Advances in Soil Amendments for Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Impact, and Future Prospects
Abstract
1. Introduction
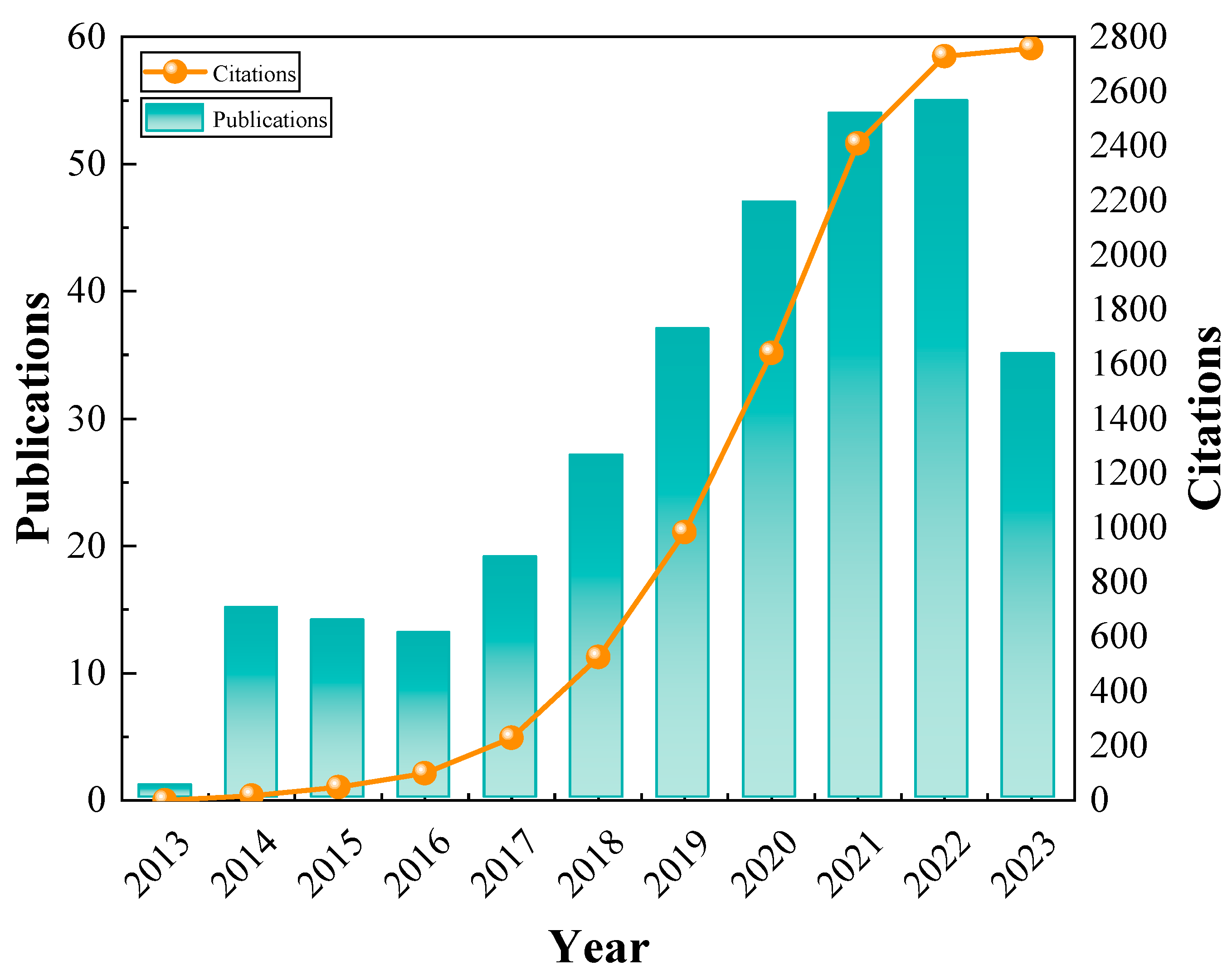
2. Bibliometric Analysis of Hotspots and Frontiers on Remediation Materials for Heavy Metal-Contaminated Soils
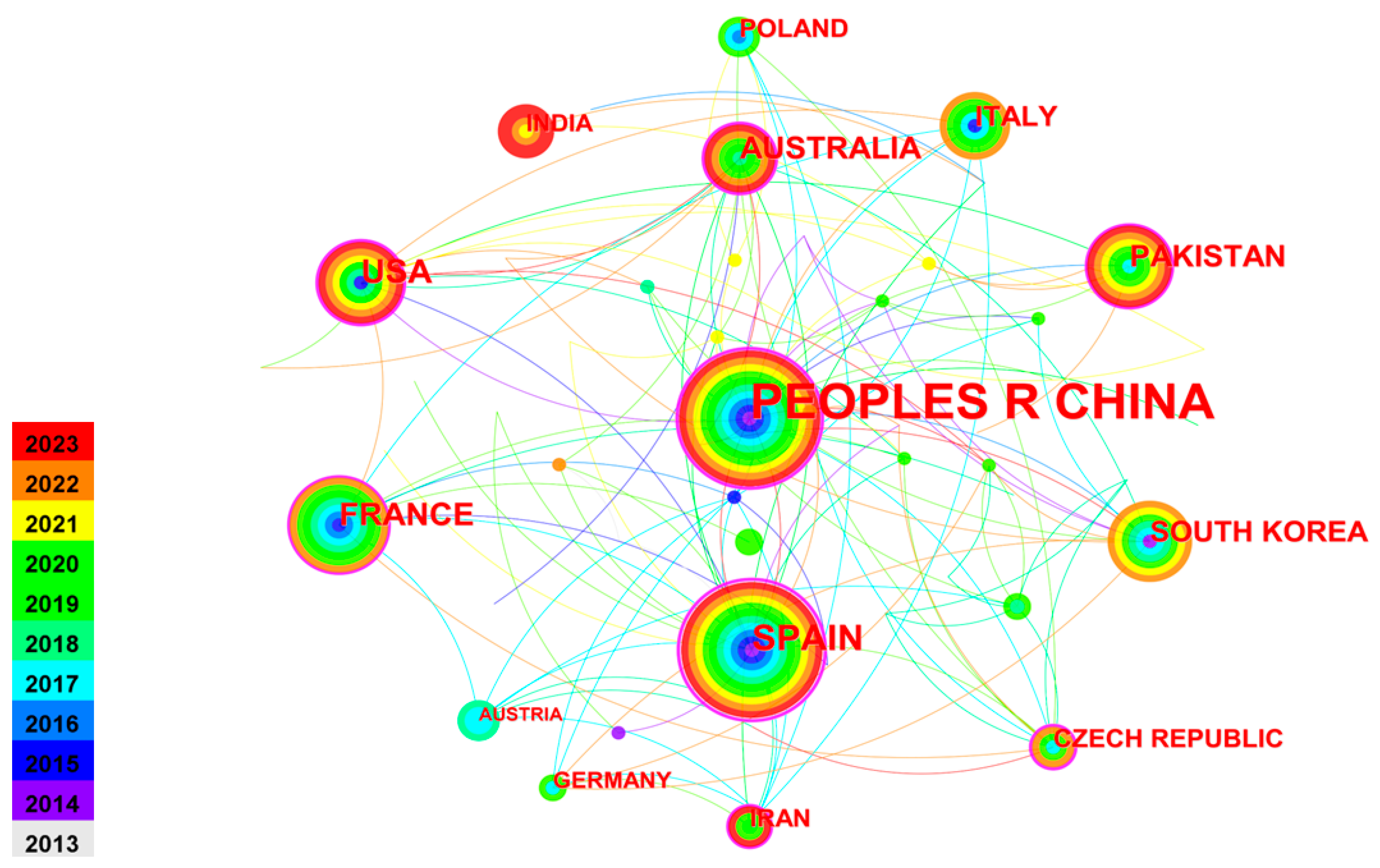
2.1. Country Co-Authorship Analysis
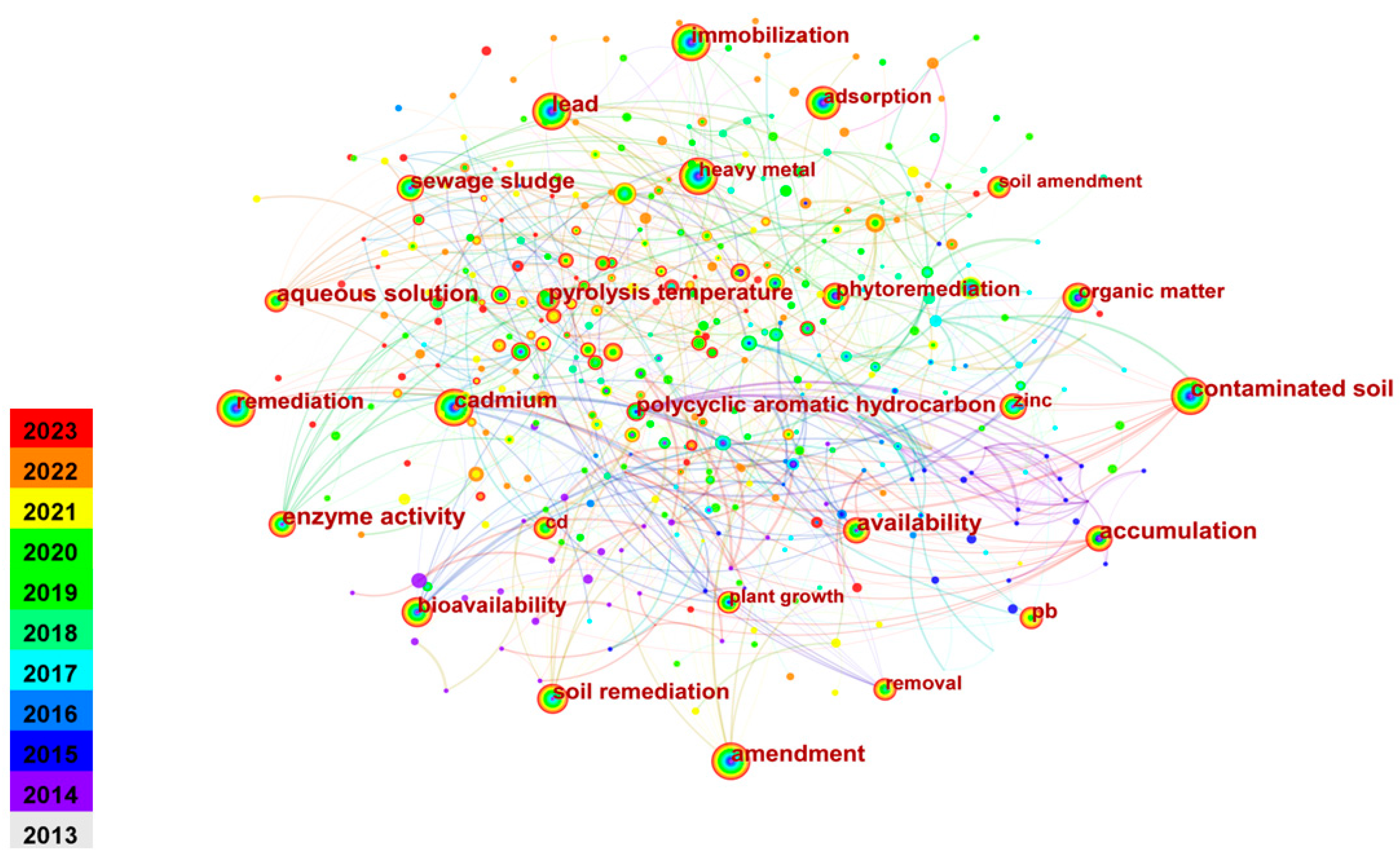
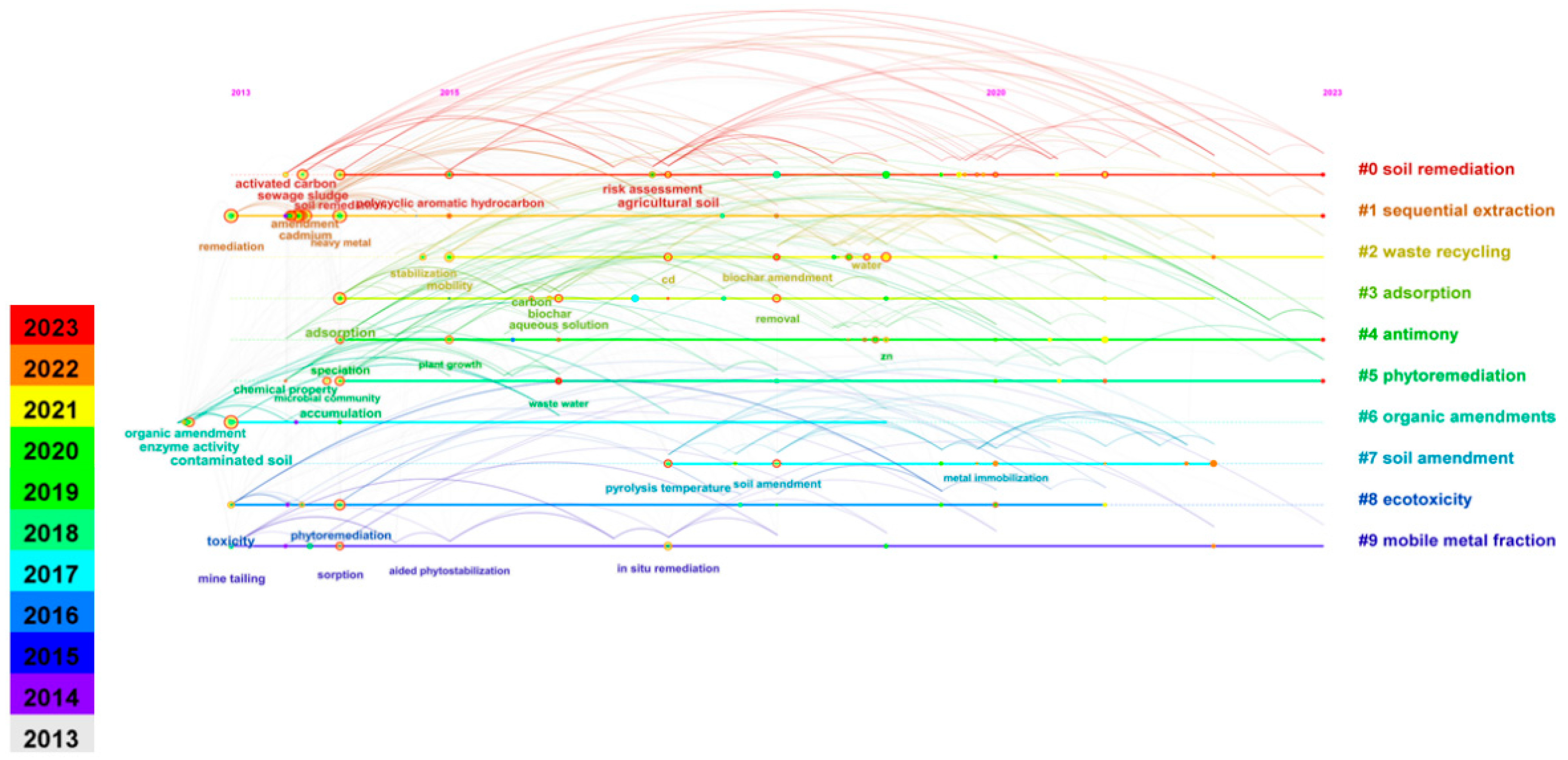
2.2. Keyword Co-Occurrence Analysis
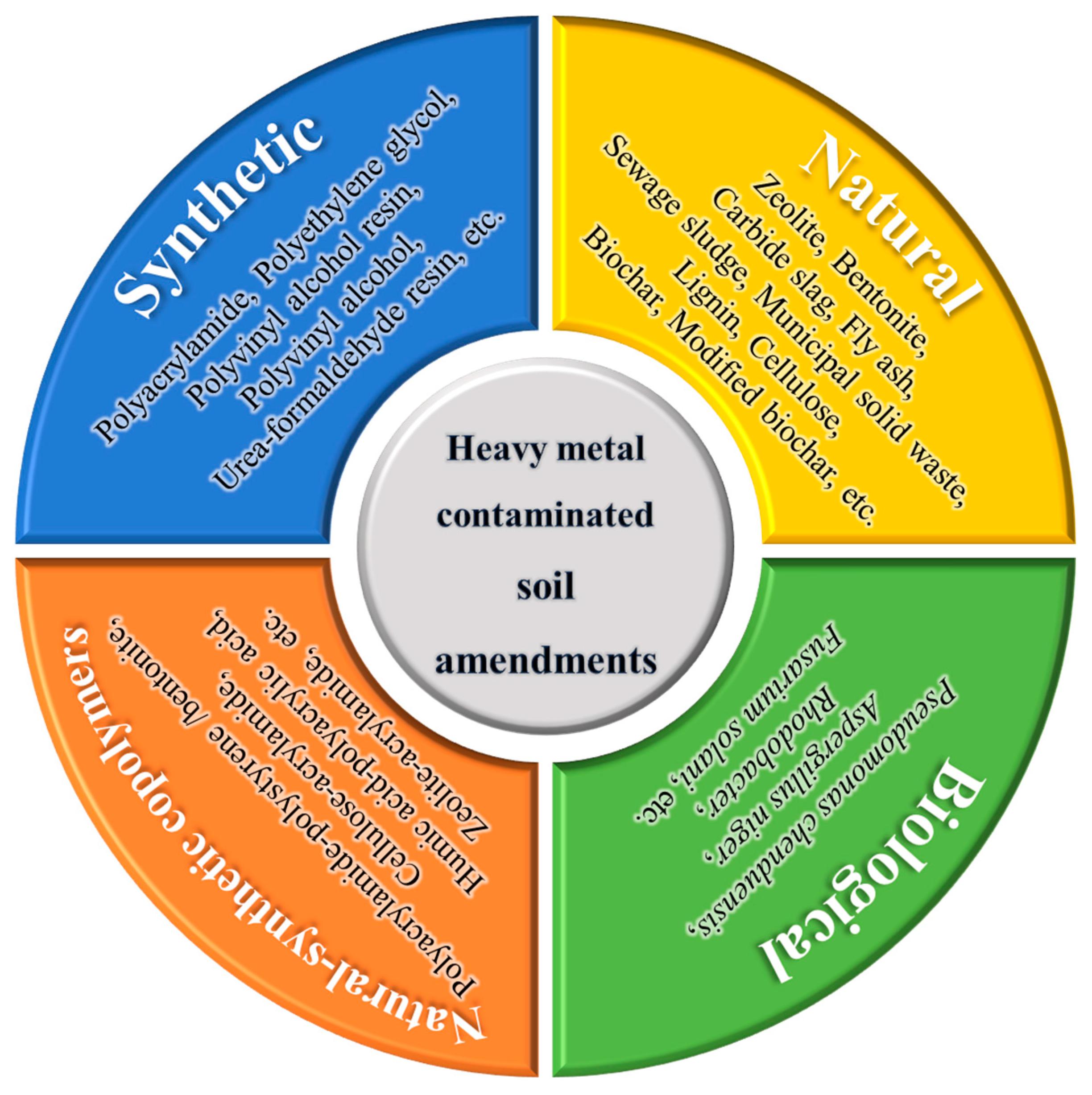
3. Natural Soil Amendments
3.1. Inorganic Soil Amendments
3.1.1. Natural Mineral
3.1.2. Inorganic Solid Waste
3.2. Organic Soil Amendments
3.2.1. Organic Solid Waste
3.2.2. Naturally Extracted Polymer Compounds
3.2.3. Organic Material
4. Synthetic Soil Amendments
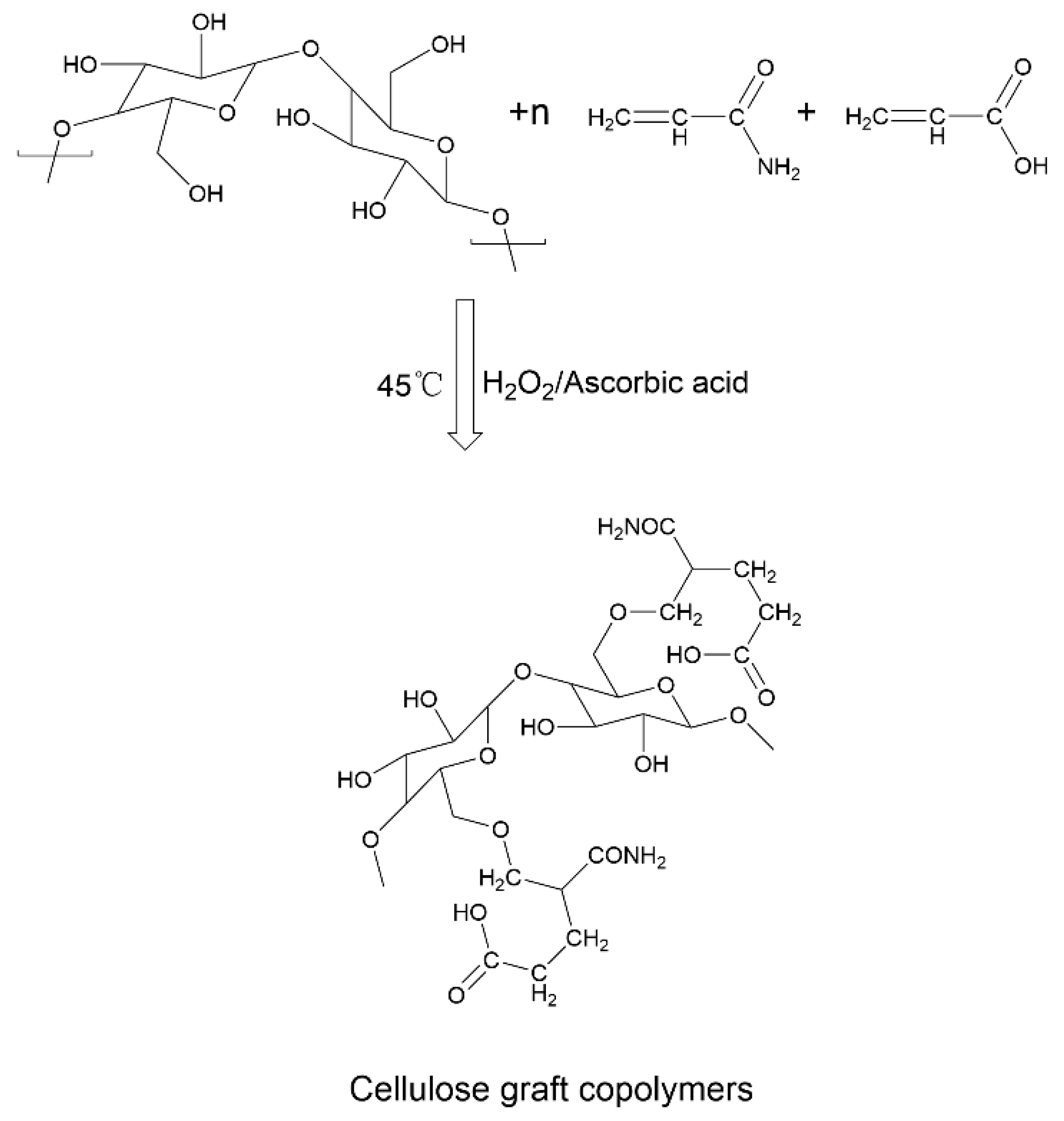
5. Natural-Synthetic Copolymer Soil Amendments
6. Biological Soil Amendments
7. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Liu, S.; Zhan, L.; Hu, L.; Du, Y. Environmental Geotechnics: State-of-the-art of Theory, Testing and Application to Practice. Chin. Civ. Eng. J. 2016, 49, 6–30. [Google Scholar] [CrossRef]
- Manzoor, M.M.; Goyal, P.; Gupta, A.P.; Gupta, S. Heavy Metal Soil Contamination and Bioremediation. In Bioremediation and Biotechnology; Bhat, R.A., Hakeem, K.R., Dervash, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 2, pp. 221–239. ISBN 978-3-030-40332-4. [Google Scholar]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Heavy Metals in Iberian Soils: Removal by Current