Occurrence, Bioaccumulation, and Trophic Transfer of Short-Chain Chlorinated Paraffins (SCCPs) in a Marine Food Web from Laizhou Bay, Bohai Sea (Eastern China)
Abstract
1. Introduction
2. Materials and Methods
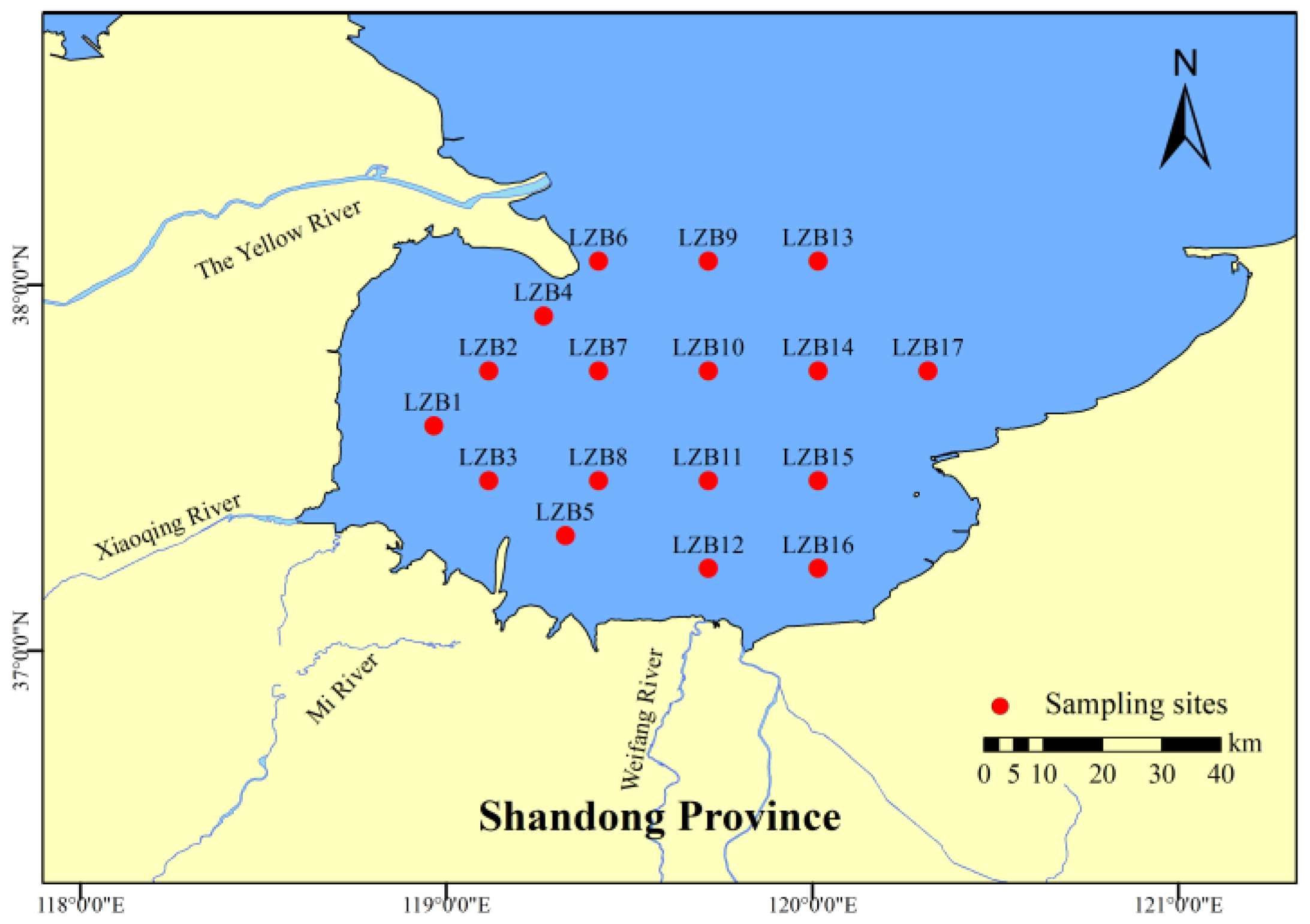
2.1. Sample Collection
2.2. Sample Pretreatments
2.3. Instrumental Analysis
2.4. Quality Assurance and Quality Control (QA/QC)
2.5. Stable Isotope Analysis and Calculations
2.5.1. Trophic Level (TL)
2.5.2. Bioaccumulation and Trophic Transfer
2.6. Dietary Risk
2.7. Statistical Analysis
3. Results and Discussion
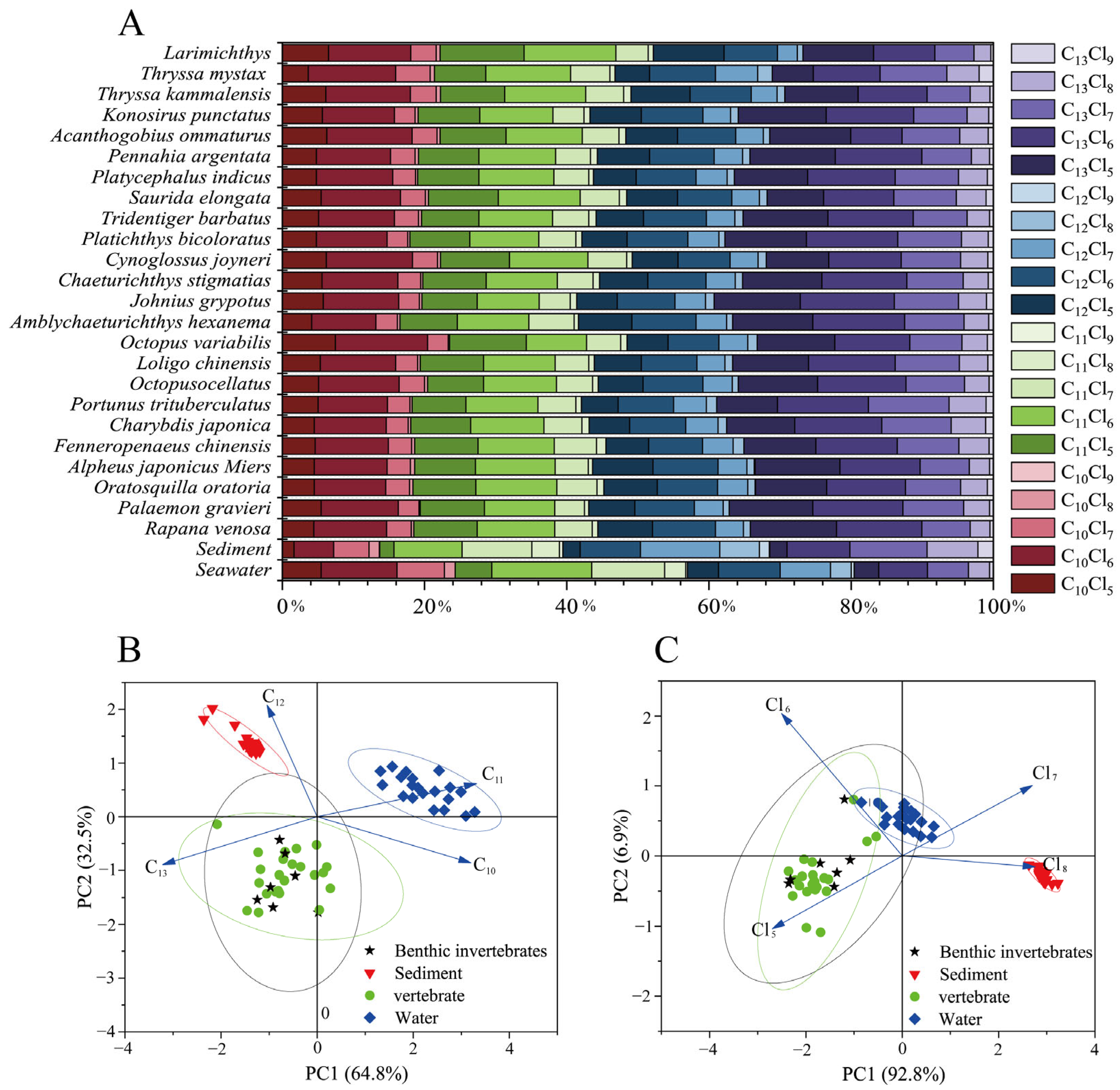
