Iodine Deficiency Exacerbates Thyroidal and Neurological Effects of Developmental Perchlorate Exposure in the Neonatal and Adult Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Tissue Collection
2.3. Perchlorate Analysis in Serum and Milk
2.4. Quantification of THs in Serum
2.4.1. T3 and T4 in Serum
2.4.2. Thyroid-Stimulating Hormone
2.5. Gene Expression in Thyroid Gland and Brain by Quantitative Real-Time PCR
2.6. Immunohistochemistry for Parvalbumin
2.6.1. Tissue Collection and Immunohistochemistry Procedures
2.6.2. Imaging and Section Selection for Cell Counting
2.6.3. Quantification of Parvalbumin-Expressing Neurons
2.7. Neurobehavioral Assessments in Adult Offspring
2.7.1. Trace Fear Conditioning
2.7.2. Prepulse Inhibition (PPI) of Acoustic Startle Response (ASR)
2.8. Statistical Analysis
3. Results
3.1. Body Weight
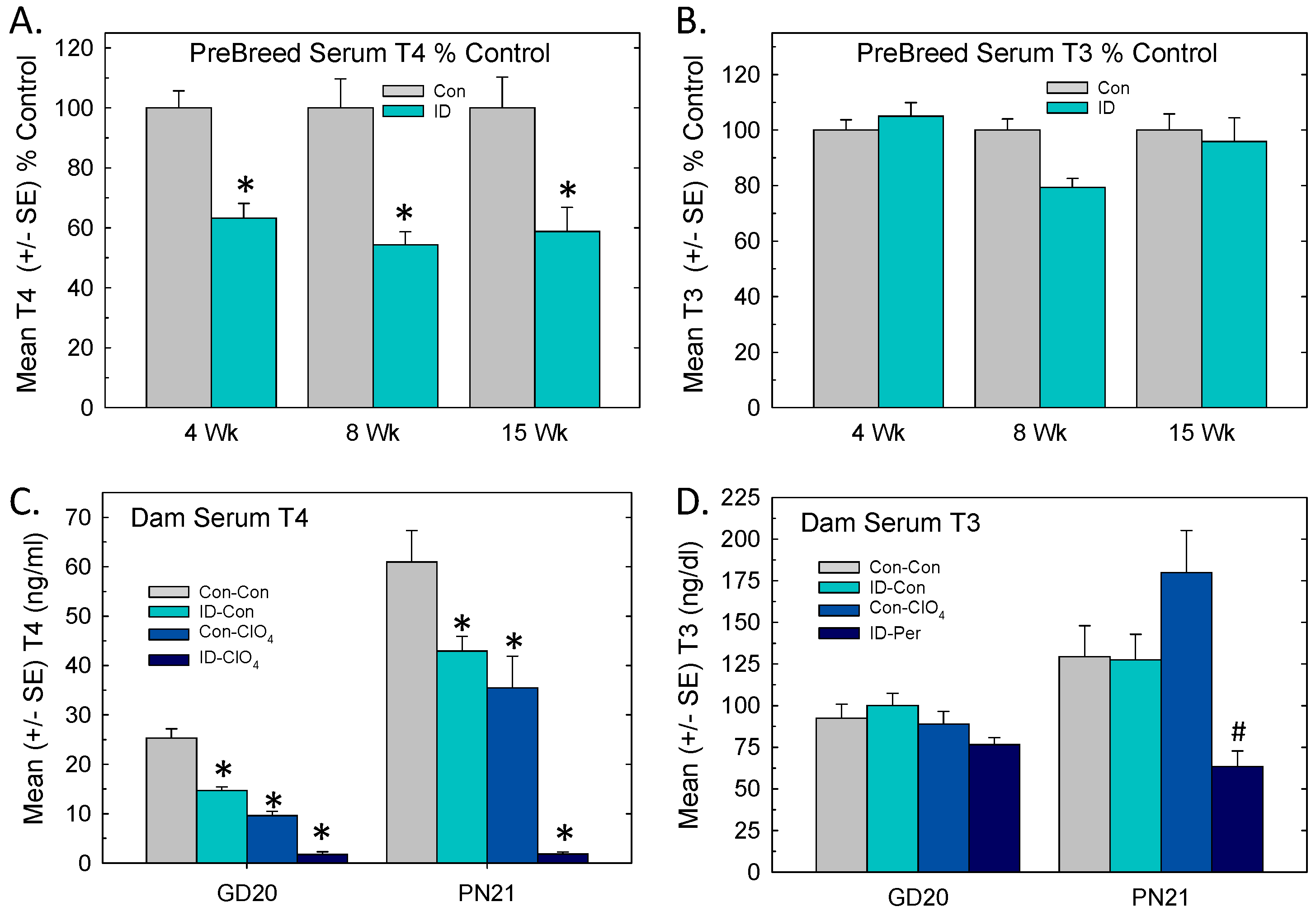
3.2. Serum Thyroid Hormones: Pre-Breeding, Gestation, Lactation
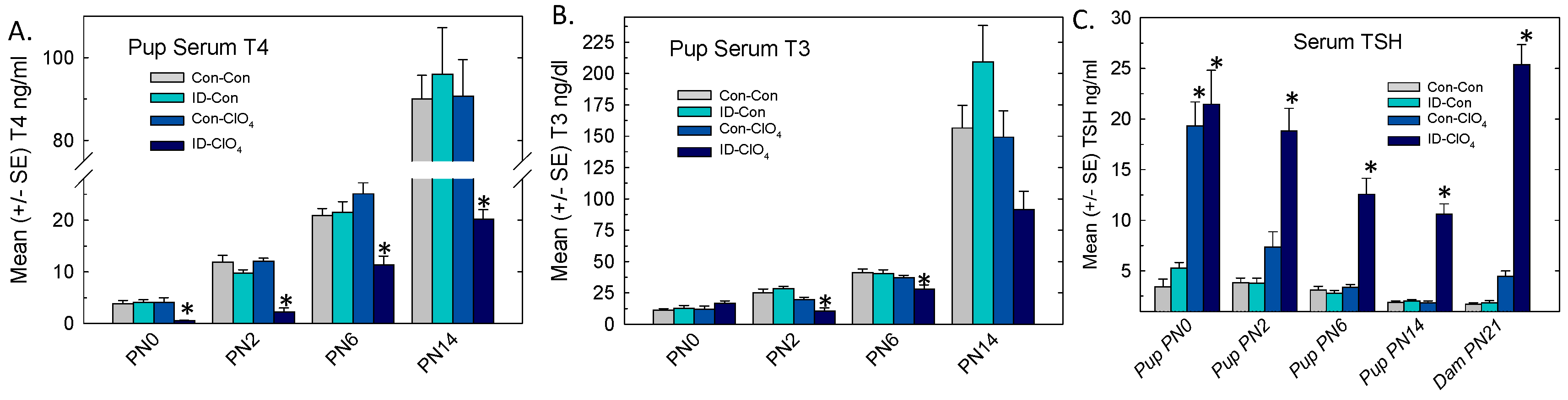
3.3. Serum Thyroid Hormones and TSH in Offspring
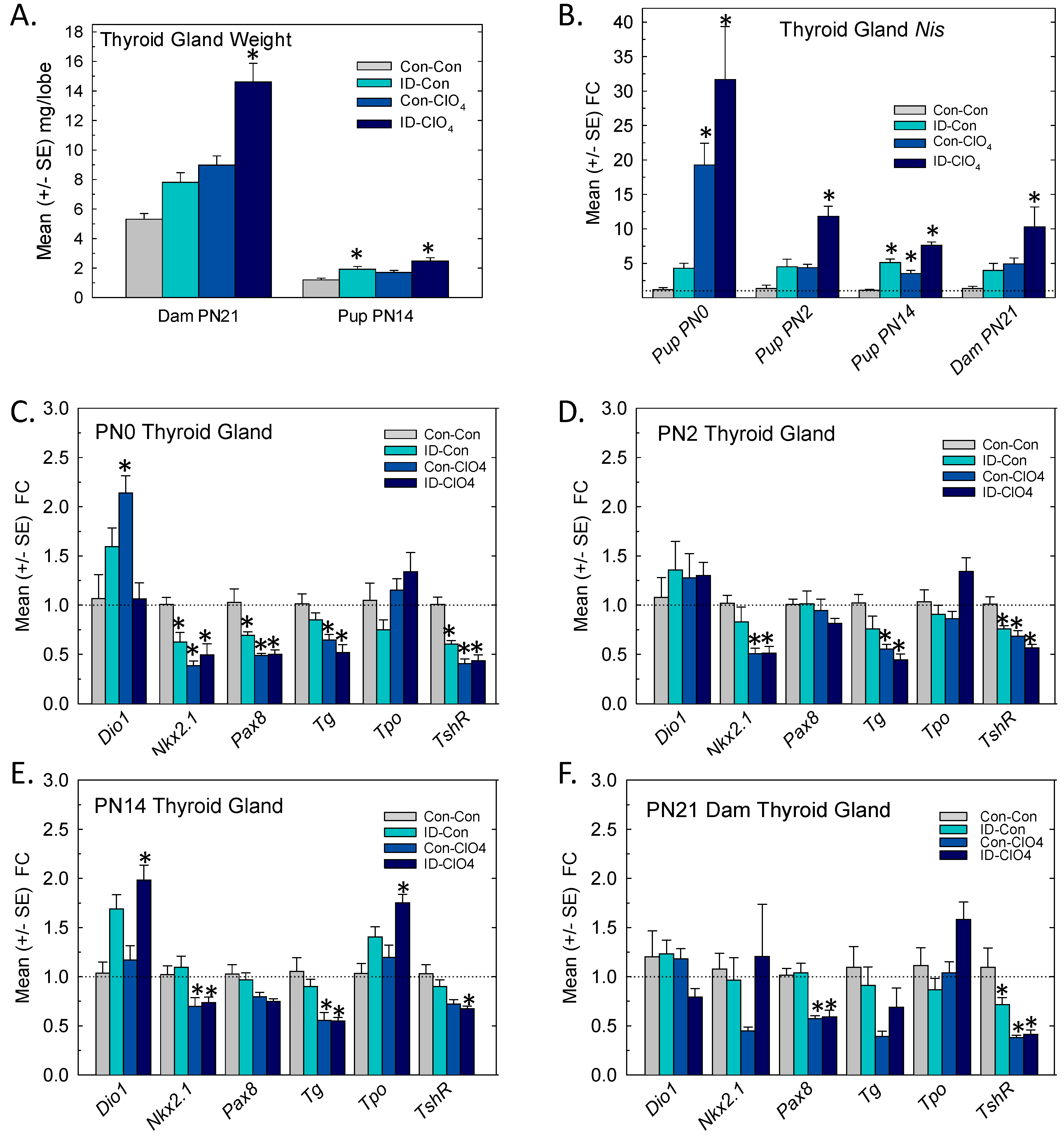
3.4. Thyroid Gland: Weight and Gene Expression
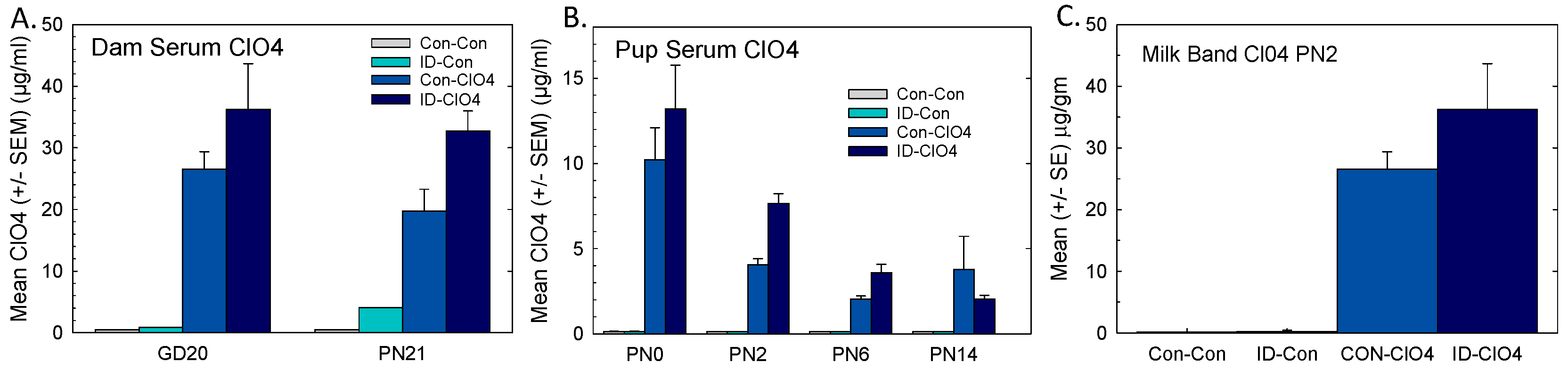
3.5. Perchlorate in Serum and Milk
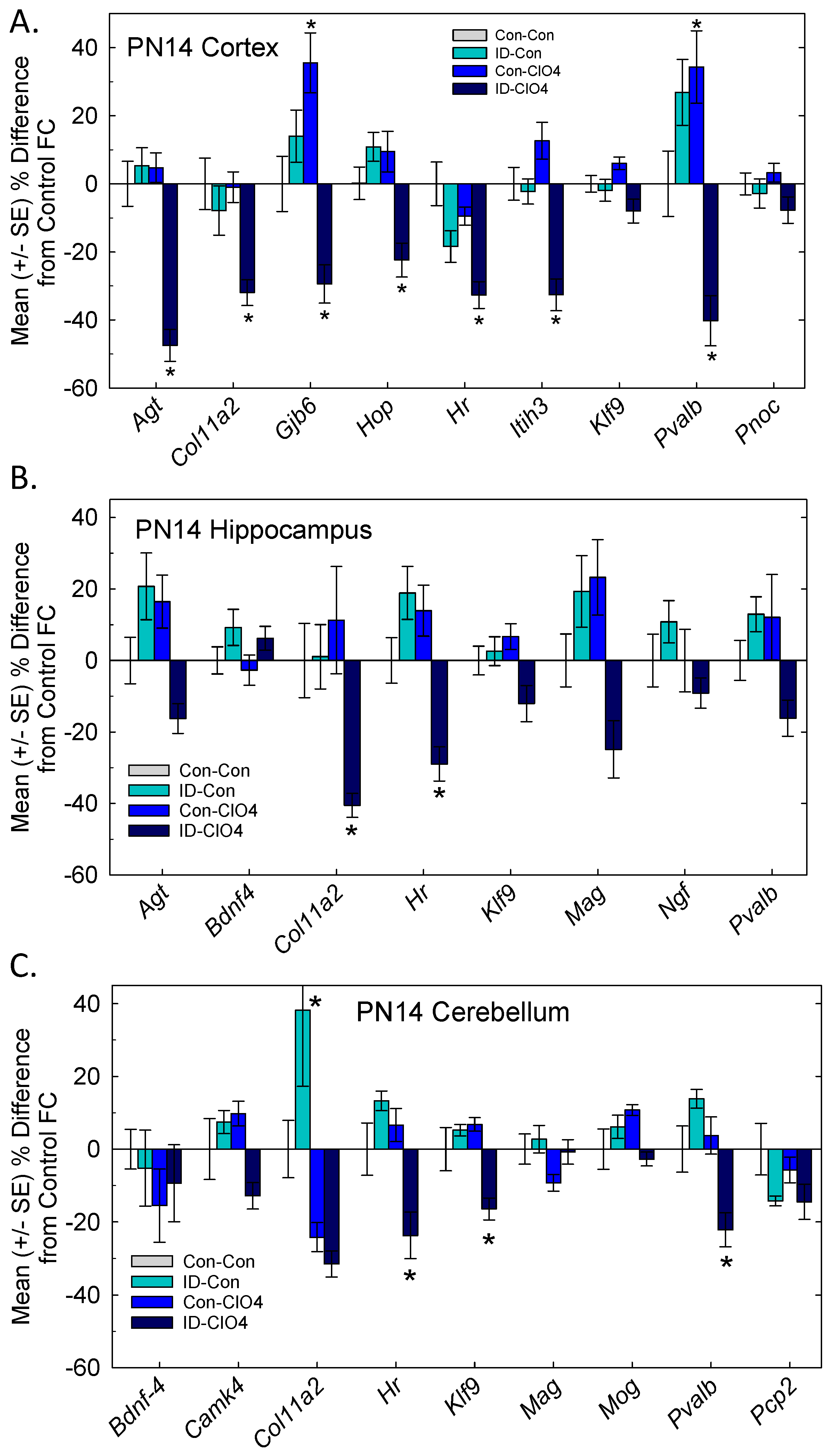
3.6. TH Action in the Brain
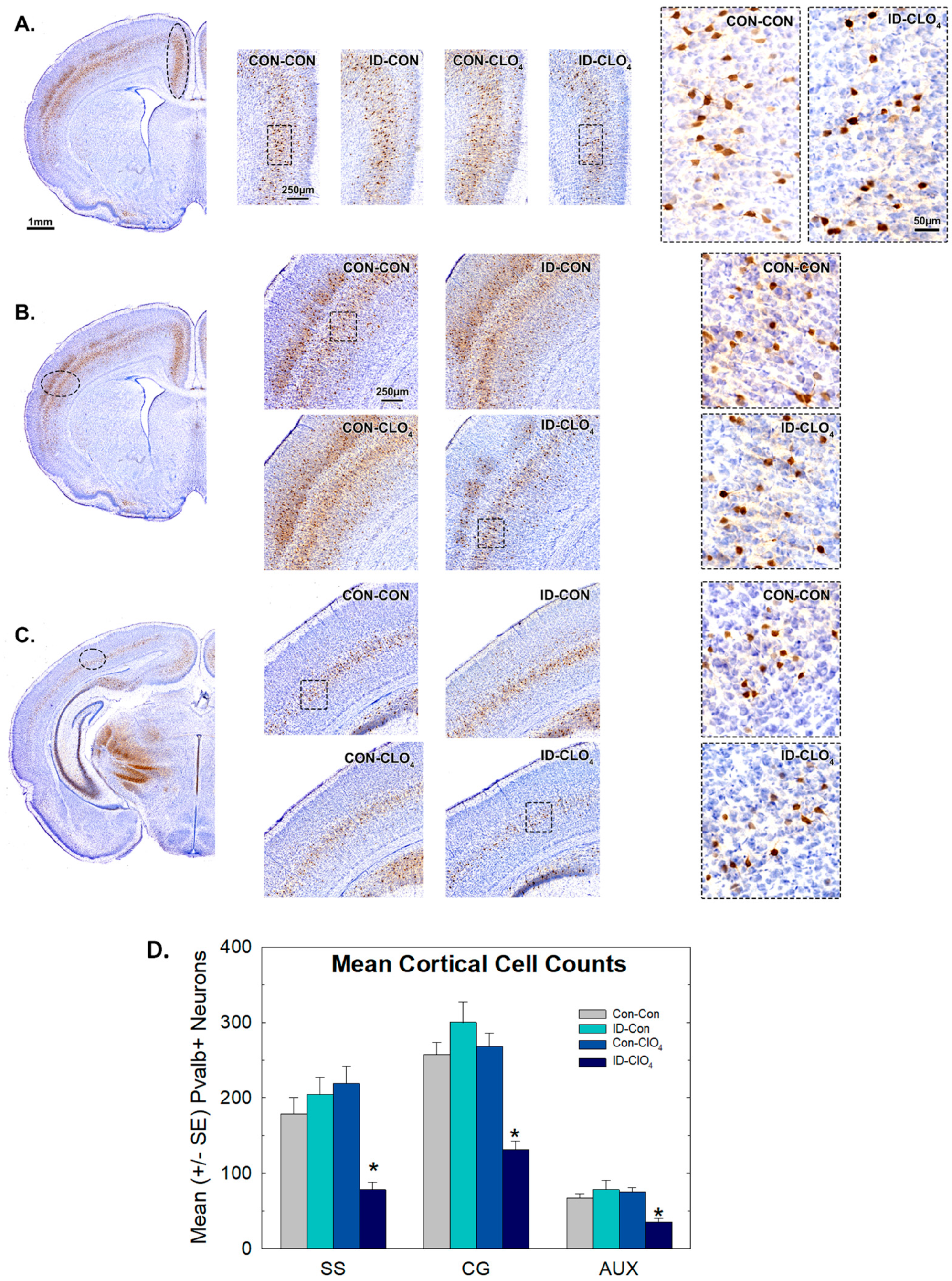
3.7. Parvalbumin Expression in Inhibitory Neurons in the Brain
3.8. Behavioral Measures in Adult Offspring
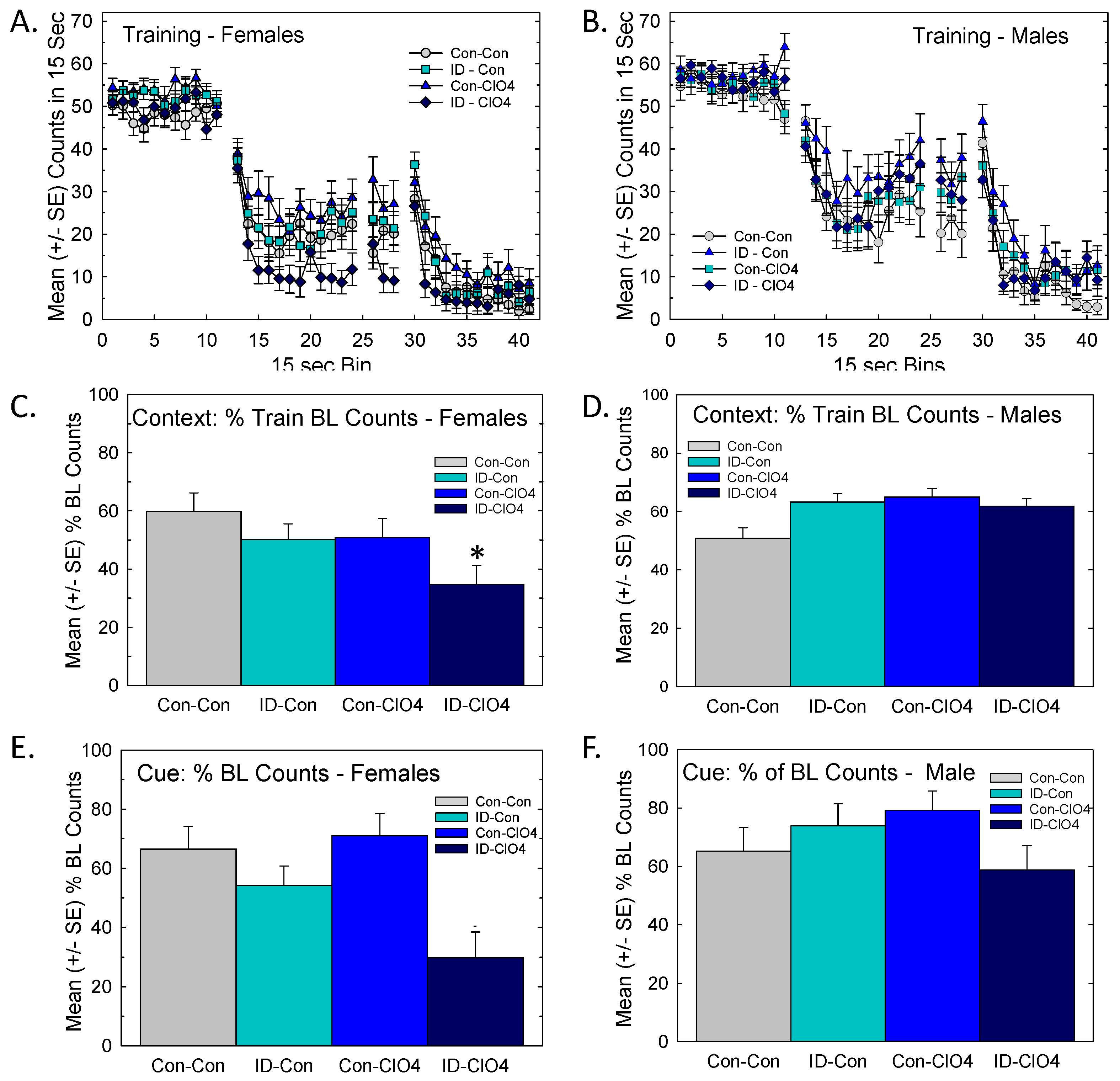
3.8.1. Distract Trace Fear Conditioning
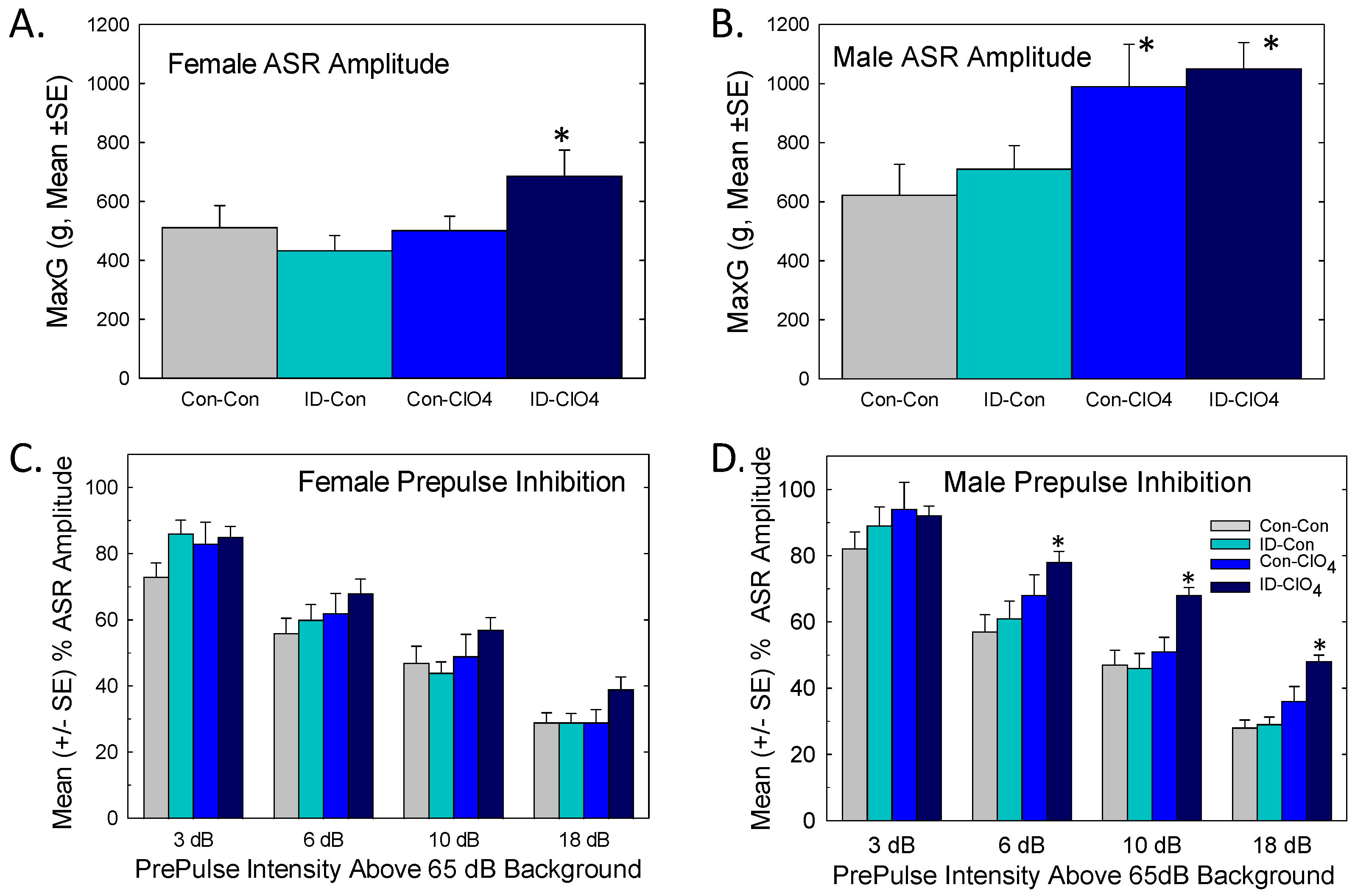
3.8.2. Acoustic Startle and Prepulse Inhibition
4. Discussion
4.1. Impact and Relevance
4.2. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delange, F. The Disorders Induced by Iodine Deficiency. Thyroid 1994, 4, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: A review. Am. J. Clin. Nutr. 2009, 89, 668S–672S. [Google Scholar] [CrossRef] [PubMed]
- Grossklaus, R.; Liesenkötter, K.-P.; Doubek, K.; Völzke, H.; Gaertner, R. Iodine Deficiency, Maternal Hypothyroxinemia and Endocrine Disrupters Affecting Fetal Brain Development: A Scoping Review. Nutrients 2023, 15, 2249. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Chapter 25—Iodine and the iodine deficiency disorders. In Present Knowledge in Nutrition, 7th ed.; Marriott, B.P., Birt, D.F., Stalling, V.A., Yates, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 429–441. [Google Scholar]
- Williams, G.R. Neurodevelopmental and Neurophysiological Actions of Thyroid Hormone. J. Neuroendocr. 2008, 20, 784–794. [Google Scholar] [CrossRef]
- Glinoer, D. Clinical and biological consequences of iodine deficiency during pregnancy. Endocr. Dev. 2007, 10, 62–85. [Google Scholar]