Adsorbents/Amendments and Prospective for Aerogels. Adv. Colloid Interface Sci. 2016, 237, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Song, Y.; Chen, L.; Ji, J.; Li, J.; Yuan, X.; Yang, Z.; Ayoko, G.A.; Frost, R.L.; Theiss, F. Human Health Risks of Heavy Metals in Paddy Rice Based on Transfer Characteristics of Heavy Metals from Soil to Rice. CATENA 2019, 175, 339–348. [Google Scholar] [CrossRef]
- He, F.; Gao, J.; Pierce, E.; Strong, P.J.; Wang, H.; Liang, L. In Situ Remediation Technologies for Mercury-Contaminated Soil. Environ. Sci. Pollut. Res. 2015, 22, 8124–8147. [Google Scholar] [CrossRef]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil Organic Matter as Sole Indicator of Soil Degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef]
- Sungur, A.; Soylak, M.; Yilmaz, E.; Yilmaz, S.; Ozcan, H. Characterization of Heavy Metal Fractions in Agricultural Soils by Sequential Extraction Procedure: The Relationship Between Soil Properties and Heavy Metal Fractions. Soil Sediment Contam. Int. J. 2015, 24, 1–15. [Google Scholar] [CrossRef]
- Minnikova, T.V.; Denisova, T.V.; Mandzhieva, S.S.; Kolesnikov, S.I.; Minkina, T.M.; Chaplygin, V.A.; Burachevskaya, M.V.; Sushkova, S.N.; Bauer, T.V. Assessing the Effect of Heavy Metals from the Novocherkassk Power Station Emissions on the Biological Activity of Soils in the Adjacent Areas. J. Geochem. Explor. 2017, 174, 70–78. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, Y. Progress of Research and Utilization of Soil Amendments. Ecol. Environ. 2008, 1282–1289. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Basic Concepts on Heavy Metal Soil Bioremediation. Eur. J. Miner. Process. Environ. Prot. 2003, 3, 58–66. [Google Scholar]
