Abstract
In aquatic ecosystems, the interaction between heavy metals and dissolved organic carbon (DOC) plays a pivotal role in modifying the bioavailability of these metals. This study, employing a toxicokinetic–toxicodynamic model, delves into the interactive effects of humic acid (HA), a significant component of DOC, on the bioaccumulation and toxicity of copper (Cu) in the estuarine economic bivalve Sinonovacula constricta. Utilizing the stable isotope 65Cu as a tracer, we evaluated Cu uptake in S. constricta under varied DOC concentrations in a controlled laboratory setting. Our findings reveal that at DOC concentrations below 3.05 mg L−1, the bioavailability of Cu is reduced due to shifts in the speciation distribution of Cu, resulting in decreased bioaccumulation within S. constricta. Conversely, at DOC levels exceeding 3.05 mg L−1, the formation of colloidal Cu–HA complexes allows its entry into the bivalves’ digestive system. Moreover, toxicity assays demonstrate an increase in S. constricta survival rates with higher DOC concentrations, suggesting a protective effect of DOC against Cu toxicity. The integration of accumulation and toxicity data infers that Cu–HA complexes, when ingested via the digestive tract, exhibit lower toxicity compared to Cu directly assimilated from the water phase. These findings emphasize the need to consider environmental DOC levels in assessing Cu pollution risks and provide insights for managing heavy metal toxicity in estuarine aquaculture.
1. Introduction
As a prevalent heavy metal contaminant in China’s coastal waters [1,2], copper (Cu) is readily absorbed and utilized by marine organisms, particularly bivalves, leading to significant accumulation in their tissues [3,4]. This accumulation escalates with increasing Cu levels in the water, particularly in heavy metal-polluted estuarine areas [5]. Furthermore, Cu is more toxic to bivalves than many other heavy metals [6]; when tissue concentrations exceed certain threshold levels, it can result in toxic effects [7,8], thereby posing substantial risks to marine bivalve populations.
It is worth noting that the chemical composition of seawater, such as dissolved organic matter (DOM), can greatly influence the bioavailability of dissolved Cu [9,10]. DOM is typically characterized by the concentration of dissolved organic carbon (DOC). According to the concept of the widely used biotic ligand model (BLM), aquatic organisms have the highest utilization rate for free ion metals in water, while DOC competes with free ion metals for binding, thereby reducing the proportion of free metals in water and consequently decreasing their bioavailability [11,12]. Theoretically, the higher the concentration of DOC in the water, the lower the utilization rate of heavy metals by organisms, and the lower toxicity to organisms. Humic acid (HA) is a significant component of DOC found in natural water bodies [13]. Roughly half of the dissolved organic matter in natural waters consists of HA [14]. Humic substances demonstrate a notably high affinity for Cu particularly due to its natural tendency to form carbonate and hydroxide complexes [15,16,17].
Previous studies have reported on the influence of DOC in water bodies, including HA and other small molecules, on the accumulation of metals in bivalves. The biokinetic (BK) model approach, which is only used with bioaccumulation, has been successfully applied in these studies [18,19,20,21]. However, there are still few studies that connect the effects of Cu–DOC complexes on the bioaccumulation and toxicity of Cu in marine bivalves [22]. The toxicokinetic–toxicodynamic (TK–TD) model offers a robust framework for examining these relationships by simulating the dynamic process of accumulation and its toxic effects in organisms [23]. In this approach, toxicokinetic (TK) models link time-dependent bioaccumulation with the concentration in the exposure medium, encompassing uptake, distribution, metabolism, elimination, and growth dilution [24]. Meanwhile, toxicodynamic (TD) models correlate the accumulated concentration with the progression of toxic effects, such as mortality.
Sinonovacula constricta, a type of clam and one of China’s four traditionally farmed bivalves, is extensively distributed along the nation’s coast and is a key contributor to the marine bivalve aquaculture industry, boasting an annual production of approximately 850,000 tons in 2022 [25]. However, since domestic S. constricta aquaculture sites are mainly located in heavily human-impacted nearshore estuaries, there is a higher risk of heavy metal accumulation and subsequent food safety issues in S. constricta aquaculture [26]. Overall, studying the accumulation process of Cu in S. constricta and its limiting factors in aquaculture waters is of great significance for food safety control and ecological risk assessment in S. constricta aquaculture.
In this study, we utilized the stable isotope 65Cu as a tracer; we not only examined the effects of varying concentrations of HA on Cu uptake in a controlled lab environment but also conducted toxicity tests. A one-compartment TK–TD model was established to elucidate the role of DOC in influencing Cu bioavailability and its resultant toxicity to S. constricta. This study could offer insights for ecological risk assessment and the management of heavy metal pollution in aquaculture settings.