- Delange, F. Epidemiology and Impact of Iodine Deficiency in Pediatrics. J. Pediatr. Endocrinol. Metab. 2005, 18, 1245–1252. [Google Scholar] [CrossRef]
- Portulano, C.; Paroder-Belenitsky, M.; Carrasco, N. The Na+/I− symporter (NIS): Mechanism and medical impact. Endocr. Rev. 2014, 35, 106–149. [Google Scholar] [CrossRef]
- Schiller, T.; Agmon, A.; Ostrovsky, V.; Shefer, G.; Knobler, H.; Zornitzki, T. Moderate Iodine Deficiency Is Common in Pregnancy but Does Not Alter Maternal and Ne-onatal Thyroid Function Tests. Front. Endocrinol. 2020, 11, 523319. [Google Scholar] [CrossRef]
- Barnett-Itzhaki, Z.; Ehrlich, D.; Troen, A.M.; Rorman, E.; Groismann, L.; Blaychfeld-Magnazi, M.; Endevelt, R.; Berman, T. Results of the national biomonitoring program show persistent iodine deficiency in Israel. Isr. J. Health Policy Res. 2022, 11, 18. [Google Scholar] [CrossRef]
- Lazarus, J.H. Iodine deficiency in Israeli pregnant women—A time for action. Isr. J. Health Policy Res. 2020, 9, 20. [Google Scholar] [CrossRef]
- Ovadia, Y.S.; Arbelle, J.E.; Gefel, D.; Brik, H.; Wolf, T.; Nadler, V.; Hunziker, S.; Zimmermann, M.B.; Troen, A.M. First Israeli National Iodine Survey Demonstrates Iodine Deficiency Among School-Aged Children and Pregnant Women. Thyroid 2017, 27, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Hatch-McChesney, A.; Lieberman, H.R. Iodine and Iodine Deficiency: A Comprehensive Review of a Re-Emerging Issue. Nutrients 2022, 14, 3474. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Ravera, S.; Reyna-Neyra, A.; Ferrandino, G.; Amzel, L.M.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 2017, 79, 261–289. [Google Scholar] [CrossRef]
- Dohán, O.; Portulano, C.; Basquin, C.; Reyna-Neyra, A.; Amzel, L.M.; Carrasco, N. The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc. Natl. Acad. Sci. USA 2007, 104, 20250–20255. [Google Scholar] [CrossRef]
- York, R.G.; Barnett, J.J.; Brown, W.R.; Garman, R.H.; Mattie, D.R.; Dodd, D. A Rat Neurodevelopmental Evaluation of Offspring, Including Evaluation of Adult and Neonatal Thyroid, from Mothers Treated with Ammonium Perchlorate in Drinking Water. Int. J. Toxicol. 2004, 23, 191–214. [Google Scholar] [CrossRef]
- Wolff, J. Perchlorate and the thyroid gland. Pharmacol. Rev. 1998, 50, 89–105. [Google Scholar]
- Stoker, T.E.; Ferrell, J.M.; Laws, S.C.; Cooper, R.L.; Buckalew, A. Evaluation of ammonium perchlorate in the endocrine disruptor screening and testing program’s male pubertal protocol: Ability to detect effects on thyroid endpoints. Toxicology 2006, 228, 58–65. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Sui, L. Developmental Exposure to Perchlorate Alters Synaptic Transmission in Hippocampus of the Adult Rat. Environ. Health Perspect. 2008, 116, 752–760. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Hassan, I.; Wood, C.; O’Shaughnessy, K.L.; Spring, S.; Thomas, S.; Ford, J. Gestational Exposure to Perchlorate in the Rat: Thyroid Hormones in Fetal Thyroid Gland, Serum, and Brain. Toxicol. Sci. 2022, 188, 117–130. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Hassan, I.; O’Shaughnessy, K.L.; Wood, C.; Stoker, T.E.; Riutta, C.; Ford, J.L. Ammonium Perchlorate: Serum Dosimetry, Neurotoxicity and Resilience of the Neonatal Rat Thyroid System. Toxicol. Sci. 2024, 198, 113–127. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Perchlorate Environmental Contamination: Toxicological Review and Risk Characterization; External Review Draft, NCEA-1-0503; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2002. [Google Scholar]
- Gilbert, M.E.; O’Shaughnessy, K.L.; Bell, K.S.; Ford, J.L. Structural Malformations in the Neonatal Rat Brain Accompany Developmental Exposure to Ammonium Perchlorate. Toxics 2023, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; McLanahan, E.; Hedge, J.; Crofton, K.; Fisher, J.; Valentín-Blasini, L.; Blount, B. Marginal iodide deficiency and thyroid function: Dose–response analysis for quantitative pharmacokinetic modeling. Toxicology 2011, 283, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.E.; Hedge, J.M.; Valentín-Blasini, L.; Blount, B.C.; Kannan, K.; Tietge, J.; Zoeller, R.T.; Crofton, K.M.; Jarrett, J.M.; Fisher, J.W. An Animal Model of Marginal Iodine Deficiency During Development: The Thyroid Axis and Neurodevelopmental Outcome. Toxicol. Sci. 2013, 132, 177–195. [Google Scholar] [CrossRef]
- Fisher, J.W.; Li, S.; Crofton, K.; Zoeller, R.T.; McLanahan, E.D.; Lumen, A.; Gilbert, M.E. Evaluation of Iodide Deficiency in the Lactating Rat and Pup Using a Biologically Based Dose-Response Model. Toxicol. Sci. 2013, 132, 75–86. [Google Scholar] [CrossRef]
- York, R.G.; Barnett, J., Jr.; Girard, M.F.; Mattie, D.R.; Bekkedal, M.V.; Garman, R.H.; Strawson, J.S. Refining the effects observed in a developmental neurobehavioral study of ammonium per-chlorate administered orally in drinking water to rats. I. Thyroid and reproductive effects. Int. J. Toxicol. 2005, 24, 403–418. [Google Scholar] [CrossRef]
- Hassan, I.; El-Masri, H.; Kosian, P.A.; Ford, J.; Degitz, S.J.; Gilbert, M.E. Neurodevelopment and Thyroid Hormone Synthesis Inhibition in the Rat: Quantitative Understanding Within the Adverse Outcome Pathway Framework. Toxicol. Sci. 2017, 160, 57–73. [Google Scholar] [CrossRef]
- Oldi, J.F.; Kannan, K. Perchlorate in human blood serum and plasma: Relationship to concentrations in saliva. Chemosphere 2009, 77, 43–47. [Google Scholar] [CrossRef]