- Peng, W.; Li, X.; Song, J.; Jiang, W.; Liu, Y.; Fan, W. Bioremediation of Cadmium- and Zinc-Contaminated Soil Using Rhodobacter Sphaeroides. Chemosphere 2018, 197, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, A.; Cui, C. Remediation of Heavy Metal-Contaminated Soils by Electrokinetic Technology: Mechanisms and Applicability. Chemosphere 2021, 265, 129071. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; He, Y.; Wu, Y.; Dian, B.; Zhang, J.; Li, T.; Jiang, M. Solidification/Stabilization of Soil Heavy Metals by Alkaline Industrial Wastes: A Critical Review. Environ. Pollut. 2022, 312, 120094. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Phytoremediation: A Multidisciplinary Approach to Clean up Heavy Metal Contaminated Soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Thomé, A.; Reddy, K.R.; Reginatto, C.; Cecchin, I. Review of Nanotechnology for Soil and Groundwater Remediation: Brazilian Perspectives. Water Air Soil Pollut. 2015, 226, 121. [Google Scholar] [CrossRef]
- O’Brien, P.L.; DeSutter, T.M.; Casey, F.X.M.; Khan, E.; Wick, A.F. Thermal Remediation Alters Soil Properties—A Review. J. Environ. Manag. 2018, 206, 826–835. [Google Scholar] [CrossRef]
- Superfund Remedy Report 17th Edition—EPA 542-R-23-001. 2023. Available online: https://www.epa.gov/remedytech/superfund-remedy-report (accessed on 1 November 2024).
