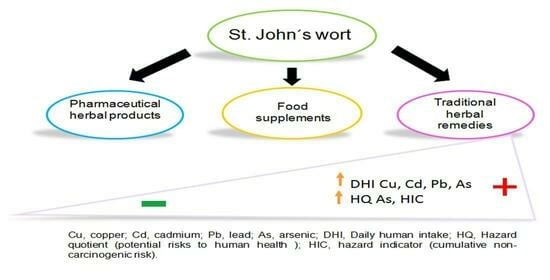
Human Health Risk Assessment of Arsenic and Other Metals in Herbal Products Containing St. John’s Wort in the Metropolitan Area of Mexico City
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Plant-Based Products
2.3. Processing of Herbal Products
2.4. Metal Contamination Status of Herbal Products Containing St. John’s Wort
2.5. Daily Human Intake Dose Calculation Equation for Cu, Pb, As, and Cd
2.6. Non-Carcinogenic Health Risk Assessment of Herbal Products
2.6.1. Human Health Risk Equation
2.6.2. Hazardousness Indicator Calculation
2.7. Statistical Analyses
3. Results
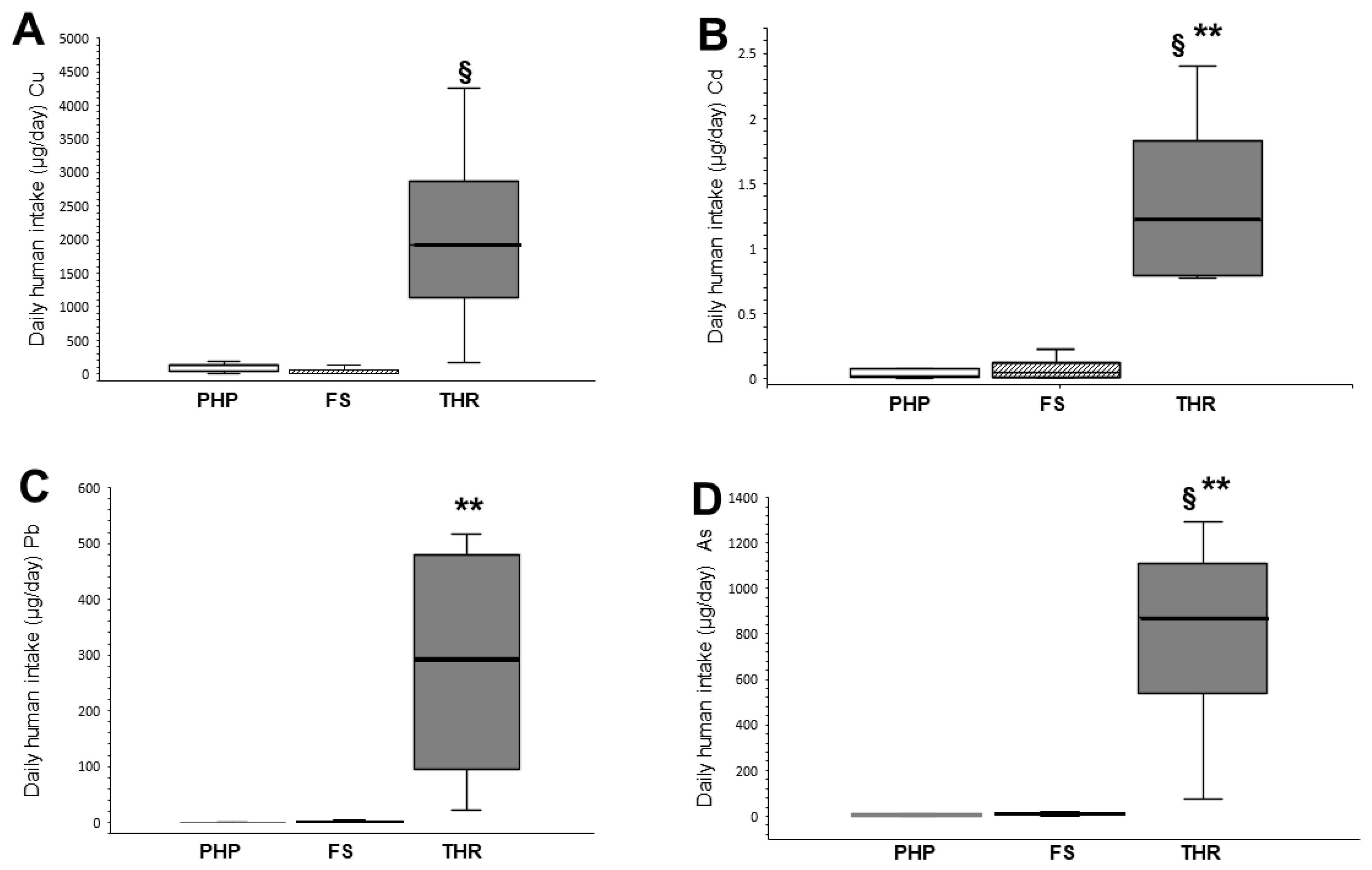
3.1. Daily Human Intake (DHI)
3.2. Non-Carcinogenic Health Risk Assessment of Herbal Products
3.2.1. Human Health Risk Estimation
3.2.2. Hazard Quotient
3.2.3. Hazard Indicator Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, G.; Purchase, D.; Mulla, S.I.; Dattatraya-Saratale, G.; Naresh-Bharagava, R. Phyroremediation of heavy metals-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2020, 249, 71–131. [Google Scholar] [CrossRef] [PubMed]
- Valdés Durán, A.; Aliaga, G.; Deckart, K.; Karas, C.; Cáceres, D.; Nario, A. The environmental geochemical baseline, background and sources of metal and metalloids present in urban, peri-urban and rural soils in the O’Higgins region, Chile. Environ. Geochem. Health 2022, 44, 3173–3189. [Google Scholar] [CrossRef] [PubMed]
- Okereafor, U.; Marhatha, M.; Mekuto, L.; Uche-Okereafor, N.; Sebola, T.; Mavumengwana, V. Toxic metals implications on agricultural soils, plants, animals, aquatic life and human health. Int. J. Environ. Res. Public Health 2020, 17, 2204. [Google Scholar] [CrossRef]
- Zhao, F.J.; Tang, Z.; Song, J.J.; Huang, X.Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phyroremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Hlihor, R.M. Medicinal plant growth in heavy metals contaminated soils: Responses to metal stress and induced risks to human health. Toxics 2022, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Mihaljev, Z.; Zivkov-Balos, M.; Cupić, Z.; Jaksić, S. Levels of some microelements and essential heavy metals in herbal teas in Siberia. Acta Pol. Pharm. 2014, 71, 385–391. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Crichton, R.R. Metal Toxicity—An introduction. In Metal Chelation in Medicine, 1st ed.; Crichton, R.R., Ward, R.J., Hider, R.C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–23. [Google Scholar]