- O’Shaughnessy, K.L.; Kosian, P.A.; Ford, J.L.; Oshiro, W.M.; Degitz, S.J.; Gilbert, M.E. Developmental Thyroid Hormone Insufficiency Induces a Cortical Brain Malfor-mation and Learning Impairments: A Cross-Fostering Study. Toxicol. Sci. 2018, 163, 101–115. [Google Scholar] [CrossRef]
- Greenwood, F.; Hunter, W.; Glover, J. The preparation of 131i-labelled human growth hormone of high specific radioactivity. Biochem. J. 1963, 89, 114–123. [Google Scholar] [CrossRef]
- Goldman, J.M.; Cooper, R.L.; Rehnberg, G.L.; Hein, J.F.; McElroy, W.K.; Gray, L.E., Jr. Effects of low subchronic doses of methoxychlor on the rat hypothalamic-pituitary re-productive axis. Toxicol. Appl. Pharmacol. 1986, 86, 474–483. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, K.L.; Wood, C.R.; Ford, R.L.; Kosian, P.A.; Hotchkiss, M.G.; Degitz, S.J.; Gilbert, M.E. Thyroid Hormone Disruption in the Fetal and Neonatal Rat: Predictive Hormone Measures and Bioindicators of Hormone Action in the Developing Cortex. Toxicol. Sci. 2018, 166, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protocol. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.H.; Gilbert, M.E. Modest Thyroid Hormone Insufficiency during Development Induces a Cellular Malformation in the Corpus Callosum: A Model of Cortical Dysplasia. Endocrinology 2007, 148, 2593–2597. [Google Scholar] [CrossRef]
- Spring, S.R.; Bastian, T.W.; Wang, Y.; Kosian, P.; Anderson, G.W.; Gilbert, M.E. Thyroid hormone-dependent formation of a subcortical band heterotopia (SBH) in the neo-natal brain is not exacerbated under conditions of low dietary iron (FeD). Neurotoxicol. Teratol. 2016, 56, 41–46. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Ramos, R.L.; McCloskey, D.P.; Goodman, J.H. Subcortical Band Heterotopia in Rat Offspring Following Maternal Hypothyroxinaemia: Structural and Functional Characteristics. J. Neuroendocr. 2014, 26, 528–541. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier Academic Press: Amsterdam, The Netherlands; London, UK, 2007; ISBN 9780123919496. [Google Scholar]
- Gilbert, M.E.; Sanchez-Huerta, K.; Wood, C. Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology 2015, 157, 774–787. [Google Scholar] [CrossRef]
- Gilbert, M.E. Impact of low-level thyroid hormone disruption induced by propylthiouracil on brain development and function. Toxicol. Sci. 2011, 124, 432–445. [Google Scholar] [CrossRef]
- Gilbert, M.E.; O’Shaughnessy, K.L.; E Thomas, S.; Riutta, C.; Wood, C.R.; Smith, A.; O Oshiro, W.; Ford, R.L.; Hotchkiss, M.G.; Hassan, I.; et al. Thyroid Disruptors: Extrathyroidal Sites of Chemical Action and Neurodevelopmental Outcome—An Examination Using Triclosan and Perfluorohexane Sulfonate. Toxicol. Sci. 2021, 183, 195–213. [Google Scholar] [CrossRef]
