Portable SpectroChip-Based Immunoassay Platform for Rapid and Accurate Melamine Quantification in Urine Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Lateral Flow Immunoassay (LFIA)
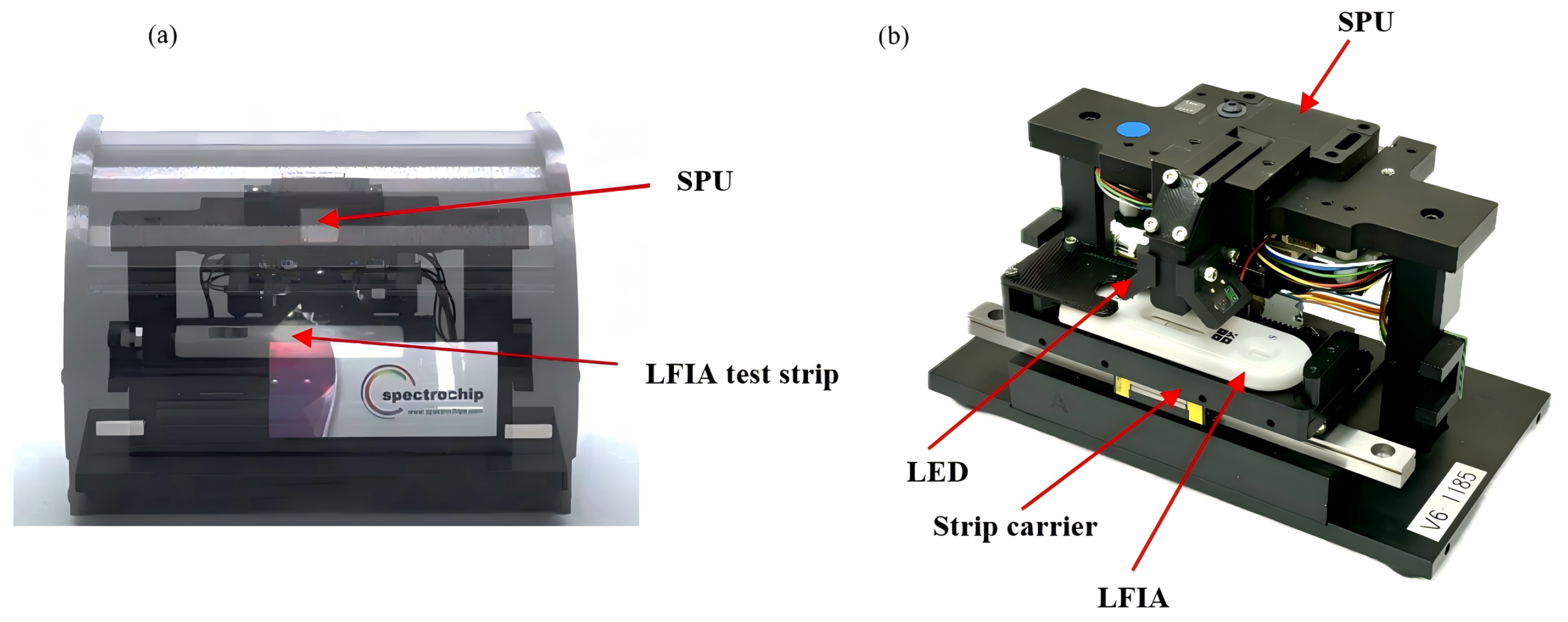
2.3. THE ONE InstantCare Platform: SpectroChip–Spectrometer Platform
