The Relationship Between PM2.5 and Eight Common Lung Diseases: A Two-Sample Mendelian Randomization Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Screening and Extraction Methods
2.3. Statistical Methods
2.4. Two-Sample MR Method
2.5. Sensitivity Analysis Methods
2.6. Analysis Software
3. Results and Analysis
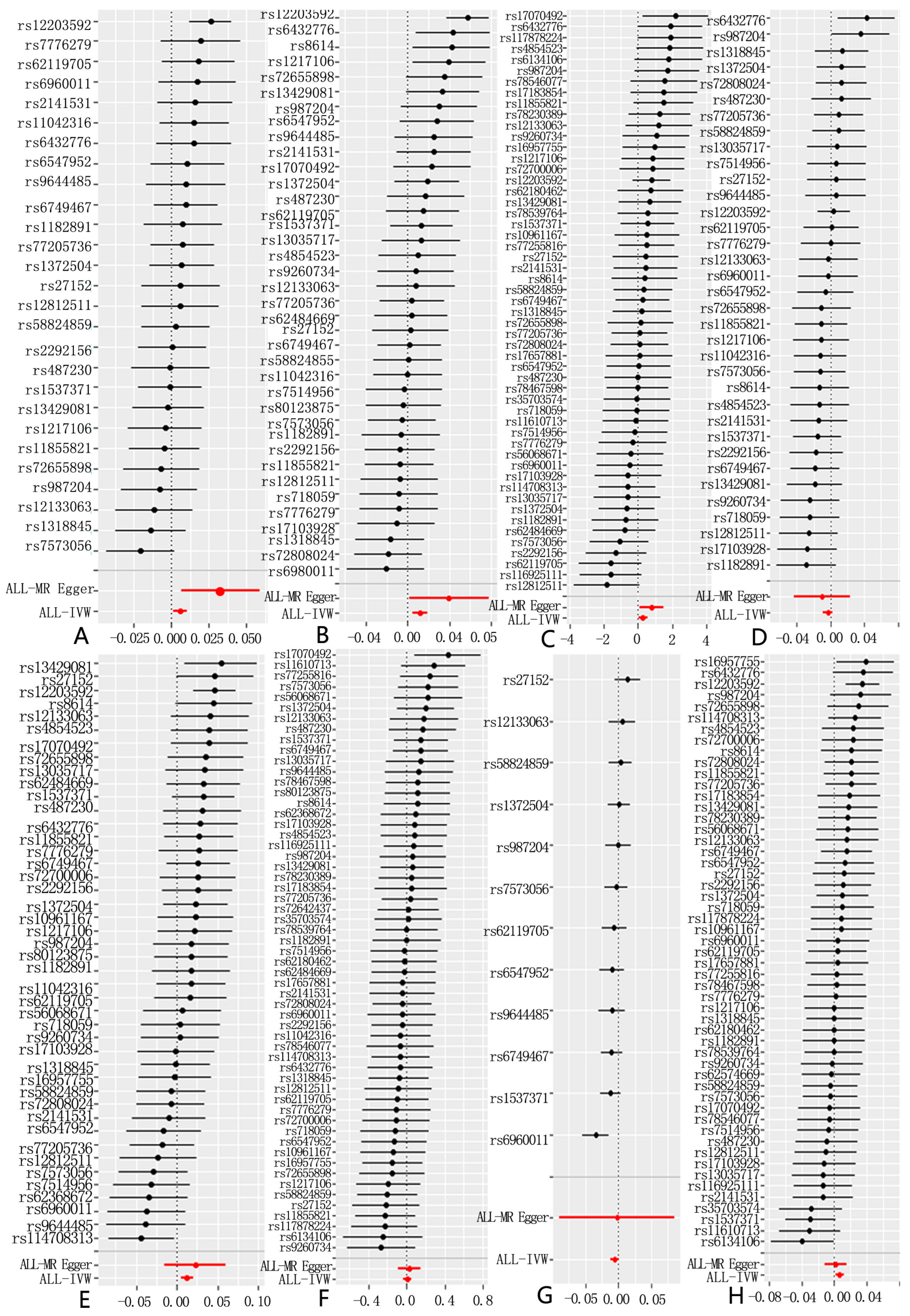
3.1. Selection of Instrumental Variables
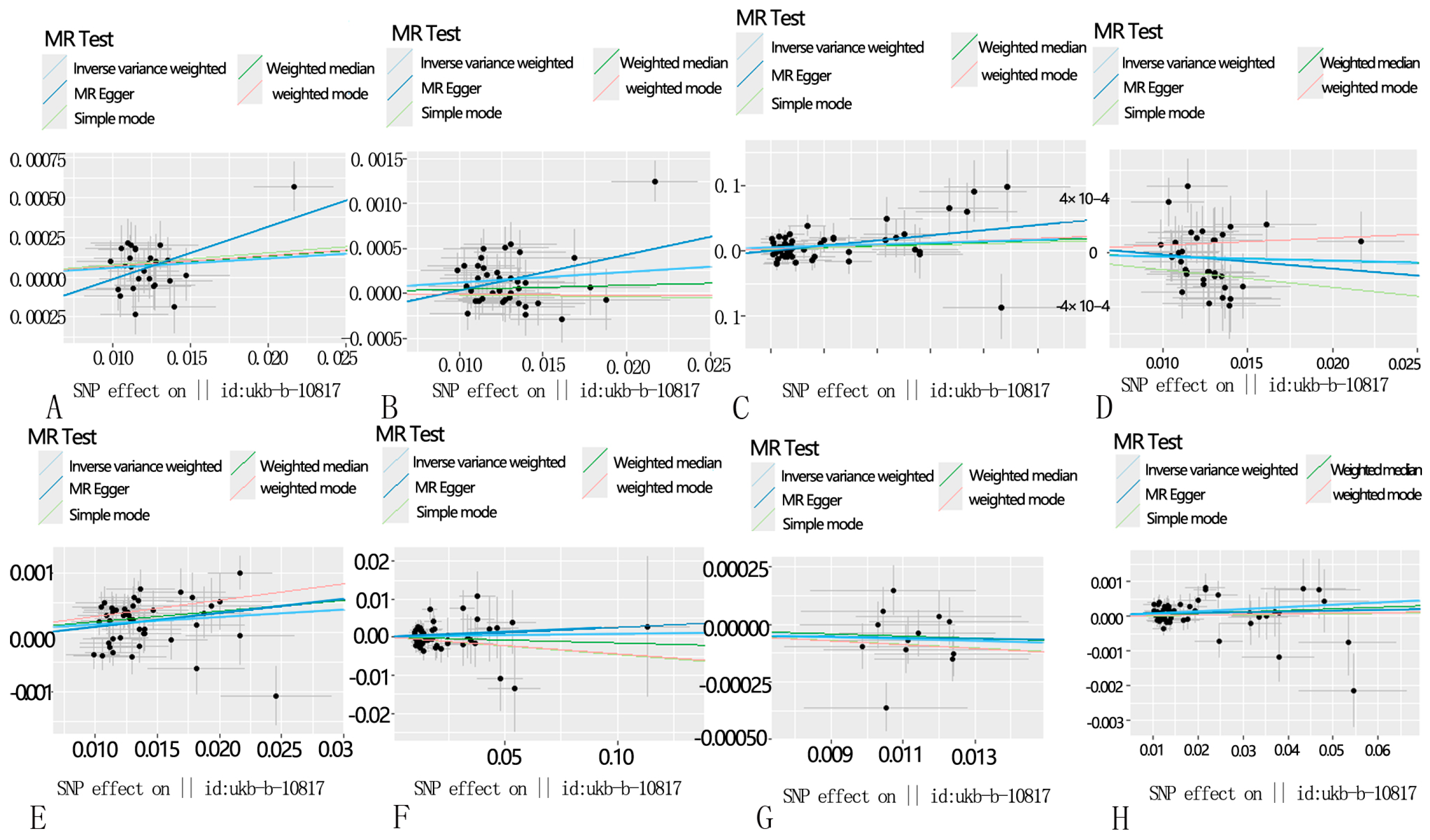
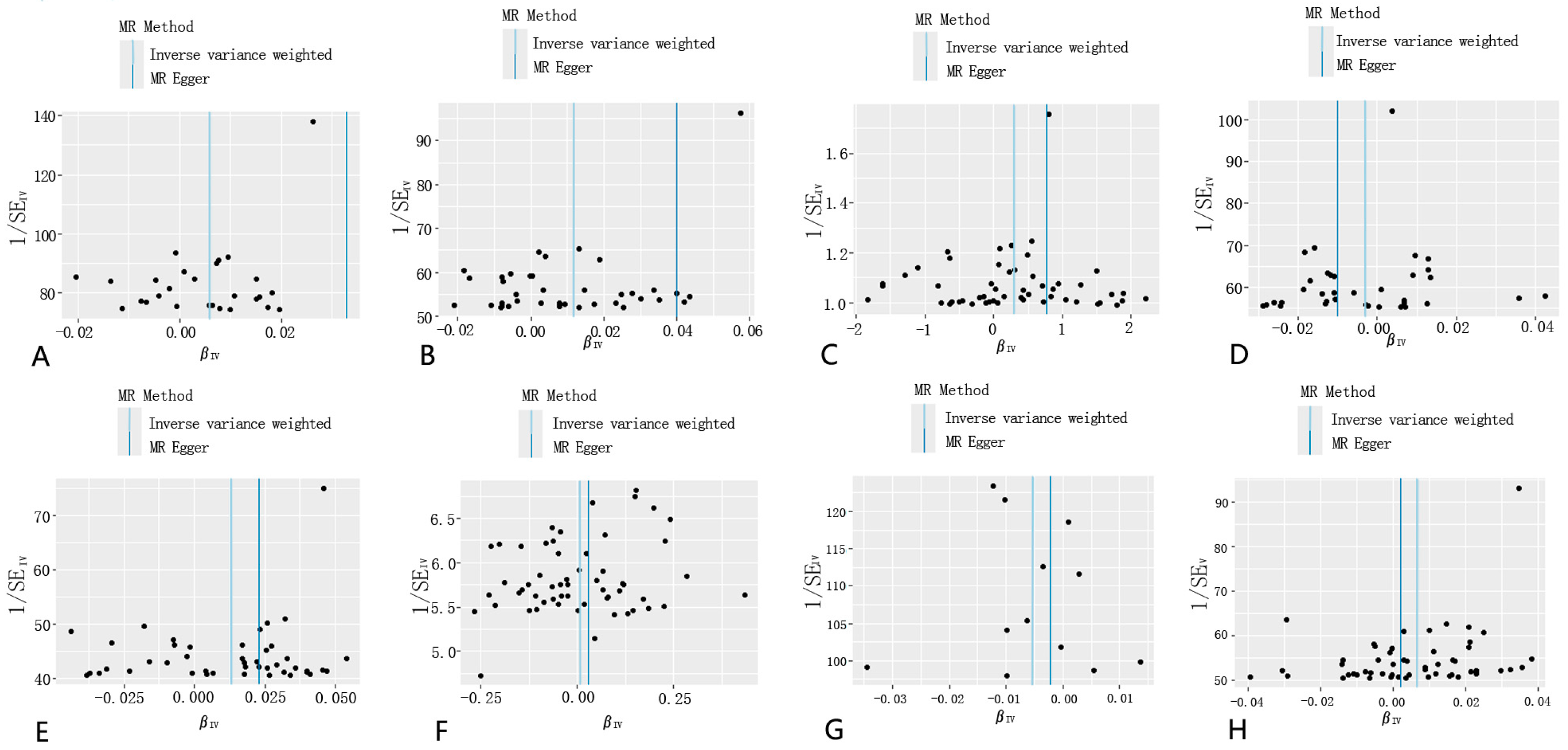
3.2. Sensitivity Analysis
3.3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, S.B.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.H.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Brunekreef, B.; Gehring, U. Meta-analysis of the effects of indoor nitrogen dioxide and gas cooking on asthma and wheeze in children. Int. J. Epidemiol. 2013, 42, 1724–1737. [Google Scholar] [CrossRef]
- Wilson, W.E.; Suh, H.H. Fine Particles and Coarse Particles: Concentration Relationships Relevant to Epidemiologic Studies. J. Air Waste Manag. Assoc. 1997, 47, 1238–1249. [Google Scholar] [CrossRef]
- Kelishadi, R.; Poursafa, P. Air pollution and non-respiratory health hazards for children. Arch. Med. Sci. 2010, 4, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Kappos, A.D.; Bruckmann, P.; Eikmann, T.; Englert, N.; Heinrich, U.; Höppe, P.; Koch, E.; Krause, G.H.M.; Kreyling, W.G.; Rauchfuss, K.; et al. Health effects of particles in ambient air. Int. J. Hydrogen Environ. Health 2004, 207, 399–407. [Google Scholar] [CrossRef]
- Coleman, N.C.; Burnett, R.T.; Ezzati, M.; Marshall, J.D.; Robinson, A.L.; Pope, C.A. Fine Particulate Matter Exposure and Cancer Incidence: Analysis of SEER Cancer Registry Data from 1992–2016. Environ. Health Perspect. 2020, 128, 107004. [Google Scholar] [CrossRef]
- Kan, H.; Chen, R.; Tong, S. Ambient air pollution, climate change, and population health in China. Environ. Int. 2012, 42, 10–19. [Google Scholar] [CrossRef]