2. Materials and Methods
2.1. Clam Acquisition and Acclimation
The razor clams S. constricta used in the experiment, with their gonads in the undeveloped stage, were collected from an aquaculture farm (23°56′02.1″ N, 117°23′39.7″ E) in Yunxiao, Fujian Province, China, in March 2021. They were temporarily cultured in a laboratory environment for 5–7 days before experiments began. The formula for the artificial seawater was adapted from previous literature [21]. The seawater was prepared by mixing sea salt with Milli-Q water to achieve a salinity of 15. This seawater composition was then utilized for all subsequent experiments. The background value of Cu in the artificial seawater is less than 1 µg L−1. During this period, the clams were fed with Chlorella sp. microalgae once a day, with a feeding amount of 2–3% of their wet weight. During this period, and throughout the entire experiment, the temperature of the seawater was consistently maintained at 20 1 °C and the dissolved oxygen content was always kept above 6 mg L−1.
2.2. Cu Accumulation and Elimination
The reconstituted seawater was filtered through 0.22 μm polypropylene membranes (Calyx Capsule) and 400 W UV lamps were used to degrade the background DOC in the filtered seawater for 8 h. The seawater was divided into 4 groups to which were added different nominal concentrations of HA (Sigma Aldrich) (0, 5, 10, 20 mg L−1); each group had 3 replicates. Following the addition of HA, the prepared seawater was sequentially filtered again through polypropylene membranes (Calyx Capsule) with pore sizes of 2 μm and subsequently 0.22 μm to effectively remove particulate matter. Prior to initiating the experiment, for each gradient of HA, 30 mL water was sampled in a brown reagent bottle for DOC determination. In this study, the stable isotope 65Cu (Trace Science International, Canada) was used as a tracer. The lowest Cu concentration (15 μg L−1) used for the experiments was commonly found in estuaries in China [4,5]. The clams were exposed to the isotope 65Cu at a nominal concentration of 15 and 150 μg L−1 (see Table S1 for measured concentrations). Seawater for exposure, spiked with 65Cu, was allowed to equilibrate for one hour prior to commencing the uptake experiments. In preparation for the exposure experiments, the 4 L polypropylene containers were soaked overnight in 5% HNO3 and then thoroughly rinsed three times with Milli-Q water.
After the start of the experiment, 22 clams were placed in the 4 L polypropylene containers filled with 4 L of prepared exposure medium and exposed for 12 h in the absence of supplemental feeding. During the initial 12 h exposure period, samples were taken every 3 h, with 3 mL of water and 2 clams for each replicate at each sample time. After the exposure, clams were transferred to clean filtered seawater for a 168 h depuration phase. The 3 mL water samples of each replicate from the same group were combined and acidified with 90 µL of 65% HNO3 for subsequent chemical analysis. In the depuration stage, the clams were provided with daily feeding, consistent with the protocol established during the acclimation period. They were then sampled at intervals of 12, 24, 48, 72, 120, and 168 h specifically for the group exposed to 15 μg L−1 of 65Cu during the uptake phase, with each sampling consisting of 2 clams per replicate. Throughout the entire duration of the experiment, all clam samples were carefully dissected, and soft tissues were rinsed twice with 1 mmol L−1 EDTA, followed by two rinses with Milli-Q water to minimize surface adsorption of 65Cu. The soft tissues were then placed in individual self-sealing bags, freeze-dried for 48 h, and weighed. The freeze-dried tissues were added to 15 mL centrifuge tubes with 1 mL of HNO3 for digestion, which was carried out at room temperature for 8–12 h, followed by hot digestion in a digestion instrument for 24 h at a temperature of 80 ℃.
2.3. Cu Toxicity Test
A short-term toxicity test was conducted under a high 65Cu concentration (nominal concentration of 300 µg L−1) for 96 h. The 4 L polypropylene containers were pre-treated by soaking overnight in 5% HNO3 and subsequently rinsed three times with Milli-Q water before use. The clams were exposed to the gradient DOC seawater prepared as mentioned above (Section 2.2). Each group had 3 replicates, and a group not spiked with any Cu was set as the control group; each replicate contained 4 L of exposure solution and 20 individuals. The accumulation experiments under 300 µg/L 65Cu (see Table S1 for measured concentrations) were conducted simultaneously and shared the same container with the toxic test. In the first 12 h of the toxicity test, sampling occurred every 3 h, with each sample comprising 3 mL of water and 2 clams. The samples were processed as described in Section 2.2. Throughout the duration of the toxicity test, the exposure medium was not replaced. The experiment results were considered reliable only when the survival rate of the control group was above 90%. Throughout the experiment, the mortality of the clams was recorded at intervals of 6–8 h, and deceased specimens were promptly removed from the containers. A clam was considered deceased if its shell remained open and it was unresponsive to touch.