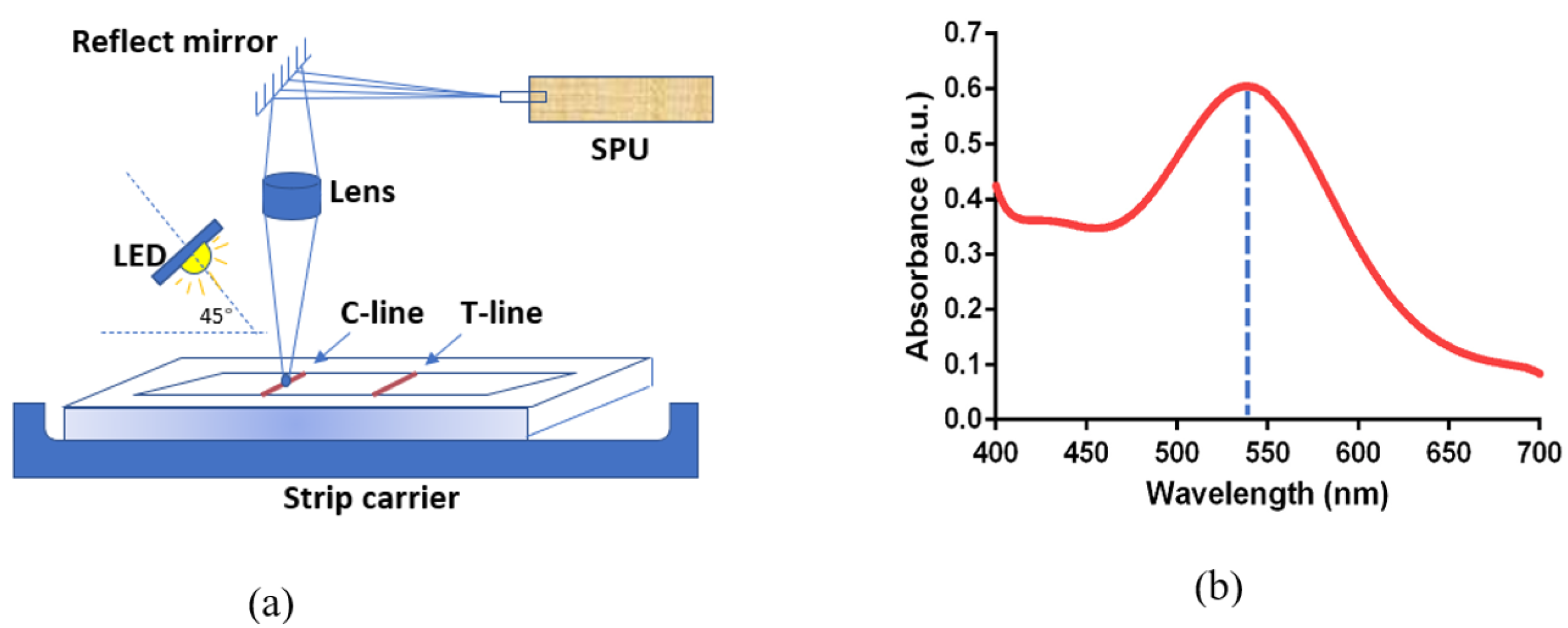
2.4. Optical Pathway and Spectral Analysis of THE ONE InstantCare Platform
2.5. Urine Collection and Measurement by LC-MS
2.6. Experimental Procedure and Statistical Analysis
3. Results
3.1. Absorbance Spectra of the Rapid Test and Concentration Model