- Burroughs Peña, M.S.; Rollins, A. Environmental Exposures and Cardiovascular Disease. Cardiol. Clin. 2017, 35, 71–86. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, H.; Zheng, R.; Li, S.; Pereira, G.; Liu, Q.; Chen, W.; Huxley, R. The burden of lung cancer mortality attributable to fine particles in China. Sci. Total Environ. 2017, 579, 1460–1466. [Google Scholar] [CrossRef]
- Hou, Q.; An, X.Q.; Wang, Y.; Guo, J.P. An evaluation of resident exposure to respirable particulate matter and health economic loss in Beijing during Beijing 2008 Olympic Games. Sci. Total Environ. 2010, 408, 4026–4032. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.-H.; Chen, T.; Liu, M.-M.; Wang, D.; Ma, Y.-N.; Ren, W.-H.; Lee, Y.L.; Zhao, Y.-D.; He, Q.-C. Gender Differences and Effect of Air Pollution on Asthma in Children with and without Allergic Predisposition: Northeast Chinese Children Health Study. PLoS ONE 2011, 6, e22470. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.R.; Favarato, G.; Atkinson, R.W. Long-term exposure to outdoor air pollution and the prevalence of asthma: Meta-analysis of multi-community prevalence studies. Air Qual. Atmos. Health 2013, 6, 57–68. [Google Scholar] [CrossRef]
- Doiron, D.; De Hoogh, K.; Probst-Hensch, N.; Fortier, I.; Cai, Y.; De Matteis, S.; Hansell, A.L. Air pollution, lung function and COPD: Results from the population-based UK Biobank study. Eur. Respir. J. 2019, 54, 1802140. [Google Scholar] [CrossRef]
- Cheung, K.; Daher, N.; Kam, W.; Shafer, M.M.; Ning, Z.; Schauer, J.J.; Sioutas, C. Spatial and temporal variation of chemical composition and mass closure of ambient coarse particulate matter (PM10–2.5) in the Los Angeles area. Atmos. Environ. 2011, 45, 2651–2662. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Li, Y.; Qian, Z.M.; Xiao, W.; Wang, X.; Rolling, C.A.; Liu, E.; Xiao, J.; Zeng, W.; et al. Short-term and long-term effects of PM2.5 on acute nasopharyngitis in 10 communities of Guangdong, China. Sci. Total Environ. 2019, 688, 136–142. [Google Scholar] [CrossRef]
