Associations Between Brominated Flame Retardant Exposure and Depression in Adults: A Cross-Sectional Study
Abstract
:1. Introduction
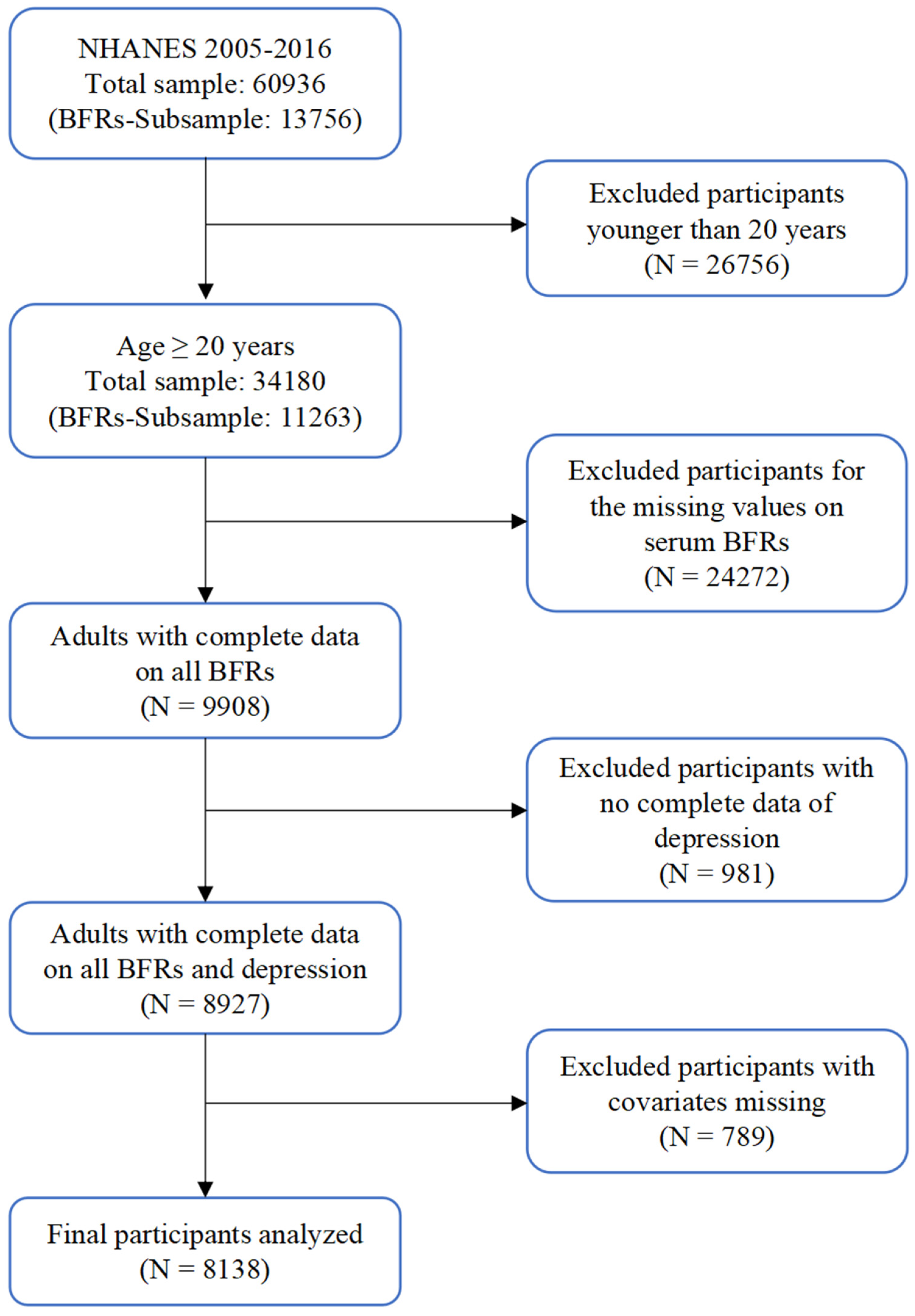
2. Methods
2.1. Study Population
2.2. Depression Definition
2.3. Measurement of Serum BFRs
2.4. Covariates
2.5. Statistical Analyses
2.6. Sensitivity Analysis
3. Results
3.1. Basic Characteristics of Participants
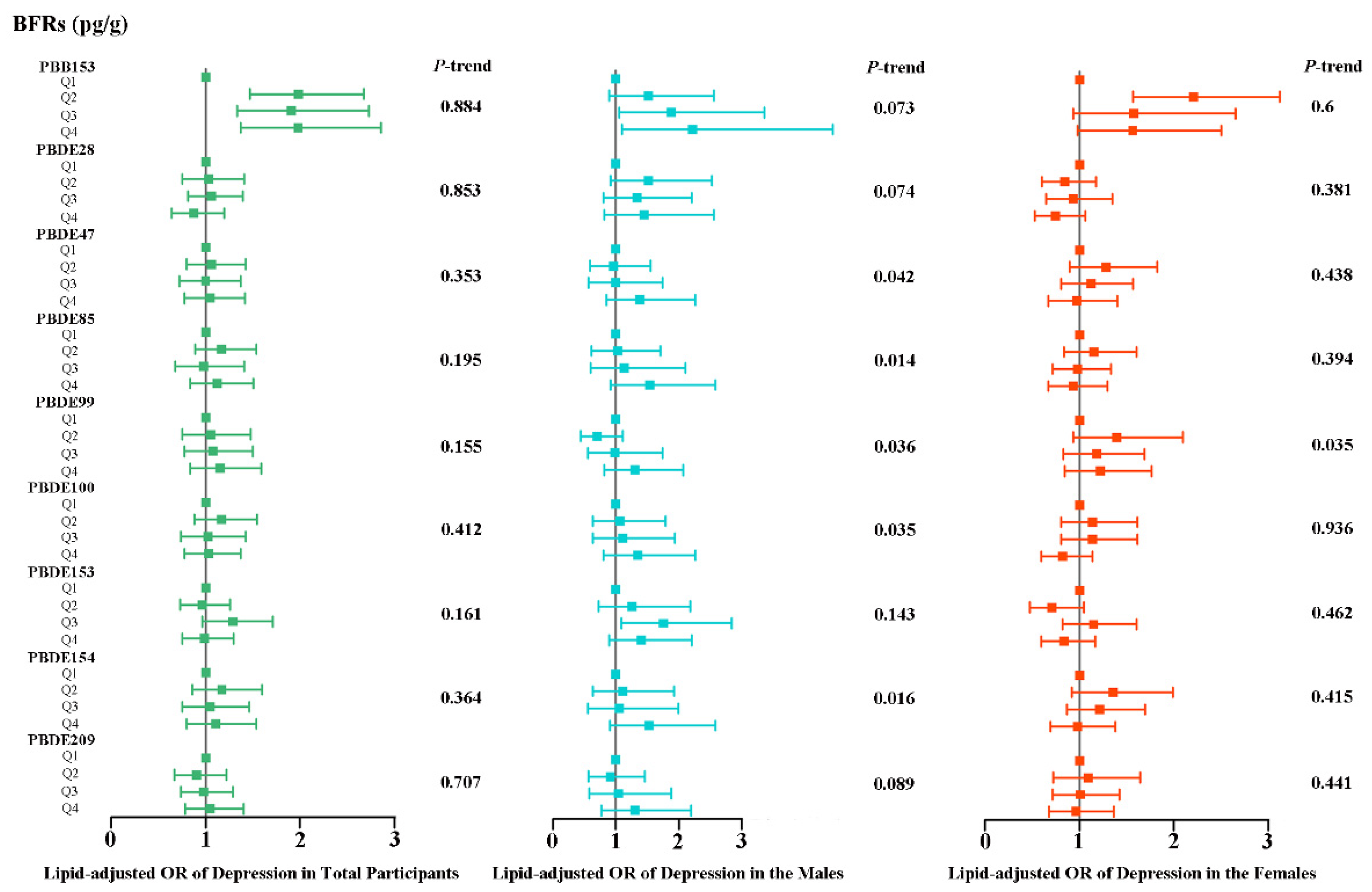
3.2. Associations of Individual BFRs with Depression
3.3. Associations of All BFRs with Depression in QGC Analyses
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef]
- Yu, G.; Bu, Q.; Cao, Z.; Du, X.; Xia, J.; Wu, M.; Huang, J. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 2016, 150, 479–490. [Google Scholar] [CrossRef]
- Li, Q.; Guo, M.; Song, H.; Cui, J.; Zhan, M.; Zou, Y.; Li, J.; Zhang, G. Size distribution and inhalation exposure of airborne particle-bound polybrominated diphenyl ethers, new brominated flame retardants, organophosphate esters, and chlorinated paraffins at urban open consumption place. Sci. Total Environ. 2021, 794, 148695. [Google Scholar] [CrossRef] [PubMed]
- Muenhor, D.; Harrad, S.; Ali, N.; Covaci, A. Brominated flame retardants (BFRs) in air and dust from electronic waste storage facilities in Thailand. Environ. Int. 2010, 36, 690–698. [Google Scholar] [CrossRef]
- Ma, Y.; Stubbings, W.A.; Jin, J.; Cline-Cole, R.; Abdallah, M.A.; Harrad, S. Impact of Legislation on Brominated Flame Retardant Concentrations in UK Indoor and Outdoor Environments: Evidence for Declining Indoor Emissions of Some Legacy BFRs. Environ. Sci. Technol. 2024, 58, 4237–4246. [Google Scholar] [CrossRef] [PubMed]