3.2. Reproducibility, Interference Testing, and Validation of Melamine Detection Using THE ONE InstantCare Platform
3.2.1. Reproducibility Analysis
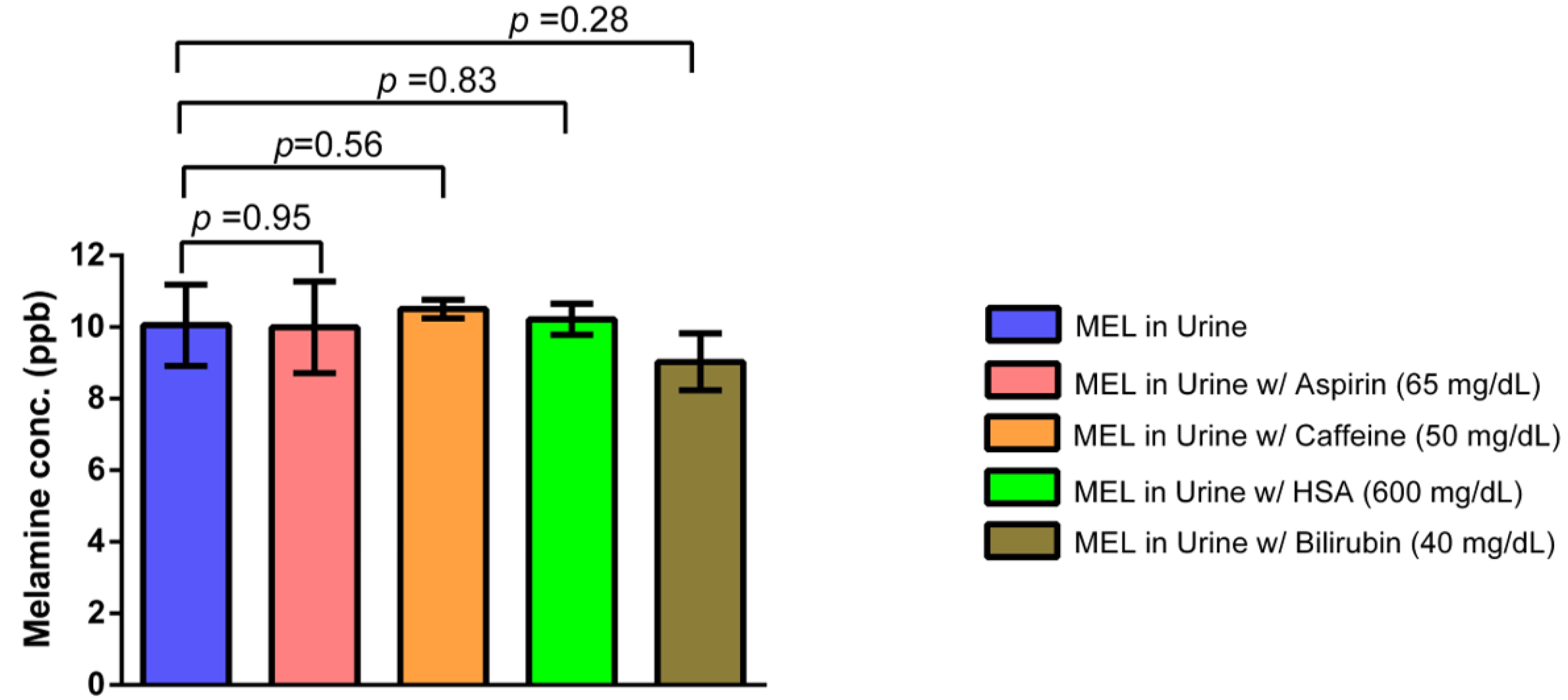
3.2.2. Interference Study
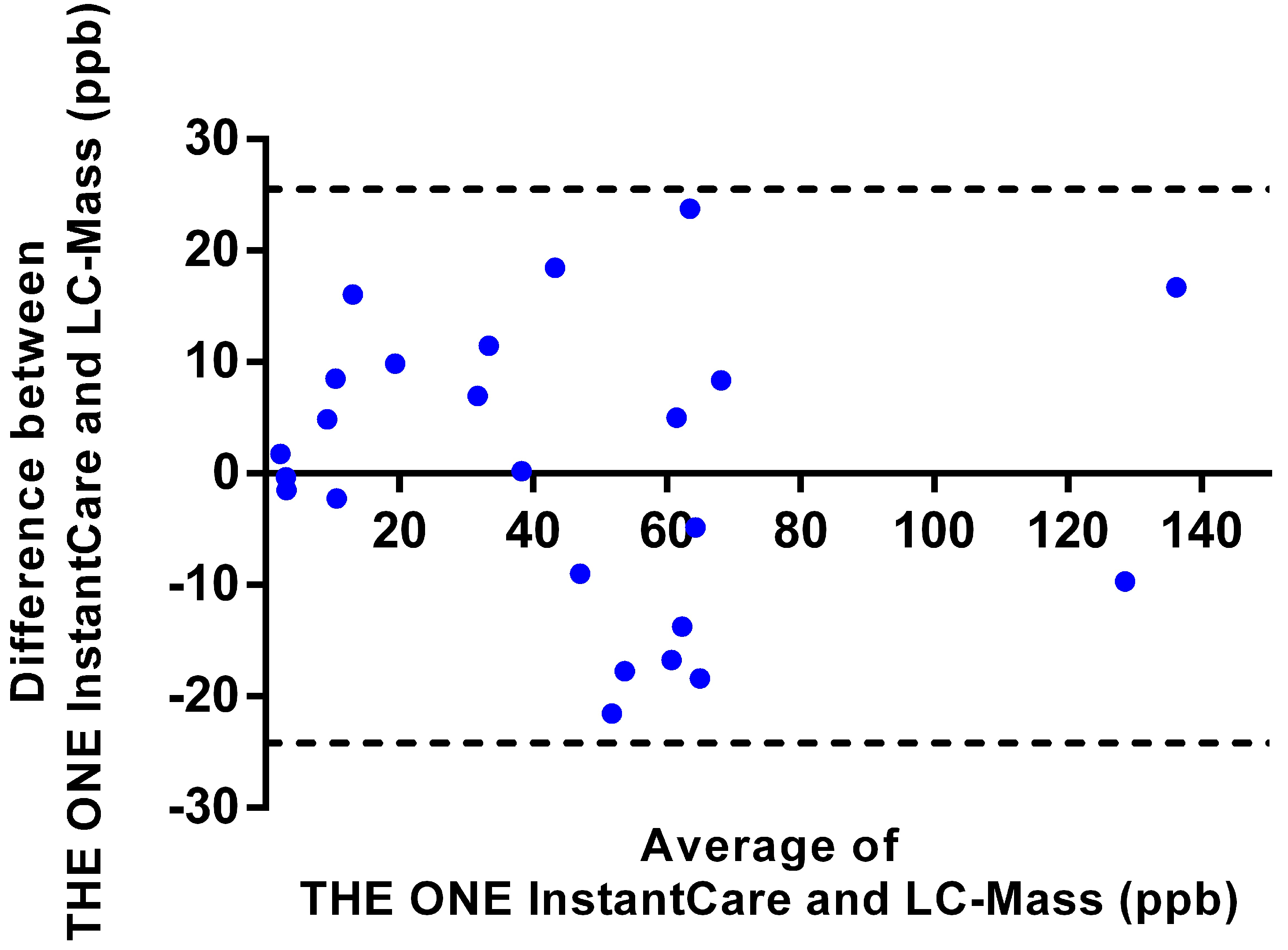
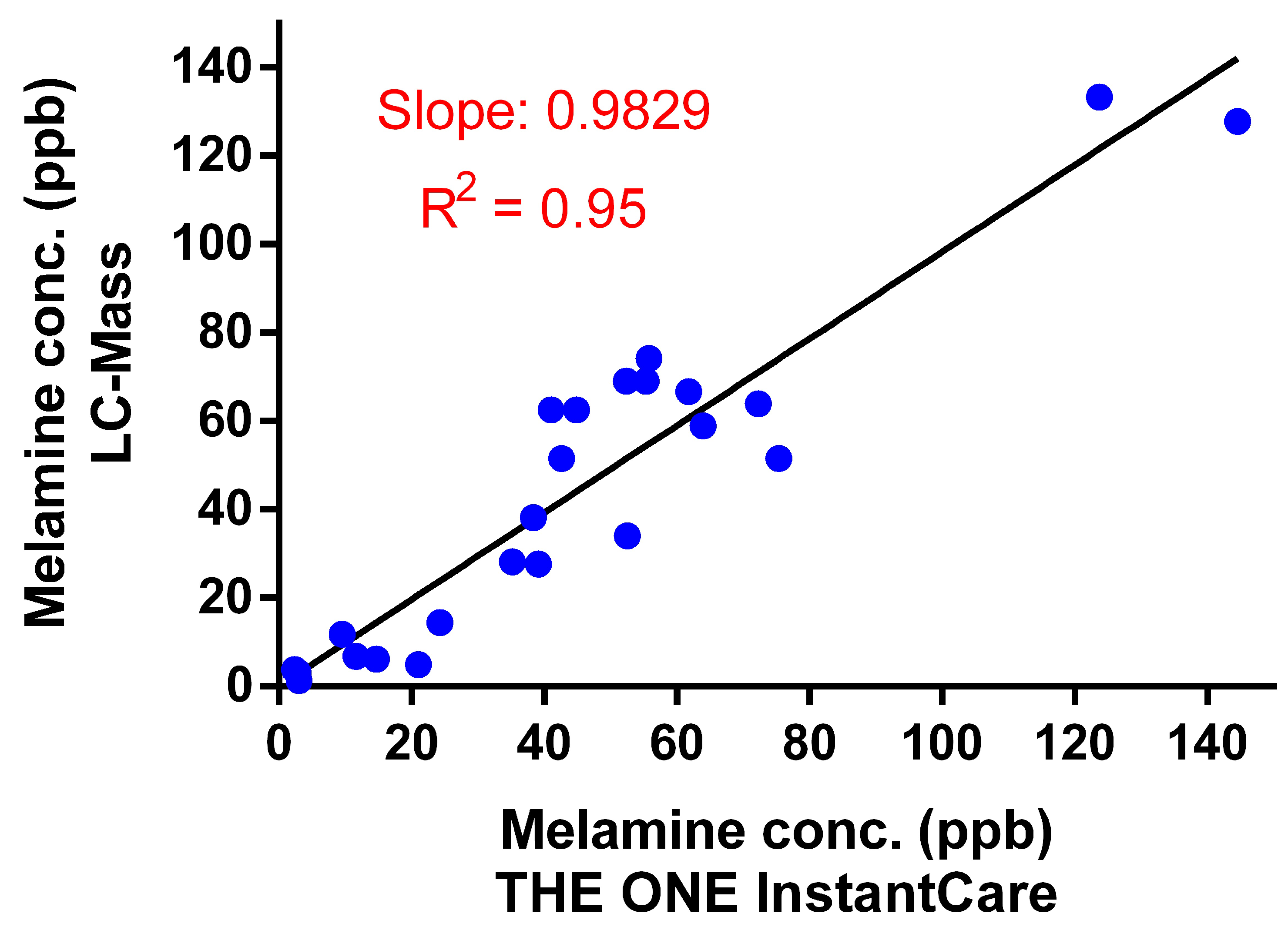
3.2.3. Validation with Human Urine Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Toxicological and Health Aspects of Melamine and Cyanuric Acid. In WHO Report; World Health Organization (WHO): Geneva, Switzerland, 2009; pp. 1–66. Available online: http://www.who.int/foodsafety/publications/chem/Melamine_report09.pdf (accessed on 10 October 2024).
