The Release and Migration of Cr in the Soil under Alternating Wet–Dry Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Column Leaching Experiment
2.2.1. Soil Column Preparation
- Peristaltic pump: The peristaltic pump is used to regulate the rate of liquid transfer.
- Automatic collector: The automatic collector collects leachate.
2.2.2. Contaminated Soil Preparation
2.2.3. Simulated Acid Rain Preparation
2.2.4. Leaching Experiment
- After filling the soil column, deionized water was introduced from the bottom, with a volume calculated as 40% of the weight of the soil inside the column. It was allowed to saturate for 24 h.
- The pH of the rainwater was adjusted to 3 using solutions of HCl and KOH. The peristaltic pump flow rate was set to 1.55 mL/min. Ninety-three milliliters of this adjusted rainwater were introduced from the top into the soil column. The leachate was collected using the automatic collector, and the leaching process was allowed to complete. Afterward, the soil column was left to dry for 24 h. This process was repeated the following day and continued until a total of 465 mL of rainwater had been introduced (over 5 days). This completed the simulation of a year’s rainfall in the Panzhihua region. The soil column was allowed to dry for an additional 7 days.
- After a 7-day dry period, the second year’s rainfall was simulated in the same manner, introducing a total of 465 mL of rainwater. After completing the leaching process, a drying period of 15 days was allowed.
- After the 15-day dry period, the total rainfall for the third year was simulated in the same manner, introducing a total of 465 mL of rainwater. Upon completion of the leaching process, all leaching stages for this soil column were finished.
- As the leaching process progressed, the pH, Eh, TOC, EC, Cr concentration, and Cr(VI) concentration of the leachate were continuously monitored.
2.2.5. Sample Processing and Testing
2.3. Chromium Content
2.4. Sequential Extraction Experiment
2.5. Cr(Ⅵ) Determination
2.6. Total Organic Carbon
3. Results
3.1. Leachate
3.1.1. Chromium Concentration
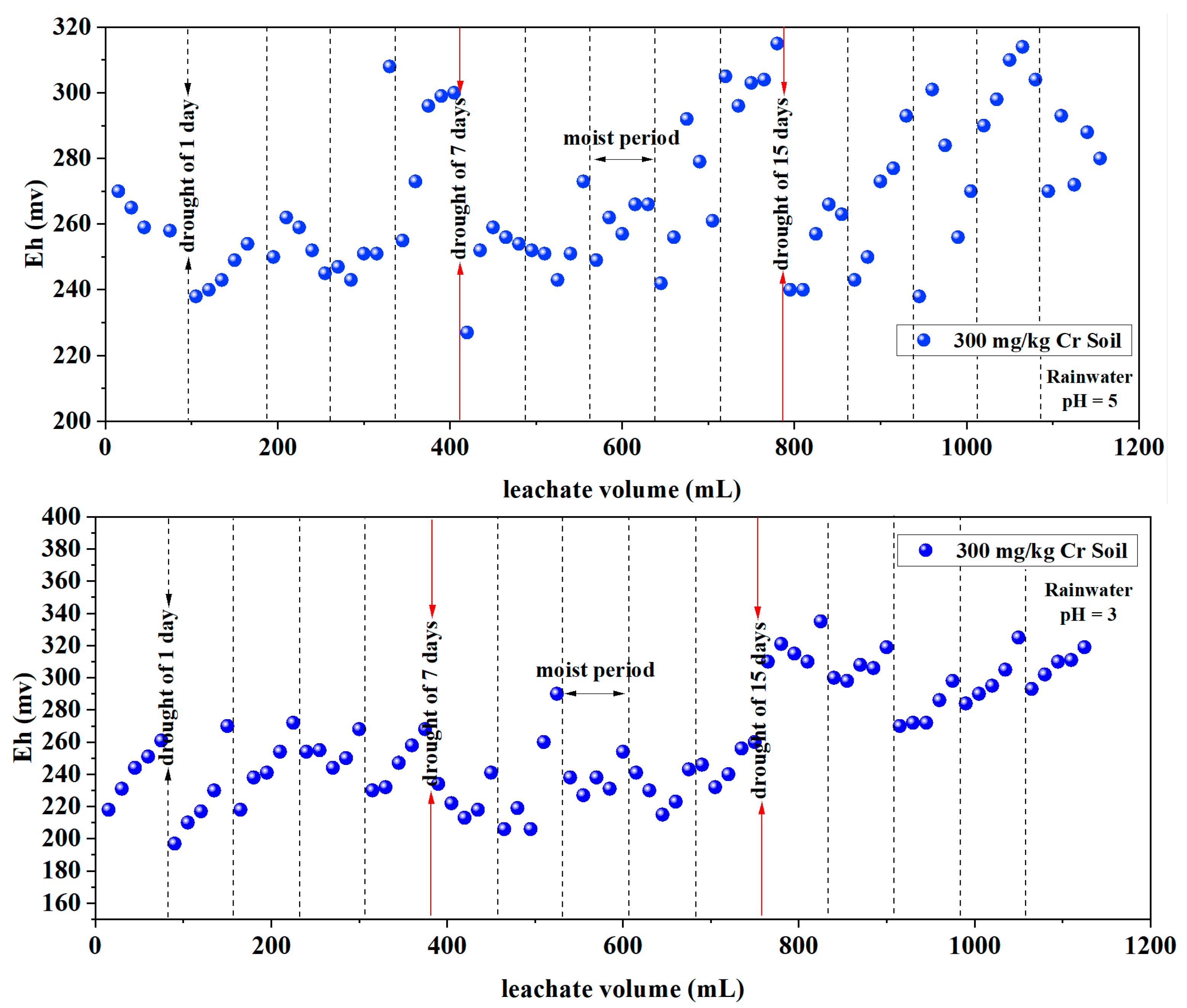
3.1.2. Redox Potential
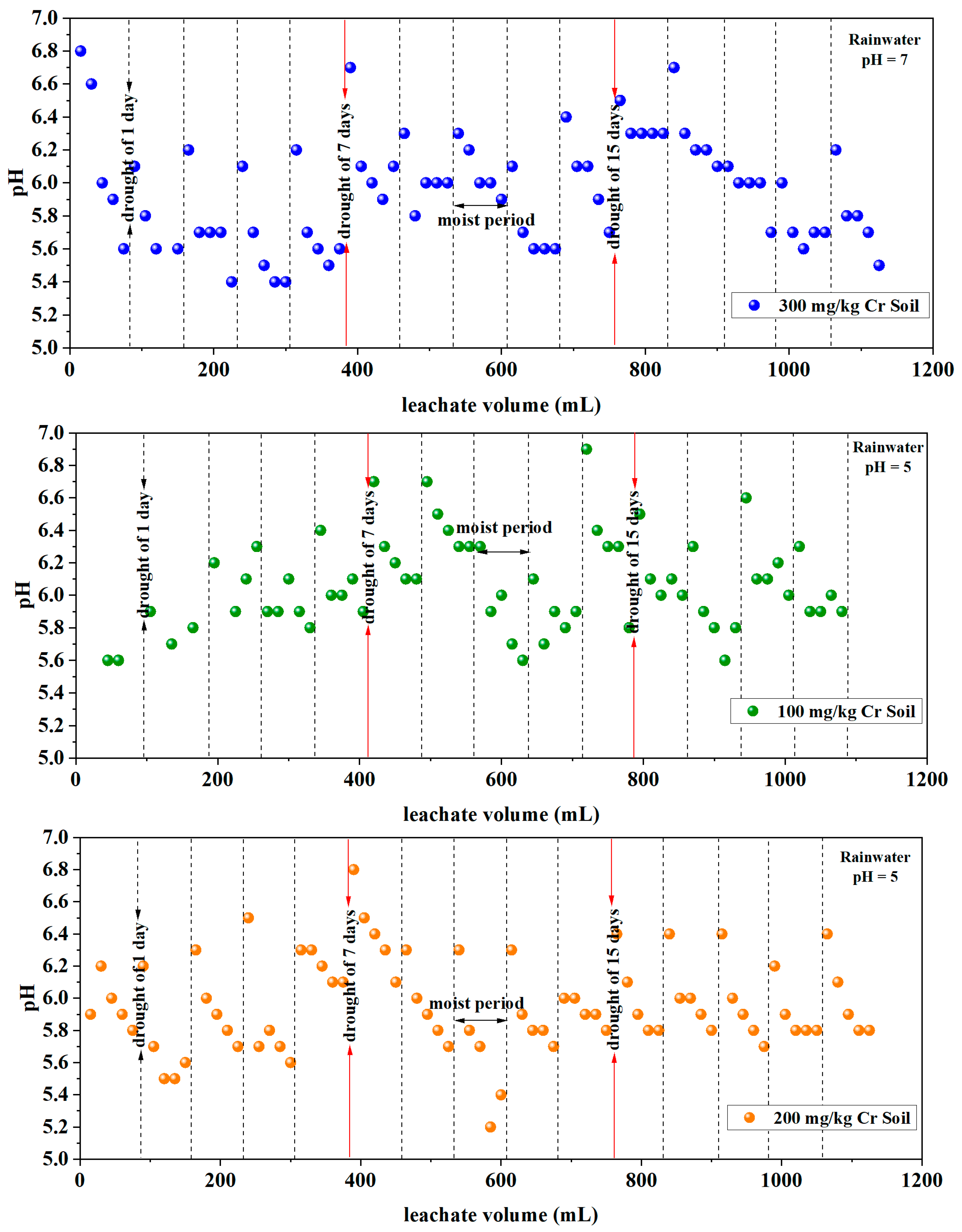
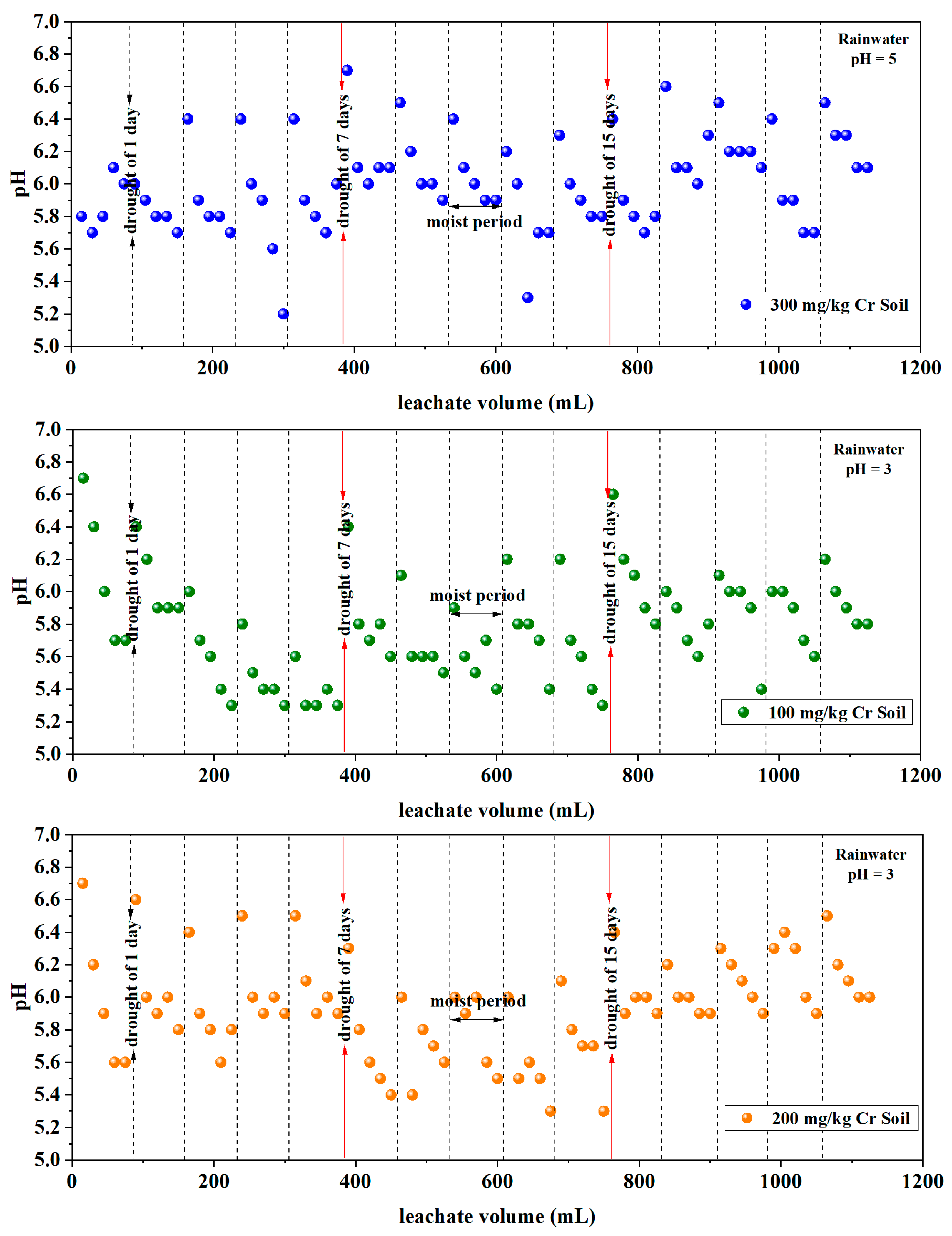
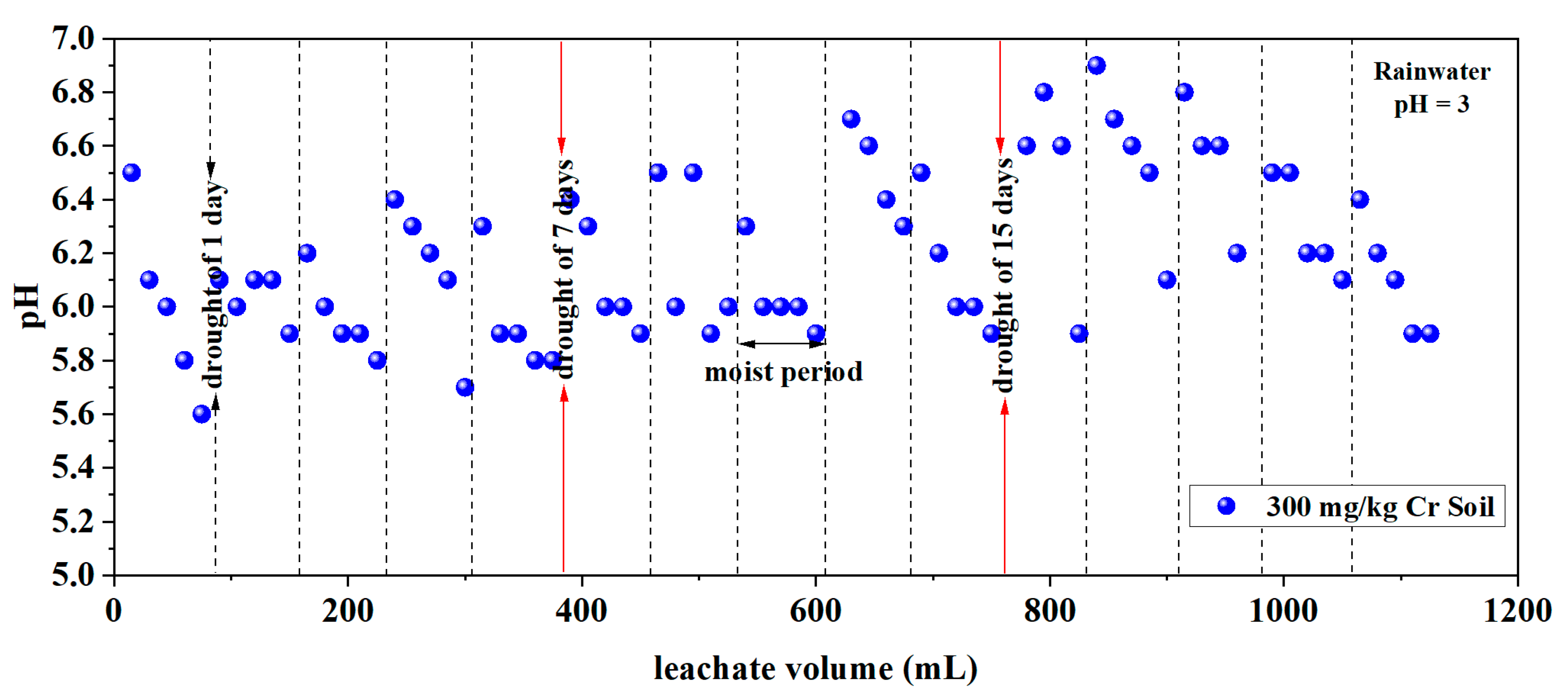
3.1.3. pH
3.1.4. Electrical Conductivity
3.1.5. Total Organic Carbon
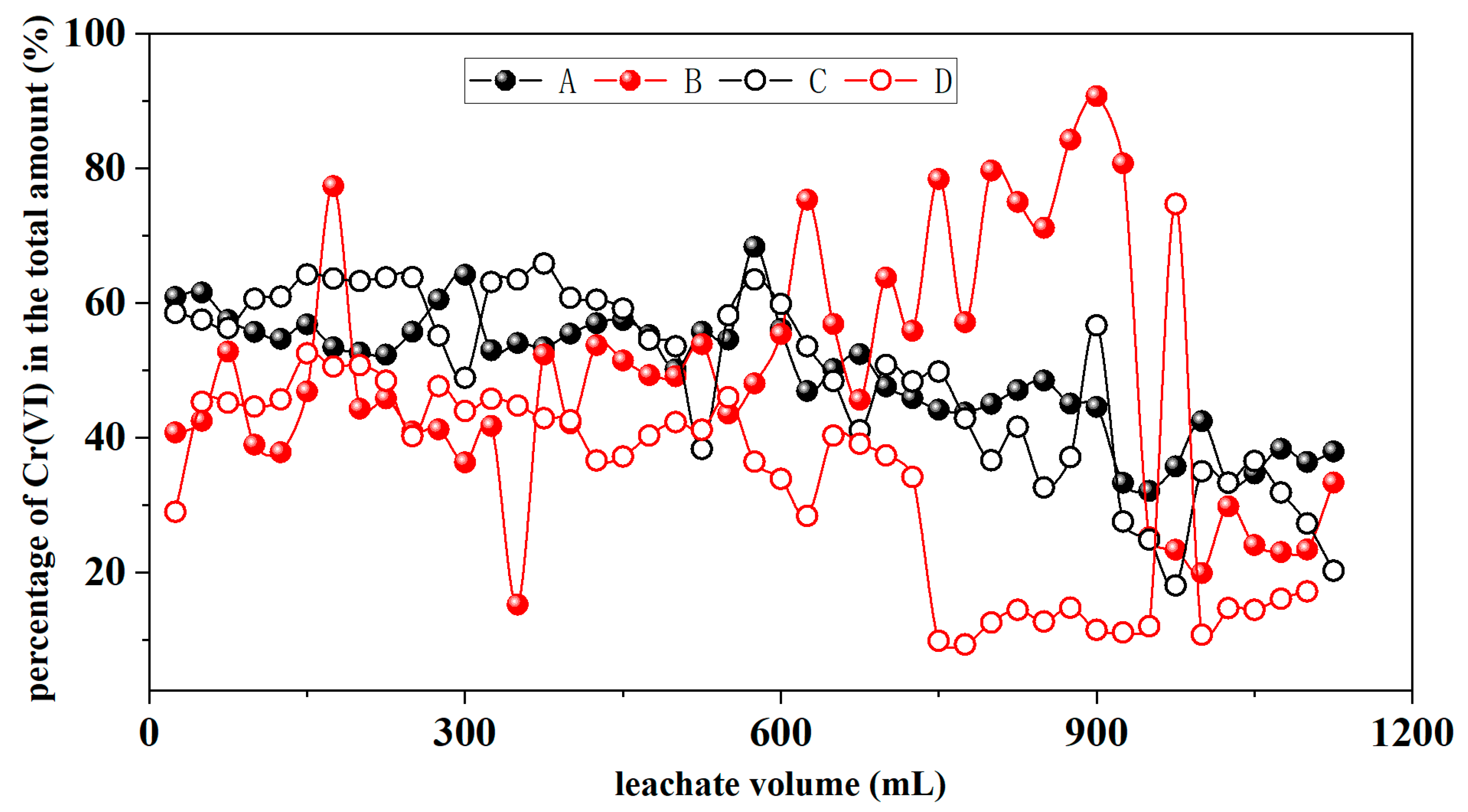
3.1.6. Cr(VI)