- Geyer, M.A.; Krebs-Thomson, K.; Braff, D.L.; Swerdlow, N.R. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology 2001, 156, 117–154. [Google Scholar] [CrossRef]
- Swerdlow, N.R.; Braff, D.L.; Geyer, M.A. Sensorimotor gating of the startle reflex: What we said 25 years ago, what has happened since then, and what comes next. J. Psychopharmacol. 2016, 30, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw-Hill Book Co.: New York, NY, USA, 1960. [Google Scholar]
- Wagenmakers, E.-J.; Farrell, S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 2004, 11, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Dempster, A.P.; Patel, C.M.; Selwyn, M.R.; Roth, A.J. Statistical and computational aspects of mixed model analysis. Appl. Stat. 1984, 33, 203–214. [Google Scholar] [CrossRef]
- Boyes, W.K.; Degn, L.; George, B.J.; Gilbert, M.E. Moderate perinatal thyroid hormone insufficiency alters visual system function in adult rats. NeuroToxicology 2018, 67, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Lavaroni, S.; Mori, A.; Ohta, M.; Saito, J.; Pietrarelli, M.; Singer, D.S.; Kimura, S.; Katoh, R.; Kawaoi, A.; et al. Autoregulation of thyroid-specific gene transcription by thyroglobulin. Proc. Natl. Acad. Sci. USA 1998, 95, 8251–8256. [Google Scholar] [CrossRef] [PubMed]
- Perron, B.; Rodriguez, A.; Leblanc, G.; Pourcher, T. Cloning of the mouse sodium iodide symporter and its expression in the mammary gland and other tissues. J. Endocrinol. 2001, 170, 185–196. [Google Scholar] [CrossRef]
- Royland, J.E.; Parker, J.S.; Gilbert, M.E. A Genomic Analysis of Subclinical Hypothyroidism in Hippocampus and Neocortex of the Developing Rat Brain. J. Neuroendocr. 2008, 20, 1319–1338. [Google Scholar] [CrossRef]
- Levitt, P.; Eagleson, K.L.; Powell, E.M. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004, 27, 400–406. [Google Scholar] [CrossRef]
- Kourdougli, N.; Suresh, A.; Liu, B.; Juarez, P.; Lin, A.; Chung, D.T.; Sams, A.G.; Gandal, M.J.; Martínez-Cerdeño, V.; Buonomano, D.V.; et al. Improvement of sensory deficits in fragile X mice by increasing cortical interneuron activity after the critical period. Neuron 2023, 111, 2863–2880.e6. [Google Scholar] [CrossRef]
- Nomura, T.; Musial, T.F.; Marshall, J.J.; Zhu, Y.; Remmers, C.L.; Xu, J.; Nicholson, D.A.; Contractor, A. Delayed Maturation of Fast-Spiking Interneurons Is Rectified by Activation of the TrkB Receptor in the Mouse Model of Fragile X Syndrome. J. Neurosci. 2017, 37, 11298–11310. [Google Scholar] [CrossRef]