2.4. Chemical Analysis
The Cu concentrations in water and organisms were determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS, NexION 2000). A calibration standard (Agilent 5188-6525) was used for calibration and quality control by measuring a control standard for every 10 samples. An internal standard Ge was used during the measurement, and it was checked every 10 samples to ensure the stability of the internal standard between 90% and 110%. The obtained results were corrected using the internal standard. The standard reference material (SRM 1566b, oyster tissue) was digested and analyzed, and the results showed that the Cu recovery rate was within the range of 90–110%.
For the analysis of DOC, water samples were treated with H3PO4 to lower their pH to below 2 and then preserved in amber glass bottles at 4 °C. DOC concentrations were quantified using a Shimadzu TOC-Vcph total organic carbon analyzer.
2.5. Toxicokinetic–Toxicodynamic Modeling and Parameters Estimation
2.5.1. Toxicokinetics
To simulate the 65Cu accumulation and elimination process in S. constricta, a one-compartment toxicokinetic model was established, drawing on methodologies established in previous studies [7,8]:
represents the accumulated concentration of 65Cu in clams at time t (µg g−1); (µg L−1) is the Cu concentration in the water; is the uptake rate constant (L g−1 d−1); and is the elimination rate constant (d−1). is the uptake rate of the clams and changes with the variation of Cu concentration ( in the water. and have the following relationship:
2.5.2. Toxicodynamics
The survivorship of clams in the toxicity experiments was characterized through the application of the toxicodynamic model, drawing on methodologies established in previous studies [7,8]:
represents the hazard posed by Cu exposure; the mortality rate, denoted by (g µg−1 h−1), quantifies the mass of organisms that perished per microgram of Cu per hour; is the threshold concentration that causes toxicity; is the background hazard rate (h−1), which is assumed to be zero in this study due to the absence of mortality in the control group. is the survival probability of the organisms at a given time t; is the survival probability of the organisms in the negative control. The core concept of this model is that the hazard of Cu exposure begins to accumulate once the Cu concentration in the organism’s tissues surpasses the threshold (). The hazard rate is directly proportional to the excessive accumulation of Cu above the threshold, or [ − ].
2.5.3. Parameters Estimation
The TK–TD model is established and relevant parameters are calculated using the Openmodel 2.4.3 software (Neil Crout at Nottingham University). To calculate the TK model parameters, the uptake and elimination data are fitted using Equations (1) and (2); to calculate the TD model parameters, Equations (3) and (4) are used for fitting. The parameters and standard deviations for TK and TD are calculated using the Marquardt algorithm.
2.6. Data Analysis
For the accumulation experiment, samples were analyzed for both 63Cu and 65Cu, and the actual 65Cu concentration was obtained by subtracting the background value [27]:
The term [65Cu] represents the aggregate concentration of isotope 65Cu within the samples, while [63Cu] denotes the background level of isotope 65Cu. Here, 65F refers to the natural isotopic abundance of 65Cu, which is valued at 0.3085.
To evaluate variations in Cu concentrations across various treatment groups within different experiment groups, we utilized one-way ANOVA (R software version 4.3.2) to determine the statistical significance of these disparities. A significance threshold was established at p < 0.05. For identifying specific differences between pairs of treatments, Tukey’s HSD post-hoc test was conducted for analysis. The median lethal concentrations (LC50) were calculated by the “ecotox” package of R software (version 4.3.2).
3. Results and Discussion
3.1. Cu Toxicokinetics
The toxicokinetics of Cu in S. constricta were investigated using a one-compartment model, which effectively fitted the experimental data, as shown in Figure 1. During the Cu accumulation and elimination phases, the clams consistently displayed a significant increase in Cu concentration during the accumulation stage, followed by a marked decrease during the elimination stage, across the entire nominal HA concentration range from 0 to 20 mg L−1. Given the negligible changes in the body weight of the clams across all groups within the approximately 8-day experiment, the influence of growth on the variations in 65Cu concentration in clams was not included in the modeling.
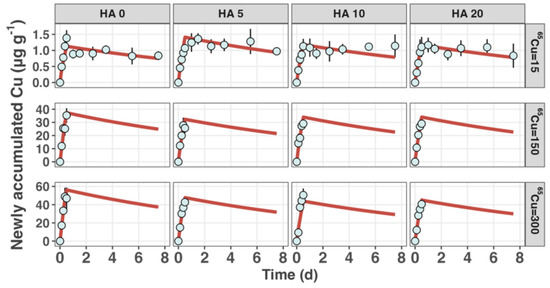
Figure 1.