- Aaseth, J.O. Toxic and essential metals in human health and disease 2021. Biomolecules 2022, 12, 1375. [Google Scholar] [CrossRef]
- Crisponi, G. Essential and toxic metal ions in human health and disease; from chemical features to clinical roles. Curr. Med. Chem. 2021, 18, 7187–7189. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometal 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwase, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy metal contaminations in herbal medicines: Determination, comprehensive risk assessments, and solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Melucci, D.; Locatelli, M. Toxic metals in herbal medicines. A review. Curr. Bioact. Compd. 2014, 10, 181–188. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E. Chronic cadmium exposure and cardiovascular disease in adults. J. Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 2020, 55, 726–729. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Ba, Q.; Wan, H. Effects of cadmium exposure on the immune system and immunoregulation. Front Immunol. 2021, 12, 695484. [Google Scholar] [CrossRef]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of heavy metals and recent advances in their removal: A review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- WHO Global Report on Traditional and Complementary Medicine 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?sequence=1&isAllowed=y (accessed on 27 January 2023).
- Fathinezhad, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Depression and Treatment with Effective Herbs. Curr. Pharm. Des. 2019, 25, 738–745. [Google Scholar] [CrossRef]
- Hypericum perforatum (St John’s Wort), CABI Compendium. CABI International. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.28268 (accessed on 1 February 2023).
- The Editors of Encyclopaedia Britannica. “Saint-John’s-Wort”. Encyclopedia Britannica, 31 May 2020. Available online: https://www.britannica.com/plant/Hypericum (accessed on 27 January 2023).
- WHO Monographs on Selected Medicinal Plants, Volume 2. 2002. Available online: http://apps.who.int/iris/bitstream/handle/10665/42052/9241545372.pdf?sequence=2 (accessed on 27 January 2023).
- Klemow, K.M.; Bartlow, A.; Crawford, J.; Kocher, N.; Shah, J.; Ritsick, M. Medical Attributes of St. John’s Wort (Hypericum perforatum). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Oxfordshire, UK, 2011. [Google Scholar]
- St. John’s Wort. [Updated 2022 May 19]. In StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557465/ (accessed on 26 January 2023).