- Tyan, Y.C.; Yang, M.H.; Jong, S.B.; Wang, C.K.; Shiea, J. Melamine contamination. Anal. Bioanal. Chem. 2009, 395, 729–735. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Toxicological and Health Aspects of Melamine and Cyanuric Acid: Report of a WHO Expert Meeting in Collaboration with FAO, Supported by Health Canada, Ottawa, Canada, 1–4 December 2008; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Wang, I.J.; Wu, Y.N.; Wu, W.C.; Leonardi, G.; Sung, Y.J.; Lin, T.J.; Wang, C.L.; Kuo, C.F.; Wu, K.Y.; Cheng, W.C. The association of clinical findings and exposure profiles with melamine associated nephrolithiasis. Arch. Dis. Child. 2009, 94, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Deng, Y. Melamine-associated urinary stone. Int. J. Surg. 2016, 36 Pt D, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.G.; Thomas, J.D.; Osterloh, J.D. Melamine toxicity. J. Med. Toxicol. 2010, 6, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.D. Dried blood spots for global health diagnostics and surveillance: Opportunities and challenges. Am. J. Trop. Med. Hyg. 2018, 99, 256. [Google Scholar] [CrossRef]
- Chen, B.; Liu, X.; Li, S.; Zhou, Y.; Jiang, Q. Melamine exposure assessment in children with nephrolithiasis. Pediatr. Nephrol. 2009, 24, 2065–2067. [Google Scholar] [CrossRef]
- Gossner, C.M.E.; Schlundt, J.; Ben Embarek, P.; Hird, S.; Lo-Fo-Wong, D.; Ocampo Beltran, J.J.; Teoh, K.N.; Tritscher, A. The melamine incident: Implications for international food and feed safety. Environ. Health Perspect. 2009, 117, 1803–1808. [Google Scholar] [CrossRef]
- Chen, J.-S. A worldwide food safety concern in 2008—Melamine-contaminated infant formula in China caused urinary tract stone in 290,000 children in China. Chin. Med. J. 2009, 122, 243–244. [Google Scholar] [CrossRef]
- Braekevelt, E.; Lau, B.P.-Y.; Feng, S.; Ménard, C.; Tittlemier, S.A. Determination of melamine, ammeline, ammelide and cyanuric acid in infant formula purchased in Canada by liquid chromatography-tandem mass spectrometry. Food Addit. Contam. 2011, 28, 698–704. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. Inter-day and inter-individual variability in urinary concentrations of melamine and cyanuric acid. Environ. Int. 2019, 123, 375–381. [Google Scholar] [CrossRef]
- Mast, R.W.; Jeffcoat, A.R.; Sadler, B.M.; Kraska, R.C.; Friedman, M.A. Metabolism, disposition and excretion of [14C] melamine in male Fischer 344 rats. Food Chem. Toxicol. 1983, 21, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Baynes, R.E.; Smith, G.; Mason, S.E.; Barrett, E.; Barlow, B.M.; Riviere, J.E. Pharmacokinetics of melamine in pigs following intravenous administration. Food Chem. Toxicol. 2008, 46, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Hau, A.K.-C.; Kwan, T.H.; Li, P.K.-T. Melamine toxicity and the kidney. J. Am. Soc. Nephrol. 2009, 20, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.P.H.; Chiang, C.F.; Chiang, P.H.; Wen, C.P. Toxicological analysis points to a lower tolerable daily intake of melamine in food. Regul. Toxicol. Pharmacol. 2009, 55, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Hsieh, T.J.; Chen, B.H.; Liu, C.C.; Wu, M.T. A crossover study of noodle soup consumption in melamine bowls and total melamine excretion in urine. JAMA Intern. Med. 2013, 173, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-Y.; Wu, C.-F.; Liu, C.-C.; Chen, B.-H.; Huang, S.-P.; Chou, Y.-H.; Chang, A.-W.; Lee, H.-H.; Pan, C.-H.; Wu, W.-J. High melamine migration in daily-use melamine-made tableware. J. Hazard. Mater. 2011, 188, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Hsieh, T.-J.; Wu, C.-F.; Lee, C.-H.; Tsai, Y.-C.; Huang, T.-Y.; Wen, S.-C.; Lee, C.-H.; Chien, T.-M.; Lee, Y.-C.; et al. Interrelationship of environmental melamine exposure, biomarkers of oxidative stress and early kidney injury. J. Hazard. Mater. 2020, 396, 122726. [Google Scholar] [CrossRef]