65Cu accumulation and elimination in the razor clam S. constricta. The average newly accumulated 65Cu (µg g−1) over a 12 h exposure period under varying concentrations of humic acid (HA) (nominal concentrations are 0, 5, 10, and 20 mg L−1) and 65Cu (nominal concentrations are 15, 150, and 300 µg L−1). The duration of the depuration period is 7 days. Error bars represent standard deviations from the mean (n = 6), and the depicted curves represent model fits.
Table 1 presents the best-fit model parameters for Cu uptake and elimination, calculated at three nominal 65Cu concentrations (15, 150, and 300 µg L−1) across various HA concentrations (0, 5, 10, and 20 mg L−1). Pan and Wang (2009) [28] investigated Cu biokinetics in five marine bivalves using the isotope 67Cu, determining and values. Their results showed values ranging from 0.053 to 0.326 L g−1 h−1. In contrast, our findings at the 15 µg L−1 exposure level indicated higher values, ranging from 0.269 to 0.307 L g−1 h−1. Additionally, Zhong et al. (2012) [20] reported a Cu uptake rate constant of only 0.083 L g−1 h−1 for P. viridis at a salinity of 33, potentially indicating reduced Cu bioavailability at higher salinity [29,30].

Table 1.
Best-fit values of the model parameters.
The uptake rate constants represent values measured at nominal 65Cu concentrations of 15, 150, and 300 μg L−1, with each level corresponding to varied concentrations of humic acid (HA). A consistent value for the elimination rate constant was applied across varying levels of HA and 65Cu concentrations; was estimated to be 0.0582 ± 0.0139 d−1. Presented values are means ± standard deviation.
The elimination rate constant () is viewed as independent of external environmental factors, relating solely to the organism’s biology. Thus, remains unaffected by changes in 65Cu concentration or HA levels in the water, leading to its consistent value in parameter calculations. The for S. constricta is 0.0582 ± 0.0139 d−1, slightly above that of the oyster Saccostrea cucullata (0.032 ± 0.021 d−1) but below that of other bivalves [28,30].
3.2. Cu Uptake at Different DOC Levels
Table 1 displays the variations in the uptake rate constant () in response to different concentrations of HA at three 65Cu exposure concentrations. The actual DOC concentrations have been determined as 0.58, 3.05, 5.61, and 8.98 mg L−1 for each HA treatment (Table 2). In general, the s initially decreased slightly with increasing HA concentrations before stabilizing between each Cu exposure treatment. Specifically, at a DOC concentration of 0.58 mg L−1, the was 0.307, 0.625, and 0.481 L g−1 h−1 which slightly decreased to 0.274, 0.578, and 0.424 L g−1 h−1 at 8.98 mg L−1 DOC for three Cu exposure treatments, respectively. In a similar context, Sánchez-Marín et al. (2016) [31] also observed a decrease in for Mytilus edulis in the presence of HA, with declining from 1.939 ± 0.213 to 0.958 ± 0.068 L g−1 h1, mirroring the trend we found in our study. It is worth noting that Chen (2017) [22] reported that the of Cu for Potamocorbula laevis decreased from 0.0184 to 0.0156 L g−1 h−1 then increased to 0.0533 L g−1 h−1 as the HA concentration varied from 0.12 mg L−1 to 10.3 mg L−1. The author observed that precipitation occurred during the experimental processes, which may have formed complexes with Cu and been inadvertently ingested by the clams, thus being absorbed through the digestive tract. That might be the reason for increasing significantly when exposed to high HA concentrations.

Table 2.
Measured concentrations of DOC (mg L−1) in the exposure seawater of different HA levels used in the experiments.
Figure 2 depicts the 12 h Cu accumulation in S. constricta, highlighting the impact of HA concentration at each Cu exposure treatment. At 65Cu exposure levels of 15 µg L−1 and 150 µg L−1, there was a significant reduction in the 65Cu accumulation in clams from 1.39 ± 0.25 and 35.3 ± 5.49 µg g−1 to 1.07 ± 0.27 and 25.7 ± 3.77 µg g−1, respectively, as the actual DOC concentration significantly increased from 0.58 to 3.05 mg L−1 (one-way ANOVA, p < 0.05). This aligns with the findings of Kamunde and MacPhail (2011) [32], who also reported the reduced bioaccumulation of Cu in rainbow trout after the introduction of HA, highlighting the role of HA in lowering the bioavailability of waterborne Cu, thereby reducing its accumulation in aquatic organisms. A plausible explanation is that the addition of HA to the exposure medium significantly reduces the proportion of free Cu2+ ions, thereby decreasing the bioavailability of Cu.