3.1. Homologs Profiles of SCCPs in Laizhou Bay
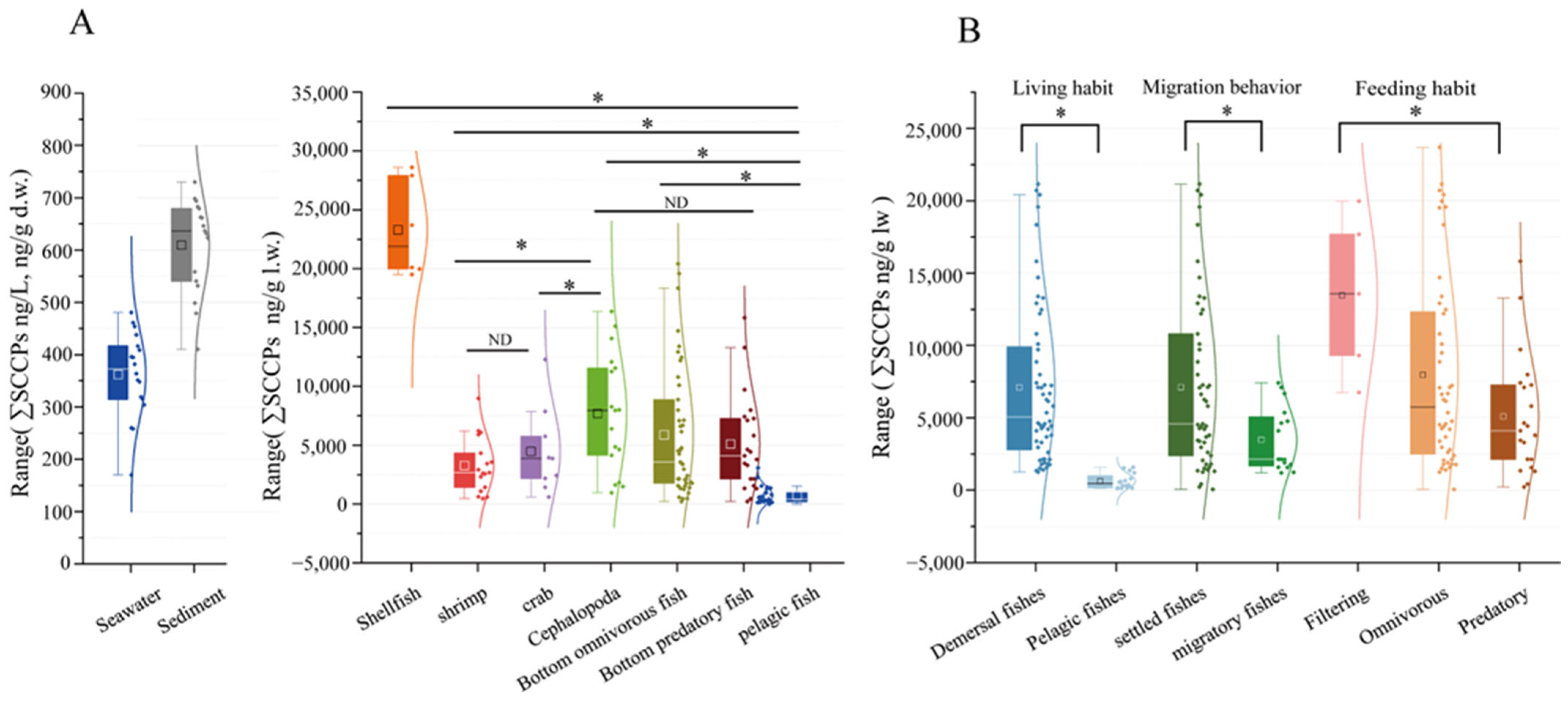
3.2. SCCP Concentrations in Organisms
3.3. Bioaccumulation and Biota-Sediment Accumulation of SCCPs
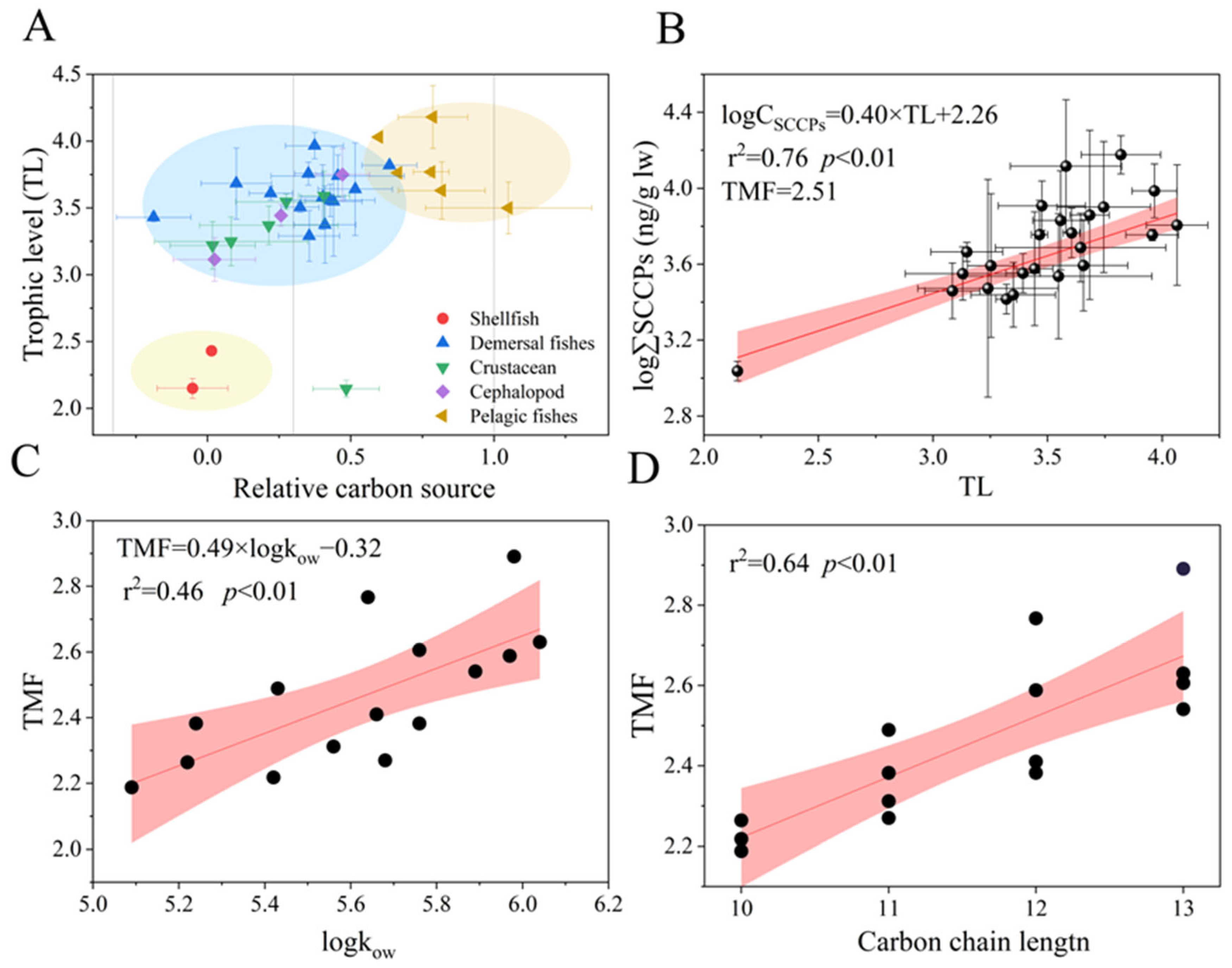
3.4. Trophic Transfer of SCCPs in the Food Web
3.5. Dietary Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glüge, J.; Wang, Z.Y.; Bogdal, C.; Scheringer, M.; Hungerbühler, K. Global production, use, and emission volumes of short-chain chlorinated paraffins—A minimum scenario. Sci. Total Environ. 2016, 573, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.Q.; Zhang, H.J.; Wang, J.; Xu, J.Z.; Yuan, H.P.; Gao, Y.; Su, F.; Chen, J.P. Release and Gas-Particle Partitioning Behaviors of Short-Chain Chlorinated Paraffins (SCCPs) During the Thermal Treatment of Polyvinyl Chloride Flooring. Environ. Sci. Technol. 2017, 51, 9005–9012. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Report of the Coference of the Parties to the Stockholm Convention on Persisent Origanic Pollutants on the Work of its Eighth Meeting. Stockholm Convention on Peresistent Organic Pollutants, Geneva. 2017. Available online: http://wedocs.umnep.org/bitstream/handle/20.500.11822/31829/RUEPIRDA.pdf?sequence=1 (accessed on 11 February 2023).