- Desogus, P.; Manca, P.P.; Orrù, G. Heavy Metal Leaching of Contaminated Soils from a Metallurgical Plant. Int. J. Min. Reclam. Environ. 2013, 27, 202–214. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, Z.; Pang, Y.; Wang, Y.; Cai, Z. Coupling Bioleaching and Electrokinetics to Remediate Heavy Metal Contaminated Soils. Bull. Environ. Contam. Toxicol. 2015, 94, 519–524. [Google Scholar] [CrossRef]
- Moon, D.H.; Chang, Y.-Y.; Lee, M.; Koutsospyros, A.; Koh, I.-H.; Ji, W.H.; Park, J.-H. Assessment of Soil Washing for Heavy Metal Contaminated Paddy Soil Using FeCl3 Washing Solutions. Environ. Geochem. Health 2021, 43, 3343–3350. [Google Scholar] [CrossRef]
- Chen, C.-P.; Cheng, C.-H.; Huang, Y.-H.; Chen, C.-T.; Lai, C.-M.; Menyailo, O.V.; Fan, L.-J.; Yang, Y.-W. Converting Leguminous Green Manure into Biochar: Changes in Chemical Composition and C and N Mineralization. Geoderma 2014, 232–234, 581–588. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.-L.; Liu, S.-Y. Application of Biomass By-Product Lignin Stabilized Soils as Sustainable Geomaterials: A Review. Sci. Total Environ. 2020, 728, 138830. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, M.; Abollino, O.; Buoso, S.; Giacomino, A.; La Gioia, C.; Mentasti, E. Accumulation of Heavy Metals from Contaminated Soil to Plants and Evaluation of Soil Remediation by Vermiculite. Chemosphere 2011, 82, 169–178. [Google Scholar] [CrossRef] [PubMed]
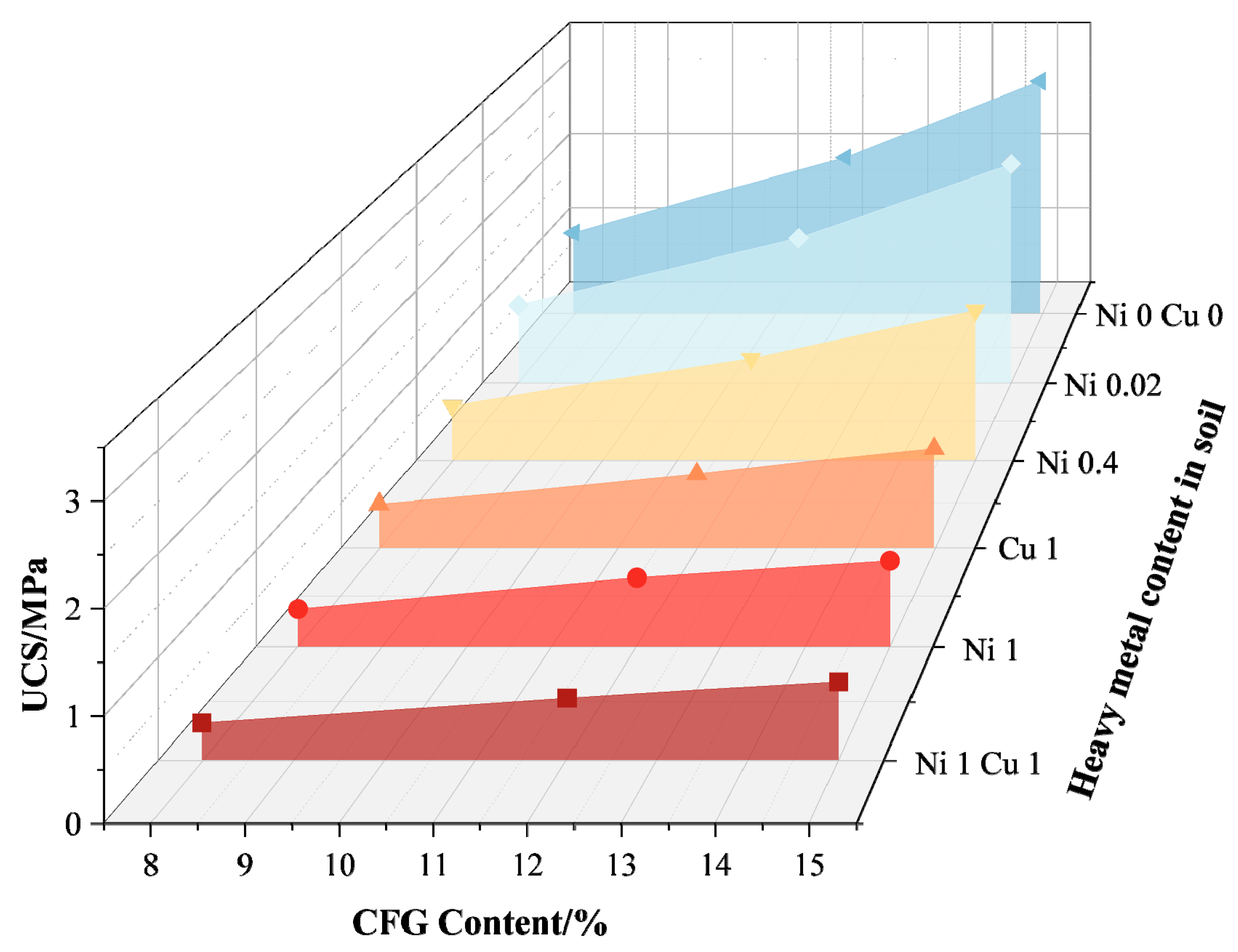
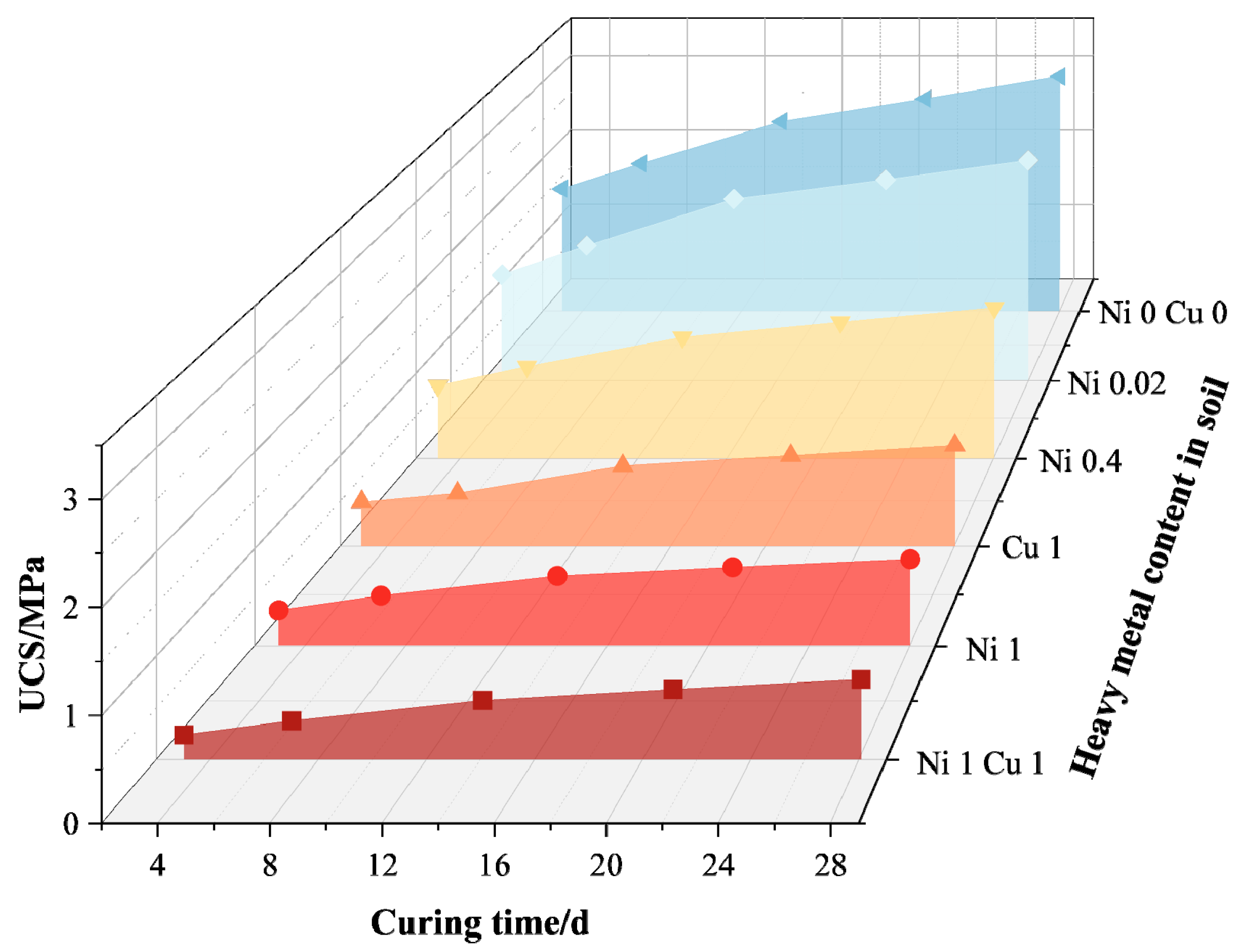
- Wang, Q.; Li, M.; Yang, J.; Cui, J.; Zhou, W.; Guo, X. Study on Mechanical and Permeability Characteristics of Nickel-Copper-Contaminated Soil Solidified by CFG. Environ. Sci. Pollut. Res. 2020, 27, 18577–18591. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Hussain, Z.; Khan, A.; Khan, M.A.; Rab, A.; Asif, M.; Shah, M.A.; Muhammad, A. The Effects of Organic Amendments on Heavy Metals Bioavailability in Mine Impacted Soil and Associated Human Health Risk. Sci. Hortic. 2020, 262, 109067. [Google Scholar] [CrossRef]
- Saravanan, D.; Gomathi, T.; Sudha, P.N. Sorption Studies on Heavy Metal Removal Using Chitin/Bentonite Biocomposite. Int. J. Biol. Macromol. 2013, 53, 67–71. [Google Scholar] [CrossRef]
- Bian, R.; Joseph, S.; Cui, L.; Pan, G.; Li, L.; Liu, X.; Zhang, A.; Rutlidge, H.; Wong, S.; Chia, C.; et al. A Three-Year Experiment Confirms Continuous Immobilization of Cadmium and Lead in Contaminated Paddy Field with Biochar Amendment. J. Hazard. Mater. 2014, 272, 121–128. [Google Scholar] [CrossRef]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.; Zhang, X.; Zheng, J.; et al. Biochar Soil Amendment as a Solution to Prevent Cd-Tainted Rice from China: Results from a Cross-Site Field Experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Anionic Polyacrylamide Influence on the Lead(II) Ion Accumulation in Soil—The Study on Montmorillonite. J. Environ. Health Sci. Eng. 2020, 18, 599–607. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Xiang, K.; Chen, J.; Zhang, Y.; Wang, J.; Sun, J.; Li, Y. A Multifunctional Microspheric Soil Conditioner Based on Chitosan-Grafted Poly(acrylamide-co-acrylic acid)/Biochar. Langmuir 2022, 38, 5717–5729. [Google Scholar] [CrossRef]
- Li, L.; Lin, Q.; Li, X.; Li, T.; He, X.; Li, D.; Tao, Y. Dynamics and Potential Roles of Abundant and Rare Subcommunities in the Bioremediation of Cadmium-Contaminated Paddy Soil by Pseudomonas Chenduensis. Appl. Microbiol. Biotechnol. 2019, 103, 8203–8214. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Khandelwal, M.; Du, K.; Zhou, J. Knowledge Mapping of Research Progress in Blast-Induced Ground Vibration from 1990 to 2022 Using CiteSpace-Based Scientometric Analysis. Environ. Sci. Pollut. Res. 2023, 30, 103534–103555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Ji, X.; Yun, C.; Wang, M.; Luo, X. The Knowledge Domain and Emerging Trends in Phytoremediation: A Scientometric Analysis with CiteSpace. Environ. Sci. Pollut. Res. 2020, 27, 15515–15536. [Google Scholar] [CrossRef] [PubMed]
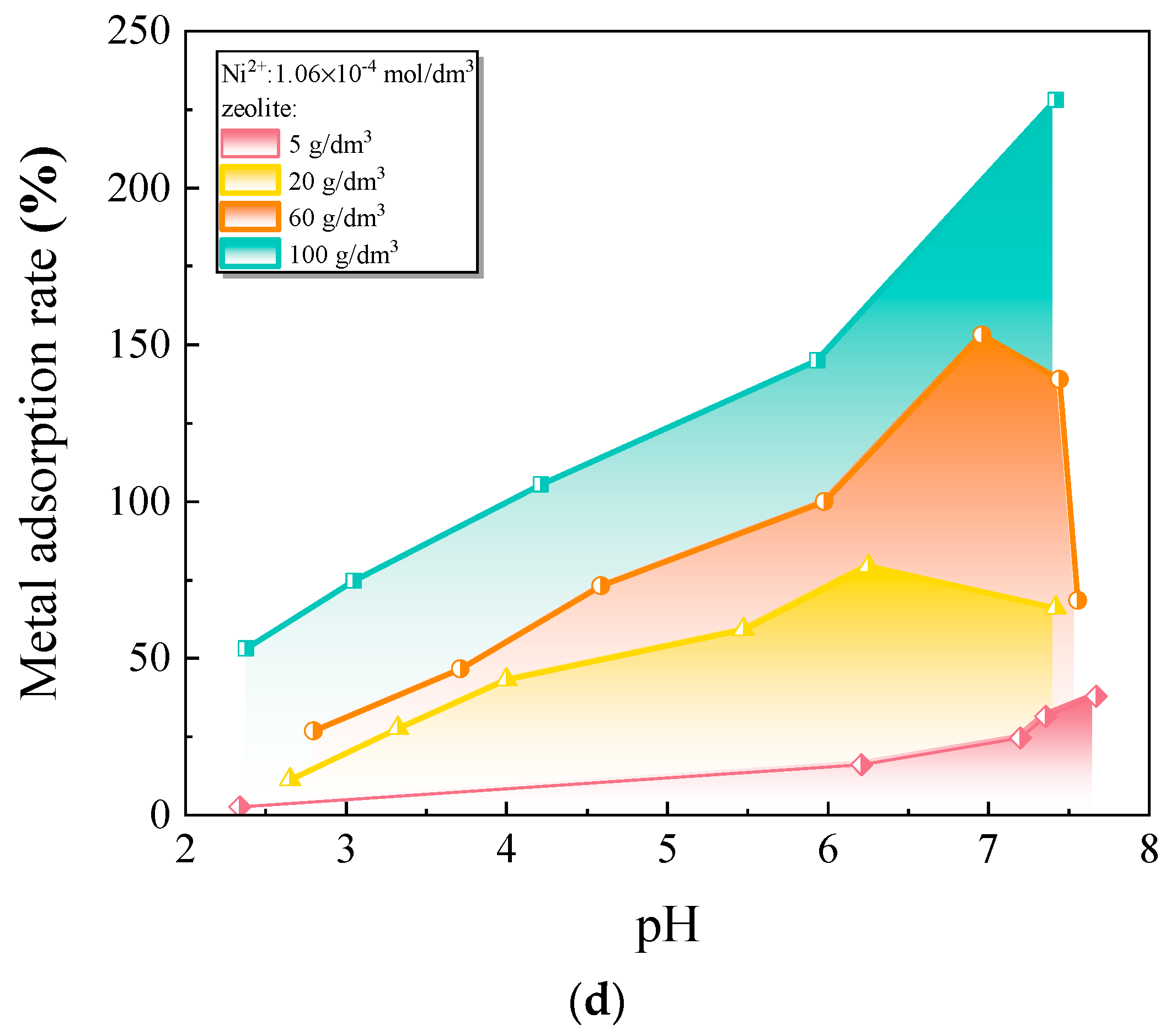
- Misaelides, P. Application of Natural Zeolites in Environmental Remediation: A Short Review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Kuldeyev, E.; Seitzhanova, M.; Tanirbergenova, S.; Tazhu, K.; Doszhanov, E.; Mansurov, Z.; Azat, S.; Nurlybaev, R.; Berndtsson, R. Modifying Natural Zeolites to Improve Heavy Metal Adsorption. Water 2023, 15, 2215. [Google Scholar] [CrossRef]
- Kordala, N.; Wyszkowski, M. Zeolite Properties, Methods of Synthesis, and Selected Applications. Molecules 2024, 29, 1069. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.; Xu, Y.; Qin, X.; Huang, Q.; Wang, L.; Sun, Y. Remediation of Heavy Metal-Polluted Agricultural Soils Using Clay Minerals: A Review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Majumder, S.; Jha, A.K. Removal of Cr and Mn from Aqueous Medium Using Bentonites and Their Derivatives. J. Chem. Sci. 2020, 132, 135. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In Situ Stabilization Remediation of Cadmium (Cd) and Lead (Pb) Co-Contaminated Paddy Soil Using Bentonite. Appl. Clay Sci. 2015, 105–106, 200–206. [Google Scholar] [CrossRef]
- Kumar Jha, A. Application of Bentonite Mineral in Removal of Heavy Metals. Acta Scie Agric. 2020, 4, 63–64. [Google Scholar] [CrossRef]
- Liu, X.; Hicher, P.; Muresan, B.; Saiyouri, N.; Hicher, P.-Y. Heavy Metal Retention Properties of Kaolin and Bentonite in a Wide Range of Concentration and Different pH Conditions. Appl. Clay Sci. 2016, 119, 365–374. [Google Scholar] [CrossRef]
- Li, W.; Ni, P.; Yi, Y. Comparison of Reactive Magnesia, Quick Lime, and Ordinary Portland Cement for Stabilization/Solidification of Heavy Metal-Contaminated Soils. Sci. Total Environ. 2019, 671, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Gabaldón, C.; Marzal, P. Sorption Characteristics of Heavy Metal Ions by a Natural Zeolite: Sorption of Heavy Metal Ions by Zeolite. J. Chem. Technol. Biotechnol. 2005, 80, 477–481. [Google Scholar] [CrossRef]