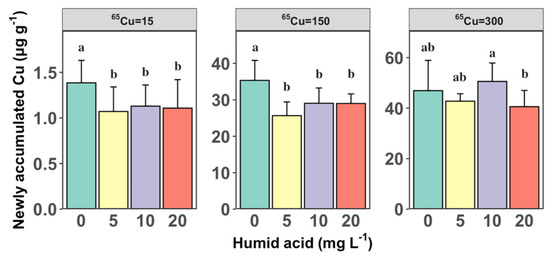
Figure 2.
The average newly accumulated Cu (µg g−1) at 12 h under varying concentrations of humic acid (HA) (0, 5, 10, and 20 mg L−1) and three levels of 65Cu exposure (15, 150, and 300 µg L−1). Error bars represent standard deviations from the mean (n = 6). different letters (e.g., “a” vs. “b”) signify that there are significant differences between these groups, with a p-value less than 0.05.
Moreover, there was no significant change in the levels of 65Cu accumulation in the clams when the actual DOC concentration increased from 3.05 to 8.98 mg L−1 (one-way ANOVA, p > 0.05). This trend is similar to the variation in the s in relation to the concentration of DOC across each Cu exposure concentration. One possible interpretation of our findings is that when the actual concentration of DOC exceeds a threshold of 3.05 mg L−1, there is an augmented formation of colloids as a result of Cu binding with HA. While we did not observe precipitation during our experiments, as noted in a previous study [22], some studies suggest that the introduction of HA into aquatic systems can lead to colloidal metal–HA formation. These complexes could be ingested through the digestive pathways of clams, potentially leading to a rise in the overall Cu concentration within the organisms as this bound form of Cu is taken up. For example, Sánchez-Marín et al. [31] found that HA did not significantly affect Cu accumulation in the overall soft tissues of the mussel Mytilus edulis. In their research, like ours, the modeling method employed in their study illustrates that colloidal metal, presented as HA, can be absorbed through the gut of marine mussels. Similar conclusions were also reported in an earlier study [33].
The elimination rate constant (), like the salinity variation in the previous report [29,30], could fit well with the depuration across varying concentrations of HA, suggesting that unlike the uptake process, the elimination rate was not dependent on HA varying. The constant elimination rate implies that the observed differences in Cu accumulation are primarily driven by variations in uptake rates rather than changes in elimination dynamics.
The relationship between the uptake rate and the waterborne Cu concentration adheres to the Michaelis–Menten equation (Table 3). For varying actual DOC concentrations of 0.58, 3.05, 5.61, and 8.98 mg L−1, the maximal Cu uptake rates () were calculated as 211, 202, 142, and 176 µg g−1 h−1, respectively. The half-saturation constants (Kms), indicative of the Cu concentration at which the uptake rate is half of , were determined to be 346, 230, 135, and 204 µg L−1.

Table 3.
The 96 h median lethal concentration (LC50) of Cu in the clam S. constricta determined at four different humic acid (HA) levels (0, 5, 10, and 20 mg L−1). The relationship between uptake rates of Cu and Cu concentrations in exposure solution ([Cu], ug L−1) are described by the Michaelis–Menten equation.
3.3. Toxicodynamics and Effects of DOC
Figure 3 shows the dynamics of survivorship of S. constricta when exposed to 300 µg L−1 65Cu in conjunction with a range of DOC concentrations (0, 3.05, 5.61, and 8.98 mg L−1) over a period of 96 h. There was a corresponding increase in the LC50s of Cu from 72 µg L−1 to 493 µg L−1. Previous studies also reported adding HA in the exposure medium can effectively enhance the Cu LC50s in aquatic animals [13,34,35], which is consistent with our observation. Moreover, the 96 h survival rate of S. constricta climbed from approximately 0% to about 50% with DOC concentrations from 0.58 mg L−1 to 8.98 mg L−1. This trend also indicates that an increase in HA concentration can significantly mitigate the toxic effects of Cu on S. constricta.
