) | 43 (22.87%) | 188 | 654 (93.30%) | 47 (6.70%) | 701 | ||
| 99th percentile | 500 (97.47%) | 13 (2.53%) | 513 | 85 (45.21%) | 103 (54.79%) | 188 | 585 (83.45%) | 116 (16.55%) | 701 | ||
| Inflammatory Biomarker | %Change (95% CI) | ||
|---|---|---|---|
| Non-Hispanic White | Asian | All Participants | |
| CRP | |||
| Annual average exposure 1 | 3.49% (−0.21%, 7.33%) * | 0.74% (−1.65%, 3.17%) | 1.45% (−0.36%, 3.30%) |
| Intensity of exposure peaks 2 | 0.63% (−0.55%, 1.81%) | 0.65% (−0.80%, 2.12%) | 0.43% (−0.37%, 1.23%) |
| Frequency of exposure peaks 3 | 1.62% (−0.86%, 4.16%) | −0.16% (−2.82%, 2.58%) | 0.68% (−0.97%, 2.35%) |
| IL-6 | |||
| Annual average exposure 1 | 2.33% (−0.03%, 4.75%) * | 0.26% (−1.18%, 1.72%) | 0.88% (−0.25%, 2.03%) |
| Intensity of exposure peaks 2 | 0.44% (−0.31%, 1.20%) | 0.24% (−0.63%, 1.13%) | 0.36% (−0.14%, 0.87%) |
| Frequency of exposure peaks 3 | 1.75% (0.16%, 3.36%) ** | 0.11% (−1.51%, 1.76%) | 1.01% (−0.03%, 2.05%) * |
| TNF-RII | |||
| Annual average exposure 1 | 1.72% (0.59%, 2.87%) *** | −0.04% (−0.75%, 0.68%) | 0.41% (−0.13%, 0.96%) |
| Intensity of exposure peaks 2 | 0.63% (0.27%, 0.99%) **** | 0.07% (−0.36%, 0.51%) | 0.37% (0.13%, 0.61%) *** |
| Frequency of exposure peaks 3 | 0.29% (−0.48%, 1.08%) | −0.05% (−0.86%, 0.76%) | 0.02% (−0.48%, 0.52%) |
| Blood Lipid Indicator | Change in mg/dL (95% CI) | ||
| Non-Hispanic White | Asian | All participants | |
| LDL | |||
| Annual average exposure 1 | 0.59 (−0.50, 1.68) | −0.70 (−1.47, 0.06) * | −0.27 (−0.87, 0.34) |
| Intensity of exposure peaks 2 | 0.14 (−0.22, 0.49) | −0.40 (−0.88, 0.08) # | −0.03 (−0.29, 0.24) |
| Frequency of exposure peaks 3 | 0.22 (−0.63, 1.07) | −0.79 (−1.65, 0.07) * | −0.10 (−0.68, 0.47) |
| HDL | |||
| Annual average exposure 1 | −1.19 (−1.92, −0.47) *** | −0.52 (−0.90, −0.14) *** | −0.66 (−0.99, −0.33) **** |
| Intensity of exposure peaks 2 | −0.44 (−0.67, −0.20) **** | −0.31 (−0.54, −0.08) *** | −0.40 (−0.54, −0.25) **** |
| Frequency of exposure peaks 3 | −0.29 (−0.88, 0.31) | −0.75 (−1.17, −0.33) **** | −0.44 (−0.76, −0.12) *** |
| Blood Lipid Indicator | % Change (95% CI) | ||
| Non-Hispanic White | Asian | All participants | |
| Triglycerides | |||
| Annual average exposure 1 | −2.28% (−4.13%, −0.38%) ** | 0.77% (−0.41%, 1.96%) | −0.18% (−1.11%, 0.76%) |
| Intensity of exposure peaks 2 | −0.76% (−1.37%, −0.14%) ** | 0.32% (−0.39%, 1.03%) | −0.39% (−0.80%, 0.02%) * |
| Frequency of exposure peaks 3 | −0.37% (−1.79%, 1.08%) | 1.18% (−0.13%, 2.50%) * | 0.30% (−0.59%, 1.19%) |
| Total cholesterol | |||
| Annual average exposure 1 | −0.67% (−1.56%, 0.23%) | −0.49% (−1.01%, 0.04%) * | −0.52% (−0.94%, −0.09%) ** |
| Intensity of exposure peaks 2 | −0.31% (−0.60%, −0.02%) ** | −0.26% (−0.58%, 0.05%) # | −0.30% (−0.49%, −0.11%) *** |
| Frequency of exposure peaks 3 | −0.12% (−0.79%, 0.55%) | −0.62% (−1.20%, −0.04%) ** | −0.23% (−0.63%, 0.18%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Lane, K.J.; Chomitz, V.R.; Griffiths, J.K.; Brugge, D. The Exposure Peaks of Traffic-Related Ultrafine Particles Associated with Inflammatory Biomarkers and Blood Lipid Profiles. Toxics 2024, 12, 147. https://doi.org/10.3390/toxics12020147
Lin C, Lane KJ, Chomitz VR, Griffiths JK, Brugge D. The Exposure Peaks of Traffic-Related Ultrafine Particles Associated with Inflammatory Biomarkers and Blood Lipid Profiles. Toxics. 2024; 12(2):147. https://doi.org/10.3390/toxics12020147
Chicago/Turabian StyleLin, Cheng, Kevin J. Lane, Virginia R. Chomitz, Jeffrey K. Griffiths, and Doug Brugge. 2024. "The Exposure Peaks of Traffic-Related Ultrafine Particles Associated with Inflammatory Biomarkers and Blood Lipid Profiles" Toxics 12, no. 2: 147. https://doi.org/10.3390/toxics12020147
APA StyleLin, C., Lane, K. J., Chomitz, V. R., Griffiths, J. K., & Brugge, D. (2024). The Exposure Peaks of Traffic-Related Ultrafine Particles Associated with Inflammatory Biomarkers and Blood Lipid Profiles. Toxics, 12(2), 147. https://doi.org/10.3390/toxics12020147