- Feiteiro, J.; Mariana, M.; Cairrão, E. Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021, 285, 117475. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Feng, Q.; Zhang, J.; Wang, X.; Zhao, L.; Fan, Y.; Hu, P.; Wei, P.; Bu, Q.; Cao, Z. Global patterns of human exposure to flame retardants indoors. Sci. Total Environ. 2024, 912, 169393. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; La Guardia, M.J.; Luellen, D.R.; Harvey, E.; Mainor, T.M.; Hale, R.C. Do temporal and geographical patterns of HBCD and PBDE flame retardants in U.S. fish reflect evolving industrial usage? Environ. Sci. Technol. 2011, 45, 8254–8261. [Google Scholar] [CrossRef]
- de Boer, J.; Harrad, S.; Sharkey, M. The European Regulatory Strategy for flame retardants—The right direction but still a risk of getting lost. Chemosphere 2024, 347, 140638. [Google Scholar] [CrossRef] [PubMed]
- UNEP Persistent Organic Pollutants (POPs) and Pesticides. Available online: https://www.unep.org/cep/persistent-organic-pollutants-pops-and-pesticides (accessed on 3 December 2024).
- Wu, M.; Wu, D.; Wang, C.; Guo, Z.; Li, B.; Zuo, Z. Hexabromocyclododecane exposure induces cardiac hypertrophy and arrhythmia by inhibiting miR-1 expression via up-regulation of the homeobox gene Nkx2.5. J. Hazard. Mater. 2016, 302, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Jia, H.; Chen, R.; Pan, K.; Fan, Z.; Su, C.; Wu, Z.; Zhang, T. Associations between exposure to brominated flame retardants and metabolic syndrome and its components in U.S. adults. Sci. Total Environ. 2023, 858 Pt 2, 159935. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tanguay, R.L.; Xiao, Y.; Haggard, D.E.; Ge, X.; Jia, Y.; Zheng, Y.; Dong, Q.; Huang, C.; Lin, K. TBBPA exposure during a sensitive developmental window produces neurobehavioral changes in larval zebrafish. Environ. Pollut. 2016, 216, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Al-Mousa, F.; Michelangeli, F. Some commonly used brominated flame retardants cause Ca2+-ATPase inhibition, beta-amyloid peptide release and apoptosis in SH-SY5Y neuronal cells. PLoS ONE 2012, 7, e33059. [Google Scholar] [CrossRef]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human health risk associated with brominated flame-retardants (BFRs). Environ. Int. 2015, 74, 170–180. [Google Scholar] [CrossRef]
- Liang, S.; Xu, F.; Tang, W.; Zhang, Z.; Zhang, W.; Liu, L.; Wang, J.; Lin, K. Brominated flame retardants in the hair and serum samples from an e-waste recycling area in southeastern China: The possibility of using hair for biomonitoring. Environ. Sci. Pollut. Res. Int. 2016, 23, 14889–14897. [Google Scholar] [CrossRef] [PubMed]
- de Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Wu, P.; Ding, C.; Yan, M.; Qian, B.; Wang, W.; Sun, P.; Zhao, J. Perfluorooctane sulfonate induces apoptosis via activation of FoxO3a and upregulation of proapoptotic Bcl-2 proteins in PC12 cells. J. Toxicol. Sci. 2019, 44, 657–666. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.; Qu, J.; You, H.; Liu, D. New understanding of novel brominated flame retardants (NBFRs): Neuro(endocrine) toxicity. Ecotoxicol. Environ. Saf. 2021, 208, 111570. [Google Scholar] [CrossRef] [PubMed]
- WHO Depression. 2024. Available online: https://www.who.int/health-topics/depression#tab=tab_1 (accessed on 3 December 2024).