3.2. Soil
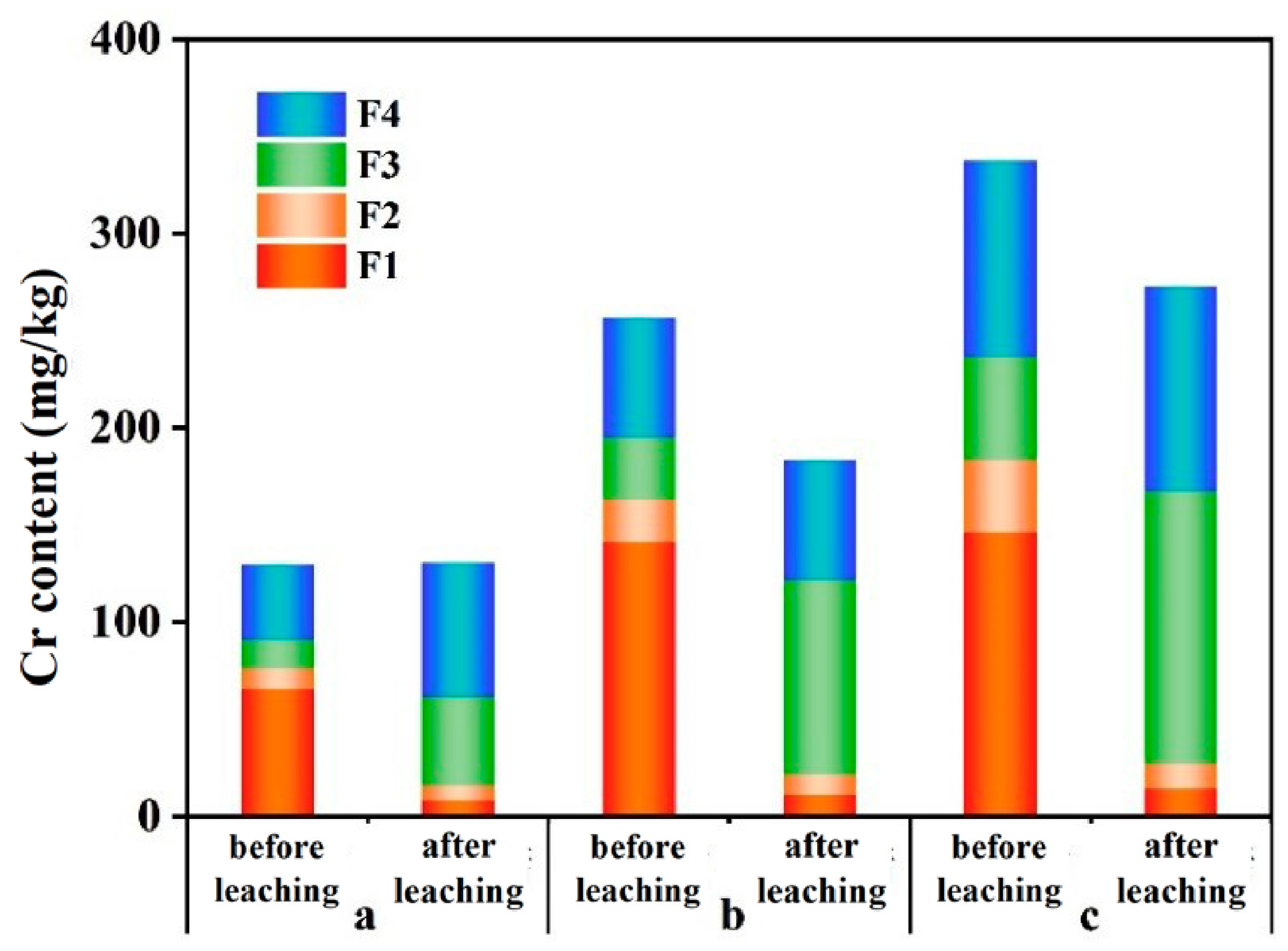
3.2.1. Chromium Content
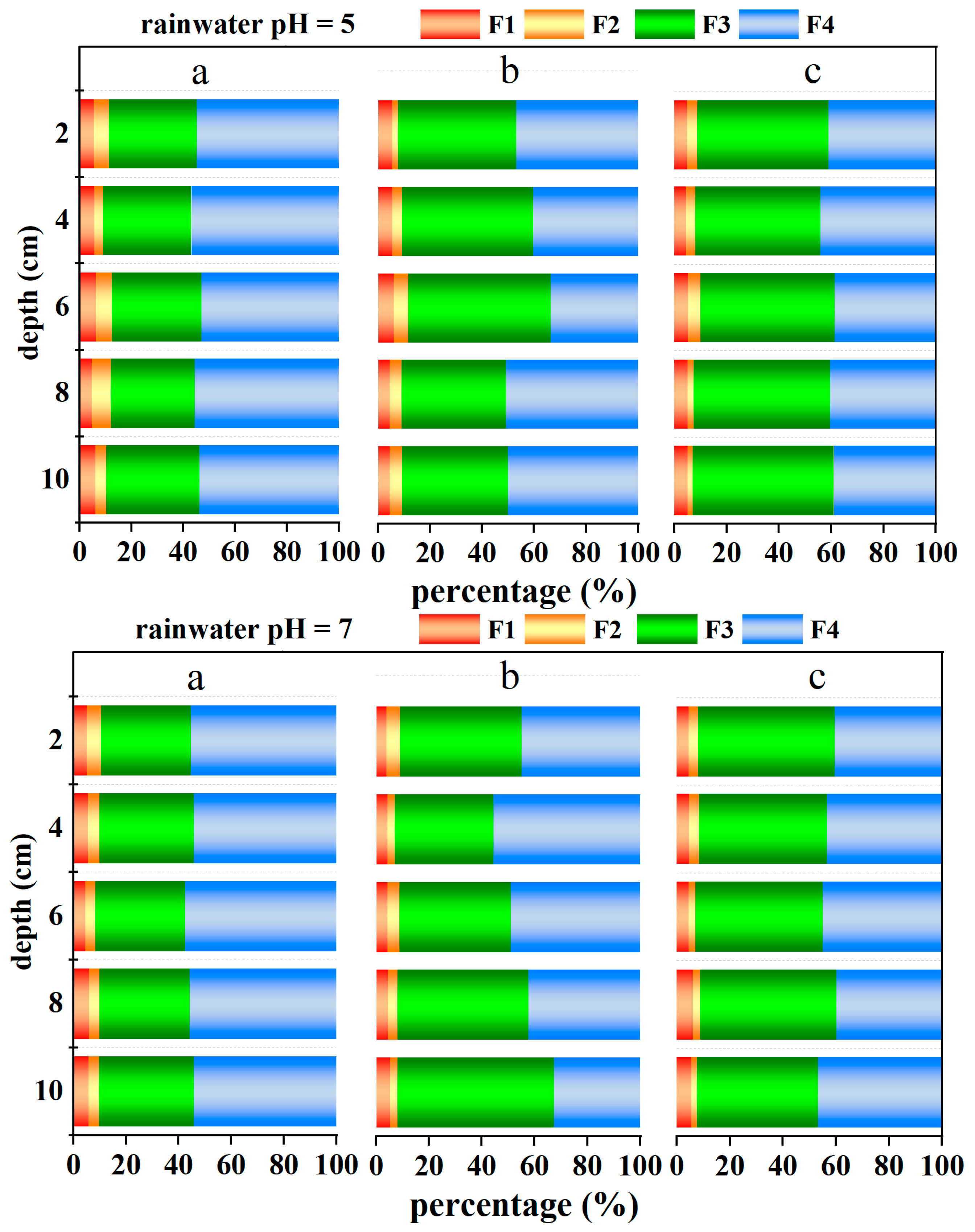
3.2.2. Sequential Extraction
3.2.3. Total Organic Carbon
4. Discussion
4.1. Release of Cr from Soil to Leachate
4.2. Variation in Cr in Different Soil Fractions
4.3. Redox of Cr under Alternating Wet–Dry Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wang, R.; Fan, L.; Chen, T.; Bai, Y.; Yu, Q.; Liu, Y. Assessment of multiple exposure to chemical elements and health risks among residents near Huodehong lead-zinc mining area in Yunnan, Southwest China. Chemosphere 2017, 174, 613–627. [Google Scholar] [CrossRef]
- Luo, X.; Yu, L.; Wang, C.; Yin, X.; Mosa, A.; Lv, J.; Sun, H. Sorption of vanadium (V) onto natural soil colloids under various solution pH and ionic strength conditions. Chemosphere 2017, 169, 609–617. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Bolan, N.; Wang, H. Characteristics and applications of biochar for remediating Cr(VI)-contaminated soils and wastewater. Environ. Geochem. Health 2020, 42, 1543–1567. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawai, K.; Moribe, M.; Niinae, M. Recovery of Cr as Cr(III) from Cr(VI)-contaminated kaolinite clay by electrokinetics coupled with a permeable reactive barrier. J. Hazard. Mater. 2014, 278, 297–303. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Alvarez, C.C.; Bravo Gómez, M.E.; Hernández Zavala, A. Hexavalent chromium: Regulation and health effects. J. Trace Elem. Med. Biol. 2021, 65, 126729. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef]
- Huang, L.; Yu, C.; Hopke, P.; Lioy, P.; Buckley, B.; Shin, J.; Fan, Z. Measurement of Soluble and Total Hexavalent Chromium in the Ambient Airborne Particles in New Jersey. Aerosol Air Qual. Res. 2014, 14, 1939–1949. [Google Scholar] [CrossRef]
- Wani, K.I.; Naeem, M.; Aftab, T. Chromium in plant-soil nexus: Speciation, uptake, transport and sustainable remediation techniques. Environ. Pollut. 2022, 315, 120350. [Google Scholar] [CrossRef]
- de Souza Braz, A.M.; Fernandes, A.R.; Ferreira, J.R.; Alleoni, L.R.F. Distribution coefficients of potentially toxic elements in soils from the eastern Amazon. Environ. Sci. Pollut. Res. 2013, 20, 7231–7242. [Google Scholar] [CrossRef]
- Fonseca, B.; Teixeira, A.; Figueiredo, H.; Tavares, T. Modelling of the Cr(VI) transport in typical soils of the North of Portugal. J. Hazard. Mater. 2009, 167, 756–762. [Google Scholar] [CrossRef]