- Liu, C.-C.; Wu, C.-F.; Chen, B.-H.; Huang, S.-P.; Goggins, W.; Lee, H.-H.; Chou, Y.-H.; Wu, W.-J.; Huang, C.-H.; Shiea, J.; et al. Low exposure to melamine increases the risk of urolithiasis in adults. Kidney Int. 2011, 80, 746–752. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Melamine detection in milk and dairy products: Traditional analytical methods and recent developments. Food Anal. Methods 2018, 11, 128–147. [Google Scholar] [CrossRef]
- Liang, W.; Wei, Y.; Gao, M.; Yan, X.; Zhu, X.; Guo, W. Detection of melamine adulteration in milk powder by using optical spectroscopy technologies in the last decade—A review. Food Anal. Methods 2020, 13, 2059–2069. [Google Scholar] [CrossRef]
- Panuwet, P.; Nguyen, J.V.; Wade, E.L.; D’Souza, P.E.; Ryan, P.B.; Barr, D.B. Quantification of melamine in human urine using cation-exchange based high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 887, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chin, W.S. Rapid and sensitive SERS detection of melamine in milk using Ag nanocube array substrate coupled with multivariate analysis. Food Chem. 2021, 357, 129717. [Google Scholar] [CrossRef]
- Dikici, E.; Acet, B.Ö.; Acet, Ö.; Odabaşı, M. “Lab-on-pol” colormatic sensor platforms: Melamine detection with color change on melamine imprinted membranes. Microchem. J. 2023, 188, 108468. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, X.; Zhang, Y.; Song, B.; Liu, L.; Zhang, J.; Shi, H. Highly sensitive and rapid detection of melamine in milk products by planar waveguide fluorescence immunosensor (PWFI). Sens. Actuators B Chem. 2014, 194, 114–119. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, R.; Hou, Y.; Qin, Y.; Li, S.; Yang, S.; Gao, Z. DNA hydrogels combined with microfluidic chips for melamine detection. Anal. Chim. Acta 2022, 1228, 340312. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Deng, H.-H.; Hong, L.; Wu, Z.-Q.; Wang, S.; Liu, A.-L.; Lin, X.-H.; Xia, X.-H. Bare gold nanoparticles as facile and sensitive colorimetric probe for melamine detection. Analyst 2012, 137, 5382–5386. [Google Scholar] [CrossRef]
- Song, J.; Wu, F.; Wan, Y.; Ma, L.-H. Visual test for melamine using silver nanoparticles modified with chromotropic acid. Microchim. Acta 2014, 181, 1267–1274. [Google Scholar] [CrossRef]
- Aramendía, M.A.; Marinas, A.; Marinas, J.M.; Sanchez, E.; Urbano, F.J.; Guillou, C.; Moreno Rojas, J.M.; Moalem, M.; Rallo, L. A nuclear magnetic resonance (1H and 13C) and isotope ratio mass spectrometry (δ13C, δ2H and δ18O) study of Andalusian olive oils. Rapid Commun. Mass Spectrom. 2010, 24, 1457–1466. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Thiagarajan, S.; Chen, S.-M. Detection of melamine in milk powder and human urine. J. Agric. Food Chem. 2010, 58, 4537–4544. [Google Scholar] [CrossRef]