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; de Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 9, 90. [Google Scholar] [CrossRef]
- Simon, G.E.; Moise, N.; Mohr, D.C. Management of Depression in Adults: A Review. JAMA 2024, 332, 141–152. [Google Scholar] [CrossRef]
- Parker, G.; Brotchie, H. Gender differences in depression. Int. Rev. Psychiatry 2010, 22, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, G.S. Depression in the elderly. Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef]
- Seiffge-Krenke, I. Depression in children and adolescents: Prevalence, diagnosis, etiology, gender differences and therapeutic approaches. Prax. Kinderpsychol. Kinderpsychiatr. 2007, 56, 185–205. [Google Scholar] [CrossRef]
- Medeiros, G.C.; Prueitt, W.L.; Minhajuddin, A.; Patel, S.S.; Czysz, A.H.; Furman, J.L.; Mason, B.L.; Rush, A.J.; Jha, M.K.; Trivedi, M.H. Childhood maltreatment and impact on clinical features of major depression in adults. Psychiatry Res. 2020, 293, 113412. [Google Scholar] [CrossRef]
- Dagher, R.K.; Bruckheim, H.E.; Colpe, L.J.; Edwards, E.; White, D.B. Perinatal Depression: Challenges and Opportunities. J. Womens Health 2021, 30, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, S.; Huang, H.; Xu, L. Neurotoxicity of melittin: Role of mitochondrial oxidative phosphorylation system in synaptic plasticity dysfunction. Toxicology 2023, 497–498, 153628. [Google Scholar] [CrossRef]
- Yi, W.; Xuan, L.; Zakaly, H.M.H.; Markovic, V.; Miszczyk, J.; Guan, H.; Zhou, P.K.; Huang, R. Association between per- and polyfluoroalkyl substances (PFAS) and depression in U.S. adults: A cross-sectional study of NHANES from 2005 to 2018. Environ. Res 2023, 238 Pt 2, 117188. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, Z.; Ma, X.; Wang, X.; Chen, L.; Luo, Y.; Cao, X.; Yu, S.; Wang, X.; Cao, Y.; et al. The association between polycyclic aromatic hydrocarbons exposure and neuropsychiatric manifestations in perimenopausal women: A cross-sectional study. J. Affect. Disord. 2024, 344, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ 2012, 184, E191–E196. [Google Scholar] [CrossRef] [PubMed]
- Levis, B.; Sun, Y.; He, C.; Wu, Y.; Krishnan, A.; Bhandari, P.M.; Neupane, D.; Imran, M.; Brehaut, E.; Negeri, Z.; et al. Accuracy of the PHQ-2 Alone and in Combination With the PHQ-9 for Screening to Detect Major Depression: Systematic Review and Meta-analysis. JAMA 2020, 323, 2290–2300. [Google Scholar] [CrossRef]
- Wu, Y.; Song, J.; Zhang, Q.; Yan, S.; Sun, X.; Yi, W.; Pan, R.; Cheng, J.; Xu, Z.; Su, H. Association between organophosphorus pesticide exposure and depression risk in adults: A cross-sectional study with NHANES data. Environ. Pollut. 2023, 316 Pt 1, 120445. [Google Scholar] [CrossRef]
- CDC National Health and Nutrition Examination Survey (NHANES). Anthropometry Procedures Manual. 2016. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/public/2015/manuals/2016_Anthropometry_Procedures_Manual.pdf (accessed on 3 December 2024).
- NHANES—National Health and Nutrition Examination Survey. 2020. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2015/DataFiles/BFRPOL_I.htm (accessed on 3 December 2024).
- Sjödin, A.; Jones, R.S.; Caudill, S.P.; Wong, L.Y.; Turner, W.E.; Calafat, A.M. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the national health and nutrition examination survey: 2003–2008. Environ. Sci. Technol. 2014, 48, 753–760. [Google Scholar] [CrossRef]
- Jain, R.B. Analysis of self-reported versus biomarker based smoking prevalence: Methodology to compute corrected smoking prevalence rates. Biomarkers 2017, 22, 476–487. [Google Scholar] [CrossRef]
- NHANES—National Health and Nutrition Examination Survey. 2019. Available online: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2015/DataFiles/COT_I.htm (accessed on 3 December 2024).