- Liu, L.H.; Ma, W.L.; Huo, C.Y.; Li, W.L.; Gao, C.J.; Li, H.L.; Li, Y.F.; Chan, H.M. Occurrence, sources and human exposure assessment of SCCPs in indoor dust of northeast China. Environ. Pollut. 2017, 225, 232–243. [Google Scholar] [CrossRef]
- Lee, S.; Choo, G.; Ekpe, O.D.; Kim, J.; Oh, J.E. Short-chain chlorinated paraffins in various foods from Republic of Korea: Levels, congener patterns, and human dietary exposure. Environ. Pollut. 2020, 263, 114520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Cui, Y.; Wang, P.W.; Li, S.S.; Jiang, W.; Luo, N.N.; Wang, Z.H.; Chen, X.F.; Ding, L. Short-chain chlorinated paraffins in soil, sediment, and seawater in the intertidal zone of Shandong Peninsula, China: Distribution and composition. Chemosphere 2019, 220, 452–458. [Google Scholar] [CrossRef]
- Chen, H.; Han, X.; Liang, B.; Deng, M.; Du, B.; Zeng, L. Spatial distribution, homologue patterns and ecological risks of chlorinated paraffins in mangrove sediments along the South China Coast. Environ. Pollut. 2022, 294, 118623. [Google Scholar] [CrossRef]
- Casà, M.V.; van Mourik, L.M.; Weijs, L.; Mueller, J.; Nash, S.B. First detection of short-chain chlorinated paraffins (SCCPs) in humpback whales (Megaptera novaeangliae) foraging in Antarctic waters. Environ. Pollut. 2019, 250, 953–959. [Google Scholar] [CrossRef]
- Jiang, W.Y.H.; Chen, H.; Huang, T.; Lian, L.L.; Li, J.X.; Jia, C.H.; Gao, H.; Mao, X.X.; Ma, J.M. Tagged sources of short-chain chlorinated paraffins in China’s marine environment and fish. Chemosphere 2019, 229, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.Y.H.; Huang, T.; Chen, H.; Lian, L.L.; Liang, X.X.; Jia, C.H.; Gao, H.; Mao, X.X.; Zhao, Y.; Ma, J.M. Contamination of short-chain chlorinated paraffins to the biotic and abiotic environments in the Bohai Sea. Environ. Pollut. 2018, 233, 114–124. [Google Scholar] [CrossRef]
- Li, H.J.; Fu, J.J.; Pan, W.X.; Wang, P.; Li, Y.M.; Zhang, Q.H.; Wang, Y.W.; Zhang, A.Q.; Liang, Y.; Jiang, G.B. Environmental behaviour of short-chain chlorinated paraffins in aquatic and terrestrial ecosystems of Ny-Alesund and London Island, Svalbard, in the Arctic. Sci. Total Environ. 2017, 590, 163–170. [Google Scholar] [CrossRef]
- Li, H.J.; Fu, J.J.; Zhang, A.G.; Zhang, Q.H.; Wang, Y.W. Occurrence, bioaccumulation and long-range transport of short-chain chlorinated paraffins on the Fildes Peninsula at King George Island, Antarctica. Environ. Int. 2016, 94, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhang, H.; Geng, N.; Ren, X.; Giesy, J.; Luo, Y.; Xing, L.; Wu, P.; Yu, Z.; Chen, J. Short-chain chlorinated paraffins (SCCPs) disrupt hepatic fatty acid metabolism in liver of male rat via interacting with peroxisome proliferator-activated receptor alpha (PPARalpha). Ecotoxicol. Environ. Saf. 2019, 181, 164–171. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Coelhan, M.; Chan, H.M.; Ma, W.; Liu, L. Relative developmental toxicity of short-chain chlorinated paraffins in Zebrafish (Danio rerio) embryos. Environ. Pollut. 2016, 219, 1122–1130. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.H.; Zhu, J.Q.; Liu, J.; Zhang, J.Y.; Zhao, M.R. Assessment of the endocrine-disrupting effects of short-chain chlorinated paraffins in in vitro models. Environ. Int. 2016, 94, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhao, Z.; Zheng, Y.; Wang, R.; Zhang, Z.; Zhang, Z.; Wang, X.; Yu, S.; Liu, L.; Huang, R.; et al. Exposure to short-chain chlorinated paraffins induces astrocyte activation via JAK2/STAT3 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 248, 114268. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Chen, L.G.; Jiang, G.; He, Q.S.; Ren, L.; Gao, B.; Cai, L.M. Bioaccumulation and biomagnification of short-chain chlorinated paraffins in marine organisms from the Pearl River Estuary, South China. Sci. Total Environ. 2019, 671, 262–269. [Google Scholar] [CrossRef]