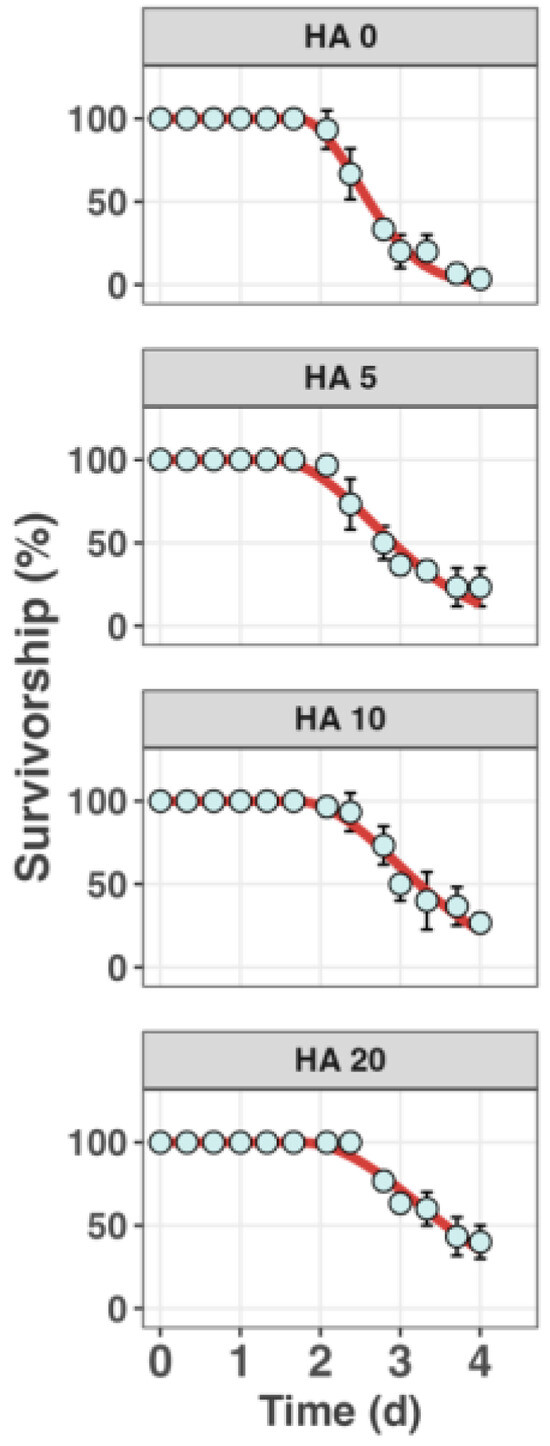
Figure 3.
Survivorship of the razor clam S. constricta when exposed to 300 µg L−1 65Cu across a gradient of humic acid (HA) (nominal concentrations are 0, 5, 10, and 20 mg L−1) for 96 h. Data points represent the mean observed survival percentages with error bars indicating standard deviation (n = 3). The lines represent the best fits from the model.
Table 1 presents the best-fit estimates of the threshold concentration () for toxicity and the killing rate () derived from the toxicodynamic (TD) model applied to the survivorship data of the clams. The of Cu increased with the DOC concentration, rising from 104 ± 9.4 µg g−1 up to 140 ± 11 µg g−1 at 8.98 mg L−1 DOC. The killing rate, , showed a decreasing trend from 10.4 ± 1.89 mg µg−1 h−1 to 5.68 ± 1.08 mg µg−1 h−1 with increased DOC concentrations, indicating that the grams of S. constricta killed by per mg of internalized Cu per hour were reduced. The calculated variations in these parameters potentially reflect a protective effect of HA against Cu toxicity. Our results are similar to the determined in a previous study which was 92 g µg−1 in P. laevis at a salinity of 15, and the s were lower than ours which was 21.8 mg µg−1 h−1 [30]. Under the three Cu exposure concentrations, when the actual concentration of HA exceeded 3.5 mg L−1, there were no significant changes in the accumulated amount of Cu over 12 h. This suggests that the threshold concentration did not increase in a regular pattern with rising HA levels, and the killing rate decreased as the HA concentration increased. This may indicate that the toxicity of Cu–DOC complexes absorbed through the digestive tract is lower than that of Cu absorbed directly from the water.
4. Conclusions
In conclusion, our study demonstrated that the impact of HA on the bioaccumulation and toxicity of Cu for S. constricta manifests in two main ways: it alters the distribution of Cu species and affects the uptake pathways of Cu. When the actual DOC concentration is below a certain threshold, it primarily reduces Cu bioavailability by altering its speciation, thus diminishing Cu bioaccumulation. However, at higher DOC concentrations, HA may promote the formation of colloidal Cu–HA complexes in the solution, which could then be ingested by S. constricta. Moreover, beyond the commonly acknowledged mechanism of diminishing Cu accumulation and toxicity for bivalves through the formation of Cu–DOC complexes, we propose that the Cu–DOC complexes ingested via the digestive tract might be less toxic than Cu absorbed directly from the water. This study highlights the importance of considering both chemical and biological factors in evaluating the health of marine ecosystems and the safety of seafood.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/xxx/s1, Table S1: Measured concentrations of 65Cu (μg L−1) in the exposure seawater of different humic acid used in the accumulation experiment.
Author Contributions
Conceptualization, Y.K.; methodology, T.M.; software, Y.K.; validation, M.C., H.Q. and B.S.; formal analysis, X.L.; investigation, M.C. and T.M.; data curation, Y.K.; writing—original draft preparation, M.C. and T.M.; writing—review and editing, Y.K.; visualization, Y.K.; supervision, H.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant number 32002379, and National Natural Science Foundation of China, grant number 32172949.