- Xing, G.; Sun, X.; Li, N.; Li, X.; Wu, T.; Wang, F. New advances in lateral flow immunoassay (LFI) technology for food safety detection. Molecules 2022, 27, 6596. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Wu, K.-H.; Hung, H.-C.; Wang, J.-C.; Chang, S.-C. Magnetic nanoparticle-based lateral flow immunochromatographic strip as a reporter for rapid detection of melamine. J. Nanosci. Nanotechnol. 2018, 18, 7190–7196. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.J.; Wu, D.; Ling, J.; Huang, C.Z. Visual and light scattering spectrometric detections of melamine with polythymine-stabilized gold nanoparticles through specific triple hydrogen-bonding recognition. Chem. Commun. 2010, 46, 4893–4895. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Pan, Q.; Zhou, J.; Ren, H.; Peng, C.; Wang, Z.; Zhang, Y. A simplified fluorescent lateral flow assay for melamine based on aggregation induced emission of gold nanoclusters. Food Chem. 2022, 385, 132670. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, Y.; Yao, L.; Zhao, D.; Zheng, L.; Liu, G.; Ye, Y.; Chen, W. Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Microchim. Acta 2016, 183, 1989–1994. [Google Scholar] [CrossRef]
- Ko, C.-H.; Lee, M.-F. Design and fabrication of a microspectrometer based on silicon concave micrograting. Opt. Eng. 2011, 50, 084401. [Google Scholar] [CrossRef]
- Lin, S.-W.; Shen, C.-F.; Liu, C.-C.; Cheng, C.-M. A paper-based IL-6 test strip coupled with a spectrum-based optical reader for differentiating influenza severity in children. Front. Bioeng. Biotechnol. 2021, 9, 752681. [Google Scholar]
- Wang, T.-Y.; Lee, Y.-T.; Chen, H.-Y.; Ko, C.-H.; Hong, C.-T.; Wen, J.-W.; Yen, T.-H.; Cheng, C.-M. A paper-based analytical device for analysis of paraquat in urine and its validation with optical-based approaches. Diagnostics 2020, 11, 6. [Google Scholar] [CrossRef]
- Chiu, W.-H.; Kong, W.-Y.; Su, Y.-J.; Wen, J.-W.; Tsai, C.-M.; Hong, C.; Chen, P.-Y.; Ko, C.-H. A faster, novel technique to detect COVID-19 neutralizing antibodies. Med. Sci. Monit. 2022, 28, e935812. [Google Scholar] [CrossRef]
- Hung, K.-F.; Hung, C.-H.; Hong, C.; Chen, S.-C.; Sun, Y.-C.; Wen, J.-W.; Kuo, C.-H.; Ko, C.-H.; Cheng, C.-M. Quantitative spectrochip-coupled lateral flow immunoassay demonstrates clinical potential for overcoming coronavirus disease 2019 pandemic screening challenges. Micromachines 2021, 12, 321. [Google Scholar] [CrossRef]
- Kong, W.-Y.; Chiu, W.-H.; Tsai, C.-M.; Lin, G.-Y.; Fang, W.; Hong, C.; Ko, C.-H. A novelty ultra-micro spectrum measurement platform for melamine detection in bio-chemical application. In Proceedings of the 2023 22nd International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Kyoto, Japan, 25–29 June 2023; pp. 1037–1040. [Google Scholar]
- Liu, C.-C.; Hsieh, T.-J.; Wu, C.-F.; Tsai, Y.-C.; Huang, S.-P.; Lee, Y.-C.; Huang, T.-Y.; Shen, J.-T.; Chou, Y.-H.; Huang, C.-N. Urinary melamine excretion and increased markers of renal tubular injury in patients with calcium urolithiasis: A cross-sectional study. Environ. Pollut. 2017, 231, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Tsai, M.-T.; Chen, Y.-L.; Cheng, C.-M.; Hung, C.-C.; Wu, C.-F.; Liu, C.-C.; Hsieh, T.-J.; Shiea, J.; Chen, B.-H. Can melamine levels in 1-spot overnight urine specimens predict the total previous 24-hour melamine excretion level in school children? Clin. Chim. Acta 2013, 420, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Needleman, S.B.; Romberg, R.W. Limits of linearity and detection for some drugs of abuse. J. Anal. Toxicol. 1990, 14, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Syahrial, S.; Melinda, M.; Junidar, J.; Razali, S.; Zulhelmi, Z. Application of the Savitzky-Golay filter in multi-spectral signal processing. Deleted J. 2024, 1, 9–19. [Google Scholar] [CrossRef]