- Ma, X.D.; Zhang, H.; Wang, Z.; Yao, Z.; Chen, J.; Chen, J. Bioaccumulation and trophic transfer of short chain chlorinated paraffins in a marine food web from Liaodong Bay, North China. Environ. Sci. Technol. 2014, 48, 5964–5971. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.X.; Luo, X.J.; Tang, B.; Chen, L.G.; Liu, Y.; Mai, B.X. Bioaccumulation of short chain chlorinated paraffins in a typical freshwater food web contaminated by e-waste in south china: Bioaccumulation factors, tissue distribution, and trophic transfer. Environ. Pollut. 2017, 222, 165–174. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yuan, B.; Nyberg, E.; Yin, G.; Bignert, A.; Glynn, A.; Odland, J.O.; Qiu, Y.L.; Sun, Y.J.; Wu, Y.N.; et al. Chlorinated Paraffins in Human Milk from Urban Sites in China, Sweden, and Norway. Environ. Sci. Technol. 2020, 54, 4356–4366. [Google Scholar] [CrossRef]
- Li, H.J.; Gao, S.; Yang, M.L.; Zhang, F.; Cao, L.M.; Xie, H.Y.; Chen, X.F.; Cai, Z.W. Dietary exposure and risk assessment of short-chain chlorinated paraffins in supermarket fresh products in Jinan, China. Chemosphere 2020, 244, 125393. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Ji, C.; Xiao, P.; Tang, J. Habitat-dependent trophic transfer of legacy and emerging halogenated flame retardants in estuarine and coastal food webs near a source region. Environ. Pollut. 2022, 300, 118987. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.P.; Lu, R.F.; Xu, Q.S.; Zheng, X.B.; Zeng, Y.H.; Mai, B.X. Distinct biomagnification of chlorinated persistent organic pollutants in adjacent aquatic and terrestrial food webs. Environ. Pollut. 2022, 317, 120841. [Google Scholar] [CrossRef]
- Huang, H.T.; Gao, L.R.; Xia, D.; Qiao, L. Bioaccumulation and biomagnification of short and medium chain polychlorinated paraffins in different species of fish from Liaodong Bay, North China. Sci. Rep. 2017, 7, 10749. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, T.; Zhu, N.; Zhang, K.; Zeng, L.; Fu, J.; Wang, Y.; Jiang, G. Short chain chlorinated paraffins in mollusks from coastal waters in the Chinese Bohai Sea. Environ. Sci. Technol. 2012, 46, 6489–6496. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Qiao, L.; Xu, C.; Wang, K.; Huang, D. Bioaccessibility of short chain chlorinated paraffins in meat and seafood. Sci. Total Environ. 2019, 668, 996–1003. [Google Scholar] [CrossRef]
- Wan, Y.; Hu, J.Y.; Zhang, K.; An, L.H. Trophodynamics of polybrominated diphenyl ethers in the marine food web of Bohai Bay, North China. Environ. Sci. Technol. 2008, 42, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Hu, J.; Yang, M.; An, L.; An, W.; Jin, X.; Hattori, T.; Itoh, M. Characterization of trophic transfer for polychlorinated dibenzo-p-dioxins, dibenzofurans, non- and mono-ortho polychlorinated biphenyls in the marine food web of Bohai Bay, north China. Environ. Sci. Technol. 2005, 39, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Bekele, T.G.; Zhao, H.X.; Wang, Q.Z. Tissue distribution and bioaccumulation of organophosphate esters in wild marine fish from Laizhou Bay, North China: Implications of human exposure via fish consumption. J. Hazard. Mater. 2021, 401, 123410. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.H.; Dou, S.Z.; Huang, W. Biomagnification of methylmercury in a marine food web in Laizhou Bay (North China) and associated potential risks to public health. Mar. Pollut. Bull. 2020, 150, 110762. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, N.; Sun, S.; Ji, Y.; An, Q.; Li, X.; Li, Z.; Zhang, W. Bioaccumulation of organophosphorus flame retardants in marine organisms in Liaodong Bay and their potential ecological risks based on species sensitivity distribution. Environ. Pollut. 2023, 317, 120812. [Google Scholar] [CrossRef]
