Sex-Specific Effects of Short-Term Oral Administration of Food-Grade Titanium Dioxide Nanoparticles in the Liver and Kidneys of Adult Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Characterization
2.2. Animals and Treatment
- (1)
- Control, vehicle only (distilled water);
- (2)
- 1 mg/kg bw per day of TiO2 NPs;
- (3)
- 2 mg/kg bw per day of TiO2 NPs.
2.3. Serum Biomarkers
2.4. Histological and Histomorphometrical Analysis
2.5. Gene Expression Analysis
2.6. Data Analysis
3. Results
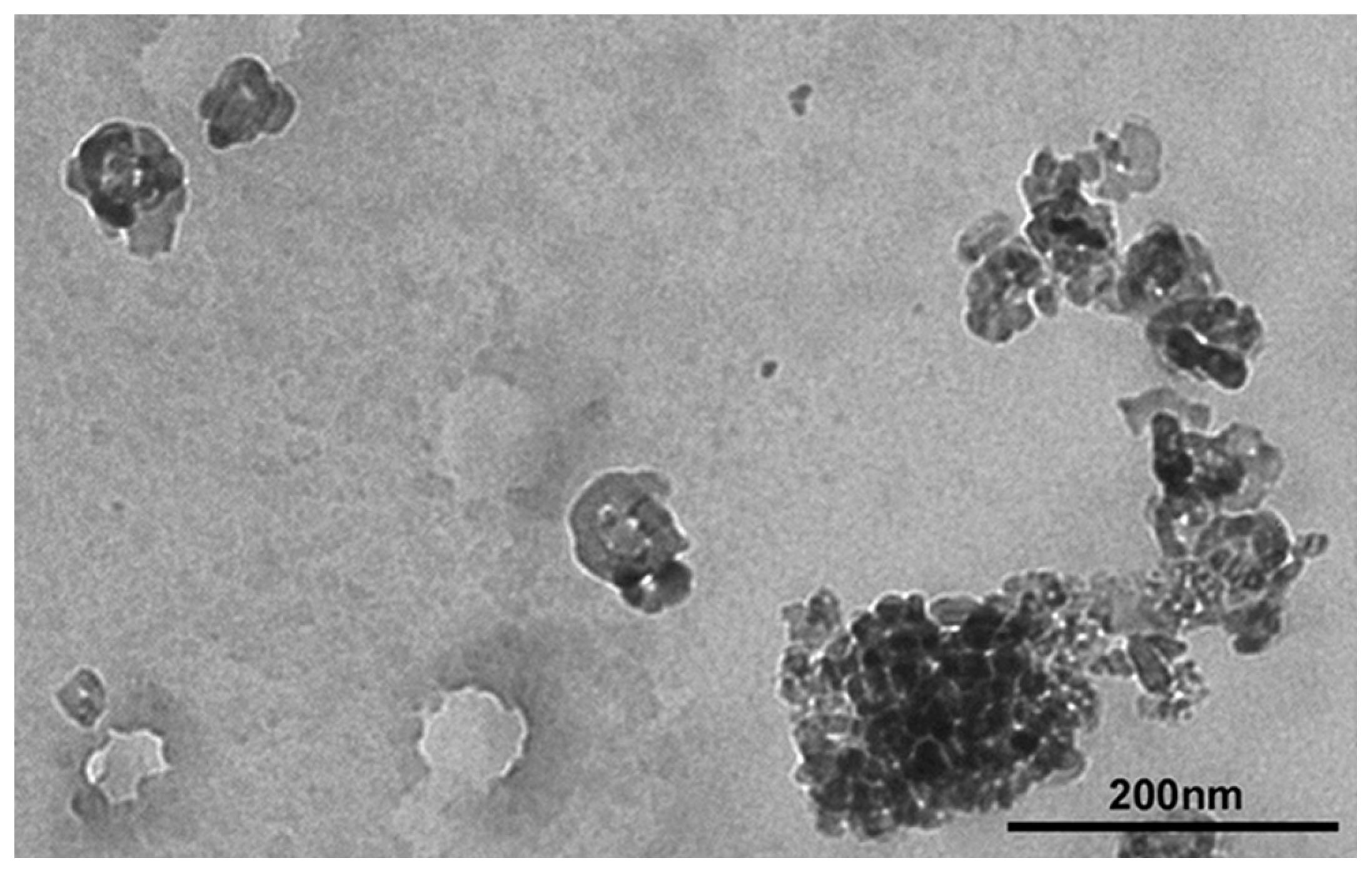