- Nobakht, S.Z.; Akaberi, M.; Mohammadpour, A.H.; Tafazoli, M.A.; Emami, S.A. Hypericum perforatum: Traditional uses, clinical trials, and drug interactions. Iran. J. Basic Med. Sci. 2022, 25, 1045–1058. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.; Srđenović, B.; Grujić, N.; Rat, M.; Gavarić, N.; Anačkov, G.; Božin, B. St. John’s Wort (Hypericum Spp.)–Relation between the biological source and medical properties. In Hypericum: Botanical Sources, Medical Properties and Health Effects, 1st ed.; Davis, H.R., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 53–80. [Google Scholar]
- Rojas, P.; Ruiz-Sánchez, E.; Ríos, C.; Ruiz-Chow, A.; Reséndiz-Albor, A.A. A health risk assessment of lead and other metals in pharmaceutical herbal products and dietary supplements containing Ginkgo biloba in the Mexico City Metropolitan Area. Int. J. Environ. Res. Public Health 2021, 18, 8285. [Google Scholar] [CrossRef] [PubMed]
- California Office of Environmental Health Hazard Assessment. Proposition 65 No Significant Risk Levels (NSRLs) and Maximum Allowable Dose Levels (MADLs). (Revised 2021). Available online: https://oehha.ca.gov/proposition-65/general-info/current-proposition-65-no-significant-risk-levels-nsrls-maximum (accessed on 14 February 2023).
- American Herbal Product Association. AHPA Guidance Policy. Heavy Metals (Revised 2012). Available online: www.ahpa.org/Files/Document%20Library/AHPAGuidancePolicies/AHPA_Guidance_Heavy_Metals.pdf (accessed on 1 February 2023).
- Natural and Non-Prescription Health Products (Canada). Quality of Natural Health Products Guide. 2015. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/prodnatur/legislation/docs/eq-paq-eng.pdf (accessed on 14 February 2023).
- US EPA. Exposure Factors Handbook 2011 Edition (Final Report), 1st ed.; United States Environmental Protection Agency: Washington, DC, USA, 2011; pp. 1–56.
- US EPA. Integrated Risk Information System; United States Environmental Protection Agency: Washington, DC, USA, 2008. Available online: https://iris.epa.gov/AtoZ/?list_type=alpha (accessed on 5 February 2023).
- Morales Guerrero, J.C.; Camacho Parra, M.E.; García Morales, C.; Juárez Ramos, P.; Flores Sánchez, J.J. ¿Hay riesgo de efectos adversos por el consumo de nutrimentos a partir de productos alimenticios adicionados en México? Nutr. Hosp. 2018, 35, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Bourges, H.E.; Casanueva, E.; Rosado, J.L. Recomendaciones de ingestión de nutrimentos para la población mexicana. In Bases Fisiológicas, I. Apéndice 2, 1st ed.; Casanueva, E., Rosado, J.L., Eds.; Editorial Médica Panamericana: Mexico City, Mexico, 2005; pp. 1–372. [Google Scholar]
- Thomford, N.E.; Senthebane, D.S.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Jaiswal, A.; Verma, A.; Jaiswal, P. Detrimental effects of heavy metals in soil, plants, and aquatic ecosystems and in humans. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 183–197. [Google Scholar] [CrossRef] [PubMed]
- García-Rico, I.; Leyva-Pérez, J.; Jara-Marini, M.E. Content and daily intake of copper, zinc, lead, cadmium, and mercury from dietary supplements in Mexico. Food Chem. Toxicol. 2007, 45, 1599–1605. [Google Scholar] [CrossRef]
- Tschinkel, P.F.S.; Melo, E.S.P.; Pereira, H.S.; Silva, K.R.N.; Arakaki, D.G.; Lima, N.V.; Fernandes, M.R.; Leite, L.C.S.; Melo, E.S.P.; Melnikov, P.; et al. The hazardous level of heavy metals in different medicinal plants and their decoctions in water: A public health problem in Brazil. Biomed. Res. Int. 2020, 2020, 1465051. [Google Scholar] [CrossRef]
- Mikulski, M.A.; Wichman, M.D.; Simmons, D.L.; Pham, A.N.; Clottey, V.; Fuortes, L.J. Toxic metals in ayurvedic preparations from a public health lead poisoning cluster investigation. Int. J. Occup. Environ. Health 2017, 23, 187–192. [Google Scholar] [CrossRef]
