PM2.5 Exposure as a Risk Factor for Optic Nerve Health in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Levels of Air Pollution
2.3. Measurement of Optic Disc Diameters
2.4. Statistical Analyses
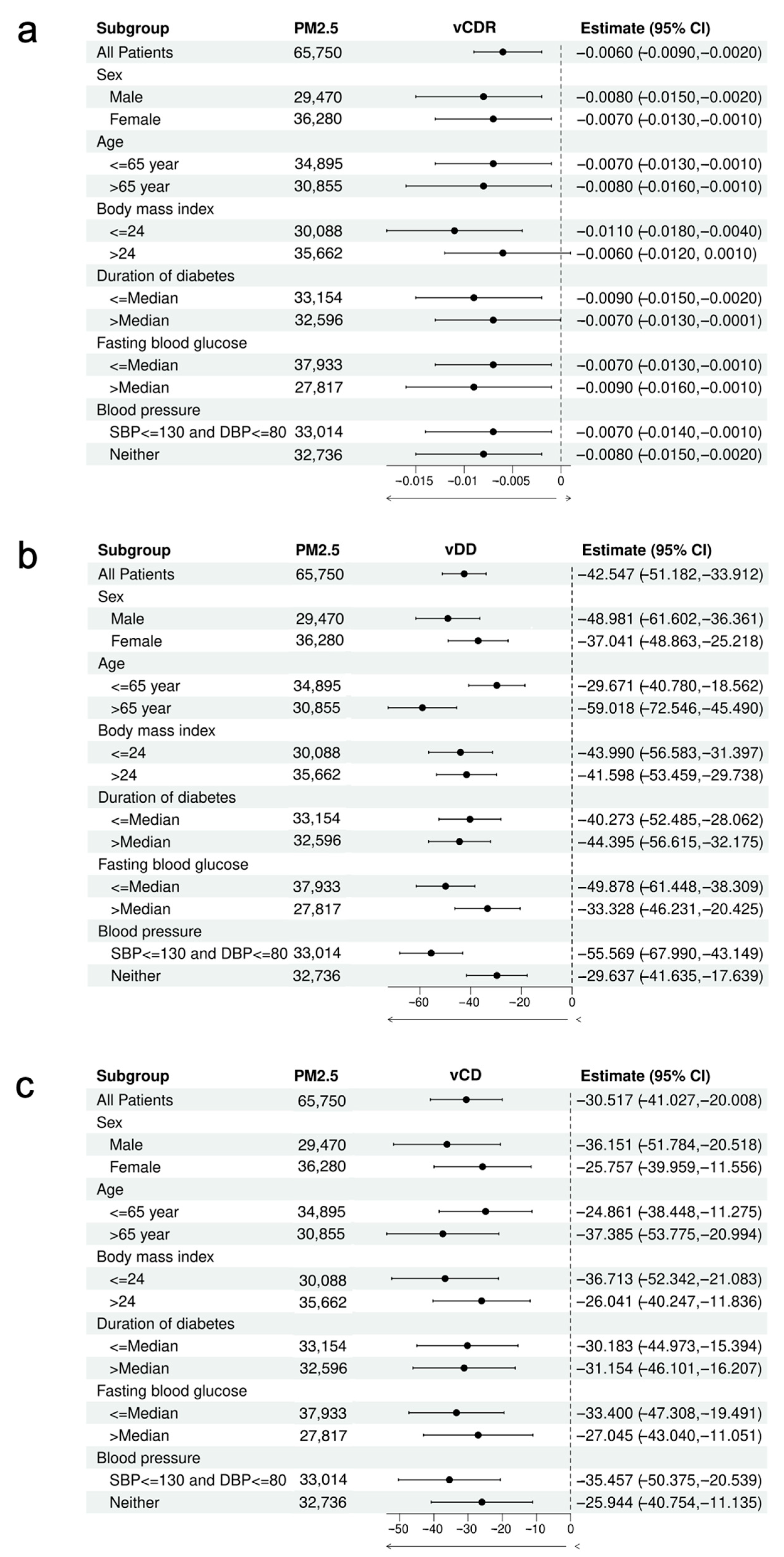
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Linear Regression Results of the Models
| Model 1 β (SE) | Model 2 β (SE) | Model 3 β (SE) | Model 4 β (SE) | |
|---|---|---|---|---|
| PM2.5 (per 10 µg/m3 increase) | −0.005 (0.002) * | −0.005 (0.002) * | −0.005 (0.002) * | −0.008 (0.002) *** |
| Age (per year increase) | 1.98 × 10−4 (6.3 × 10−5) ** | 1.82 × 10−4 (6.4 × 10−5) ** | 1.84 × 10−4 (6.4 × 10−5) ** | 1.71 × 10−4 (6.4 × 10−5) ** |
| Gender (male vs. female) | 0.016 (0.001) *** | 0.016 (0.001) *** | 0.016 (0.001) *** | 0.016 (0.001) *** |
| Duration of diabetes (per year increase) | 0.0002 (0.0001) * | 0.0002 (0.0001) | 0.0002 (0.0001) | 0.0001 (0.0001) |
| BMI (per unit increase) | −0.001 (0.0002) *** | −0.001 (0.0002) *** | −0.001 (0.0002) *** | |
| Alcohol drinking (yes vs. no) | −0.002 (0.003) | −0.002 (0.003) | −0.002 (0.003) | |
| Cigarette smoking (yes vs. no) | 0.002 (0.002) | 0.002 (0.002) | 0.002 (0.002) | |
| Physical exercise (per unit increase) | −1.75 × 10−⁶ (4.00 × 10−⁶) | −1.68 × 10−⁶ (4.00 × 10−⁶) | −1.59 × 10−⁶ (4.00 × 10−⁶) | |
| Fasting blood glucose (per unit increase) | 0.002 (0.004) | 0.002 (0.004) | ||
| Blood pressure (per mmHg increase) | −5.67 × 10−⁶ (7.6 × 10−5) | −3.48 × 10−⁶ (7.6 × 10−5) | ||
| O3 (per 10 µg/m3 increase) | 0.012 (0.005) * | |||
| NO2 (per 10 µg/m3 increase) | 4.84 × 10−4 (0.001) |
| Model 1 β (SE) | Model 2 β (SE) | Model 3 β (SE) | Model 4 β (SE) | |
|---|---|---|---|---|
| PM2.5 (per 10 µg/m3 increase) | −47.794 (3.837) *** | −48.755 (3.868) *** | −48.262 (3.875) *** | −42.547 (4.406) *** |
| Age (per year increase) | −1.114 (0.117) *** | −1.031 (0.118) *** | −1.046 (0.118) *** | −1.019 (0.118) *** |
| Gender (male vs. female) | 8.048 (1.738) *** | 4.966 (1.838) ** | 5.037 (1.839) ** | 5.213 (1.84) ** |