- Kuhlman, S.J.; Olivas, N.D.; Tring, E.; Ikrar, T.; Xu, X.; Trachtenberg, J.T. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 2013, 501, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; van der Lei, M.B.; Kitiashvili, M.; Mamcarz, M.; Oliveira, M.M.; Longo, F.; Klann, E. Deletion of Fmr1 in parvalbumin-expressing neurons results in dysregulated translation and selective behavioral deficits associated with fragile X syndrome. Mol. Autism 2022, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, J.; Moss, J.; Wen, Z.; Sun, G.J.; Hsu, D.; Zhong, C.; Davoudi, H.; Christian, K.M.; Toni, N.; et al. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat. Neurosci. 2013, 16, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.E.; O’Shaughnessy, K.L.; Axelstad, M. Regulation of Thyroid-disrupting Chemicals to Protect the Developing Brain. Endocrinology 2020, 161, bqaa106. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Ren, J.; Flamant, F. Thyroid hormone action during GABAergic neuron maturation: The quest for mechanisms. Front. Endocrinol. 2023, 14, 1256877. [Google Scholar] [CrossRef]
- Schweizer, U.; Fabiano, M. Selenoproteins in brain development and function. Free. Radic. Biol. Med. 2022, 190, 105–115. [Google Scholar] [CrossRef]
- Gilbert, M.E.; Sui, L.; Walker, M.J.; Anderson, W.; Thomas, S.; Smoller, S.N.; Schon, J.P.; Phani, S.; Goodman, J.H. Thyroid hormone insufficiency during brain development reduces parvalbumin immuno-reactivity and inhibitory function in the hippocampus. Endocrinology 2007, 148, 92–102. [Google Scholar] [CrossRef]
- Berbel, P.; Marco, P.; Cerezo, J.; DeFelipe, J. Distribution of parvalbumin immunoreactivity in the neocortex of hypothyroid adult rats. Neurosci. Lett. 1996, 204, 65–68. [Google Scholar] [CrossRef]
- Mayerl, S.; Chen, J.; Salveridou, E.; Boelen, A.; Darras, V.M.; Heuer, H. Thyroid Hormone Transporter Deficiency in Mice Impacts Multiple Stages of GABAergic In-terneuron Development. Cereb Cortex 2022, 32, 329–341. [Google Scholar] [CrossRef]
- Esclassan, F.; Coutureau, E.; Di Scala, G.; Marchand, A.R. A Cholinergic-Dependent Role for the Entorhinal Cortex in Trace Fear Conditioning. J. Neurosci. 2009, 29, 8087–8093. [Google Scholar] [CrossRef]
- Bangasser, D.A.; Waxler, D.E.; Santollo, J.; Shors, T.J. Trace Conditioning and the Hippocampus: The Importance of Contiguity. J. Neurosci. 2006, 26, 8702–8706. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, M.R.; Kwapis, J.L.; Helmstetter, F.J. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiol. Learn. Mem. 2012, 97, 452–464. [Google Scholar] [CrossRef] [PubMed]
- McEchron, M.D.; Bouwmeester, H.; Tseng, W.; Weiss, C.; Disterhoft, J.F. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 1998, 8, 638–646. [Google Scholar] [CrossRef]
- Han, C.J.; O’Tuathaigh, C.M.; van Trigt, L.; Quinn, J.J.; Fanselow, M.S.; Mongeau, R.; Koch, C.; Anderson, D.J. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA 2003, 100, 13087–13092. [Google Scholar] [CrossRef]