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef] [PubMed]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef]
- Höglund, P.; Hakelind, C.; Nordin, S. Severity and prevalence of various types of mental ill-health in a general adult population: Age and sex differences. BMC Psychiatry 2020, 20, 209. [Google Scholar] [CrossRef]
- CDC. Polybrominated Diphenyl Ethers (PBDEs). 2024. Available online: https://wwwn.cdc.gov/TSP/substances/ToxSubstance.aspx?toxid=183 (accessed on 3 December 2024).
- Keil, A. The Qgcomp Package: G-Computation on Exposure Quantiles. 2023. Available online: https://cran.r-project.org/web/packages/qgcomp/vignettes/qgcomp-vignette.html (accessed on 3 December 2024).
- Berk, M.; Williams, L.J.; Andreazza, A.C.; Pasco, J.A.; Dodd, S.; Jacka, F.N.; Moylan, S.; Reiner, E.J.; Magalhaes, P.V. Pop, heavy metal and the blues: Secondary analysis of persistent organic pollutants (POP), heavy metals and depressive symptoms in the NHANES National Epidemiological Survey. BMJ Open 2014, 4, e005142. [Google Scholar] [CrossRef]
- Fu, X.; Li, H.; Song, L.; Cen, M.; Wu, J. Association of urinary heavy metals co-exposure and adult depression: Modification of physical activity. Neurotoxicology 2023, 95, 117–126. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, H. Joint association of heavy metals and polycyclic aromatic hydrocarbons exposure with depression in adults. Environ. Res. 2024, 242, 117807. [Google Scholar] [CrossRef]
- Kim, J.H.; Moon, N.; Ji, E.; Moon, H.B. Effects of postnatal exposure to phthalate, bisphenol a, triclosan, parabens, and per- and poly-fluoroalkyl substances on maternal postpartum depression and infant neurodevelopment: A korean mother-infant pair cohort study. Environ. Sci. Pollut. Res. Int. 2023, 30, 96384–96399. [Google Scholar] [CrossRef] [PubMed]
- Sobrian, S.K.; Marr, L.; Ressman, K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, L.; Bai, Y.; Zhou, R.; Chen, L. Hypothalamic-pituitary-adrenal axis hyperactivity accounts for anxiety- and depression-like behaviors in rats perinatally exposed to bisphenol A. J. Biomed. Res. 2015, 29, 250–258. [Google Scholar]
- Wang, J.; Dai, G.D. Comparative Effects of Brominated Flame Retardants BDE-209, TBBPA, and HBCD on Neurotoxicity in Mice. Chem. Res. Toxicol. 2022, 35, 1512–1518. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, Z.; Zhu, H.; Li, N.; Zhao, H.; Mi, W.; Jiang, W.; Hu, X.; Ye, L. Tris-(2,3-dibromopropyl) isocyanurate induces depression-like behaviors and neurotoxicity by oxidative damage and cell apoptosis in vitro and in vivo. J. Toxicol. Sci. 2015, 40, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Hu, Z.; Wang, H.; Zhu, H.; Dong, Z.; Jiang, W.; Zhao, H.; Li, N.; Mi, W.; Wang, W.; et al. Tris-(2,3-Dibromopropyl) Isocyanurate, a New Emerging Pollutant, Impairs Cognition and Provokes Depression-Like Behaviors in Adult Rats. PLoS ONE 2015, 10, e0140281. [Google Scholar] [CrossRef]
- Cao, M.; Xu, T.; Zhang, H.; Wei, S.; Wang, H.; Song, Y.; Guo, X.; Chen, D.; Yin, D. BDE-47 Causes Depression-like Effects in Zebrafish Larvae via a Non-Image-Forming Visual Mechanism. Environ. Sci. Technol. 2023, 57, 9592–9602. [Google Scholar] [CrossRef]
- Hendriks, H.S.; Westerink, R.H. Neurotoxicity and risk assessment of brominated and alternative flame retardants. Neurotoxicol. Teratol. 2015, 52, 248–269. [Google Scholar] [CrossRef]