- Harris, E.S.; Cao, S.; Littlefield, B.A. Heavy metal and pesticide content in commonly prescribed individual raw Chinese Herbal Medicines. Sci. Total Environ. 2011, 409, 4297–4305. [Google Scholar] [CrossRef]
- Li, X.; Chi, W.; Tian, H. Probabilistic ecological risk assessment of heavy metals in western Laizhou Bay, Shandong Province, China. PLoS ONE 2018, 14, e0213011. [Google Scholar] [CrossRef]
- Singh, N.K.; Raghubanshi, A.S.; Upadhyay, A.K. Arsenic and other heavy metal accumulation in plants and algae growing naturally in contaminated area of West Bengal, India. Ecotox. Environ. Saf. 2016, 130, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Mulaudzi, R.B.; Tshikalange, T.E.; Olowoyo, J.O. Antimicrobial activity, cytotoxicity evaluation and heavy metal content of five commonly used South African herbal mixtures. South Afr. J. Bot. 2017, 112, 314–318. [Google Scholar] [CrossRef]
- Kin, K.A.; Dickson, R.; Amponsah, I. The heavy metal contents of some selected medicinal plants sampled from different geographical locations. Pharmacogn. Res. 2013, 5, 103. [Google Scholar] [CrossRef]
- Leal, A.S.; Prado, G.; Gomes, T.C.B. Determination of metals in medicinal plants highly consumed in Brazil. Braz. J. Pharm. Sci. 2013, 499, 599–607. [Google Scholar] [CrossRef]
- Yee, S.K.; Chu, S.S.; Xu, Y.M.; Choo, P.L. Regulatory control of Chinese proprietary medicines in Singapore. Health Policy 2005, 71, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Alinia-Ahandani, E.; Sheydaei, M.; Akram, M.; Selamoglu, Z.; Alizadeh-Terepoei, Z.; Alinia-Ahandani, M. Heavy metals concentrations in some roadsides with different traffic volumes in Rasht City-Iran. Op. Acc. J. Bio. Sci. Res. 2021, 7, 1–4. [Google Scholar] [CrossRef]
- Alinia-Ahandani, E.; Alizadeh-Terepoei, Z.; Sheydaei, M.; Peysepar-Balalami, F. Assessment of soil on some heavy metals and its pollution in Roodsar-Iran. Biomed. J. Sci. Tech. Res. 2020, 28, 21977–21979. [Google Scholar] [CrossRef]
- Asiminicesei, D.-M.; Vasilachi, I.C.; Gavrilescu, M. Heavy metal contamination of medicinal plants and potential implications on human health. Rev. Chim. 2020, 71, 16–36. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Han, H.; Du, R.; Wang, X. Physiological and molecular mechanisms of plant responses to copper stress. Int. J. Mol. Sci. 2022, 23, 12950. [Google Scholar] [CrossRef]
- Chen, Y.; Moore, K.L.; Miller, A.J.; McGrath, S.P.; Ma, J.F.; Zhao, F.-J. The role of nodes in arsenic storage and distribution in rice. J. Exp. Bot. 2015, 66, 3717–3724. [Google Scholar] [CrossRef]
- Moore, K.L.; Chen, Y.; van de Meene, A.M.L.; Hughes, L.; Liu, W.J.; Geraki, T.; Mosselmans, F.; McGrath, S.P.; Grovenor, C.; Zhao, F.J. Combined NanoSIMS and synchrotron X-ray fluorescence reveals distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol. 2014, 201, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Skuza, L.; Szucko-Kaciuba, I.; Filip, E.; Bozek, I. Natural molecular mechanisms of plant hyperaccumulation and hypertolerance heavy metals. Int. J. Mol. Sci. 2022, 23, 9335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Z.; Zhang, Y.; Wei, Y.; Jiang, Z. Effects of lead stress on the growth, physiology, and cellular structure of privet seedings. PLoS ONE 2018, 13, e0191139. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Kong, Y.C.; Zain, N.A.M. Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant Sambung nyawa (Gynura procumbens (Lour.) Merr). Molecules 2017, 22, 1623. [Google Scholar] [CrossRef]