- Panuwet, P.; Wade, E.L.; Nguyen, J.V.; Montesano, M.A.; Needham, L.L.; Barr, D.B. Quantification of Cyanuric Acid Residue in Human Urine Using High Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2010, 878, 2916–2922. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Chen, S.-K.; Lin, T.-J.; Wang, I.-J.; Kao, Y.-M.; Shih, D.Y.-C. Determination of urine melamine by validated isotopic ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1776–1782. [Google Scholar] [CrossRef]
- Fremlin, L.J.; Pelzing, M. Melamine and cyanuric acid detection in 5 min using LC-MS. Appl. Noteb. 2009, 28–29. [Google Scholar]


| Parameters | Specifications |
|---|---|
| Spectral Resolution | 5 nm |
| Wavelength Range | 300–1000 nm |
| Spectral Accuracy | ±0.5 nm |
| Stray Light | 0.04% |
| Spectral Dispersion Principle | Flat-field micro concave grating chip |
| Image Sensor | AR0130 CMOS |
| Spectral Pixel | 1280 pixel |
| Optical Angle | 24 degrees |
| A/D Conversion | 12 bits |
| Analog Gain | 1∼32 |
| Dynamic Range | 6000 (37.78 dB) |
| Dimensions (W × D × H) | 44 mm × 34 mm × 8.7 mm |
| Parameters | Specifications |
|---|---|
| Data Interface | mini-USB |
| Working Temperature | °C |
| Readout Time | 2 min |
| Immunoassay | LFIA |
| Dimensions (W × D × H) | 15 cm × 8 cm × 10 cm |
| Weight | 490 g |
| Power Interface | micro-USB |
| Power Consumption | 250 mW |
| Methods/Platform | Pre-Processing | LOD (ppb) | Read Time (min) | Portable | Cost |
|---|---|---|---|---|---|
| THE ONE InstantCare | Directly use | 1.91 | 10 | YES | LOW |
| HPLC | Extraction with buffer (HCOOH/NaCOOH); Centrifugation at 4 °C and treatment with ACN; centrifugation at 4 °C and treatment with CHCl2 | 145 | 60–120 | NO | HIGH |
| LC-MS | Extraction from an Oasis MCX SPE cartridge; Dried with nitrogen | 0.8 | 60–120 | NO | HIGH |
| GC-MS | Extraction with ACN/H2O; Clean up with CARB/SCX cartridges; derivatization with BSTFA-TMCS | 1–10 | 60–120 | NO | HIGH |
| SERS | Directly use | 500 | <30 | YES | LOW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, C.-H.; Kong, W.-Y.; Kabiso, A.C.; Chiu, W.-H.; Tadesse, A.B.; Hong, C.; Wu, C.-F.; Lin, H.-H. Portable SpectroChip-Based Immunoassay Platform for Rapid and Accurate Melamine Quantification in Urine Samples. Toxics 2024, 12, 870. https://doi.org/10.3390/toxics12120870
Ko C-H, Kong W-Y, Kabiso AC, Chiu W-H, Tadesse AB, Hong C, Wu C-F, Lin H-H. Portable SpectroChip-Based Immunoassay Platform for Rapid and Accurate Melamine Quantification in Urine Samples. Toxics. 2024; 12(12):870. https://doi.org/10.3390/toxics12120870
Chicago/Turabian StyleKo, Cheng-Hao, Wei-Yi Kong, Abel Chernet Kabiso, Wei-Huai Chiu, Ashenafi Belihu Tadesse, Chitsung Hong, Chia-Fang Wu, and Hung-Hsun Lin. 2024. "Portable SpectroChip-Based Immunoassay Platform for Rapid and Accurate Melamine Quantification in Urine Samples" Toxics 12, no. 12: 870. https://doi.org/10.3390/toxics12120870
APA StyleKo, C.-H., Kong, W.-Y., Kabiso, A. C., Chiu, W.-H., Tadesse, A. B., Hong, C., Wu, C.-F., & Lin, H.-H. (2024). Portable SpectroChip-Based Immunoassay Platform for Rapid and Accurate Melamine Quantification in Urine Samples. Toxics, 12(12), 870. https://doi.org/10.3390/toxics12120870