- Jean-Soro, L.; Bordas, F.; Bollinger, J.C. Column leaching of chromium and nickel from a contaminated soil using EDTA and citric acid. Environ. Pollut. 2012, 164, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Wang, F.; Yan, C.; Tian, Z.; Chen, H.; Zhou, B.; Yuan, R.; Yao, J. Leaching behavior of metals from iron tailings under varying pH and low-molecular-weight organic acids. J. Hazard. Mater. 2020, 383, 121136. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Tan, J.; Li, W.; Singh, B.P.; Yang, X.; Bolan, N.; Chen, X.; Xu, S.; Bao, Y.; et al. An overview of the direct and indirect effects of acid rain on plants: Relationships among acid rain, soil, microorganisms, and plants. Sci. Total Environ. 2023, 873, 162388. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, X.; Chen, C. Leaching Behavior of Heavy Metals and Transformation of Their Speciation in Polluted Soil Receiving Simulated Acid Rain. PLoS ONE 2012, 7, e49664. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, Z.; Guo, Z.; Wang, M.; Zhu, H.; Han, X. Effect of simulated acid rain on leaching and transformation of vanadium in paddy soils from stone coal smelting area. Process. Saf. Environ. Prot. 2017, 109, 697–703. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Huang, J.; Chen, G.; Nie, X.; Ma, W.; Yao, H.; Zhen, J.; Zeng, G. Aging effect on the leaching behavior of heavy metals (Cu, Zn, and Cd) in red paddy soil. Environ. Sci. Pollut. Res. 2015, 22, 11467–11477. [Google Scholar] [CrossRef]
- Li, J.; Jia, C.; Lu, Y.; Tang, S.; Shim, H. Multivariate analysis of heavy metal leaching from urban soils following simulated acid rain. Microchem. J. 2015, 122, 89–95. [Google Scholar] [CrossRef]
- Zhai, H.; Xue, M.; Du, Z.; Wang, D.; Zhou, F.; Feng, P.; Liang, D. Leaching behaviors and chemical fraction distribution of exogenous selenium in three agricultural soils through simulated rainfall. Ecotoxicol. Environ. Saf. 2019, 173, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Z.; Hu, Y.; Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695, 133893. [Google Scholar] [CrossRef]
- Wang, H.; Ju, C.; Zhou, M.; Chen, J.; Kan, X.; Dong, Y.; Hou, H. Acid rain-dependent detailed leaching characteristics and simultaneous immobilization of Pb, Zn, Cr, and Cd from hazardous lead-zinc tailing. Environ. Pollut. 2022, 307, 119529. [Google Scholar] [CrossRef]