- Sobariu, D.L.; Fertu, D.I.T.; Diaconu, M.; Pavel, L.V.; Hlihor, R.M.; Dragoi, E.N.; Curteanu, S.; Lenz, M.; Corvini, P.F.X.; Gavrilescu, M. Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. N. Biotechnol. 2017, 39 Pt A, 125–134. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Nica Badea, D. Determination of potentially toxic heavy metals (Pb, Hg, Cd) in popular medicinal herbs in the coal power plant tea. Rev. Chim. 2015, 66, 1132–1136. [Google Scholar]
- Diaconu, D.; Diaconu, R.; Navrotescu, T. Estimation of heavy metals in medicinal plants and their infusions. Ovidius Univ. Ann Chem. 2012, 23, 115–120. [Google Scholar] [CrossRef][Green Version]
- Muntean, N.; Muntean, E.; Creta, C.; Duda, M. Heavy metals in some commercial herbal teas. ProEnvironment 2013, 6, 591–594. [Google Scholar]
- Ghazala, Y.; Fizza, I.; Muniba, I.; Vania, M. Monitoring and risk assessment due to presence of heavy metals and pesticides in tea samples. Food Sci. Technol. 2018, 38, 625–628. [Google Scholar] [CrossRef]
- Miroslawski, J.; Paukszto, A. Determination of the cadmium, chromium, nickel and lead ions relays in selected polish medicinal plants and their infusion. Biol. Trace Elem. Res. 2018, 182, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Samuel Nakamura, K.; Hodge, F.S. Occurrence and risk of metal(loid)s in Thelesperma megapotamicum tea plant. Plants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.J.; Kumar, M.; Ramteke, P.W.; Gupta, R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2021, 28, 43. [Google Scholar] [CrossRef] [PubMed]
- Leblebici, S.; Bahtiyar, S.D.; Özyurt, M.S. Determination of the amount of heavy metal in some medicinal plants sold in herbalist in Kütahya. DPU J. Grad. Sch. Nat. Appl. Sci. Mehmet Akif Ersoy Univ. 2012, 29, 1–6. [Google Scholar]
- Ergün, N.; Yolcu, H.; Karanlik, S.; Dikkaya, E. Heavy metal accumulation and mineral contents of some plants on Amanos Mountains (Hatay), Turkey. BIBAD Biyol. Bilim. Arast. Derg. 2010, 3, 121–127. [Google Scholar]
- Bin, C.; Xiaoru, W.; Lee, F.S.C. Pyrolisis coupled with atomic absorption spectrometry for determination of mercury in Chinese medicinal materials. Anal. Chim. Acta 2001, 447, 161–169. [Google Scholar] [CrossRef]
- Geneva, M. Metal uptake by Saint John’s wort (Hypericum perforatum L.) grown on industrially polluted soil. In Proceedings of the 6th Conference on Aromatic and Medicinal Plants of Southeast European Countries, Antalaya, Turkey, 18–22 April 2010. [Google Scholar]
- Đurović, D.; Bulat, Z.; Buha, A.; Matovic, V. Cadmium, mercury and lead in Hypericum perforatum L. collected in Western Serbia. In Proceedings of the 16th International Conference on Heavy Metals in the Environment, Rome, Italy, 23–27 September 2012. [Google Scholar]
- Fischer, A.; Brodziak-Dopierała, B.; Loska, K.; Stojko, J. The assessment of toxic metals in plants used in cosmetics and cosmetology. Int. J. Environ. Res. Public Health 2017, 14, 1280. [Google Scholar] [CrossRef]
- Barbulescu, A.; Berbes, L.; Dumitriu, C.S. Impact of soil pollution on Melliferous plants. Toxics 2022, 10, 239. [Google Scholar] [CrossRef]
- Scotti, F.; Löbel, K.; Booker, A.; Heinrich, M. St. John’s wort (Hypericum perforatum) products- How variable is the primary material? Front. Plant Sci. 2019, 9, 1973. [Google Scholar] [CrossRef]
- Zobayed, S.; Saxena, P.K. Production of St. John’s wort plants under controlled environment for maximizing biomass and secondary metabolites. In Vitro Cell. Dev. Biol. Plant 2004, 40, 108–114. [Google Scholar] [CrossRef]
- Shelar, M.; Gawade, V.; Bhujbal, S. A review on heavy metal contamination in herbals. J. Pharm. Res. Int. 2021, 33, 7–16. [Google Scholar] [CrossRef]
- Clemens, S. Safer food through plant science: Reducing toxic element accumulation in crops. J. Exp. Bot. 2019, 70, 5537–5557. [Google Scholar] [CrossRef]