- Zhao, S.; Meng, Z.; Fan, X.; Jing, R.; Yang, J.; Shao, Y.; Liu, X.; Wu, M.; Zhang, Q.; Liu, A. Removal of Heavy Metals from Soil by Vermiculite Supported Layered Double Hydroxides with Three-Dimensional Hierarchical Structure. Chem. Eng. J. 2020, 390, 124554. [Google Scholar] [CrossRef]
- Colombani, N.; Mastrocicco, M.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Batch and Column Experiments on Nutrient Leaching in Soils Amended with Italian Natural Zeolitites. CATENA 2015, 127, 64–71. [Google Scholar] [CrossRef]
- Miraki, H.; Shariatmadari, N.; Ghadir, P.; Jahandari, S.; Tao, Z.; Siddique, R. Clayey Soil Stabilization Using Alkali-Activated Volcanic Ash and Slag. J. Rock Mech. Geotech. Eng. 2022, 14, 576–591. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, W.; Wang, X.; Pan, H.; Zhao, Q.; Wang, D. Utilization of Municipal Solid Waste Incineration Fly Ash with Red Mud-Carbide Slag for Eco-Friendly Geopolymer Preparation. J. Clean. Prod. 2022, 340, 130820. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Lu, Y.; Zhu, D. The Influence of Industrial Solid Waste in Conjunction with Lepidolite Tailings on the Mechanical Properties and Microstructure of Cemented Backfill Materials. Constr. Build. Mater. 2024, 419, 135422. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Wan, S.; Hu, B.; Tong, J.; Sun, H.; Chen, Y.; Zhang, J.; Hou, H. Immobilizatiaon of Heavy Metals in Municipal Solid Waste Incineration Fly Ash with Red Mud-Coal Gangue. J. Mater. Cycles Waste Manag. 2020, 22, 1953–1964. [Google Scholar] [CrossRef]
- Gu, Y.; Li, J.-L.; Peng, J.-K.; Xing, F.; Long, W.-J.; Khayat, K.H. Immobilization of Hazardous Ferronickel Slag Treated Using Ternary Limestone Calcined Clay Cement. Constr. Build. Mater. 2020, 250, 118837. [Google Scholar] [CrossRef]
- Toksöz Hozatlıoğlu, D.; Yılmaz, I. Shallow Mixing and Column Performances of Lime, Fly Ash and Gypsum on the Stabilization of Swelling Soils. Eng. Geol. 2021, 280, 105931. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Liu, X.; Gao, H.; Song, S.; Lu, J. Enhancing Mechanical Properties of Micaceous Weathered Granitic Soil: A Synergetic Approach Using Natural Fibers and Fly Ash. Bull. Eng. Geol. Environ. 2024, 83, 257. [Google Scholar] [CrossRef]
- Nayak, A.K.; Raja, R.; Rao, K.S.; Shukla, A.K.; Mohanty, S.; Shahid, M.; Tripathi, R.; Panda, B.B.; Bhattacharyya, P.; Kumar, A.; et al. Effect of Fly Ash Application on Soil Microbial Response and Heavy Metal Accumulation in Soil and Rice Plant. Ecotoxicol. Environ. Saf. 2015, 114, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zheng, X.; Liu, S.; Al-Tabbaa, A. Comparison of Reactive Magnesia- and Carbide Slag-Activated Ground Granulated Blastfurnace Slag and Portland Cement for Stabilisation of a Natural Soil. Appl. Clay Sci. 2015, 111, 21–26. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Yang, J.; Guo, X.; Zhou, W. Strength and Mechanism of Carbonated Solidified Clay with Steel Slag Curing Agent. KSCE J. Civ. Eng. 2021, 25, 805–821. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, B.; Zhang, G.; Zhang, X. Sewage Sludge as Barrier Material for Heavy Metals in Waste Landfill. Arch. Environ. Prot. 2016, 42, 52–58. [Google Scholar] [CrossRef]
- Goodarzi, A.R.; Movahedrad, M. Stabilization/Solidification of Zinc-Contaminated Kaolin Clay Using Ground Granulated Blast-Furnace Slag and Different Types of Activators. Appl. Geochem. 2017, 81, 155–165. [Google Scholar] [CrossRef]
- Nguyen Phan, B.; Sekine, R.; Hayano, K.; Yamauchi, H. Assessment of Consistency and Strength Properties of Clays Treated with Paper Sludge Ash-Based Stabilizers Using the Water Absorption and Retention Rate. Constr. Build. Mater. 2022, 351, 128936. [Google Scholar] [CrossRef]
- Gómez, M.; Peisino, L.E.; Kreiker, J.; Gaggino, R.; Cappelletti, A.L.; Martín, S.E.; Uberman, P.M.; Positieri, M.; Raggiotti, B.B. Stabilization of Hazardous Compounds from WEEE Plastic: Development of a Novel Core-Shell Recycled Plastic Aggregate for Use in Building Materials. Constr. Build. Mater. 2020, 230, 116977. [Google Scholar] [CrossRef]
- Chen, R.; Cai, G.; Dong, X.; Pu, S.; Dai, X.; Duan, W. Green Utilization of Modified Biomass By-Product Rice Husk Ash: A Novel Eco-Friendly Binder for Stabilizing Waste Clay as Road Material. J. Clean. Prod. 2022, 376, 134303. [Google Scholar] [CrossRef]
- Sanito, R.C.; Bernuy-Zumaeta, M.; You, S.-J.; Wang, Y.-F. A Review on Vitrification Technologies of Hazardous Waste. J. Environ. Manag. 2022, 316, 115243. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-S.; Cho, Y.-C.; Lin, Y.-P. Regeneration of Heavy Metal Contaminated Soils for Cement Production by Cement Kiln Co-Processing. Resour. Conserv. Recycl. 2022, 176, 105909. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of Fly Ash Incorporation in Soil Systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Singh, J.S.; Pandey, V.C. Fly Ash Application in Nutrient Poor Agriculture Soils: Impact on Methanotrophs Population Dynamics and Paddy Yields. Ecotoxicol. Environ. Saf. 2013, 89, 43–51. [Google Scholar] [CrossRef]
- Kováčik, P.; Macák, M.; Ducsay, L.; Halčínová, M.; Jančich, M. Effect of Ash-Fly Ash Mixture Application on Soil Fertility. J. Elem. 2011, 16, 215–225. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Jha, S.K.; Ram, L.C. Impact of Heavy Metals in Indian Fly Ashes on Its Application as Soil Ameliorant. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2568–2574. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, S.; Yuan, Z. Adoption of Solid Organic Waste Composting Products: A Critical Review. J. Clean. Prod. 2020, 272, 122712. [Google Scholar] [CrossRef]
- Atiyeh, R.; Lee, S.; Edwards, C.; Arancon, N.; Metzger, J. The Influence of Humic Acids Derived from Earthworm-Processed Organic Wastes on Plant Growth. Bioresour. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef]