- Yang, L.; Wei, T.; Li, S.; Lv, Y.; Miki, T.; Yang, L.; Nagasaka, T. Immobilization persistence of Cu, Cr, Pb, Zn ions by the addition of steel slag in acidic contaminated mine soil. Hazard. Mater. 2021, 412, 125176. [Google Scholar] [CrossRef]
- Cederkvist, K.; Ingvertsen, S.T.; Jensen, M.B.; Holm, P.E. Behaviour of chromium(VI) in stormwater soil infiltration systems. Appl. Geochem. 2013, 35, 44–50. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, T.; Yang, Y.; Jackson, D. Leaching of cadmium, chromium, copper, lead, and zinc from two slag dumps with different environmental exposure periods under dynamic acidic condition. Ecotoxicol. Environ. Saf. 2014, 104, 43–50. [Google Scholar] [CrossRef]
- Li, Z.; Wu, L.; Zhang, H.; Luo, Y.; Christie, P. Effects of soil drying and wetting-drying cycles on the availability of heavy metals and their relationship to dissolved organic matter. J. Soils Sediments 2015, 15, 1510–1519. [Google Scholar] [CrossRef]
- Pang, X.; Chen, C.; Sun, J.; Zhan, H.; Xiao, Y.; Cai, J.; Yu, X.; Liu, Y.; Long, L.; Yang, G. Effects of complex pollution by microplastics and heavy metals on soil physicochemical properties and microbial communities under alternate wetting and drying conditions. J. Hazard. Mater. 2023, 458, 131989. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Barsanti, M.; Garcia-Tenorio, R.; Schirone, A.; Rozmaric, M.; Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.A.; Delbono, I.; Conte, F.; Godoy, J.M.D.O.; Heijnis, H.; et al. Challenges and limitations of the 210Pb sediment dating method: Results from an IAEA modelling interlaboratory comparison exercise. Quat. Geochronol. 2020, 59, 101093. [Google Scholar] [CrossRef]
- Cheng, Q.; Lou, G.; Huang, W.; Li, X. Assessment and potential sources of metals in the surface sediments of the Yellow River Delta, Eastern China. Environ. Sci. Pollut. Res. 2017, 24, 17446–17454. [Google Scholar] [CrossRef] [PubMed]
- Christophoridis, C.; Dedepsidis, D.; Fytianos, K. Occurrence and distribution of selected heavy metals in the surface sediments of Thermaikos Gulf, N. Greece. Assessment using pollution indicators. J. Hazard. Mater. 2009, 168, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.; Ruiz, E.; Alonso-Azcárate, J.; Rincón, J. Heavy metal distribution and chemical speciation in tailings and soils around a Pb–Zn mine in Spain. J. Environ. Manag. 2009, 90, 1106–1116. [Google Scholar] [CrossRef]