- Peng, G.J.; Tian, J.S.; Gao, X.X.; Zhou, Y.Z.; Qin, X.M. Research on the Pathological Mechanism and Drug Treatment Mechanism of Depression. Curr. Neuropharmacol. 2015, 13, 514–523. [Google Scholar] [CrossRef]
- He, M.C.; Shi, Z.; Sha, N.N.; Chen, N.; Peng, S.Y.; Liao, D.F.; Wong, M.S.; Dong, X.L.; Wang, Y.J.; Yuan, T.F.; et al. Paricalcitol alleviates lipopolysaccharide-induced depressive-like behavior by suppressing hypothalamic microglia activation and neuroinflammation. Biochem. Pharmacol. 2019, 163, 1–8. [Google Scholar] [CrossRef]
- Guan, W.; Xu, D.W.; Ji, C.H.; Wang, C.N.; Liu, Y.; Tang, W.Q.; Gu, J.H.; Chen, Y.M.; Huang, J.; Liu, J.F.; et al. Hippocampal miR-206-3p participates in the pathogenesis of depression via regulating the expression of BDNF. Pharmacol. Res. 2021, 174, 105932. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Wang, Q.; Guo, Y.; Li, N.; Ma, M.; Zhou, B. The neurotoxicity of DE-71: Effects on neural development and impairment of serotonergic signaling in zebrafish larvae. J. Appl. Toxicol. 2016, 36, 1605–1613. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carmassi, C.; Mucci, F.; Marazziti, D. Depression, Serotonin and Tryptophan. Curr. Pharm. Des. 2016, 22, 949–954. [Google Scholar] [CrossRef]
- Cardon, I.; Grobecker, S.; Jenne, F.; Jahner, T.; Rupprecht, R.; Milenkovic, V.M.; Wetzel, C.H. Serotonin effects on human iPSC-derived neural cell functions: From mitochondria to depression. Mol. Psychiatry 2024, 29, 2689–2700. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Qiao, S.; Ma, W.; Cai, J.; Zhang, X.; Zhang, Z. Melatonin administration alleviates 2,2,4,4-tetra-brominated diphenyl ether (PBDE-47)-induced necroptosis and secretion of inflammatory factors via miR-140-5p/TLR4/NF-κB axis in fish kidney cells. Fish. Shellfish. Immunol. 2022, 128, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Sun, Q.; Qiu, H.; Yang, K.; Xiao, B.; Xia, T.; Wang, A.; Gao, H.; Zhang, S. Melatonin protects against developmental PBDE-47 neurotoxicity by targeting the AMPK/mitophagy axis. J. Pineal Res. 2023, 75, e12871. [Google Scholar] [CrossRef]
- Won, E.; Na, K.S.; Kim, Y.K. Associations between Melatonin, Neuroinflammation, and Brain Alterations in Depression. Int. J. Mol. Sci. 2021, 23, 305. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Monti, J.M.; Burman, D.; Karthikeyan, R.; BaHammam, A.S.; Spence, D.W.; Brown, G.M.; Narashimhan, M. Clarifying the role of sleep in depression: A narrative review. Psychiatry Res. 2020, 291, 113239. [Google Scholar] [CrossRef]
- Dehdari Ebrahimi, N.; Parsa, S.; Nozari, F.; Shahlaee, M.A.; Maktabi, A.; Sayadi, M.; Sadeghi, A.; Azarpira, N. Protective effects of melatonin against the toxic effects of environmental pollutants and heavy metals on testicular tissue: A systematic review and meta-analysis of animal studies. Front. Endocrinol. 2023, 14, 1119553. [Google Scholar] [CrossRef]