- Fekri, M.; Kaveh, S. Heavy Metal Accumulation in Soil after Application of Organic Wastes. Arab. J. Geosci. 2013, 6, 463–467. [Google Scholar] [CrossRef]
- Liu, K. Major Factors Influencing Cadmium Uptake from the Soil into Wheat Plants. Ecotoxicol. Environ. Saf. 2015, 113, 207–213. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in Straw Decomposition Rate and Soil Microbial Community Composition after Straw Addition in Different Long-Term Fertilization Soils. Appl. Soil. Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Wu, L.; Ma, H.; Zhao, Q.; Zhang, S.; Wei, W.; Ding, X. Changes in Soil Bacterial Community and Enzyme Activity Under Five Years Straw Returning in Paddy Soil. Eur. J. Soil. Biol. 2020, 100, 103215. [Google Scholar] [CrossRef]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land Application of Organic Waste—Effects on the Soil Ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Griffin, R.A.; Jurinak, J.J. Test of a New Model for the Kinetics of Adsorption-Desorption Processes. Soil Sci. Soc. Am. J. 1973, 37, 869–872. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Guo, Z.; Xu, L.; Zhao, H.; Zhao, P.; Ma, C.; Yi, K.; Jia, X. Revealing the Underlying Molecular Basis of Phosphorus Recycling in the Green Manure Crop Astragalus Sinicus. J. Clean. Prod. 2022, 341, 130924. [Google Scholar] [CrossRef]
- Su, Y.; Lv, J.L.; Yu, M.; Ma, Z.H.; Xi, H.; Kou, C.L.; He, Z.C.; Shen, A.L. Long-term Decomposed Straw Return Positively Affects the Soil Microbial Community. J. Appl. Microbiol. 2020, 128, 138–150. [Google Scholar] [CrossRef]
- Cai, G.; Zhang, T.; Liu, S.; Li, J.; Jie, D. Stabilization Mechanism and Effect Evaluation of Stabilized Silt with Lignin Based on Laboratory Data. Mar. Georesour. Geotechnol. 2016, 34, 331–340. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S. Application of Lignin-Based by-Product Stabilized Silty Soil in Highway Subgrade: A Field Investigation. J. Clean. Prod. 2017, 142, 4243–4257. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, S.; Zhan, H.; Ma, C.; Cai, G. Durability of Silty Soil Stabilized with Recycled Lignin for Sustainable Engineering Materials. J. Clean. Prod. 2020, 248, 119293. [Google Scholar] [CrossRef]
- He, L.; Dai, Z.; Liu, X.; Tang, C.; Xu, J. Effect of Alkaline Lignin on Immobilization of Cadmium and Lead in Soils and the Associated Mechanisms. Chemosphere 2021, 281, 130969. [Google Scholar] [CrossRef]
- Haddad, S.A.; Lemanowicz, J.; Abd El-Azeim, M.M. Cellulose Decomposition in Clay and Sandy Soils Contaminated with Heavy Metals. Int. J. Environ. Sci. Technol. 2019, 16, 3275–3290. [Google Scholar] [CrossRef]
- Jamshaid, A.; Hamid, A.; Muhammad, N.; Naseer, A.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Shah, N.S. Cellulose-Based Materials for the Removal of Heavy Metals from Wastewater—An Overview. ChemBioEng Rev. 2017, 4, 240–256. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C. Cellulose-g-poly-(acrylamide-co-acrylic acid) Polymeric Bioadsorbent for the Removal of Toxic Inorganic Pollutants from Wastewaters. Carbohydr. Polym. 2020, 228, 115396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, H.; Guo, S. Preparation of Cellulose/Chitin Blend Materials and Influence of Their Properties on Sorption of Heavy Metals. Sustainability 2021, 13, 6460. [Google Scholar] [CrossRef]
- Cretoiu, M.S.; Korthals, G.W.; Visser, J.H.M.; van Elsas, J.D. Chitin Amendment Increases Soil Suppressiveness toward Plant Pathogens and Modulates the Actinobacterial and Oxalobacteraceal Communities in an Experimental Agricultural Field. Appl. Environ. Microbiol. 2013, 79, 5291–5301. [Google Scholar] [CrossRef]
- Hui, C.; Jiang, H.; Liu, B.; Wei, R.; Zhang, Y.; Zhang, Q.; Liang, Y.; Zhao, Y. Chitin Degradation and the Temporary Response of Bacterial Chitinolytic Communities to Chitin Amendment in Soil under Different Fertilization Regimes. Sci. Total Environ. 2020, 705, 136003. [Google Scholar] [CrossRef]
- Duan, Y.; Freyburger, A.; Kunz, W.; Zollfrank, C. Lignin/Chitin Films and Their Adsorption Characteristics for Heavy Metal Ions. ACS Sustain. Chem. Eng. 2018, 6, 6965–6973. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A Review of Biochars’ Potential Role in the Remediation, Revegetation and Restoration of Contaminated Soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using Biochar for Remediation of Soils Contaminated with Heavy Metals and Organic Pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Taraqqi-A-Kamal, A.; Atkinson, C.J.; Khan, A.; Zhang, K.; Sun, P.; Akther, S.; Zhang, Y. Biochar Remediation of Soil: Linking Biochar Production with Function in Heavy Metal Contaminated Soils. Plant Soil Environ. 2021, 67, 183–201. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Adhikari, S.; Ma, H.; Kim, D.-H.; Bai, Y.; Hou, D. Progress and Future Prospects in Biochar Composites: Application and Reflection in the Soil Environment. Crit. Rev. Environ. Sci. Technol. 2021, 51, 219–271. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Li, X.; Wang, J. Mechanisms of Biochar Effects on Thermal Properties of Red Soil in South China. Geoderma 2018, 323, 41–51. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative Distribution of Pb2+ Sorption Mechanisms by Sludge-Derived Biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, B.; Shafi, M.; Ma, J.; Guo, J.; Wu, J.; Ye, Z.; Liu, D.; Jin, H. Effects of Biochar on Growth, and Heavy Metals Accumulation of Moso Bamboo (Phyllostachy pubescens), Soil Physical Properties, and Heavy Metals Solubility in Soil. Chemosphere 2019, 219, 510–516. [Google Scholar] [CrossRef]
- Githinji, L. Effect of Biochar Application Rate on Soil Physical and Hydraulic Properties of a Sandy Loam. Arch. Agron. Soil Sci. 2014, 60, 457–470. [Google Scholar] [CrossRef]