- Bohn, H.L. Redox Potentials. Soil Sci. 1971, 112, 39–45. [Google Scholar] [CrossRef]
- Frohne, T.; Diaz-Bone, R.A.; Du Laing, G.; Rinklebe, J. Impact of systematic change of redox potential on the leaching of Ba, Cr, Sr, and V from a riverine soil into water. J. Soils Sediments 2015, 15, 623–633. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Díaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2021, 73, e13203. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tong, J.; Hu, B.; Wei, W. Adsorption and desorption for dynamics transport of hexavalent chromium (Cr(VI)) in soil column. Environ. Sci. Pollut. Res. 2018, 25, 459–468. [Google Scholar] [CrossRef]
- Yu, J.; Klarup, D. Extraction kinetics of copper, zinc, iron, and manganese from contaminated sediment using disodium ethylenediaminetetraacetate. Water Air Soil Pollut. 1994, 75, 205. [Google Scholar] [CrossRef]
- Dhillon, S.K.; Dhillon, K.S.; Kohli, A.; Khera, K.L. Evaluation of leaching and runoff losses of selenium from seleniferous soils through simulated rainfall. J. Plant Nutr. Soil Sci. 2008, 171, 187–192. [Google Scholar] [CrossRef]
- Li, J.; Kosugi, T.; Riya, S.; Hashimoto, Y.; Hou, H.; Terada, A.; Hosomi, M. Pollution potential leaching index as a tool to assess water leaching risk of arsenic in excavated urban soils. Ecotoxicol. Environ. Saf. 2018, 147, 72–79. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Y.; Yang, K.; Rouff, A.; Elzinga, E.; Huang, J. Leaching characteristics of vanadium in mine tailings and soils near a vanadium titanomagnetite mining site. J. Hazard. Mater. 2014, 264, 498–504. [Google Scholar] [CrossRef]
- Fendorf, S.E. Surface Reactions of Chromium in Soils and Waters. Geoderma 1995, 67, 55–71. [Google Scholar] [CrossRef]
- Merckx, R.; Brans, K.; Smolders, E. Decomposition of dissolved organic carbon after soil drying and rewetting as an indicator of metal toxicity in soils. Soil Biol. Biochem. 2001, 33, 235–240. [Google Scholar] [CrossRef]
- Conesa, H.M.; María-Cervantes, A.; Álvarez-Rogel, J.; González-Alcaraz, M.N. Influence of soil properties on trace element availability and plant accumulation in a Mediterranean salt marsh polluted by mining wastes: Implications for phytomanagement. Sci. Total Environ. 2011, 409, 4470–4479. [Google Scholar] [CrossRef]