| Duration of diabetes (per year increase) | −0.139 (0.159) | −0.112 (0.16) | −0.07 (0.161) | −0.048 (0.161) |
| BMI (per unit increase) | 0.578 (0.288) * | 0.619 (0.29) * | 0.591 (0.291) * | |
| Alcohol drinking (yes vs. no) | −3.172 (4.706) | −3.277 (4.707) | −2.708 (4.712) | |
| Cigarette smoking (yes vs. no) | −17.318 (3.831) *** | −17.614 (3.835) *** | −16.807 (3.846) *** | |
| Physical exercise (per unit increase) | 0.005 (0.007) | 0.005 (0.007) | 0.005 (0.007) | |
| Fasting blood glucose (per unit increase) | −1.653 (0.676) * | −1.691 (0.676) * | ||
| Blood pressure (per mmHg increase) | 0.045 (0.14) | 0.04 (0.14) | ||
| O3 (per 10 µg/m3 increase) | −24.308 (8.92) ** | |||
| NO2 (per 10 µg/m3 increase) | −22.143 (1.587) *** |
| Model 1 β (SE) | Model 2 β (SE) | Model 3 β (SE) | Model 4 β (SE) | |
|---|---|---|---|---|
| PM2.5 (per 10 µg/m3 increase) | −26.815 (4.669) *** | −27.105 (4.707) *** | −26.988 (4.716) *** | −30.517 (5.362) *** |
| Age (per year increase) | 0.033 (0.142) | 0.037 (0.143) | 0.033 (0.143) | 0.016 (0.144) |
| Gender (male vs. female) | 32.056 (2.115) *** | 30.871 (2.237) *** | 30.9 (2.239) *** | 30.791 (2.24) *** |
| Duration of diabetes (per year increase) | 0.309 (0.194) | 0.271 (0.195) | 0.282 (0.196) | 0.268 (0.196) |
| BMI (per unit increase) | −1.282 (0.351) *** | −1.266 (0.354) *** | −1.248 (0.354) *** | |
| Alcohol drinking (yes vs. no) | −4.246 (5.727) | −4.292 (5.729) | −4.644 (5.735) | |
| Cigarette smoking (yes vs. no) | −3.482 (4.663) | −3.582 (4.667) | −4.08 (4.681) | |
| Physical exercise (per unit increase) | −0.001 (0.008) | −0.001 (0.008) | −0.001 (0.008) | |
| Fasting blood glucose (per unit increase) | −0.451 (0.822) | −0.427 (0.822) | ||
| Blood pressure (per mmHg increase) | −0.009 (0.17) | −0.007 (0.17) | ||
| O3 (per 10 µg/m3 increase) | 15.013 (10.856) | |||
| NO2 (per 10 µg/m3 increase) | −7.327 (1.935) *** |
References