- Berihun, T.; Tadele, M.; Kebede, F. The Application of Biochar on Soil Acidity and Other Physico-chemical Properties of Soils in Southern Ethiopia. J. Plant Nutr. Soil Sci. 2017, 180, 381–388. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, K.-R.; Kim, H.-J.; Yoon, J.-H.; Yang, J.E.; Ok, Y.S.; Owens, G.; Kim, K.-H. Effect of Biochar on Heavy Metal Immobilization and Uptake by Lettuce (Lactuca sativa L.) in Agricultural Soil. Environ. Earth Sci. 2015, 74, 1249–1259. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.-J.; Lee, M.-E.; Chung, J.W. Comparison of Heavy Metal Immobilization in Contaminated Soils Amended with Peat Moss and Peat Moss-Derived Biochar. Environ. Sci. Process. Impacts 2016, 18, 514–520. [Google Scholar] [CrossRef]
- Egene, C.E.; Van Poucke, R.; Ok, Y.S.; Meers, E.; Tack, F.M.G. Impact of Organic Amendments (Biochar, Compost and Peat) on Cd and Zn Mobility and Solubility in Contaminated Soil of the Campine Region after Three Years. Sci. Total Environ. 2018, 626, 195–202. [Google Scholar] [CrossRef]
- Huang, D.; Liu, L.; Zeng, G.; Xu, P.; Huang, C.; Deng, L.; Wang, R.; Wan, J. The Effects of Rice Straw Biochar on Indigenous Microbial Community and Enzymes Activity in Heavy Metal-Contaminated Sediment. Chemosphere 2017, 174, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Igalavithana, A.D.; Lee, S.-E.; Lee, Y.H.; Tsang, D.C.W.; Rinklebe, J.; Kwon, E.E.; Ok, Y.S. Heavy Metal Immobilization and Microbial Community Abundance by Vegetable Waste and Pine Cone Biochar of Agricultural Soils. Chemosphere 2017, 174, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Abulaiti, A.; She, D.; Liu, Z.; Sun, X.; Wang, H. Application of Biochar and Polyacrylamide to Revitalize Coastal Saline Soil Quality to Improve Rice Growth. Environ. Sci. Pollut. Res. 2022, 30, 18731–18747. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Fijałkowska, G.; Szewczuk-Karpisz, K.; Herda, K.; Chibowski, S. Ionic Polyacrylamides as Stability-Modifying Substances of Soil Mineral Suspensions Containing Heavy Metal Impurities. Processes 2022, 10, 1473. [Google Scholar] [CrossRef]
- Rabiee, A.; Gilani, M.; Jamshidi, H.; Baharvand, H. Synthesis and Characterization of a Calcium- and Sodium-Containing Acrylamide-Based Polymer and Its Effect on Soil Strength. J. Vinyl Addit. Technol. 2013, 19, 140–146. [Google Scholar] [CrossRef]
- Asghari, S.; Abbasi, F.; Neyshabouri, M.R. Effects of Soil Conditioners on Physical Quality and Bromide Transport Properties in a Sandy Loam Soil. Biosyst. Eng. 2011, 109, 90–97. [Google Scholar] [CrossRef]
- Dhiman, J.; Prasher, S.O.; ElSayed, E.; Patel, R.M.; Nzediegwu, C.; Mawof, A. Effect of Hydrogel Based Soil Amendments on Heavy Metal Uptake by Spinach Grown with Wastewater Irrigation. J. Clean. Prod. 2021, 311, 127644. [Google Scholar] [CrossRef]
- Dhiman, J.; Prasher, S.O.; ElSayed, E.; Patel, R.; Nzediegwu, C.; Mawof, A. Use of Polyacrylamide Superabsorbent Polymers and Plantain Peel Biochar to Reduce Heavy Metal Mobility and Uptake by Wastewater-Irrigated Potato Plants. Trans. ASABE 2020, 63, 11–28. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Zhang, Y.; Sun, T.; An, S.; Jia, H. Effects of Amendments on Physicochemical Properties and Respiration Rate of Soil from the Arid Region of Northwest China. Sustainability 2021, 13, 5332. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, K.; Chen, J.; Shi, X.; Li, X.; Ma, Y.; Fang, G.; Xu, S. Changes in Heavy Metal Mobility and Availability in Contaminated Wet-Land Soil Remediated Using Lignin-Based Poly(acrylic acid). J. Hazard. Mater. 2019, 368, 459–467. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, A.; Zhao, Y.; Wang, Q.; Chen, Y.; Li, M.; Xing, W. Competitive Adsorption of Hg2+, Pb2+ and Co2+ Ions on Polyacrylamide/Attapulgite. Desalination 2011, 270, 269–274. [Google Scholar] [CrossRef]
- Basuki, K.T.; Swantomo, D.; Sigit; Sanyoto, N.T. Characterization of Chitosan-Acrylamide Hydrogels as Soil Conditioner. AMR 2015, 1112, 414–417. [Google Scholar] [CrossRef]
- Niu, Y.; Li, H. Controlled Release of Urea Encapsulated by Starch-g-poly(vinyl acetate). Ind. Eng. Chem. Res. 2012, 51, 12173–12177. [Google Scholar] [CrossRef]
- Mahdavi, A.E.B.; Panahpour, E.; Kalbasi, R.J.; Gholami, A. Preparation, Characterization, and Application of Polyacrylamide-Polystyrene/Bentonite Nanocomposite as an Effective Immobilizing Adsorbent for Remediation of Soil. ChemistrySelect 2020, 5, 4538–4547. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent Advances in Soil Remediation Technology for Heavy Metal Contaminated Sites: A Critical Review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Verma, S.; Kuila, A. Bioremediation of Heavy Metals by Microbial Process. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Teng, Y.; Li, Z.; Ma, L.Q. Bioremediation of Oily Sludge-Contaminated Soil by Stimulating Indigenous Microbes. Environ. Geochem. Health 2010, 32, 23–29. [Google Scholar] [CrossRef]
- Ren, W.-X.; Li, P.-J.; Geng, Y.; Li, X.-J. Biological Leaching of Heavy Metals from a Contaminated Soil by Aspergillus niger. J. Hazard. Mater. 2009, 167, 164–169. [Google Scholar] [CrossRef]
- Basyal, B.; Emery, S.M. An Arbuscular Mycorrhizal Fungus Alters Switchgrass Growth, Root Architecture, and Cell Wall Chemistry across a Soil Moisture Gradient. Mycorrhiza 2021, 31, 251–258. [Google Scholar] [CrossRef]


| Selected Remedy | Number | Percent |
|---|---|---|
| Treatment | 48 | 37% |
| In Situ Treatment | 37 | 28% |
| Thermal Treatment | 14 | 11% |
| Soil Vapor Extraction | 13 | 10% |
| Solidification/Stabilization | 8 | 6% |
| Chemical Treatment | 5 | 4% |
| Bioremediation | 3 | 2% |
| Flushing | 2 | 2% |
| Multi-phase Extraction | 2 | 2% |
| Soil Amendments | 2 | 2% |
| Ex Situ Treatment | 17 | 13% |
| Solidification/Stabilization | 7 | 5% |
| Physical Separation | 6 | 5% |
| Thermal Treatment | 2 | 2% |
| Containment/Disposal | 90 | 69% |
| Monitored Natural Attenuation | 1 | 1% |
| Institutional Controls | 98 | 75% |
| Other | 21 | 16% |
| Soil Amendments | Classification | Experiment Types | Heavy Metals | Remediation Effectiveness | Reference |
|---|---|---|---|---|---|