- Mitchell, K.; Trakal, L.; Sillerova, H.; Avelar-González, F.J.; Guerrero-Barrera, A.L.; Hough, R.; Beesley, L. Mobility of As, Cr and Cu in a contaminated grassland soil in response to diverse organic amendments; a sequential column leaching experiment. Appl. Geochem. 2018, 88, 95–102. [Google Scholar] [CrossRef]
- Sarpong, L.; Boadi, N.O.; Akoto, O. Metal Fractionation and Leaching in Soils from a Gold Mining Area in the Equatorial Rainforest Zone. J. Chem. 2023, 2023, 3542165. [Google Scholar] [CrossRef]
- Masscheleyn, P.H.; Pardue, J.H.; DeLaune, R.D.; Patrick, J.W. Chromium redox chemistry in a Lower Mississippi Valley bottomland hardwood wetland. Environ. Sci. Technol. 1992, 26, 1217–1226. [Google Scholar] [CrossRef]
- Banks, M.K.; Schwab, A.P.; Henderson, C. Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere 2006, 62, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Eckbo, C.; Okkenhaug, G.; Hale, S.E. The effects of soil organic matter on leaching of hexavalent chromium from concrete waste: Batch and column experiments. J. Environ. Manag. 2022, 309, 114708. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, J.; Brown, S.D.; Snape, C.E. Coals as sorbents for the removal and reduction of hexavalent chromium from aqueous waste streams. Fuel 2002, 81, 691–698. [Google Scholar] [CrossRef]




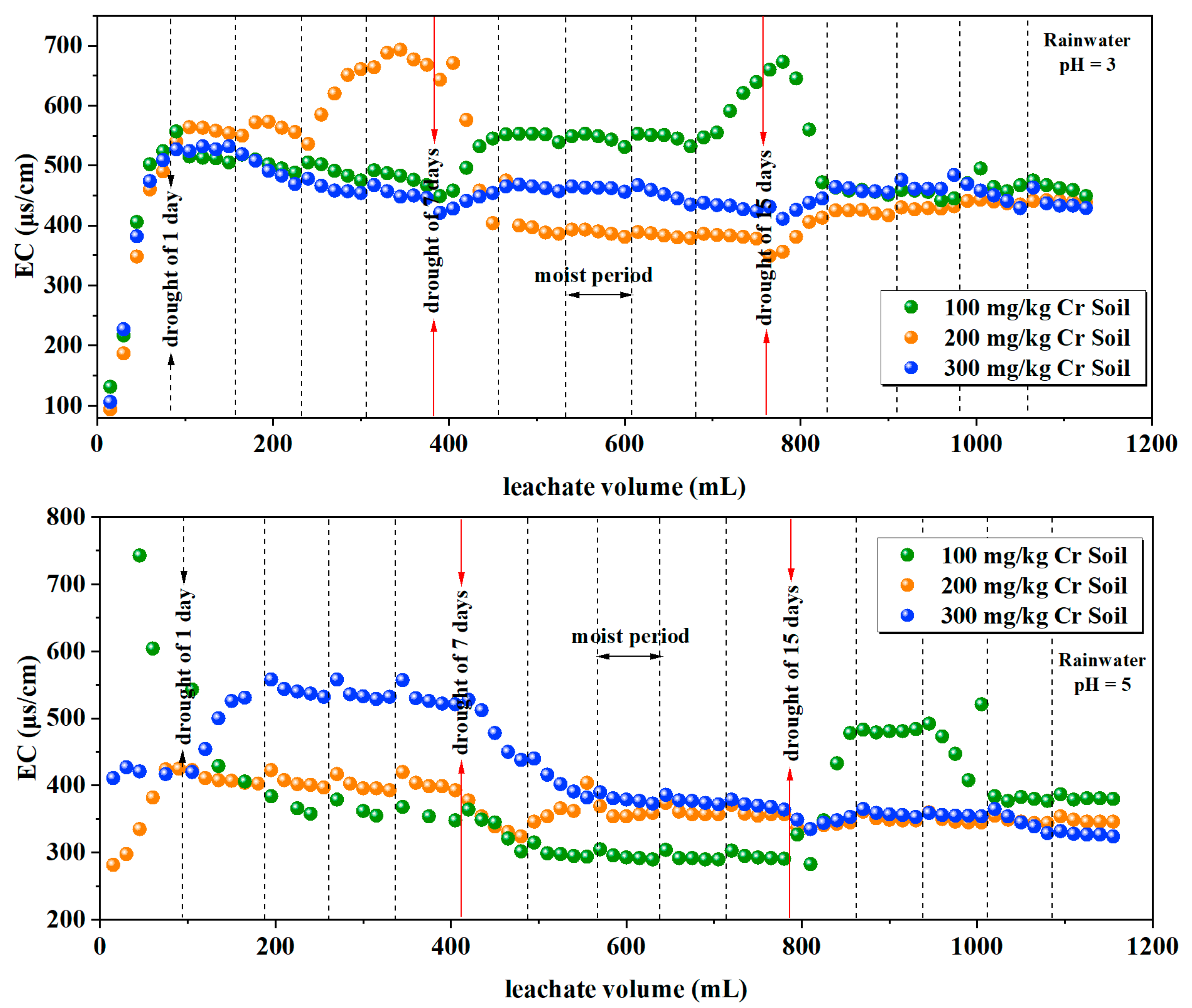
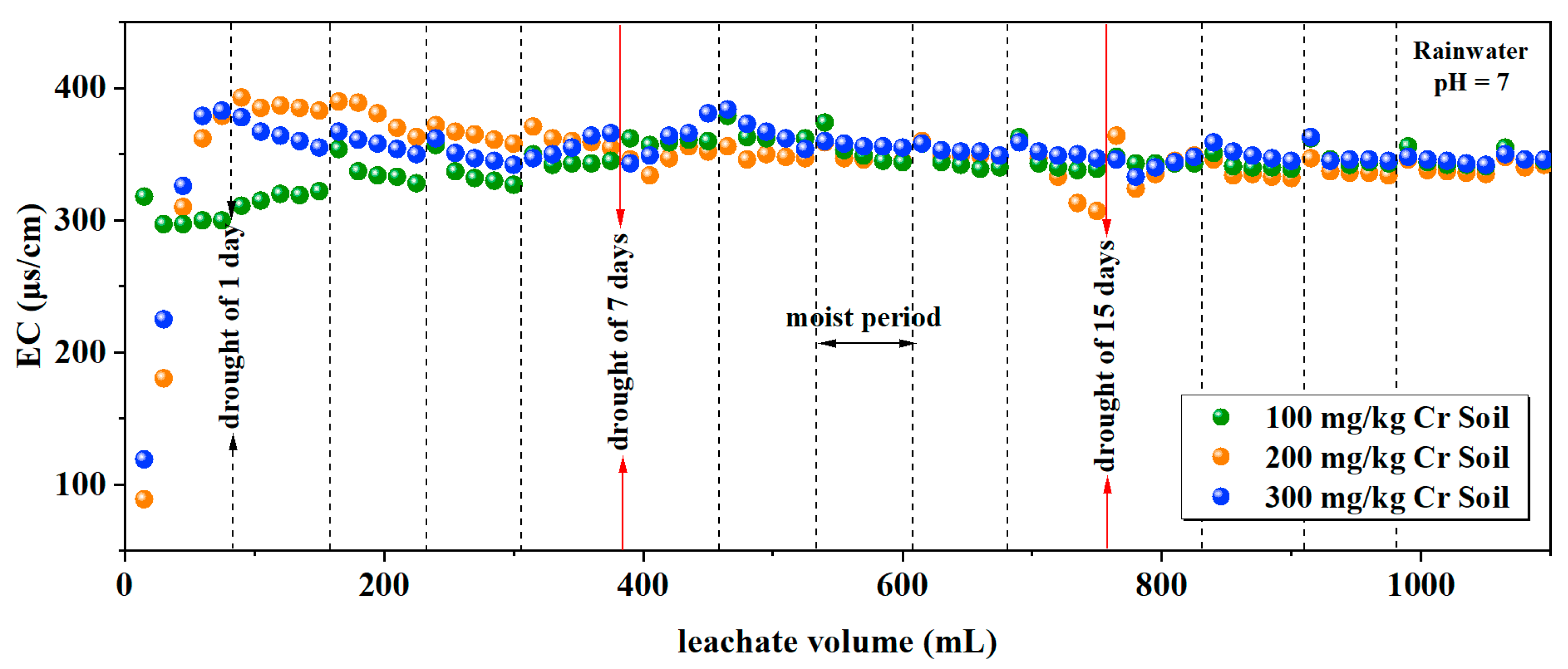


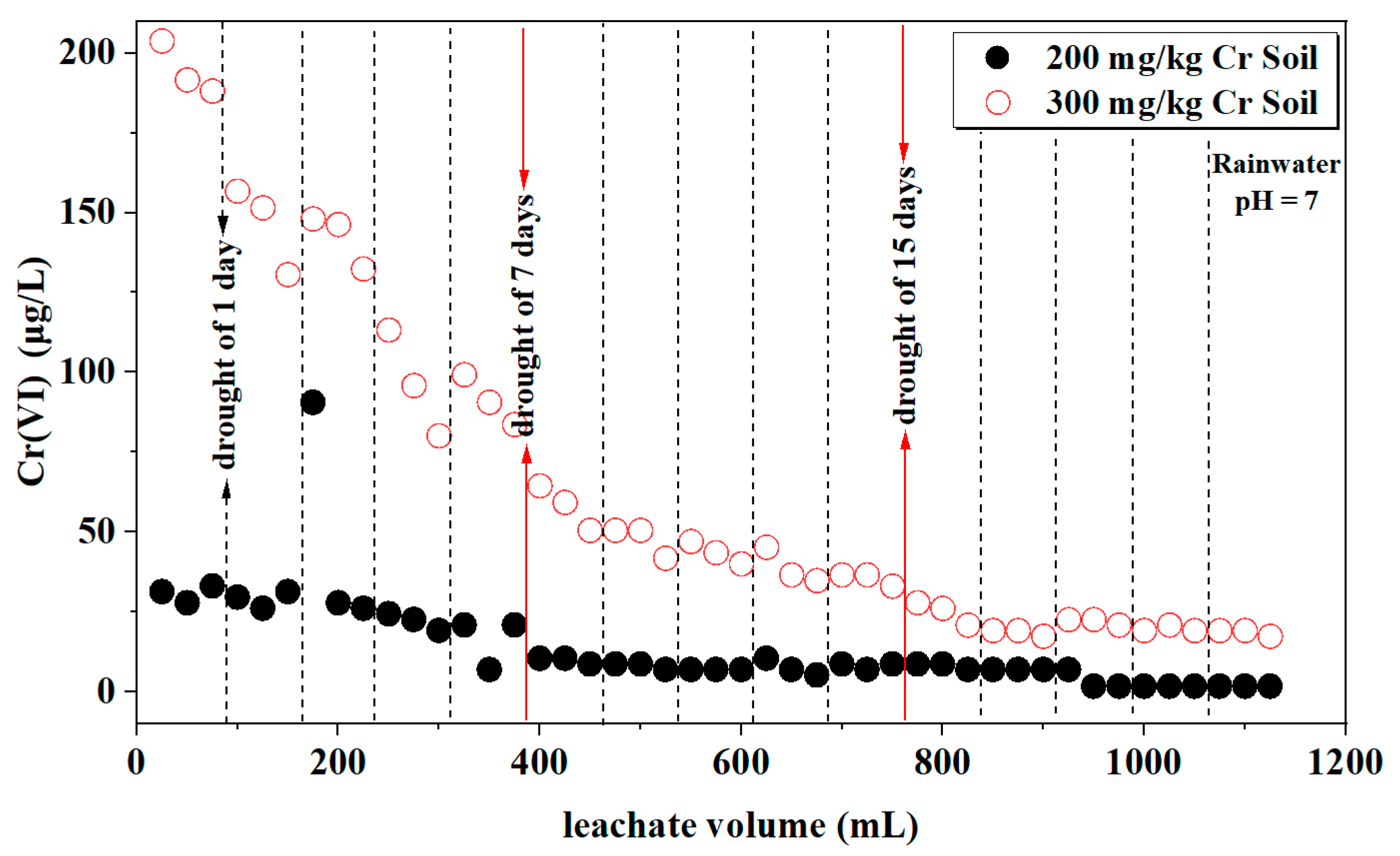
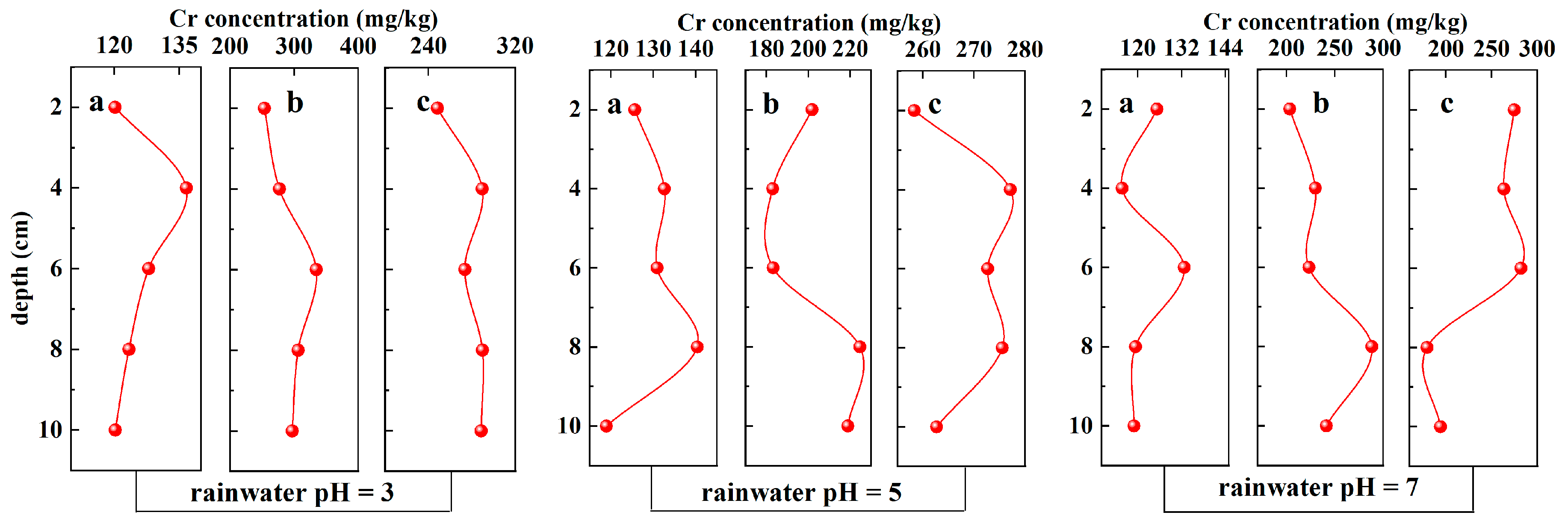

| Rainwater pH | Soil Cr Content (mg/kg) | Soil Column Mass (g) | Soil Mass(g) | Distilled Water Volume (mL) |
|---|---|---|---|---|
| 3 | 100 | 354.2 | 93.8 | 37.5 |
| 200 | 354.2 | 97.6 | 39 | |
| 300 | 354.2 | 93 | 37.1 | |
| 5 | 100 | 362.4 | 84.8 | 34 |
| 200 | 361.0 | 86.4 | 34.4 | |
| 300 | 354.2 | 91.8 | 36.4 | |
| 7 | 100 | 354.2 | 94 | 37.6 |
| 200 | 354.2 | 96 | 38.4 | |
| 300 | 354.2 | 95 | 38 |
| n | Mean | Std. Deviation | p-Value * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rainwater pH | 3 | 224 | 664 | 8.02 | 73.43 | 98.69 | 107.71 | 0.014 | <0.001 |
| 5 | 241 | 115.31 | 122.75 | ||||||
| 7 | 225 | 83.56 | 99.23 | ||||||
| Initial Soil Cr Content | 100 mg/kg | 213 | 664 | 14.87 | 73.43 | 24.02 | 107.71 | <0.001 | |
| 200 mg/kg | 227 | 70.22 | 96.68 | ||||||
| 300 mg/kg | 224 | 132.37 | 133.02 | ||||||
| n | Mean | Std. Deviation | p-Value * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rainwater pH | 3 | 225 | 663 | 245.07 | 255.7 | 33.58 | 33.14 | <0.001 | <0.001 |