- Cui, Q.; Han, D.; Qin, H.; Li, H.; Liu, Y.; Guo, W.; Song, M.; Li, J.; Sun, Y.; Luo, J.; et al. Investigating the levels, spatial distribution, and trophic transfer patterns of short-chain chlorinated paraffins in the Southern Bohai Sea, China. Water Res. 2024, 253, 121337. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Brüchert, V.; Sobek, A.; de Wit, C.A. Temporal Trends of C8–C36 Chlorinated Paraffins in Swedish Coastal Sediment Cores over the Past 80 Years. Environ. Sci. Technol. 2017, 51, 14199–14208. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ding, C.; Su, Q.; Wang, Y.; Cui, Z.; Yin, Q.; Wang, X. A simplified method for determination of short-, medium-, and long-chain chlorinated paraffins using tetramethyl ammonium chloride as mobile phase modifier. J. Chromatogr. A 2021, 1642, 462002. [Google Scholar] [CrossRef]
- Li, H.W.; Zhang, Z.W.; Sun, Y.X.; Wang, W.W.; Xie, J.L.; Xie, C.M.; Hu, Y.X.; Gao, Y.L.; Xu, X.R.; Luo, X.J.; et al. Tetrabromobisphenol A and hexabromocyclododecanes in sediments and biota from two typical mangrove wetlands of South China: Distribution, bioaccumulation and biomagnification. Sci. Total Environ. 2021, 750, 141695. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.X.; Lu, G.H.; Liu, J.C.; Yan, Z.H.; Ma, B.N.; Zhang, Z.H.; Chen, W. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere 2015, 138, 140–147. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhen, H.J.; Wan, Y.; Gao, J.M.; An, W.; An, L.H.; Jin, F.; Jin, X.H. Trophic magnification of triphenyltin in a marine food web of Bohai Bay, north China: Comparison to tributyltin. Environ. Sci. Technol. 2006, 40, 3142–3147. [Google Scholar] [CrossRef]
- Diao, J.Y.; Chen, Z.W.; Wang, T.Y.; Su, C.H.; Sun, Q.P.; Guo, Y.J.; Zheng, Z.; Wang, L.; Li, P.; Liu, W.H.; et al. Perfluoroalkyl substances in marine food webs from South China Sea: Trophic transfer and human exposure implication. J. Hazard. Mater. 2022, 431, 128602. [Google Scholar] [CrossRef]
- Wu, Y.N.; Zhao, Y.F.; Li, J.G. The Fifth China Total Diet Study; China Science Publishing & Media Ltd. (CSPM): Beijing, China, 2018. [Google Scholar]
- Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. 2024. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20200803134940 (accessed on 15 March 2024).
- Xia, D.; Gao, L.; Zheng, M.; Sun, Y.; Qiao, L.; Huang, H.; Zhang, H.; Fu, J.; Wu, Y.; Li, J.; et al. Identification and evaluation of chlorinated nonane paraffins in the environment: A persistent organic pollutant candidate for the Stockholm Convention? J. Hazard. Mater. 2019, 371, 449–455. [Google Scholar] [CrossRef]
- Ye, Q.; Song, Q.; Zhou, J.; Wu, Y.; Zhou, Y.; Zhang, J.; Wu, W. Behavior and fate of short chain chlorinated paraffins (SCCPs) in different oxidation reactions. Chem. Eng. J. 2023, 464, 142557. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, T.; Wang, P.; Liu, Q.; Han, S.; Yuan, B.; Zhu, N.; Wang, Y.; Jiang, G. Distribution and Trophic Transfer of Short-Chain Chlorinated Paraffins in an Aquatic Ecosystem Receiving Effluents from a Sewage Treatment Plant. Environ. Sci. Technol. 2011, 45, 5529–5535. [Google Scholar] [CrossRef]
- Zeng, L.; Lam, J.C.W.; Wang, Y.; Jiang, G.; Lam, P.K.S. Temporal Trends and Pattern Changes of Short- and Medium-Chain Chlorinated Paraffins in Marine Mammals from the South China Sea over the Past Decade. Environ. Sci. Technol. 2015, 49, 11348–11355. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Qu, J.; Zhao, M.; Wu, P.; Zhu, W.; Zhou, Y.; Jin, H. Bioaccumulation and trophic magnification of short chain chlorinated paraffins in marine organisms from East China Sea. Mar. Pollut. Bull. 2021, 173, 113049. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Armitage, J.M.; Wania, F. Model-based exploration of the variability in lake trout (Salvelinus namaycush) bioaccumulation factors: The influence of physiology and trophic relationships. Environ. Toxicol. Chem. 2019, 38, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xia, X.; Liu, R.; Wang, Z.; Zhai, Y.; Lin, H.; Wen, W.; Li, Y.; Wang, D.; Yang, Z.; et al. Dietary Uptake Patterns Affect Bioaccumulation and Biomagnification of Hydrophobic Organic Compounds in Fish. Environ. Sci. Technol. 2019, 53, 4274–4284. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Zhou, Q.; Lu, S.; Liu, R.; Cui, L.; Yin, D.; Mayer, P.; Jiang, G. Bioavailability of organochlorine compounds in aqueous suspensions of fullerene: Evaluated with medaka (Oryzias latipes) and negligible depletion solid-phase microextraction. Chemosphere 2010, 80, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.D.; Zhang, H.J.; Yao, Z.W.; Zhao, X.F.; Wang, L.X.; Wang, Z.; Chen, J.P.; Chen, J.W. Bioaccumulation and trophic transfer of polybrominated diphenyl ethers (PBDEs) in a marine food web from Liaodong Bay, North China. Mar. Pollut. Bull. 2013, 74, 110–115. [Google Scholar] [CrossRef]