- Bowen, C.; Childers, G.; Perry, C.; Martin, N.; McPherson, C.A.; Lauten, T.; Santos, J.; Harry, G.J. Mitochondrial-related effects of pentabromophenol, tetrabromobisphenol A, and triphenyl phosphate on murine BV-2 microglia cells. Chemosphere 2020, 255, 126919. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Hong, H.; Lu, X.; Lu, Q.; Huang, C.; Cui, Z. Potential therapeutic effects and pharmacological evidence of sinomenine in central nervous system disorders. Front. Pharmacol. 2022, 13, 1015035. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cheng, X.; Zhao, M.; Zhao, T.; Zhang, M.; Shi, Z.; Yue, X.; Geng, Y.; Gao, J.; Wang, C.; et al. Gypenoside-14 Reduces Depression via Downregulation of the Nuclear Factor Kappa B (NF-kB) Signaling Pathway on the Lipopolysaccharide (LPS)-Induced Depression Model. Pharmaceuticals 2023, 16, 1152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, Q.; Li, Q.; Huo, A.R.; Shen, T.T.; Cao, J.Q.; Liu, C.F.; Liu, T.; Luo, W.F.; Cong, Q.F. Botulinum neurotoxin A ameliorates depressive-like behavior in a reserpine-induced Parkinson’s disease mouse model via suppressing hippocampal microglial engulfment and neuroinflammation. Acta Pharmacol. Sin. 2023, 44, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Aimuzi, R.; Wang, Y.; Luo, K.; Jiang, Y. Exposure to phthalates, phenols, and parabens mixture and alterations in sex steroid hormones among adolescents. Chemosphere 2022, 302, 134834. [Google Scholar] [CrossRef] [PubMed]
- Habib, P.; Beyer, C. Regulation of brain microglia by female gonadal steroids. J. Steroid Biochem. Mol. Biol. 2015, 146, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Meng, L.; Li, Y.; Li, A.; Turyk, M.E.; Yang, R.; Wang, P.; Xiao, K.; Zhao, J.; Zhang, J.; et al. Associations between the exposure to persistent organic pollutants and type 2 diabetes in East China: A case-control study. Chemosphere 2020, 241, 125030. [Google Scholar] [CrossRef]


| Catalogs | Total Population | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Depression | Depression | Depression | |||||||
| No | Yes | p-Value | No | Yes | p-Value | No | Yes | p-Value | |
| Number of subjects (%) a | 7422 (91.2) | 716 (8.8) | 3736 (94.0) | 239 (6.0) | 3686 (88.5) | 477 (11.5) | |||
| Age (%) a | 0.028 * | 0.262 | 0.073 | ||||||
| 20–40 years | 2590 (34.9) | 242 (33.8) | 1328 (35.5) | 76 (31.8) | 1262 (34.2) | 166 (34.8) | |||
| 40–60 years | 2390 (32.2) | 264 (36.9) | 1177 (31.5) | 87 (36.4) | 1213 (32.9) | 177 (37.1) | |||
| ≥60 years | 2442 (32.9) | 210 (29.3) | 1231 (32.9) | 76 (31.8) | 1211 (32.9) | 134 (28.1) | |||
| Race (%) a | <0.001 * | 0.617 | <0.001 * | ||||||
| Mexican American | 1157 (15.6) | 114 (15.9) | 587 (15.7) | 39 (16.3) | 570 (15.5) | 75 (15.7) | |||
| Other Hispanic | 675 (9.1) | 101 (14.1) | 331 (8.9) | 27 (11.3) | 344 (9.3) | 74 (15.5) | |||
| Non-Hispanic White | 3353 (45.2) | 299 (41.8) | 1690 (45.2) | 100 (41.8) | 1663 (45.1) | 199 (41.7) | |||
| Non-Hispanic Black | 1542 (20.8) | 156 (21.8) | 773 (20.7) | 53 (22.2) | 769 (20.9) | 103 (21.6) | |||
| Other race | 695 (9.4) | 46 (6.4) | 355 (9.5) | 20 (8.4) | 340 (9.2) | 26 (5.5) | |||
| Educational level (%) a | <0.001 * | <0.001 * | <0.001 * | ||||||
| Below high school | 1714 (23.1) | 247 (34.5) | 916 (24.5) | 87 (36.4) | 798 (21.6) | 160 (33.5) | |||
| High school | 1684 (22.7) | 172 (24.0) | 893 (23.9) | 62 (25.9) | 791 (21.5) | 110 (23.1) | |||
| Above high school | 4024 (54.2) | 297 (41.5) | 1927 (51.6) | 90 (37.7) | 2097 (56.9) | 207 (43.4) | |||
| Marital status (%) a | <0.001 * | <0.001 * | <0.001 * | ||||||
| Married/living with partner | 4591 (61.9) | 334 (46.6) | 2486 (66.5) | 128 (53.6) | 2105 (57.1) | 206 (43.2) | |||
| Widowed/divorced/separated/never married | 2831 (38.1) | 382 (53.4) | 1250 (33.5) | 111 (46.4) | 1581 (42.9) | 271 (56.8) | |||