- Kloog, I.; Ridgway, B.; Koutrakis, P.; Coull, B.A.; Schwartz, J.D. Long- and Short-Term Exposure to PM2.5 and Mortality: Using Novel Exposure Models. Epidemiology 2013, 24, 555–561. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Sanderson, E. Multivariable Mendelian Randomization and Mediation. Cold Spring Harb. Perspect. Med. 2021, 11, a038984. [Google Scholar] [CrossRef]
- Visscher, P.M.; Brown, M.A.; McCarthy, M.I.; Yang, J. Five Years of GWAS Discovery. Am. J. Hum. Genet. 2012, 90, 7–24. [Google Scholar] [CrossRef]
- Eeftens, M.; Beelen, R.; De Hoogh, K.; Bellander, T.; Cesaroni, G.; Cirach, M.; Declercq, C.; Dėdelė, A.; Dons, E.; De Nazelle, A.; et al. Development of Land Use Regression Models for PM2.5, PM2.5 Absorbance, PM 10 and PM coarse in 20 European Study Areas; Results of the ESCAPE Project. Environ. Sci. Technol. 2012, 46, 11195–11205. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G. CRP CHD Genetics Collaboration Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ognjenovic, J.; Karandur, D.; Miller, K.; Merk, A.; Subramaniam, S.; Kuriyan, J. A molecular mechanism for the generation of ligand-dependent differential outputs by the epidermal growth factor receptor. eLife 2021, 10, e73218. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian Randomization Analysis With Multiple Genetic Variants Using Summarized Data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, L.; Ma, F.; Xue, S.; Sun, T.; Xu, Z. Effects of Selenium on Chronic Kidney Disease: A Mendelian Randomization Study. Nutrients 2022, 14, 4458. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.L.; Burgess, S. Efficient Design for Mendelian Randomization Studies: Subsample and 2-Sample Instrumental Variable Estimators. Am. J. Epidemiol. 2013, 178, 1177–1184. [Google Scholar] [CrossRef]