3.1. Characterization of TiO2 NP Suspensions
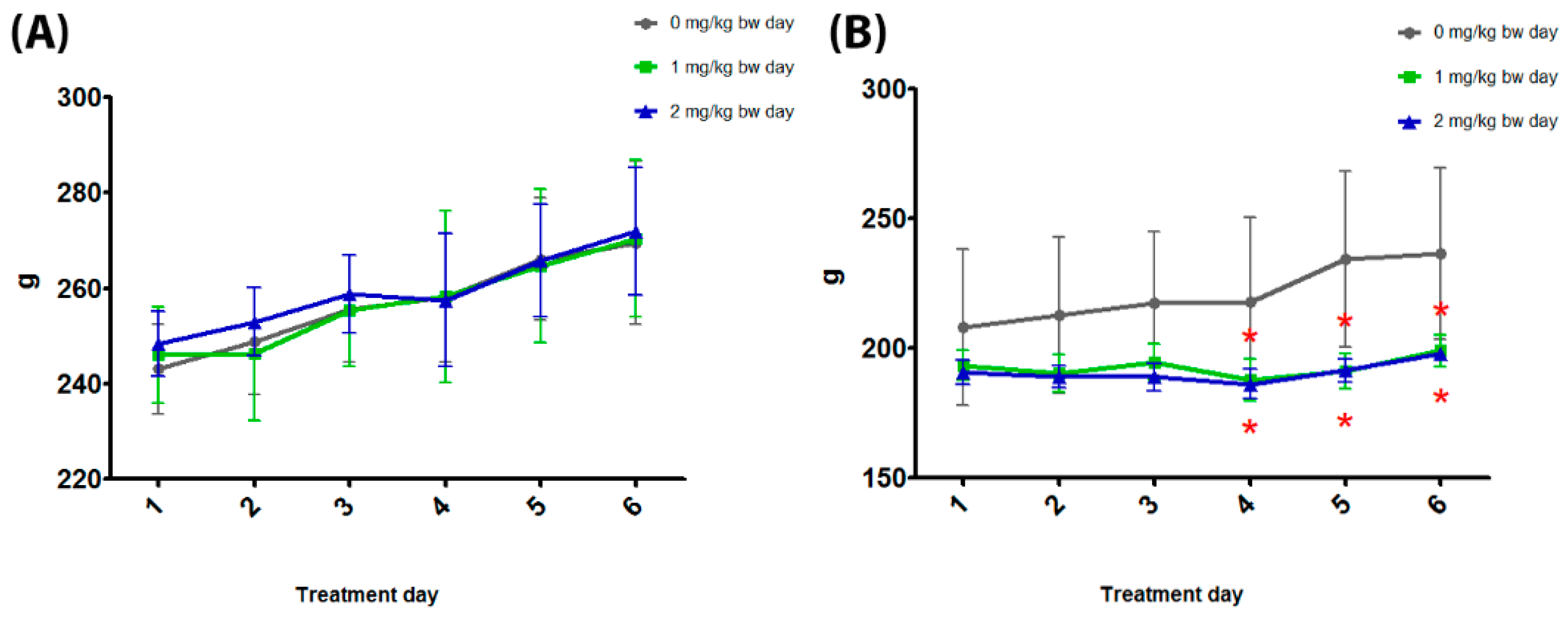
3.2. General Toxicity
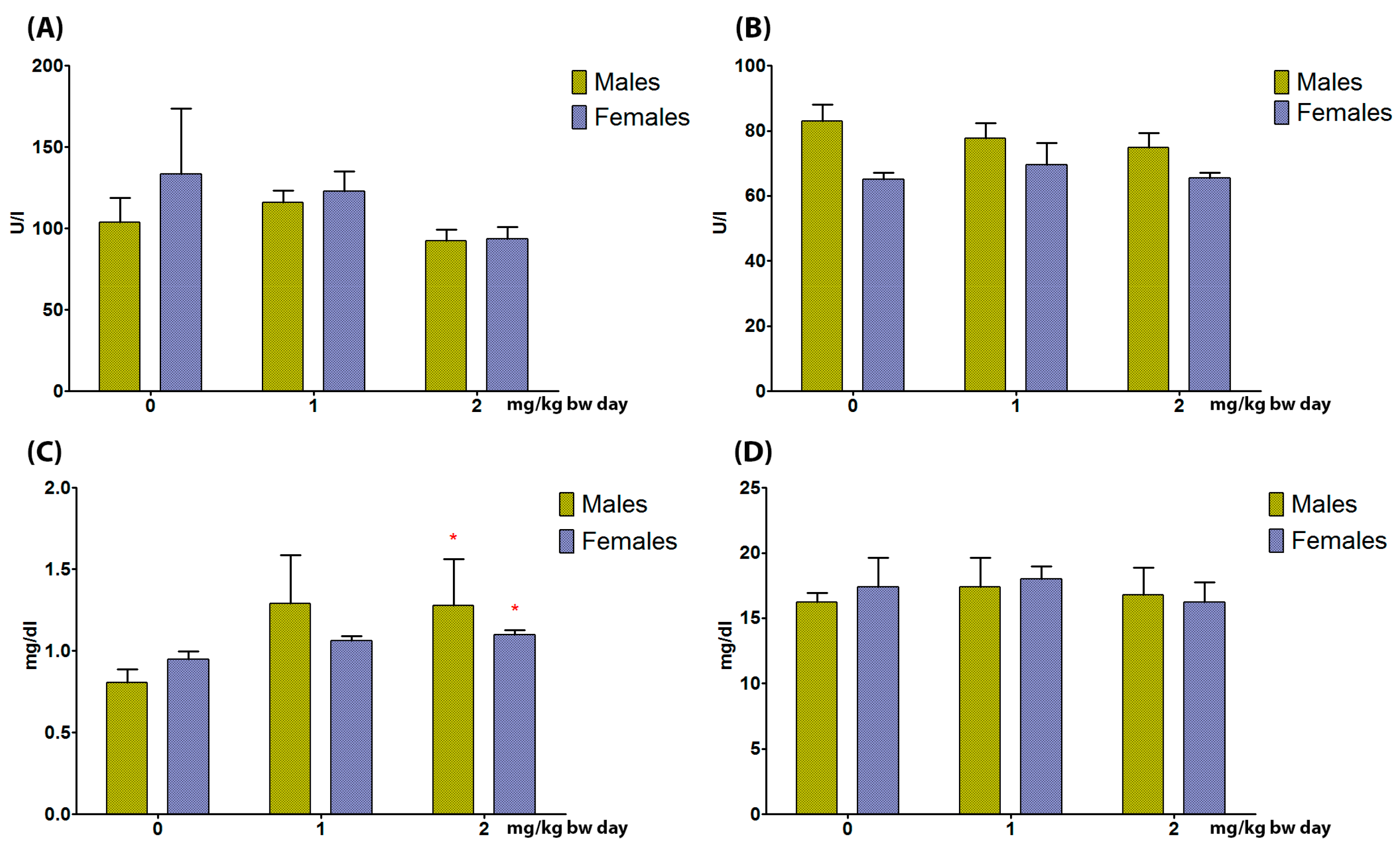
3.3. Serum Biomarkers
3.3.1. Liver
3.3.2. Kidneys
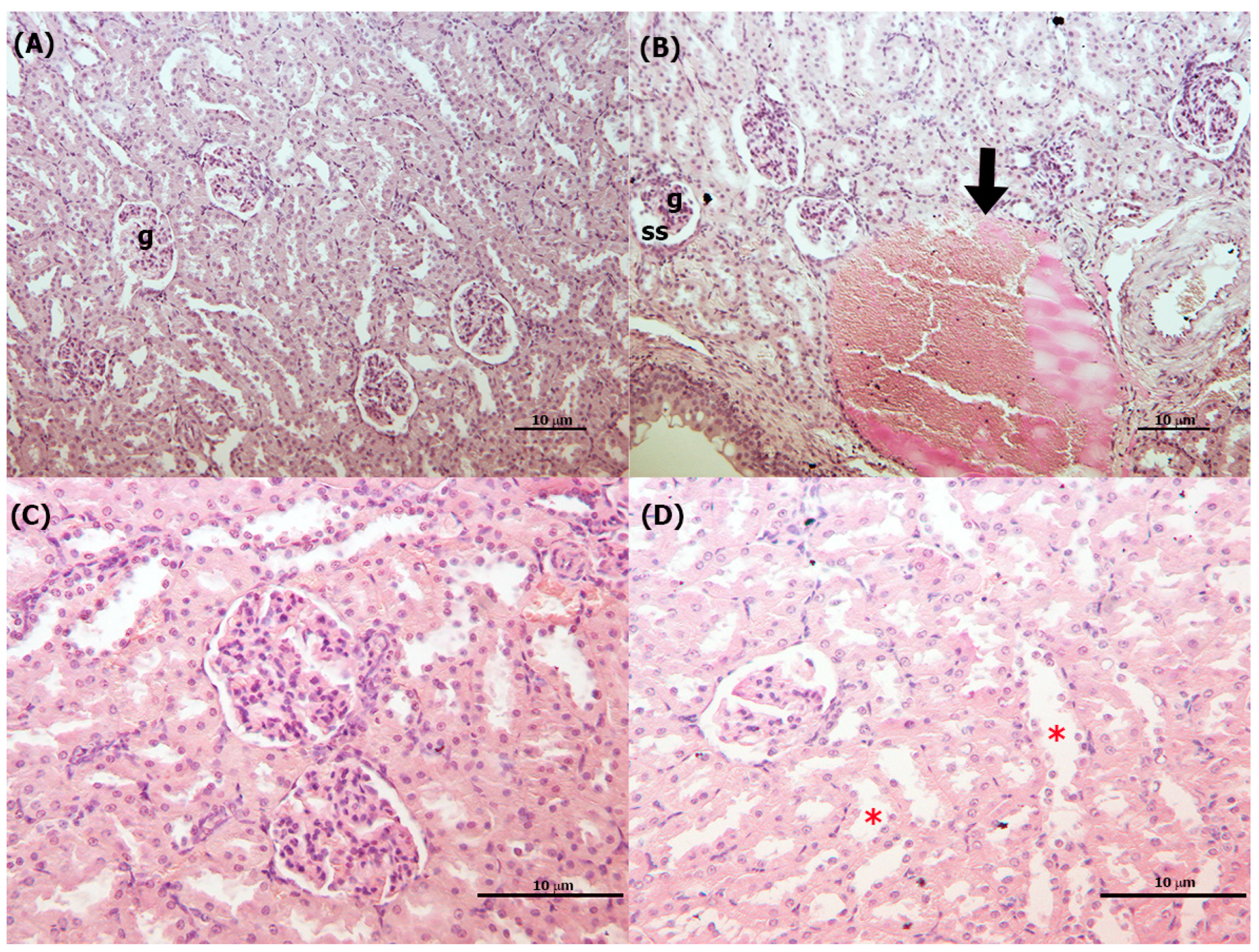
3.4. Histopathological Analysis
3.4.1. Liver
3.4.2. Kidneys
3.5. Gene Expression Analysis
3.5.1. Liver
3.5.2. Kidneys
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansoor, A.; Khurshid, Z.; Khan, M.T.; Mansoor, E.; Butt, F.A.; Jamal, A.; Palma, P.J. Medical and Dental Applications of Titania Nanoparticles: An Overview. Nanomaterials 2022, 12, 3670. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO(2) nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- Titanium Dioxide: Are There Health Risks? 2021. Available online: https://www.bfr.bund.de/en/titanium_dioxide__are_there_health_risks_-241091.html (accessed on 23 July 2023).
- EFSA. EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the french agency for food, environmental and occupational health and safety (ANSES). EFSA J. 2019, 17, e05714. [Google Scholar]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cheng, B.; Yang, Y.; Cao, A.; Liu, J.; Du, L.; Liu, Y.; Zhao, Y.; Wang, H. Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum. Small 2013, 9, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Flavourings, (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar]
- Commission Regulation (EU) 2022/63 of 14 January 2022 Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the Food Additive Titanium Dioxide (E 171) (Text with EEA Relevance); Official Journal of the European Union: Luxembourg, 2022.