- Kim, Y.; Knowles, S.; Manley, J.; Radoias, V. Long-run health consequences of air pollution: Evidence from Indonesia’s forest fires of 1997. Econ. Hum. Biol. 2017, 26, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, S. Air Quality and Early-Life Mortality. J. Hum. Resour. 2009, 44, 916–954. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.; Manley, J.; Radoias, V. Air Pollution and Long Term Mental Health. Atmosphere 2020, 11, 1355. [Google Scholar] [CrossRef]
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicol. Environ. Saf. 2019, 174, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G. Cut particulate air pollution, save lives. Bmj 2021, 375, n2561. [Google Scholar] [CrossRef]
- Thiankhaw, K.; Chattipakorn, N.; Chattipakorn, S.C. PM2.5 exposure in association with AD-related neuropathology and cognitive outcomes. Environ. Pollut. 2022, 292 Pt. A, 118320. [Google Scholar] [CrossRef]
- Li, W.; Lin, G.; Xiao, Z.; Zhang, Y.; Li, B.; Zhou, Y.; Ma, Y.; Chai, E. A review of respirable fine particulate matter (PM2.5)-induced brain damage. Front. Mol. Neurosci. 2022, 15, 967174. [Google Scholar] [CrossRef]
- Palacios, N. Air pollution and Parkinson’s disease—Evidence and future directions. Rev. Environ. Health 2017, 32, 303–313. [Google Scholar] [CrossRef]
- Sadun, A.A.; Wang, M.Y. Abnormalities of the optic disc. Handb. Clin. Neurol. 2011, 102, 117–157. [Google Scholar] [CrossRef]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of diabetes on cognitive function and brain structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef]
- Luo, A.; Xie, Z.; Wang, Y.; Wang, X.; Li, S.; Yan, J.; Zhan, G.; Zhou, Z.; Zhao, Y.; Li, S. Type 2 diabetes mellitus-associated cognitive dysfunction: Advances in potential mechanisms and therapies. Neurosci. Biobehav. Rev. 2022, 137, 104642. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qian, Y.; Lin, Q.; Chen, Z.; Xiang, Z.; Cui, L.; Sun, J.; Qin, X.; Xu, Y.; Lu, L.; et al. Increased serum 12-hydroxyeicosatetraenoic acid levels are correlated with an increased risk of diabetic retinopathy in both children and adults with diabetes. Acta Diabetol. 