- The LifeLines Cohort Study; The InterAct Consortium; kConFab/AOCS Investigators; Endometrial Cancer Association Consortium; Ovarian Cancer Association Consortium; PRACTICAL consortium; Day, F.R.; Thompson, D.J.; Helgason, H.; Chasman, D.I.; et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 2017, 49, 834–841. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Klein, M.; Flanders, W.D.; Mulholland, J.A.; Tolbert, P.E.; Strickland, M.J. Air Pollution and Acute Respiratory Infections Among Children 0–4 Years of Age: An 18-Year Time-Series Study. Am. J. Epidemiol. 2014, 180, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-S.; Chiu, H.-F.; Liou, S.-H.; Yang, C.-Y. Short-Term Effects of Fine Particulate Air Pollution on Hospital Admissions for Respiratory Diseases: A Case-Crossover Study in a Tropical City. J. Toxicol. Environ. Health Part A 2014, 77, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.K.; Balmes, J.R.; Bruckner, T.A.; Mortimer, K.M.; Margolis, H.G.; Pratt, B.; Hammond, S.K.; Lurmann, F.W.; Tager, I.B. Short-Term Effects of Air Pollution on Wheeze in Asthmatic Children in Fresno, California. Environ. Health Perspect. 2010, 118, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Pun, V.C.; Kazemiparkouhi, F.; Manjourides, J.; Suh, H.H. Long-Term PM2.5 Exposures and Respiratory, Cancer and Cardiovascular Mortality in American Older Adults. Am. J. Epidemiol. 2017, 186, 961–969. [Google Scholar] [CrossRef]