| Vermiculite | Natural mineral | Pot experiments | Cu, Cr, Ni | Significantly reduce the absorption of metal pollutants by mustard and spinach plants. | [25] |
| Cement, Fly ash, Desulfurization gypsum | Inorganic solid waste | Solidification/ Stabilization | Cu, Ni | Significantly increase the compressive strength and permeability of contaminated soils. | [26] |
| Vermicompost, Leaf compost, Spent mushroom compost | Organic solid waste | Greenhouse experiments | Cd, Cr, Pb, Mn | Decrease the absorption of Cd, Cr, Pb, and Mn by plants, promoting plant growth. | [27] |
| Lignin, Chitin | Naturally extracted polymer compounds | Kinetics experiments, Adsorption experiments | Cu, Fe | Show high adsorption capacity for metal ions, especially at low concentrations. | [28] |
| Biochar | Organic material | Field experiments | Cd, Pb | Significantly increase the pH value and total organic carbon content of the soil and effectively immobilize Cd and Pb in the soil. | [29,30] |
| Polyacrylamide | Synthetic | Adsorption experiments | Pb, Cr | Immobilize Pb and Cr on the surface of clay minerals. | [31] |
| Chitosan-grafted poly(acrylamide-co-acrylic acid)/biochar | Natural-synthetic copolymer | Kinetics experiments, Adsorption experiments | Cu | Effectively increase the adsorption capacity of soils for heavy metals and improve the water retention of soils. | [32] |
| Pseudomonas chenduensis | Biological | Pot experiments | Cu, Cd, Pb, Zn | Enhance the role of the microbial community in transforming Cd components and reduce Cd accumulation in rice grains and roots. | [33] |
| Ranking | Count | Centrality | Countries |
|---|---|---|---|
| 1 | 155 | 0.57 | People’s Republic of China |
| 2 | 35 | 0.64 | Spain |
| 3 | 25 | 0.27 | USA |
| 4 | 21 | 0.17 | France |
| 5 | 20 | 0.25 | Australia |
| 6 | 18 | 0.1 | Pakistan |
| 7 | 16 | 0.06 | South Korea |
| 8 | 15 | 0.01 | Italy |
| 9 | 11 | 0.21 | Czech Republic |
| 10 | 11 | 0.01 | Poland |
| Ranking | Count | Centrality | Keywords |
|---|---|---|---|
| 1 | 191 | 0.02 | Heavy metal |
| 2 | 105 | 0.07 | Stabilization |
| 3 | 79 | 0.04 | Fly ash |
| 4 | 78 | 0.11 | Immobilization |
| 5 | 59 | 0.07 | MSWI fly ash |
| 6 | 57 | 0.13 | Stabilization/solidification |
| 7 | 53 | 0.15 | Solidification/stabilization |
| 8 | 49 | 0.11 | Cement |
| 9 | 44 | 0.15 | Portland cement |
| 10 | 40 | 0.09 | Behavior |
| Keywords | Year | Strength | Begin | End | 2013–2023 |
|---|---|---|---|---|---|
| organic amendment | 2013 | 4.34 | 2013 | 2018 | ▃▃▃▃▃▃▂▂▂▂▂ |
| mine tailing | 2013 | 3.03 | 2013 | 2017 | ▃▃▃▃▃▂▂▂▂▂▂ |
| black carbon | 2014 | 2.93 | 2014 | 2019 | ▂▃▃▃▃▃▃▂▂▂▂ |
| pollution | 2021 | 2.48 | 2021 | 2023 | ▂▂▂▂▂▂▂▂▃▃▃ |
| health risk | 2021 | 2.38 | 2021 | 2023 | ▂▂▂▂▂▂▂▂▃▃▃ |
| trace element | 2017 | 2.37 | 2017 | 2018 | ▂▂▂▂▃▃▂▂▂▂▂ |
| impact | 2019 | 2.22 | 2019 | 2020 | ▂▂▂▂▂▂▃▃▂▂▂ |
| plant | 2019 | 2.22 | 2019 | 2020 | ▂▂▂▂▂▂▃▃▂▂▂ |
| risk assessment | 2017 | 2.1 | 2017 | 2019 | ▂▂▂▂▃▃▃▂▂▂▂ |
| fractionation | 2014 | 2.05 | 2018 | 2019 | ▂▂▂▂▂▃▃▂▂▂▂ |
| paddy soil | 2020 | 2.01 | 2020 | 2023 | ▂▂▂▂▂▂▂▃▃▃▃ |
| copper | 2014 | 2 | 2014 | 2017 | ▂▃▃▃▃▂▂▂▂▂▂ |
| Cluster-ID | Count | Silhouette | Year | Top Terms (LLR) |
|---|---|---|---|---|
| 0 | 47 | 0.685 | 2018 | lead-zinc smelting slag; heavy metal; solid waste (SW); material characteristics; cementitious property |
| 1 | 45 | 0.635 | 2017 | electrolytic manganese residue; calorimetry; building materials; fly ash; APC residues |
| 2 | 40 | 0.685 | 2020 | red mud; sewage sludge; cotreatment NBSP; arsenic-laden spent media; environmental risk assessment |
| 3 | 34 | 0.866 | 2014 | calcining pretreatment; sulfates; pickling liquor; hazard-free treatment; Cu/Zn |
| 4 | 34 | 0.714 | 2018 | potentially toxic elements; cement; leaching pattern; hazardous waste management; immobilization mechanisms |
| 5 | 31 | 0.783 | 2018 | municipal solid waste; fly ash; MSWI fly ash; hazardous waste; arsenic contaminated soil |
| 6 | 30 | 0.885 | 2015 | tobermorite; air pollution control residues; heavy metal speciation; reconstructed slag; vitrification |
| 7 | 24 | 0.639 | 2020 | pre-treatment; cementitious materials; solidification and stabilization; cement kiln co-processing; XPS |
| 8 | 23 | 0.782 | 2016 | MSWI fly ash; compressive strength; stabilization; hydration products; uncertainty |
| 9 | 18 | 0.793 | 2016 | heavy metal immobilization; alkali-activated technology; nano-alumina; synergistic effect; gelation |
| Industry Source | Name of Solid Waste | References |
|---|---|---|
| Electric power industry | Slag, desulfurization gypsum, fly ash, etc. | [15,26,48,49] |
| Non-ferrous metal mining industry | Tailings, coal gangue, limestone, gypsum, etc. | [50,51,52,53] |
| Thermal industry | Fly ash, slag, dust, etc. | [15,48,52,54,55] |
| Metal processing and smelting industry | Blast furnace slag, steel slag, dust, sludge, etc. | [56,57,58,59] |
| Paper printing industry | Deinking residue, plastic debris, tailings, etc. | [50,60,61] |
| Other industries | Waste clay, nuclear waste residue, etc. | [62,63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Huang, X.; Li, M.; Lu, Z.; Ling, X. Advances in Soil Amendments for Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Impact, and Future Prospects. Toxics 2024, 12, 872. https://doi.org/10.3390/toxics12120872
Nie X, Huang X, Li M, Lu Z, Ling X. Advances in Soil Amendments for Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Impact, and Future Prospects. Toxics. 2024; 12(12):872. https://doi.org/10.3390/toxics12120872
Chicago/Turabian StyleNie, Xinyi, Xianhuai Huang, Man Li, Zhaochi Lu, and Xinhe Ling. 2024. "Advances in Soil Amendments for Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Impact, and Future Prospects" Toxics 12, no. 12: 872. https://doi.org/10.3390/toxics12120872
APA StyleNie, X., Huang, X., Li, M., Lu, Z., & Ling, X. (2024). Advances in Soil Amendments for Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Impact, and Future Prospects. Toxics, 12(12), 872. https://doi.org/10.3390/toxics12120872