- Baran, S.E.; Armstrong, C.E.; Niren, D.C.; Conrad, C.D. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learn. Mem. 2010, 17, 267–278. [Google Scholar] [CrossRef]
- Glover, E.M.; Jovanovic, T.; Norrholm, S.D. Estrogen and Extinction of Fear Memories: Implications for Posttraumatic Stress Disorder Treatment. Biol. Psychiatry 2015, 78, 178–185. [Google Scholar] [CrossRef]
- Ter Horst, J.P.; Carobrez, A.P.; van der Mark, M.H.; de Kloet, E.R.; Oitzl, M.S. Sex differences in fear memory and extinction of mice with forebrain-specific disruption of the mineralocorticoid receptor. Eur. J. Neurosci. 2012, 36, 3096–3102. [Google Scholar] [CrossRef]
- Baker-Andresen, D.; Flavell, C.R.; Li, X.; Bredy, T.W. Activation of BDNF signaling prevents the return of fear in female mice. Learn. Mem. 2013, 20, 237–240. [Google Scholar] [CrossRef]
- Fenton, G.; Halliday, D.; Mason, R.; Stevenson, C. Medial prefrontal cortex circuit function during retrieval and extinction of associative learning under anesthesia. Neuroscience 2014, 265, 204–216. [Google Scholar] [CrossRef]
- Bárez-López, S.; Montero-Pedrazuela, A.; Bosch-García, D.; Venero, C.; Guadaño-Ferraz, A. Increased anxiety and fear memory in adult mice lacking type 2 deiodinase. Psychoneuroendocrinology 2017, 84, 51–60. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Chan, P.-Y.S.; Hsu, S.-C.; Liu, C.-Y. Meta-analysis of sensorimotor gating in patients with autism spectrum disorders. Psychiatry Res. 2018, 262, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, J.S.; Bosch, D.; Alves, N.; Daros, A.; Ure, R.J.; Schmid, S. GABA receptors and prepulse inhibition of acoustic startle in mice and rats. Eur. J. Neurosci. 2010, 31, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Popelář, J.; Rybalko, N.; Burianová, J.; Schwaller, B.; Syka, J. The effect of parvalbumin deficiency on the acoustic startle response and prepulse inhibition in mice. Neurosci. Lett. 2013, 553, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Morrissey, M.D.; Mahadevan, V.; Cajanding, J.D.; Woodin, M.A.; Yeomans, J.S.; Takehara-Nishiuchi, K.; Kim, J.C. Parvalbumin and GAD65 Interneuron Inhibition in the Ventral Hippocampus Induces Distinct Behavioral Deficits Relevant to Schizophrenia. J. Neurosci. 2014, 34, 14948–14960. [Google Scholar] [CrossRef]
- Buuse, M.V.D.; Eikelis, N. Estrogen increases prepulse inhibition of acoustic startle in rats. Eur. J. Pharmacol. 2001, 425, 33–41. [Google Scholar] [CrossRef]
- Fisher, J.; Housand, C.; Mattie, D.; Nong, A.; Moreau, M.; Gilbert, M. Towards translating in vitro measures of thyroid hormone system disruption to in vivo responses in the pregnant rat via a biologically based dose response (BBDR) model. Toxicol. Appl. Pharmacol. 2023, 479, 116733. [Google Scholar] [CrossRef]