- Perrelli, M.; Wu, R.; Liu, D.; Lucchini, R.G.; Del Bosque-Plata, L.; Vergare, M.J.; Akhter, M.P.; Ott, J.; Cragnoli, C. Heavy metals as risk factors for human diseases-a Bayesian network approach. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 9275–9310. [Google Scholar] [CrossRef]
- Masarovičová, E.; KráĬov, K.; Kummerová, M. Principles of classification of medicinal plants as hyperaccumulators or excluders. Acta Physiol. Plant. 2010, 32, 823–829. [Google Scholar] [CrossRef]
- Norton, G.J.; Williams, P.N.; Adomako, E.E.; Price, A.H.; Zhu, Y.; Zhao, F.-J.; McGrath, S.; Deacon, C.M.; Villada, A.; Sommella, A.; et al. Lead rice: Analysis of baseline lead levels in market and field collected rice grains. Sci. Total Environ. 2014, 485, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.O.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problema leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]

| Pharmaceutical Herbal Products | ||||
|---|---|---|---|---|
| Product Code | Origin * | Label Statement (Main Compounds) | Formulation Presentation | Therapeutic Uses |
| P1 | Mexico | St. John’s wort (Hypericum perforatum) | Herbal drug (drops) | Treatment of depressive state |
| P2 | Mexico | St. John´s wort (Hypericum perforatum) 150 mg (dry extract of the aerial parts), equivalent to not less than 0.40 mg and not more than 0.54 mg of Hypericin | Herbal drug (tablets) | Auxiliary in the treatment of mild and moderate depressive states |
| P3 | Germany | St. John´s wort (Hypericum perforatum) (Hypericum) 300 mg (flower dry extract) | Herbal drug (tablets) | Auxiliary in the treatment of mild and moderate depressive states |
| P4 | Spain | St. John´s wort (Hypericum perforatum) (Hypericum) 275 mg (flower powder) | Herbal drug (capsules) | Auxiliary in the treatment of nervousness, anxiety, mild depression and sleep disorders |
| P5 | USA | St. John´s wort (Hypericum perforatum) (Hypericum) 300 mg (dry extract) | Herbal drug (tablets) | Treatment of depressive states (listlessness, apathy, loss of self-esteem) and anxiety |
| P6 | Switzerland | St. John´s wort (Hypericum perforatum) 250 mg (dry extract) equivalent to 0.5 mg hypericin | Herbal drug (tablets) | Antidepressant for the treatment of mild and moderate depression, in the treatment of transitory alterations of mood, sadness, melancholy, discouragement, lack of personal interest |
| P7 | Mexico | St. John´s wort—Artemisia vulgaris | Herbal drug (drops) | Depression |
| Food Supplements | ||||
| Product Code | Origin * | Label Statement (Main Compounds) | Formulation Presentation | Therapeutic Uses |
| S1 | Mexico | St. John´s wort (Perforatum SD) Tribulus terrestres Stachys officinalis Euphorbi lathyris | Dietary supplement (drops) | Depression treatment assistant |
| S2 | Mexico | St. John’s wort (Tagetes lucida) Tribulus terrestris Tumera aphrodisiaca L. Curcuma longa Apple vinegar | Dietary supplement (drops) | Anxiety |
| S3 | Mexico | St. John’s wort (Hypericum perforatum) standardized to 0.3% Hypericin | Dietary supplement (drops) | Natural antidepressant, it promotes a general sense of well-being. It is a supplement to strengthen the nervous system |
| S4 | Mexico | St. John’s wort (Hypericum perforatum L.) | Dietary supplement (capsules) | Helps in the control of anxiety, anguish, depression, and irritability |
| S5 | Mexico | St. John’s wort (Hiperycum perforatum), water, cane alcohol | Dietary supplement (drops) | Help in stress management Help in falling asleep |
| S6 | Mexico | St. John’s wort, White Sapote, Valerian Root, Maca, Royal Jelly, California Damiana | Dietary supplement (drops) | Helps in the control of pressure, anxiety, lack of sleep, and anguish |