2022, 59, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, S.; Xu, Y.; Lu, L.; Zou, H. Unique composition of ocular surface microbiome in the old patients with dry eye and diabetes mellitus in a community from Shanghai, China. BMC Microbiol. 2024, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, W.; Shi, S.; Zhu, S.; Wang, P.; Chen, R.; Xiao, Q.; Xue, T.; Geng, G.; Zhang, Q.; et al. Evaluating the spatiotemporal ozone characteristics with high-resolution predictions in mainland China, 2013–2019. Environ. Pollut. 2022, 299, 118865. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Wang, W.; Zhang, H.; Shi, S.; Xue, T.; Lin, J.; Zhang, Y.; Liu, M.; Chen, R.; et al. Mortality burden due to ambient nitrogen dioxide pollution in China: Application of high-resolution models. Environ. Int. 2023, 176, 107967. [Google Scholar] [CrossRef]
- Long, T.; Xu, Y.; Zou, H.; Lu, L.; Yuan, T.; Dong, Z.; Dong, J.; Ke, X.; Ling, S.; Ma, Y. A Generic Pixel Pitch Calibration Method for Fundus Camera via Automated ROI Extraction. Sensors 2022, 22, 8565. [Google Scholar] [CrossRef]
- Dai, Y.; Jonas, J.B.; Ling, Z.; Sun, X. Ophthalmoscopic-Perspectively Distorted Optic Disc Diameters and Real Disc Diameters. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7076–7083. [Google Scholar] [CrossRef]
- Dong, D.; Xu, X.; Xu, W.; Xie, J. The Relationship Between the Actual Level of Air Pollution and Residents’ Concern about Air Pollution: Evidence from Shanghai, China. Int. J. Environ. Res. Public Health 2019, 16, 4784. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Tan, J.; Li, M. Concentration prediction and spatial origin analysis of criteria air pollutants in Shanghai. Environ. Pollut. 2023, 327, 121535. [Google Scholar] [CrossRef]
- Zhang, S.; Routledge, M.N. The contribution of PM(2.5) to cardiovascular disease in China. Environ. Sci. Pollut. Res. Int. 2020, 27, 37502–37513. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, T.; He, W.; Liu, L.; Li, H.; Liu, C.; Zhou, Y.; Hong, J.; Cao, L.; Lu, Y.; et al. Associations of fine particulate matter and constituents with pediatric emergency room visits for respiratory diseases in Shanghai, China. Int. J. Hyg. Environ. Health 2021, 236, 113805. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, S.; Qian, S.E.; Cai, M.; Li, H.; Wang, C.; Zou, H.; Chen, L.; Vaughn, M.G.; McMillin, S.E.; et al. Ambient air pollution associated with incidence and dynamic progression of type 2 diabetes: A trajectory analysis of a population-based cohort. BMC Med. 2022, 20, 375. [Google Scholar] [CrossRef] [PubMed]
- Markeviciute, A.; Huang-Lung, J.; Zemaitiene, R.; Grzybowski, A. A Review of Ambient Air Pollution as a Risk Factor for Posterior Segment Ocular Diseases. J. Clin. Med. 2023, 12, 3842. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Tong, B.; Xiang, Y.; Li, S.-R.; Tan, Z.-X.; Xiang, H.-X.; Fu, L.; Wang, H.; Zhao, H.; Xu, D.-X. Acute 1-NP exposure induces inflammatory responses through activating various inflammatory signaling pathways in mouse lungs and human A549 cells. Ecotoxicol. Environ. Saf. 2020, 189, 109977. [Google Scholar] [CrossRef]
- Vajaranant, T.S.; Hallak, J.; Espeland, M.A.; Pasquale, L.R.; Klein, B.E.; Meuer, S.M.; Rapp, S.R.; Haan, M.N.; Maki, P.M. An Association Between Large Optic Nerve Cupping and Cognitive Function. Am. J. Ophthalmol. 2019, 206, 40–47. [Google Scholar] [CrossRef]
- Kang, Y.J.; Tan, H.; Lee, C.Y.; Cho, H. An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv. Sci. 2021, 8, e2101251. [Google Scholar] [CrossRef]
- Mac Nair, C.E.; Nickells, R.W. Neuroinflammation in Glaucoma and Optic Nerve Damage. Prog. Mol. Biol. Transl. Sci. 2015, 134, 343–363. [Google Scholar] [CrossRef]
- Gayraud, L.; Mortamais, M.; Schweitzer, C.; de Hoogh, K.; Cougnard-Grégoire, A.; Korobelnik, J.-F.; Delyfer, M.-N.; Rougier, M.-B.; Leffondré, K.; Helmer, C.; et al. Association of long-term exposure to ambient air pollution with retinal neurodegeneration: The prospective Alienor study. Environ. Res. 2023, 232, 116364. [Google Scholar] [CrossRef]