- Zheng, G.M.; Wan, Y.; Hu, J.Y. Intrinsic Clearance of Xenobiotic Chemicals by Liver Microsomes: Assessment of Trophic Magnification Potentials. Environ. Sci. Technol. 2016, 50, 6343–6353. [Google Scholar] [CrossRef]
- Hou, R.; Huang, Q.Y.; Pan, Y.F.; Lin, L.; Liu, S.; Li, H.X.; Xu, X.R. Novel Brominated Flame Retardants (NBFRs) in a Tropical Marine Food Web from the South China Sea: The Influence of Hydrophobicity and Biotransformation on Structure-Related Trophodynamics. Environ. Sci. Technol. 2022, 56, 3147–3158. [Google Scholar] [CrossRef]
- Hu, H.M.; Zhao, M.R.; Guo, Y.M.; Zhou, Y.D.; Li, T.J.; Zhu, W.B.; Jin, H.B. Occurrence, bioaccumulation and potential risk of polyhalogenated carbazoles in marine organisms from the East China Sea. Sci. Total Environ. 2022, 807, 150643. [Google Scholar] [CrossRef]
- Thomann, R.V. Bioaccumulation model of organic-chemical distribution in aquatic food-chains. Environ. Sci. Technol. 1989, 23, 699–707. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yin, G.; Du, X.Y.; Xu, M.Y.; Qiu, Y.L.; Ahlqvist, P.; Chen, Q.F.; Zhao, J.F. Short-chain chlorinated paraffins (SCCPs) in a freshwater food web from Dianshan Lake: Occurrence level, congener pattern and trophic transfer. Sci. Total Environ. 2018, 615, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Xu, K.H.; Lyu, L.; Ding, C.H.; Zhao, Y.R.; Wang, X. Identification and yield of metabolites of chlorinated paraffins incubated with chicken liver microsomes: Assessment of their potential to convert into metabolites. J. Hazard. Mater. 2023, 455, 131640. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Cui, Z.F.; Ding, C.H.; Su, Q.Q.; Lin, X.X.; Wang, W.L.; Yin, Q.M.; Wang, X. Differential Accumulation of Short-, Medium-, and Long-Chain Chlorinated Paraffin in Free-Range Laying Hens from an E-Waste Recycling Area. J. Agric. Food Chem. 2021, 69, 10329–10337. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Zhao, H.X.; Lehmler, H.J.; Cai, X.Y.; Chen, J.W. Antibiotic Pollution in Marine Food Webs in Laizhou Bay, North China: Trophodynamics and Human Exposure Implication. Environ. Sci. Technol. 2017, 51, 2392–2400. [Google Scholar] [CrossRef]
- McKinney, M.A.; McMeans, B.C.; Tomy, G.T.; Rosenberg, B.; Ferguson, S.H.; Morris, A.; Muir, D.C.G.; Fisk, A.T. Trophic Transfer of Contaminants in a Changing Arctic Marine Food Web: Cumberland Sound, Nunavut, Canada. Environ. Sci. Technol. 2012, 46, 9914–9922. [Google Scholar] [CrossRef]
| Species | Sample Number | SCCP Concentrations | TL | LogBAF (L/kg w.w.) | BSAF (l.w./TOC) | Feeding Habits a | Living Habitat b | ||
|---|---|---|---|---|---|---|---|---|---|
| (ng/g l.w.) | (ng/g d.w.) | (ng/g w.w.) | |||||||
| Shellfish | |||||||||
| Scapharca subcrenata | 3 | 18,446 ± 1771 | 163 ± 63 | 56 ± 21 | 2.43 ± 0.92 | 2.19 | 0.40 | FL/DE | BE |
| Rapana venosa | 7 | 24,756 ± 14,080 | 258 ± 126 | 59 ± 30 | 2.15 ± 0.56 | 2.15 | 0.50 | OM/DE | BE |
| Average | - | 26,247 ± 10,939 | 239 ± 121 | 75 ± 44 | - | 2.17 | 0.45 | - | - |
| Crustacean | |||||||||
| Palaemon gravieri | 3 | 4626 ± 846 | 49 ± 9 | 10 ± 2 | 3.55 ± 0.47 | 1.18 | 0.09 | PL/OM | DE |
| Oratosquilla oratoria | 9 | 3944 ± 3047 | 266 ± 221 | 53 ± 41 | 3.25 ± 1.45 | 2.17 | 0.08 | OM | DE |
| Alpheus japonicus Miers | 3 | 3554 ± 819 | 108 ± 25 | 25 ± 6 | 3.22 ± 0.49 | 1.84 | 0.07 | OM | DE |
| Fenneropenaeus chinensis | 4 | 2262 ± 1132 | 39 ± 34 | 9 ± 8 | 2.14 ± 0.32 | 1.39 | 0.03 | OM | DE |