| 5 | 213 | 265.57 | 25.52 | ||||||
| 7 | 225 | 256.96 | 35.96 | ||||||
| Initial Soil Cr Content | 100 mg/kg | 214 | 663 | 253.76 | 255.7 | 32.82 | 33.14 | 0.048 | |
| 200 mg/kg | 225 | 253.23 | 34.60 | ||||||
| 300 mg/kg | 224 | 260.02 | 31.62 | ||||||
| n | Mean | Std. Deviation | p-Value * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rainwater pH | 3 | 225 | 663 | 5.97 | 6 | 0.39 | 0.35 | 0.275 | <0.001 |
| 5 | 213 | 6.01 | 0.29 | ||||||
| 7 | 225 | 6.01 | 0.35 | ||||||
| Initial SoilCr Content | 100 mg/kg | 214 | 663 | 5.97 | 6 | 0.35 | 0.35 | 0.071 | |
| 200 mg/kg | 225 | 5.98 | 0.30 | ||||||
| 300 mg/kg | 224 | 6.04 | 0.38 | ||||||
| n | Mean | Std. Deviation | p-Value * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rainwater pH | 3 | 225 | 663 | 472.08 | 401.77 | 87.22 | 86 | <0.001 | <0.001 |
| 5 | 213 | 386.84 | 72.08 | ||||||
| 7 | 225 | 345.6 | 31.39 | ||||||
| Initial Soil Cr Content | 100 mg/kg | 214 | 663 | 395.74 | 401.77 | 99.31 | 86 | 0.173 | |
| 200 mg/kg | 225 | 403.27 | 85.32 | ||||||
| 300 mg/kg | 224 | 406.03 | 71.9 | ||||||
| n | Mean | Std. Deviation | p-Value * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rainwater pH | 3 | 135 | 405 | 11.78 | 10.37 | 11.07 | 8.19 | 0.048 | 0.451 |
| 5 | 135 | 9.57 | 4.23 | ||||||
| 7 | 135 | 9.76 | 7.68 | ||||||
| Initial Soil Cr Content | 100 mg/kg | 135 | 405 | 9.36 | 10.37 | 6.40 | 8.19 | 0.056 | |
| 200 mg/kg | 135 | 10.07 | 7.44 | ||||||
| 300 mg/kg | 135 | 11.68 | 10.16 | ||||||
| n | Mean | Std. Deviation | p-Value * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rainwater pH | 3 | 90 | 180 | 47.22 | 43.52 | 49.87 | 48.73 | 0.273 | <0.001 |
| 7 | 90 | 39.82 | 47.55 | ||||||
| Initial Soil Cr Content | 200 mg/kg | 90 | 180 | 30.25 | 43.52 | 43.34 | 48.73 | <0.001 | |
| 300 mg/kg | 90 | 56.79 | 50.41 | ||||||
| n | Mean | Std. Deviation | p-Value * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rainwater pH | 3 | 15 | 45 | 232.39 | 210.74 | 80.73 | 69.18 | <0.001 | <0.001 |
| 5 | 15 | 200.41 | 60.22 | ||||||
| 7 | 15 | 199.43 | 64.38 | ||||||
| Initial Soil Cr Content | 100 mg/kg | 15 | 45 | 125.90 | 210.74 | 7.36 | 69.18 | <0.001 | |
| 200 mg/kg | 15 | 244.35 | 46.61 | ||||||
| 300 mg/kg | 15 | 261.97 | 32.89 | ||||||
| n | Mean | Std. Deviation | p-Value * | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | Rainwater pH | 3 | 15 | 45 | 5.37 | 5.16 | 1.84 | 1.17 | 0.447 | 0.137 |