Institutional Review Board Statement
The sample collection and experimental protocols were approved by the Animal Care and Use Committee of the Fisheries College of Jimei University (Animal Ethics No. 2021-4). All animal handling and methods were performed according to the relevant guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Wang, W.-X.; Pan, K.; Tan, Q.; Guo, L.; Simpson, S.L. Estuarine Pollution of Metals in China: Science and Mitigation. Environ. Sci. Technol. 2014, 48, 9975–9976. [Google Scholar] [CrossRef]
- Pan, K.; Wang, W.-X. Trace Metal Contamination in Estuarine and Coastal Environments in China. Sci. Total Environ. 2012, 421–422, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, P.S. Trace Metal Concentrations in Aquatic Invertebrates: Why and so What? Environ. Pollut. 2002, 120, 497–507. [Google Scholar] [CrossRef]
- Hall, L.W.; Anderson, R.D.; Lewis, B.L.; Arnold, W.R. The Influence of Salinity and Dissolved Organic Carbon on the Toxicity of Copper to the Estuarine Copepod, Eurytemora affinis. Arch. Environ. Contam. Toxicol. 2008, 54, 44–56. [Google Scholar] [CrossRef]
- Weng, N.; Wang, W.-X. Variations of Trace Metals in Two Estuarine Environments with Contrasting Pollution Histories. Sci. Total Environ. 2014, 485, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Markich, S.J. Comparative Embryo/Larval Sensitivity of Australian Marine Bivalves to Ten Metals: A Disjunct between Physiology and Phylogeny. Sci. Total Environ. 2021, 789, 147988. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-G.; Wang, W.-X. Two-Compartment Toxicokinetic–Toxicodynamic Model to Predict Metal Toxicity in Daphnia magna. Environ. Sci. Technol. 2012, 46, 9709–9715. [Google Scholar] [CrossRef] [PubMed]
- Jager, T.; Albert, C.; Preuss, T.G.; Ashauer, R. General Unified Threshold Model of Survival—A Toxicokinetic-Toxicodynamic Framework for Ecotoxicology. Environ. Sci. Technol. 2011, 45, 2529–2540. [Google Scholar] [CrossRef]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of Dissolved Organic Matter on the Environmental Fate of Metals, Nanoparticles, and Colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Moffett, J.W.; Dupont, C. Cu Complexation by Organic Ligands in the Sub-Arctic NW Pacific and Bering Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 586–595. [Google Scholar] [CrossRef]
- Santore, R.C.; Toro, D.M.D.; Paquin, P.R.; Allen, H.E.; Meyer, J.S. Biotic Ligand Model of the Acute Toxicity of Metals. 2. Application to Acute Copper Toxicity in Freshwater Fish and Daphnia. Environ. Toxicol. Chem. 2001, 20, 2397–2402. [Google Scholar] [CrossRef]
- Paquin, P.R.; Gorsuch, J.W.; Apte, S.; Batley, G.E.; Bowles, K.C.; Campbell, P.G.C.; Delos, C.G.; Toro, D.M.D.; Dwyer, R.L.; Galvez, F.; et al. The Biotic Ligand Model: A Historical Overview. Comp. Biochem. Physiol. Part C 2002, 133, 3–35. [Google Scholar] [CrossRef]
- Ortego, L.S.; Benson, W.H. Effects of Dissolved Humic Material on the Toxicity of Selected Pyrethroid Insecticides. Environ. Toxicol. Chem. 1992, 11, 261–265. [Google Scholar] [CrossRef]
- Guo, L.; Coleman, C.H.; Santschi, P.H. The Distribution of Colloidal and Dissolved Organic Carbon in the Gulf of Mexico. Mar. Chem. 1994, 45, 105–119. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Li, L.; Zhen, G.; Lu, X.; Zhang, J.; Liu, H.; Zhou, Z.; Wu, Z.; Zhang, X. Prospects for Humic Acids Treatment and Recovery in Wastewater: A Review. Chemosphere 2023, 312, 137193. [Google Scholar] [CrossRef]
- Laglera, L.M.; Berg, C.M.G. van den Evidence for Geochemical Control of Iron by Humic Substances in Seawater. Limnol. Oceanogr. 2009, 54, 610–619. [Google Scholar] [CrossRef]
- Calza, P.; Vione, D.; Minero, C. The Role of Humic and Fulvic Acids in the Phototransformation of Phenolic Compounds in Seawater. Sci. Total Environ. 2014, 493, 411–418. [Google Scholar] [CrossRef]
- Pan, J.-F.; Wang, W.-X. Influences of Dissolved and Colloidal Organic Carbon on the Uptake of Ag, Cd, and Cr by the Marine Mussel Perna viridis. Environ. Pollut. 2004, 129, 467–477. [Google Scholar] [CrossRef]