| Poverty income ratio (%) a | <0.001 * | <0.001 * | <0.001 * | ||||||
| ≤1.3 | 2127 (28.7) | 379 (52.9) | 1030 (27.6) | 123 (51.5) | 1097 (29.8) | 256 (53.7) | |||
| 1.3–3.5 | 2820 (38.0) | 238 (33.2) | 1424 (38.1) | 79 (33.1) | 1396 (37.9) | 159 (33.3) | |||
| >3.5 | 2475 (33.3) | 99 (13.8) | 1282 (34.3) | 37 (15.5) | 1193 (32.4) | 62 (13.0) | |||
| Body mass index (%) a | <0.001 * | 0.062 | <0.001 * | ||||||
| <25 kg/m2 | 2155 (29.0) | 168 (23.5) | 992 (26.6) | 61 (25.5) | 1163 (31.6) | 107 (22.4) | |||
| 25–30 kg/m2 | 2537 (34.2) | 197 (27.5) | 1479 (39.6) | 80 (33.5) | 1058 (28.7) | 117 (24.5) | |||
| ≥30 kg/m2 | 2730 (36.8) | 351 (49.0) | 1265 (33.9) | 98 (41.0) | 1465 (39.7) | 253 (53.0) | |||
| Cotinine level (%) a | <0.001 * | 0.001 * | <0.001 * | ||||||
| Below LLOD | 1963 (26.4) | 121 (16.9) | 777 (20.8) | 28 (11.7) | 1186 (32.2) | 93 (19.5) | |||
| Above LLOD | 5459 (73.6) | 595 (83.1) | 2959 (79.2) | 211 (88.3) | 2500 (67.8) | 384 (80.5) | |||
| Alcohol consumption (%) a | 0.591 | 0.419 * | 0.174 | ||||||
| 12 drinks or fewer | 5352 (72.1) | 509 (71.1) | 622 (16.6) | 35 (14.6) | 1448 (39.3) | 172 (36.1) | |||
| More than 12 drinks | 2070 (27.9) | 207 (28.9) | 3114 (83.4) | 204 (85.4) | 2238 (60.7) | 305 (63.9) | |||
| Hypertension (%) a | 2594 (35.0) | 322 (45.0) | <0.001 * | 1300 (34.8) | 105 (43.9) | 0.004 * | 1294 (35.1) | 217 (45.5) | <0.001 * |
| Diabetes (%) a | 862 (11.6) | 144 (20.1) | <0.001 * | 456 (12.2) | 41 (17.2) | 0.025 * | 406 (11.0) | 103 (21.6) | <0.001 * |
| Serum BFRs (pg/g) | GM (95% CI) a | Median (IQR) b |
|---|---|---|
| PBB153 | 15.907 (15.534, 16.297) | 16.980 (7.941, 29.870) |
| PBDE28 | 7.410 (7.308, 7.516) | 7.362 (4.854, 11.200) |
| PBDE47 | 133.188 (131.236, 135.233) | 126.900 (83.860, 205.300) |
| PBDE85 | 2.753 (2.708, 2.798) | 2.549 (1.573, 4.369) |
| PBDE99 | 26.457 (25.997, 26.934) | 24.890(15.160, 41.840) |
| PBDE100 | 27.321 (26.897, 27.743) | 25.500 (16.480, 41700) |
| PBDE153 | 55.855 (55.037, 56.713) | 52.260 (34.300, 87.070) |
| PBDE154 | 2.530 (2.489, 2.573) | 2.342 (1.580, 4.005) |
| PBDE209 | 16.301 (16.119, 16.478) | 17.600 (12.030, 17.940) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Fei, Y.; Xu, Z.; Huang, R.; Jiang, Y.; Sun, L.; Wang, X.; Yu, S.; Luo, Y.; Mao, X.; et al. Associations Between Brominated Flame Retardant Exposure and Depression in Adults: A Cross-Sectional Study. Toxics 2024, 12, 918. https://doi.org/10.3390/toxics12120918
Cheng Y, Fei Y, Xu Z, Huang R, Jiang Y, Sun L, Wang X, Yu S, Luo Y, Mao X, et al. Associations Between Brominated Flame Retardant Exposure and Depression in Adults: A Cross-Sectional Study. Toxics. 2024; 12(12):918. https://doi.org/10.3390/toxics12120918
Chicago/Turabian StyleCheng, Yulan, Yue Fei, Zemin Xu, Ruiyao Huang, Yuling Jiang, Lihan Sun, Xuehai Wang, Shali Yu, Yonghua Luo, Xiaobo Mao, and et al. 2024. "Associations Between Brominated Flame Retardant Exposure and Depression in Adults: A Cross-Sectional Study" Toxics 12, no. 12: 918. https://doi.org/10.3390/toxics12120918
APA StyleCheng, Y., Fei, Y., Xu, Z., Huang, R., Jiang, Y., Sun, L., Wang, X., Yu, S., Luo, Y., Mao, X., & Zhao, X. (2024). Associations Between Brominated Flame Retardant Exposure and Depression in Adults: A Cross-Sectional Study. Toxics, 12(12), 918. https://doi.org/10.3390/toxics12120918