| S7 | Mexico | St. John’s wort (Tagetes lucida) | Dietary supplement (tablets) | Auxiliary in the treatment of stress, depression, nervousness, and anxiety |
| S8 | Mexico | St. John’s wort (Hypericum perforatum) cbp vehicle 50 mL | Dietary supplement (drops) | Natural antidepressant, auxiliary in the treatment of anxiety, melancholy and nervousness |
| S9 | Mexico | St. John´s wort (Hyperucum perforatum), White Sapote, Passion flower | Dietary supplement (capsules) | Helps in the control of pressure, anxiety, lack of sleep, anguish and headache |
| S10 | Mexico | St. John’s wort, California Damiana, White hawthorn, guarana, zarzapilla, royal jelly Vitamins: A, B1, B2, B3, B6; glutamic acid, calcium | Dietary supplement (tablets) | Removes anguish and anxiety due to depression (antidepressant) |
| S11 | Mexico | St. John´s wort (Hypericum perforatum), Turnera difusa, Crataegus oxyacantha l, flower pollen, honey bee, Lepidium meyenii, Paullinia cupana, Smilax aspera, royal jelly, vitamin A (Palmitate), vitamin B1 (Thiamine), vitamin B2 (Riboflavin), vitamin B3 (Nicotinamide), vitamin B6 (Pyridoxine), vitamin E (D alpha tocopherol), glutamic acid, calcium pantothenate | Dietary supplement (tablets) | Calms and soothes anxiety, relieves headaches, acts on the nervous system, energizing, helps in prostate problems, helps in the immune system, gives energy |
| S12 | Mexico | St. John’s wort (Hypericum perforatum) leaf and stem, gelatin, magnesium stearate | Dietary supplement (capsules) | Treatment for depression and anxiety |
| Traditional Herbal Remedies | ||||
| Product Code | Origin * | Label Statement (Main Compounds) | Formulation Presentation | Therapeutic Uses |
| T1 | Mexico | St. John´s wort (Tagetes lucida) | Traditional herbal remedy (leaves) | Auxiliary in the treatment of nervous depression, anguish and stress |
| T2 | Mexico | St. John’s wort | Traditional herbal remedy (leaves and stems) | Nerves |
| T3 | Mexico | St. John’s wort | Traditional herbal remedy (leaves and stems) | Nerves and anxiety |
| T4 | México | St. John’s wort | Traditional herbal remedy (leaves and stems) | Depression |
| Daily Human Intake (µg/Day) | Pharmaceutical Herbal Products n = 7 | Food Supplements n = 12 | Traditional Herbal Remedies n = 4 | ||||
|---|---|---|---|---|---|---|---|
| Metals | Mean ± SD | Median (25–75 Percentile) | Mean ± SD | Median (25–75 Percentile) | Mean ± SD | Median (25–75 Percentile) | p |
| Cu | 99.07 ± 71.56 | 127.04 (50.64–138.72) | 61.91 ± 96.77 | 12.7 (7.26–86.77) | 2067 ± 1721.6 | 1923.2 (809–3325) | 0.016 * 1 a 0.071 b 0.013 c |
| Cd | 0.08 ± 0.13 | 0.02 (0.01–0.08) | 0.10 ± 0.15 | 0.05 (0.01–0.13) | 1.40 ± 0.78 | 1.22 (0.79–2.02) | 0.006 * 1 a 0.006 b 0.021 c |
| Pb | 0.84 ± 0.72 | 0.76 (0.28–1.11) | 2.19 ± 1.16 | 2.3 (1.09–3) | 281.5 ± 247.11 | 294 (71–492) | 0.001 * 0.126 a 0.001 b 0.061 c |
| As | 4.29 ± 4.17 | 3 (1.10–6.88) | 9.42 ± 5.26 | 8.81 (5.01–13.23) | 776.6 ± 530.04 | 871.2 (382–1171.2) | 0.002 * 0.277 a 0.001 b 0.046 c |
| Pharmaceutical Herbal Products | ||||
|---|---|---|---|---|
| Product Code | EHDI (mg/kg/day) Cu | EHDI (mg/kg/day) Cd | EHDI (mg/kg/day) Pb | EHDI (mg/kg/day) As |
| P1 | 3.135 × 10−5 | 4.615 × 10−8 | 3.877 × 10−6 | 2.448 × 10−5 |
| P2 | 1.996 × 10−3 | 2.749 × 10−7 | 2.092 × 10−5 | 1.272 × 10−4 |
| P3 | 2.869 × 10−3 | 1.231 × 10−6 | 3.352 × 10−5 | 1.704 × 10−4 |
| P4 | 1.527 × 10−3 | 5.785 × 10−6 | 1.332 × 10−5 | 4.609 × 10−5 |
| P5 | 2.272 × 10−3 | 1.107 × 10−6 | 1.177 × 10−5 | N.D. |
| P6 | 1.954 × 10−3 | 9.231 × 10−8 | 4.677 × 10−6 | 8.446 × 10−5 |
| P7 | 1.883 × 10−5 | 1.231 × 10−7 | 2.585 × 10−6 | 9.4 × 10−6 |
| Food Supplements | ||||
| Product Code | EHDI (mg/kg/day) Cu | EHDI (mg/kg/day) Cd | EHDI (mg/kg/day) Pb | EHDI (mg/kg/day) As |