- Pan, J.-F.; Wang, W.-X. Differential Uptake of Dissolved and Particulate Organic Carbon by the Marine Mussel Perna viridis. Limnol. Oceanogr. 2004, 49, 1980–1991. [Google Scholar] [CrossRef]
- Zhong, H.; Evans, D.; Wang, W.-X. Uptake of Dissolved Organic Carbon-Complexed 65Cu by the Green Mussel Perna viridis. Environ. Sci. Technol. 2012, 46, 2383–2390. [Google Scholar] [CrossRef]
- Lorenzo, J.I.; Nieto, O.; Beiras, R. Effect of Humic Acids on Speciation and Toxicity of Copper to Paracentrotus lividus Larvae in Seawater. Aquat. Toxicol. 2002, 58, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Understanding The Effects of Water Chemistry on the Toxicity of Copper in an Estuarine Clam Potamocorbula Laevis Using the Toxicokinetic-Toxicodynamic Model. Master’s Thesis, Xiamen University, Xiamen, China, 2017. [Google Scholar]
- Wang, W.-X.; Tan, Q.-G. Applications of Dynamic Models in Predicting the Bioaccumulation, Transport and Toxicity of Trace Metals in Aquatic Organisms. Environ. Pollut. 2019, 252, 1561–1573. [Google Scholar] [CrossRef]
- Ashauer, R.; Brown, C.D. Toxicodynamic Assumptions in Ecotoxicological Hazard Models. Environ. Toxicol. Chem. 2008, 27, 1817. [Google Scholar] [CrossRef] [PubMed]
- Fisheries and Fisheries Administration Bureau of the Ministry of Agriculture. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2023. [Google Scholar]
- Ke, Y.; Wang, W.-X. Metal Accumulation, Growth and Reproduction of Razor Clam Sinonovacula Constricta Transplanted in a Multi-Metal Contaminated Estuary. Sci. Total Environ. 2018, 636, 829–837. [Google Scholar] [CrossRef]
- Croteau, M.-N.; Luoma, S.N.; Topping, B.R.; Lopez, C.B. Stable Metal Isotopes Reveal Copper Accumulation and Loss Dynamics in the Freshwater Bivalve Corbicula. Environ. Sci. Technol. 2004, 38, 5002–5009. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Wang, W.-X. Biodynamics to Explain the Difference of Copper Body Concentrations in Five Marine Bivalve Species. Environ. Sci. Technol. 2009, 43, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-G.; Lu, S.; Chen, R.; Peng, J. Making Acute Tests More Ecologically Relevant: Cadmium Bioaccumulation and Toxicity in an Estuarine Clam under Various Salinities Modeled in a Toxicokinetic–Toxicodynamic Framework. Environ. Sci. Technol. 2019, 53, 2873–2880. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Wang, W.-X.; Tan, Q.-G. Revealing the Complex Effects of Salinity on Copper Toxicity in an Estuarine Clam Potamocorbula Laevis with a Toxicokinetic-Toxicodynamic Model. Environ. Pollut. 2017, 222, 323–330. [Google Scholar] [CrossRef]
- Sánchez-Marín, P.; Aierbe, E.; Lorenzo, J.I.; Mubiana, V.K.; Beiras, R.; Blust, R. Dynamic Modeling of Copper Bioaccumulation by Mytilus Edulis in the Presence of Humic Acid Aggregates. Aquat. Toxicol. 2016, 178, 165–170. [Google Scholar] [CrossRef]
- Kamunde, C.; MacPhail, R. Effect of Humic Acid during Concurrent Chronic Waterborne Exposure of Rainbow Trout (Oncorhynchus Mykiss) to Copper, Cadmium and Zinc. Ecotoxicol. Environ. Saf. 2011, 74, 259–269. [Google Scholar] [CrossRef]
- Lorenzo, J.I.; Beiras, R.; Mubiana, V.K.; Blust, R. Copper Uptake by Mytilus Edulis in the Presence of Humic Acids. Environ. Toxicol. Chem. 2005, 24, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Zitoun, R.; Clearwater, S.J.; Hassler, C.; Thompson, K.J.; Albert, A.; Sander, S.G. Copper Toxicity to Blue Mussel Embryos (Mytilus galloprovincialis) the Effect of Natural Dissolved Organic Matter on Copper Toxicity in Estuarine Waters. Sci. Total Environ. 2019, 653, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Gale, S.L.; Ragg, N.L.C.; Sander, S.G.; Burritt, D.J.; Benedict, B.; Le, D.V.; Villas-Bôas, S.G.; Alfaro, A.C. Metabolic Regulation of Copper Toxicity during Marine Mussel Embryogenesis. Metabolites 2023, 13, 838. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).