- Goobie, G.C.; Carlsten, C.; Johannson, K.A.; Khalil, N.; Marcoux, V.; Assayag, D.; Manganas, H.; Fisher, J.H.; Kolb, M.R.J.; Lindell, K.O.; et al. Association of Particulate Matter Exposure With Lung Function and Mortality Among Patients With Fibrotic Interstitial Lung Disease. JAMA Intern. Med. 2022, 182, 1248. [Google Scholar] [CrossRef]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A.; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA A Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, H.; Zheng, R.; Li, S.; Barnett, A.G.; Zhang, S.; Zou, X.; Huxley, R.; Chen, W.; Williams, G. The association between lung cancer incidence and ambient air pollution in China: A spatiotemporal analysis. Environ. Res. 2016, 144, 60–65. [Google Scholar] [CrossRef]
- Choe, S.-A.; Kauderer, S.; Eliot, M.N.; Glazer, K.B.; Kingsley, S.L.; Carlson, L.; Awad, Y.A.; Schwartz, J.D.; Savitz, D.A.; Wellenius, G.A. Air pollution, land use, and complications of pregnancy. Sci. Total Environ. 2018, 645, 1057–1064. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Yang, L.; Sun, L.; Wen, Y.; Huang, Y.; Gao, K.; Yang, W.; Bai, F.; Ling, L.; et al. PM2.5 promotes NSCLC carcinogenesis through translationally and transcriptionally activating DLAT-mediated glycolysis reprograming. J. Exp. Clin. Cancer Res. 2022, 41, 229. [Google Scholar] [CrossRef]
- Honda, A.; Fukushima, W.; Oishi, M.; Tsuji, K.; Sawahara, T.; Hayashi, T.; Kudo, H.; Kashima, Y.; Takahashi, K.; Sasaki, H.; et al. Effects of Components of PM2.5 Collected in Japan on the Respiratory and Immune Systems. Int. J. Toxicol. 2017, 36, 153–164. [Google Scholar] [CrossRef] [PubMed]

| GWAS ID | Trait | Consortium | Sample Size | Year | Category |
|---|---|---|---|---|---|
| ukb-b-10817 | Particulate matter air pollution (PM2.5); 2010 | MRC-IEU | 423,796 | 2018 | Continuous |
| ukb-b-20208 | Diagnoses—main ICD10: J45.9 Asthma, unspecified | MRC-IEU | 463,010 | 2018 | Binary |
| ukb-b-16751 | Diagnoses—secondary ICD10: J44.9 Chronic obstructive pulmonary disease, unspecified | MRC-IEU | 463,010 | 2018 | Binary |
| ieu-b-4976 | Pneumonia | MRC-IEU | 486,484 | 2018 | Disease |