| Scomber japonicus | 10 | 5455 ± 4544 | 279 ± 427 | 118 ± 180 | 3.47 ± 0.96 | 2.51 | 0.11 | OM | DE |
| Portunus trituberculatus | 5 | 3746 ± 872 | 383 ± 237 | 112 ± 36 | 3.59 ± 0.45 | 2.49 | 0.07 | OM | DE |
| Average | - | 3017 ± 2921 | 223 ± 278 | 66 ± 102 | - | 1.93 | 0.08 | - | - |
| Cephalopoda | |||||||||
| Octopusocellatus | 5 | 3769 ± 2039 | 109 ± 71 | 24 ± 13 | 3.44 ± 0.08 | 1.82 | - | PL/OM | PE |
| Loligo chinensis | 15 | 13,018 ± 7944 | 452 ± 367 | 82 ± 50 | 3.75 ± 0.2 | 2.35 | - | PL/OM | PE |
| Octopus variabilis | 3 | 7999 ± 1111 | 274 ± 38 | 50 ± 7 | 3.12 ± 0.16 | 2.14 | - | PL/OM | PE |
| Average | - | 9814 ± 7630 | 334 ± 330 | 62 ± 48 | - | 2.10 | - | - | - |
| Pelagic fishes | |||||||||
| Konosirus punctatus | 8 | 493 ± 494 | 316 ± 316 | 133 ± 133 | 3.63 ± 0.22 | 2.56 | - | PL | PE |
| Thryssa kammalensis | 5 | 361 ± 177 | 137 ± 67 | 40 ± 20 | 3.57 ± 0.13 | 2.07 | - | PL | PE |
| Thryssa mystax | 3 | 772 ± 599 | 351 ± 272 | 93 ± 72 | 3.77 ± 0.08 | 2.41 | - | PL | PE |
| Average | - | 520 ± 445 | 274 ± 258 | 98 ± 104 | - | 2.35 | - | - | - |
| Demersal fishes | |||||||||
| Pennahia argentata | 6 | 5815 ± 1693 | 867 ± 252 | 201 ± 59 | 3.61 ± 0.6 | 2.74 | 0.12 | PD | DE |
| Acanthogobius ommaturus | 7 | 4870 ± 6088 | 173 ± 224 | 35 ± 43 | 3.64 ± 0.25 | 1.98 | 0.10 | PD | DE |
| Cynoglossus semilaevis | 3 | 8772 ± 4198 | 69 ± 55 | 18 ± 9 | 3.53 ± 0.21 | 1.71 | 0.18 | OM | DE |
| Cynoglossus joyneri | 12 | 3443 ± 1997 | 266 ± 176 | 62 ± 36 | 3.55 ± 0.67 | 2.23 | 0.07 | OM | DE |
| Callionymidae | 6 | 11,574 ± 8586 | 103 ± 76 | 35 ± 26 | 3.82 ± 0.09 | 1.98 | 0.23 | OM | DE |
| Lateolabrax japonicus | 4 | 5281 ± 2801 | 124 ± 105 | 31 ± 16 | 3.97 ± 0.74 | 1.93 | 0.11 | PD | DE |
| Johnius grypotus | 4 | 1946 ± 331 | 244 ± 71 | 67 ± 11 | 3.61 ± 0.13 | 2.27 | 0.04 | OM | DE |
| Amblychaeturichthys hexanema | 3 | 3567 ± 814 | 160 ± 36 | 33 ± 8 | 3.39 ± 0.67 | 1.96 | 0.07 | OM | DE |
| Chaeturichthys stigmatias | 12 | 11,624 ± 8963 | 157 ± 100 | 37 ± 23 | 3.58 ± 0.68 | 2.01 | 0.23 | OM | DE |
| Platichthys bicoloratus | 4 | 6776 ± 6039 | 245 ± 219 | 52 ± 46 | 3.56 ± 0.58 | 2.16 | 0.14 | OM | DE |
| Takifugu niphobles | 4 | 3835 ± 2325 | 38 ± 24 | 9 ± 5 | 3.76 ± 0.26 | 1.39 | 0.08 | OM | DE |
| Platycephalus indicus | 5 | 8098 ± 6144 | 607 ± 460 | 118 ± 90 | 3.68 ± 0.58 | 2.51 | 0.16 | PD | DE |
| Saurida elongata | 3 | 4676 ± 1037 | 234 ± 52 | 54 ± 12 | 3.74 ± 1.12 | 2.17 | 0.09 | OM | DE |
| Tridentiger barbatus | 3 | 8663 ± 2033 | 202 ± 47 | 37 ± 9 | 3.48 ± 1.18 | 2.01 | 0.17 | PD | DE |
| Average | - | 6291 ± 5711 | 244 ± 252 | 56 ± 55 | - | 2.08 | 0.13 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Han, D.; Hu, S.; Cui, Q.; Li, H.; Li, F.; Zhang, J.; Liu, Y.; Zhao, M.; Zhang, C.; et al. Occurrence, Bioaccumulation, and Trophic Transfer of Short-Chain Chlorinated Paraffins (SCCPs) in a Marine Food Web from Laizhou Bay, Bohai Sea (Eastern China). Toxics 2024, 12, 877. https://doi.org/10.3390/toxics12120877
Song M, Han D, Hu S, Cui Q, Li H, Li F, Zhang J, Liu Y, Zhao M, Zhang C, et al. Occurrence, Bioaccumulation, and Trophic Transfer of Short-Chain Chlorinated Paraffins (SCCPs) in a Marine Food Web from Laizhou Bay, Bohai Sea (Eastern China). Toxics. 2024; 12(12):877. https://doi.org/10.3390/toxics12120877
Chicago/Turabian StyleSong, Min, Dianfeng Han, Shunxin Hu, Qingkui Cui, Huanjun Li, Fan Li, Jianbai Zhang, Yongchun Liu, Mei Zhao, Cunxin Zhang, and et al. 2024. "Occurrence, Bioaccumulation, and Trophic Transfer of Short-Chain Chlorinated Paraffins (SCCPs) in a Marine Food Web from Laizhou Bay, Bohai Sea (Eastern China)" Toxics 12, no. 12: 877. https://doi.org/10.3390/toxics12120877
APA StyleSong, M., Han, D., Hu, S., Cui, Q., Li, H., Li, F., Zhang, J., Liu, Y., Zhao, M., Zhang, C., & Xu, Y. (2024). Occurrence, Bioaccumulation, and Trophic Transfer of Short-Chain Chlorinated Paraffins (SCCPs) in a Marine Food Web from Laizhou Bay, Bohai Sea (Eastern China). Toxics, 12(12), 877. https://doi.org/10.3390/toxics12120877