- Li, J.; Yuan, N.; Chu, W.K.; Cheung, C.Y.; Tang, S.; Li, F.F.; Chen, L.J.; Kam, K.W.; Young, A.L.; Ip, P.; et al. Exposure to Secondhand Smoke in Children is Associated with a Thinner Retinal Nerve Fiber Layer: The Hong Kong Children Eye Study. Am. J. Ophthalmol. 2021, 223, 91–99. [Google Scholar] [CrossRef]
- Jonas, J.B.; Schmidt, A.M.; Müller-Bergh, J.A.; Schlötzer-Schrehardt, U.M.; Naumann, G.O. Human optic nerve fiber count and optic disc size. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2012–2018. [Google Scholar]
- Ning, K.; Tran, M.; Kowal, T.J.; Mesentier-Louro, L.A.; Sendayen, B.E.; Wang, Q.; Lo, C.; Li, T.; Majumder, R.; Luo, J.; et al. Compartmentalized ciliation changes of oligodendrocytes in aged mouse optic nerve. J. Neurosci. Res. 2024, 102, e25273. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.M.; Sadaka, A.; Berry, S.; Malik, A.; Lee, A.G. Bilateral disc edema in hypertensive emergency. Can. J. Ophthalmol. 2018, 53, e113–e115. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Jonas, R.A.; Bikbov, M.M.; Wang, Y.X.; Panda-Jonas, S. Myopia: Histology, clinical features, and potential implications for the etiology of axial elongation. Prog. Retin. Eye Res. 2023, 96, 101156. [Google Scholar] [CrossRef] [PubMed]
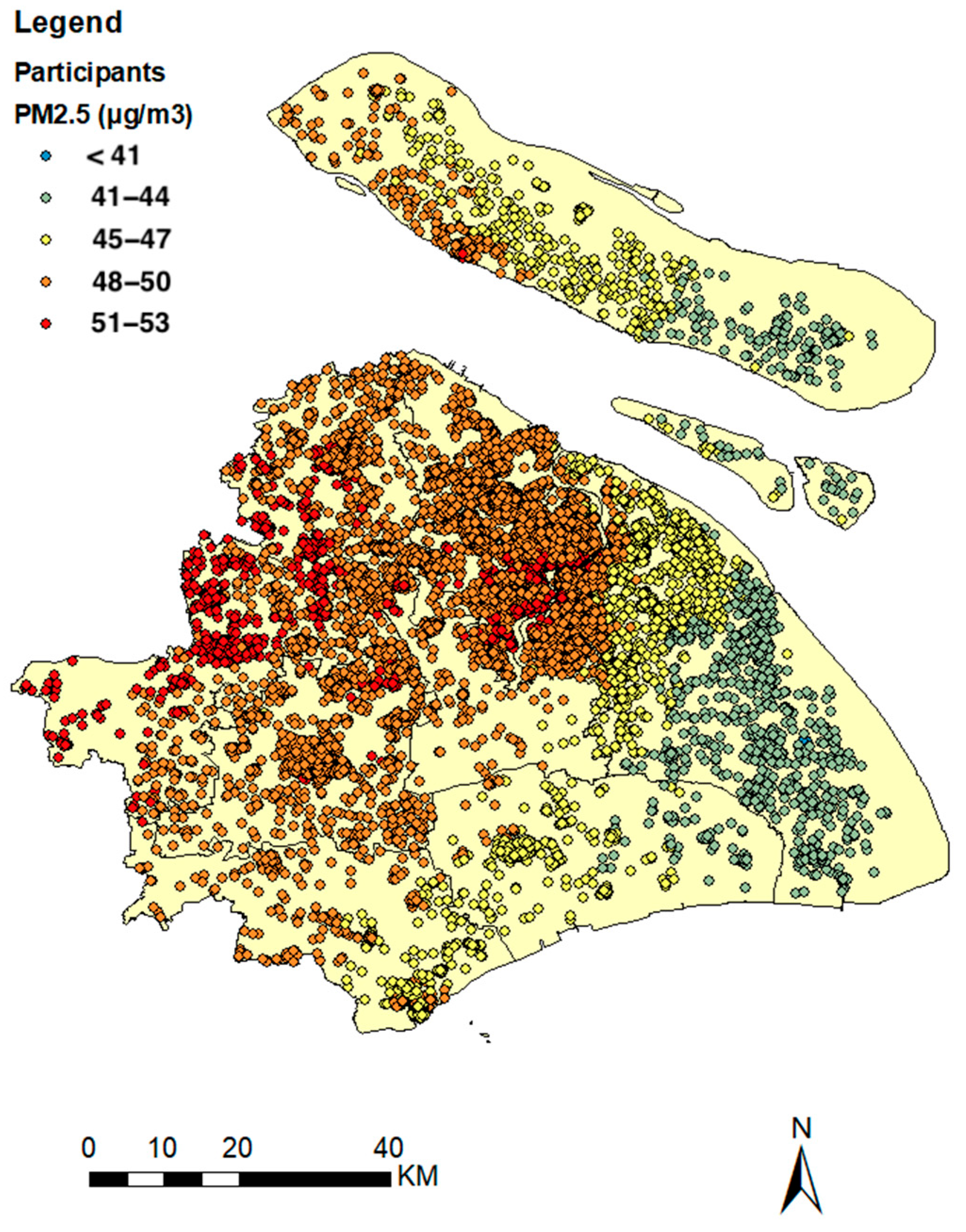
| Mean ± SD | Min | Max | |
|---|---|---|---|
| Male (%) | 29,470 (44.82) | ||
| Age (years) | 64.73 ± 7.53 | 18 | 97 |
| Duration of diabetes (years) | 8.01 ± 5.52 | 0 | 30 |
| BMI (kg/m2) | 24.52 ± 3.01 | 11.72 | 57.42 |
| SBP (mmHg) | 129.55 ± 9.09 | 84 | 220 |
| DBP (mmHg) | 78.42 ± 6.12 | 30 | 122 |
| FBG (mmol/L) | 6.89 ± 1.31 | 2.2 | 25.9 |
| Cigarette smoking (%) | 5050 (7.68) | ||
| Alcohol drinking (%) | 3056 (4.65) | ||
| Physical exercise (min/week) | 136.58 ± 128.13 | 0 | 500 |
| Variables | Mean ± SD | Percentile | ||||
|---|---|---|---|---|---|---|
| 25th | Median | 75th | Max | IQR | ||
| vCDR | 0.48 ± 0.1 | 0.42 | 0.48 | 0.54 | 0.97 | 0.12 |
| vDD (μm) | 1893.34 ± 210.64 | 1768.69 | 1885.42 | 2007.87 | 3448.8 | 239.18 |
| vCD (μm) | 916.08 ± 243.23 | 757.87 | 900.78 | 1062.9 | 2549.07 | 305.03 |
| Air pollution | ||||||