| S1 | 3.134 × 10−5 | 9.231 × 10−8 | 1.408 × 10−5 | 7.449 × 10−5 |
| S2 | 7.786 × 10−5 | 1.077 × 10−7 | 1.943 × 10−5 | 3.708 × 10−5 |
| S3 | 1.404 × 10−4 | 1.231 × 10−7 | 3.808 × 10−5 | 5.463 × 10−5 |
| S4 | 7.107 × 10−4 | 1.815 × 10−6 | 4.078 × 10−5 | 1.958 × 10−4 |
| S5 | 1.862 × 10−4 | 2.923 × 10−7 | 4.292 × 10−5 | 7.975 × 10−5 |
| S6 | 2.044 × 10−4 | 1.692 × 10−7 | 1.192 × 10−5 | 1.511 × 10−4 |
| S7 | 2.306 × 10−4 | 2.323 × 10−6 | 4.928 × 10−5 | 2.901 × 10−4 |
| S8 | 8.595 × 10−5 | 1.231 × 10−6 | 7.846 × 10−6 | 9.757 × 10−5 |
| S9 | 1.959 × 10−3 | 7.969 × 10−6 | 3.257 × 10−5 | 1.202 × 10−4 |
| S10 | 1.373 × 10−4 | 3.231 × 10−7 | 2.901 × 10−5 | 1.752 × 10−4 |
| S11 | 3.008 × 10−3 | 1.385 × 10−6 | 5.283 × 10−5 | 2.513 × 10−4 |
| S12 | 4.657 × 10−3 | 3.462 × 10−6 | 6.566 × 10−5 | 2.112 × 10−4 |
| Traditional Herbal Remedies | ||||
| Product Code | EHDI (mg/kg/day) Cu | EHDI (mg/kg/day) Cd | EHDI (mg/kg/day) Pb | EHDI (mg/kg/day) As |
| T1 | 2.548 × 10−3 | 1.192 × 10−5 | 3.385 × 10−4 | 1.12 × 10−3 |
| T2 | 6.548 × 10−2 | 2.52 × 10−5 | 7.2 × 10−3 | 1.986 × 10−2 |
| T3 | 3.683 × 10−2 | 1.231 × 10−5 | 7.938 × 10−3 | 1.063 × 10−2 |
| T4 | 2.234 × 10−2 | 3.692 × 10−5 | 1.846 × 10−3 | 1.617 × 10−2 |
| Pharmaceutical Herbal Products | |||
|---|---|---|---|
| Product Code | HQ Cd | HQ As | HIC |
| P1 | 0.00009 | 0.08159 | 0.08168 |
| P2 | 0.00055 | 0.42385 | 0.42440 |
| P3 | 0.00246 | 0.56815 | 0.57062 |
| P4 | 0.01157 | 0.15364 | 0.16521 |
| P5 | 0.00222 | N.A. | 0.00222 |
| P6 | 0.00018 | 0.28154 | 0.28172 |
| P7 | 0.00025 | 0.03133 | 0.03158 |
| Food Supplements | |||
| S1 | 0.00018 | 0.24831 | 0.24849 |
| S2 | 0.00022 | 0.12359 | 0.12381 |
| S3 | 0.00025 | 0.18210 | 0.18235 |
| S4 | 0.00363 | 0.65262 | 0.65625 |
| S5 | 0.00058 | 0.26585 | 0.26643 |
| S6 | 0.00034 | 0.50359 | 0.50393 |
| S7 | 0.00465 | 0.96708 | 0.97172 |
| S8 | 0.00246 | 0.32523 | 0.32769 |
| S9 | 0.01594 | 0.40056 | 0.41650 |
| S10 | 0.00065 | 0.58410 | 0.58475 |
| S11 | 0.00277 | 0.83774 | 0.84051 |
| S12 | 0.00692 | 0.70410 | 0.71103 |
| Traditional Herbal Remedies | |||
| T1 | 0.02385 | 3.73333 | 3.75718 |
| T2 | 0.05040 | 66.21538 | 66.26578 |
| T3 | 0.02462 | 35.44615 | 35.47077 |
| T4 | 0.07383 | 53.90769 | 53.98154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, P.; Ruiz-Sánchez, E.; Rojas, C.; García-Martínez, B.A.; López-Ramírez, A.M.; Osorio-Rico, L.; Ríos, C.; Reséndiz-Albor, A.A. Human Health Risk Assessment of Arsenic and Other Metals in Herbal Products Containing St. John’s Wort in the Metropolitan Area of Mexico City. Toxics 2023, 11, 801. https://doi.org/10.3390/toxics11090801
Rojas P, Ruiz-Sánchez E, Rojas C, García-Martínez BA, López-Ramírez AM, Osorio-Rico L, Ríos C, Reséndiz-Albor AA. Human Health Risk Assessment of Arsenic and Other Metals in Herbal Products Containing St. John’s Wort in the Metropolitan Area of Mexico City. Toxics. 2023; 11(9):801. https://doi.org/10.3390/toxics11090801
Chicago/Turabian StyleRojas, Patricia, Elizabeth Ruiz-Sánchez, Carolina Rojas, Betzabeth A. García-Martínez, Arely M. López-Ramírez, Laura Osorio-Rico, Camilo Ríos, and Aldo Arturo Reséndiz-Albor. 2023. "Human Health Risk Assessment of Arsenic and Other Metals in Herbal Products Containing St. John’s Wort in the Metropolitan Area of Mexico City" Toxics 11, no. 9: 801. https://doi.org/10.3390/toxics11090801
APA StyleRojas, P., Ruiz-Sánchez, E., Rojas, C., García-Martínez, B. A., López-Ramírez, A. M., Osorio-Rico, L., Ríos, C., & Reséndiz-Albor, A. A. (2023). Human Health Risk Assessment of Arsenic and Other Metals in Herbal Products Containing St. John’s Wort in the Metropolitan Area of Mexico City. Toxics, 11(9), 801. https://doi.org/10.3390/toxics11090801