| 5 | 15 | 5.21 | 0.61 | |||||||
| 7 | 15 | 4.91 | 0.67 | |||||||
| Initial Soil Cr Content | 100 mg/kg | 15 | 45 | 5.96 | 5.16 | 1.56 | 1.17 | 0.003 | ||
| 200 mg/kg | 15 | 4.74 | 0.60 | |||||||
| 300 mg/kg | 15 | 4.79 | 0.72 | |||||||
| F2 | Rainwater pH | 3 | 15 | 45 | 4.15 | 4.03 | 2.51 | 1.75 | 0.203 | <0.001 |
| 5 | 15 | 4.31 | 1.48 | |||||||
| 7 | 15 | 3.63 | 0.92 | |||||||
| Initial Soil Cr Content | 100 mg/kg | 15 | 45 | 5.62 | 4.03 | 1.76 | 1.75 | <0.001 | ||
| 200 mg/kg | 15 | 3.63 | 1.05 | |||||||
| 300 mg/kg | 15 | 2.84 | 1.00 | |||||||
| F3 | Rainwater pH | 3 | 15 | 45 | 43.47 | 43.66 | 8.44 | 8 | 0.967 | 0.528 |
| 5 | 15 | 43.85 | 8.19 | |||||||
| 7 | 15 | 43.65 | 7.92 | |||||||
| Initial Soil Cr Content | 100 mg/kg | 15 | 45 | 33.86 | 43.66 | 1.64 | 8 | <0.001 | ||
| 200 mg/kg | 15 | 47.41 | 6.02 | |||||||
| 300 mg/kg | 15 | 49.71 | 2.22 | |||||||
| F4 | Rainwater pH | 3 | 15 | 45 | 47.00 | 47.15 | 5.34 | 6.69 | 0.749 | 0.707 |
| 5 | 15 | 46.64 | 7.46 | |||||||
| 7 | 15 | 47.81 | 7.46 | |||||||
| Initial Soil Cr Content | 100 mg/kg | 15 | 45 | 54.57 | 47.15 | 1.76 | 6.69 | <0.001 | ||
| 200 mg/kg | 15 | 44.21 | 6.37 | |||||||
| 300 mg/kg | 15 | 42.67 | 2.67 | |||||||
| Rainwater pH | Chromium-Containing Soil (mg/kg) | Organic Matter Content (mg/L) |
|---|---|---|
| Before Leaching | 30.85 | |
| 7 | 100 | 0.65 |
| 200 | 0.88 | |
| 300 | 0.74 | |
| 5 | 100 | 0.78 |
| 200 | 0.79 | |
| 300 | 0.74 | |
| 3 | 100 | 0.77 |
| 200 | 0.58 | |
| 300 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Chen, Y.; Liang, J.; Sun, Z.; Zhao, H.; Huang, Y. The Release and Migration of Cr in the Soil under Alternating Wet–Dry Conditions. Toxics 2024, 12, 140. https://doi.org/10.3390/toxics12020140
Chen Z, Chen Y, Liang J, Sun Z, Zhao H, Huang Y. The Release and Migration of Cr in the Soil under Alternating Wet–Dry Conditions. Toxics. 2024; 12(2):140. https://doi.org/10.3390/toxics12020140
Chicago/Turabian StyleChen, Zhe, Ying Chen, Jing Liang, Zhiyu Sun, Haoren Zhao, and Yi Huang. 2024. "The Release and Migration of Cr in the Soil under Alternating Wet–Dry Conditions" Toxics 12, no. 2: 140. https://doi.org/10.3390/toxics12020140
APA StyleChen, Z., Chen, Y., Liang, J., Sun, Z., Zhao, H., & Huang, Y. (2024). The Release and Migration of Cr in the Soil under Alternating Wet–Dry Conditions. Toxics, 12(2), 140. https://doi.org/10.3390/toxics12020140