- Abt, E.; Spungen, J.; Pouillot, R.; Gamalo-Siebers, M.; Wirtz, M. Update on dietary intake of perchlorate and iodine from U.S. food and drug administration’s total diet study: 2008–2012. J. Expo. Sci. Environ. Epidemiol. 2016, 28, 21–30. [Google Scholar] [CrossRef]
- USEPA. 2019. Available online: https://www.regulations.gov/document/EPA-HQ-OW-2016-0438-0019 (accessed on 1 November 2024).
- Thompson, B.L.; Levitt, P. The clinical-basic interface in defining pathogenesis in disorders of neurodevelopmental origin. Neuron 2010, 67, 702–712. [Google Scholar] [CrossRef]
- Kast, R.J.; Levitt, P. Precision in the development of neocortical architecture: From progenitors to cortical networks. Prog. Neurobiol. 2019, 175, 77–95. [Google Scholar] [CrossRef]
- Butt, S.J.; Stacey, A.J.; Teramoto, Y.; Vagnoni, C. A role for GABAergic interneuron diversity in circuit development and plasticity of the neonatal cerebral cortex. Curr. Opin. Neurobiol. 2017, 43, 149–155. [Google Scholar] [CrossRef]
- Janickova, L.; Schwaller, B. Parvalbumin-Deficiency Accelerates the Age-Dependent ROS Production in Pvalb Neurons in vivo: Link to Neurodevelopmental Disorders. Front. Cell. Neurosci. 2020, 14, 571216. [Google Scholar] [CrossRef]
- Filice, F.; Janickova, L.; Henzi, T.; Bilella, A.; Schwaller, B. The Parvalbumin Hypothesis of Autism Spectrum Disorder. Front. Cell. Neurosci. 2020, 14, 577525. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert, M.E.; Hawks, M.G.; Bell, K.S.; Oshiro, W.; Wood, C.; George, B.J.; Thomas, R.; Ford, J. Iodine Deficiency Exacerbates Thyroidal and Neurological Effects of Developmental Perchlorate Exposure in the Neonatal and Adult Rat. Toxics 2024, 12, 842. https://doi.org/10.3390/toxics12120842
Gilbert ME, Hawks MG, Bell KS, Oshiro W, Wood C, George BJ, Thomas R, Ford J. Iodine Deficiency Exacerbates Thyroidal and Neurological Effects of Developmental Perchlorate Exposure in the Neonatal and Adult Rat. Toxics. 2024; 12(12):842. https://doi.org/10.3390/toxics12120842
Chicago/Turabian StyleGilbert, Mary E., MaryAnn G. Hawks, Kiersten S. Bell, Wendy Oshiro, Carmen Wood, Barbara Jane George, Ryne Thomas, and Jermaine Ford. 2024. "Iodine Deficiency Exacerbates Thyroidal and Neurological Effects of Developmental Perchlorate Exposure in the Neonatal and Adult Rat" Toxics 12, no. 12: 842. https://doi.org/10.3390/toxics12120842
APA StyleGilbert, M. E., Hawks, M. G., Bell, K. S., Oshiro, W., Wood, C., George, B. J., Thomas, R., & Ford, J. (2024). Iodine Deficiency Exacerbates Thyroidal and Neurological Effects of Developmental Perchlorate Exposure in the Neonatal and Adult Rat. Toxics, 12(12), 842. https://doi.org/10.3390/toxics12120842