| PM2.5 (μg/m3) | 47.88 ± 2.25 | 46.75 | 48.64 | 49.38 | 53.9 | 2.63 |
| O3 (μg/m3) | 41.72 ± 1.28 | 41.06 | 42.22 | 42.54 | 44.8 | 1.48 |
| NO2 (μg/m3) | 22.91 ± 9.41 | 14.54 | 23.82 | 30.79 | 45.1 | 16.25 |
|
Model 1 β (SE) |
Model 2 β (SE) |
Model 3 β (SE) |
Model 4 β (SE) | |
|---|---|---|---|---|
| vCDR | −0.005 (0.002) * | −0.005 (0.002) * | −0.005 (0.002) * | −0.008 (0.002) *** |
| vDD (μm) | −47.794 (3.837) *** | −48.755 (3.868) *** | −48.262 (3.875) *** | −42.547 (4.406) *** |
| vCD (μm) | −26.815 (4.669) *** | −27.105 (4.707) *** | −26.988 (4.716) *** | −30.517 (5.362) *** |
| Variables | 1-Year Average PM2.5 β (SE) | 2-Year Average PM2.5 β (SE) | 3-Year Average PM2.5 β (SE) |
|---|---|---|---|
| vCDR | −0.005 (0.002) | −0.012 (0.003) | −0.011 (0.003) |
| vDD (μm) | −28.558 (4.197) | −42.095 (6.324) | −49.725 (5.509) |
| vCD (μm) | −19.658 (5.111) | −38.589 (7.694) | −39.043 (6.704) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, T.; Cheng, M.; Ma, Y.; Zou, H.; Kan, H.; Meng, X.; Guo, Y.; Peng, Z.; Xu, Y.; Lu, L.; et al. PM2.5 Exposure as a Risk Factor for Optic Nerve Health in Type 2 Diabetes Mellitus. Toxics 2024, 12, 767. https://doi.org/10.3390/toxics12110767
Yuan T, Cheng M, Ma Y, Zou H, Kan H, Meng X, Guo Y, Peng Z, Xu Y, Lu L, et al. PM2.5 Exposure as a Risk Factor for Optic Nerve Health in Type 2 Diabetes Mellitus. Toxics. 2024; 12(11):767. https://doi.org/10.3390/toxics12110767
Chicago/Turabian StyleYuan, Tianyi, Minna Cheng, Yingyan Ma, Haidong Zou, Haidong Kan, Xia Meng, Yi Guo, Ziwei Peng, Yi Xu, Lina Lu, and et al. 2024. "PM2.5 Exposure as a Risk Factor for Optic Nerve Health in Type 2 Diabetes Mellitus" Toxics 12, no. 11: 767. https://doi.org/10.3390/toxics12110767
APA StyleYuan, T., Cheng, M., Ma, Y., Zou, H., Kan, H., Meng, X., Guo, Y., Peng, Z., Xu, Y., Lu, L., Ling, S., Dong, Z., Wang, Y., Yang, Q., Xu, W., Shi, Y., Liu, C., & Lin, S. (2024). PM2.5 Exposure as a Risk Factor for Optic Nerve Health in Type 2 Diabetes Mellitus. Toxics, 12(11), 767. https://doi.org/10.3390/toxics12110767