| ukb-b-3467 | Non-cancer illness code, self-reported: bronchitis | MRC-IEU | 462,933 | 2018 | Binary |
| ukb-b-7280 | Non-cancer illness code, self-reported: emphysema/chronic bronchitis | MRC-IEU | 462,933 | 2018 | Binary |
| ukb-a-358 | Underlying (primary) cause of death: ICD10: J84.1 Other interstitial pulmonary diseases with fibrosis | Neale Lab | 7637 | 2017 | NA |
| ukb-b-18163 | Non-cancer illness code, self-reported: bronchiectasis | MRC-IEU | 462,933 | 2018 | Binary |
| ieu-b-4954 | Lung cancer | UK Biobank | 374,687 | 2021 | Disease |
| ukb-b-19953 | Body mass index (BMI) | MRC-IEU | 461,460 | 2018 | Continuous |
| ukb-b-20261 | Ever smoked | MRC-IEU | 461,066 | 2018 | Binary |
| ebi-a-GCST90038709 | Bacterial infection | NA | 484,598 | 2021 | PMID: 33959723 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Si, J.; Wang, Y.; Zhang, H.; Zhou, F.; Lu, X.; Li, X.; Sun, D.; Wang, Z. The Relationship Between PM2.5 and Eight Common Lung Diseases: A Two-Sample Mendelian Randomization Analysis. Toxics 2024, 12, 851. https://doi.org/10.3390/toxics12120851
Jiang Y, Si J, Wang Y, Zhang H, Zhou F, Lu X, Li X, Sun D, Wang Z. The Relationship Between PM2.5 and Eight Common Lung Diseases: A Two-Sample Mendelian Randomization Analysis. Toxics. 2024; 12(12):851. https://doi.org/10.3390/toxics12120851
Chicago/Turabian StyleJiang, Yuhang, Jingwen Si, Yuhang Wang, Han Zhang, Fang Zhou, Xike Lu, Xin Li, Daqiang Sun, and Zheng Wang. 2024. "The Relationship Between PM2.5 and Eight Common Lung Diseases: A Two-Sample Mendelian Randomization Analysis" Toxics 12, no. 12: 851. https://doi.org/10.3390/toxics12120851
APA StyleJiang, Y., Si, J., Wang, Y., Zhang, H., Zhou, F., Lu, X., Li, X., Sun, D., & Wang, Z. (2024). The Relationship Between PM2.5 and Eight Common Lung Diseases: A Two-Sample Mendelian Randomization Analysis. Toxics, 12(12), 851. https://doi.org/10.3390/toxics12120851