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, srep40373. [Google Scholar] [CrossRef]
- Ammendolia, M.G.; Iosi, F.; Maranghi, F.; Tassinari, R.; Cubadda, F.; Aureli, F.; Raggi, A.; Superti, F.; Mantovani, A.; De Berardis, B. Short-term oral exposure to low doses of nano-sized TiO2 and potential modulatory effects on intestinal cells. Food Chem. Toxicol. 2017, 102, 63–75. [Google Scholar] [CrossRef]
- Tassinari, R.; Cubadda, F.; Moracci, G.; Aureli, F.; D’amato, M.; Valeri, M.; De Berardis, B.; Raggi, A.; Mantovani, A.; Passeri, D.; et al. Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: Focus on reproductive and endocrine systems and spleen. Nanotoxicology 2014, 8, 654–662. [Google Scholar] [CrossRef]
- Valentini, X.; Rugira, P.; Frau, A.; Tagliatti, V.; Conotte, R.; Laurent, S.; Colet, J.-M.; Nonclercq, D. Hepatic and Renal Toxicity Induced by TiO2 Nanoparticles in Rats: A Morphological and Metabonomic Study. J. Toxicol. 2019, 2019, 5767012. [Google Scholar] [CrossRef]
- Kim, W.Y.; Kim, J.; Park, J.D.; Ryu, H.Y.; Yu, I.J. Histological Study of Gender Differences in Accumulation of Silver Nanoparticles in Kidneys of Fischer 344 Rats. J. Toxicol. Environ. Health Part A 2009, 72, 1279–1284. [Google Scholar] [CrossRef]
- Liang, G.; Pu, Y.; Yin, L.; Liu, R.; Ye, B.; Su, Y.; Li, Y. Influence of Different Sizes of Titanium Dioxide Nanoparticles on Hepatic and Renal Functions in Rats with Correlation to Oxidative Stress. Toxicol. Environ. Health Part A 2009, 72, 740–745. [Google Scholar] [CrossRef]
- Tassinari, R.; Narciso, L.; Tait, S.; Busani, L.; Martinelli, A.; Virgilio, A.D.; Carli, F.; Deodati, A.; Rocca, C.; Maranghi, F. Juvenile Toxicity Rodent Model to Study Toxicological Effects of Bisphenol A (BPA) at Dose Levels Derived From Italian Children Biomonitoring Study. Toxicol. Sci. Off. J. Soc. Toxicol. 2019, 173, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, Y.; Scherer, N.; Grundner-Culemann, F.; Lehtimäki, T.; Mishra, B.H.; Raitakari, O.T.; Nauck, M.; Eckardt, K.-U.; Sekula, P.; et al. GCKD investigators, Genetics of osteopontin in patients with chronic kidney disease: The German Chronic Kidney Disease study. PLoS Genet. 2022, 18, e1010139. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Goligorsky, M.S. Role of VEGF in kidney development, microvascular maintenance and pathophysiology of renal disease. Korean J. Intern. Med. 2003, 18, 66–75. [Google Scholar]
- Su, H.; Lei, C.-T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 8, 405. [Google Scholar]
- Sucajtys-Szulc, E.; Goyke, E.; Korczynska, J.; Stelmanska, E.; Rutkowski, B.; Swierczynski, J. Chronic food restriction differentially affects NPY mRNA level in neurons of the hypothalamus and in neurons that innervate liver. Neurosci. Lett. 2008, 433, 174–177. [Google Scholar] [CrossRef]
- Winaver, J.; Abassi, Z. Role of neuropeptide Y in the regulation of kidney function. In NPY Family of Peptides in Neurobiology, Cardiovascular and Metabolic Disorders: From Genes to Therapeutics; Springer: Basel, Switzerland, 2006; pp. 123–132. [Google Scholar]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. In OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Tassinari, R.; Di Felice, G.; Butteroni, C.; Barletta, B.; Corinti, S.; Cubadda, F.; Aureli, F.; Raggi, A.; Narciso, L.; Tait, S.; et al. Maranghi, Hazard identification of pyrogenic synthetic amorphous silica (NM-203) after sub-chronic oral exposure in rat: A multitarget approach. Food Chem. Toxicol. 2020, 137, 111168. [Google Scholar] [CrossRef]
- Narciso, L.; Coppola, L.; Lori, G.; Andreoli, C.; Zjino, A.; Bocca, B.; Petrucci, F.; Di Virgilio, A.; Martinelli, A.; Tinari, A.; et al. Genotoxicity, biodistribution and toxic effects of silver nanoparticles after in vivo acute oral administration. NanoImpact 2020, 18, 100221. [Google Scholar]
- Tassinari, V.; De Gennaro, V.; La Sala, G.; Marazziti, D.; Bolasco, G.; Aguanno, S.; De Angelis, L.; Naro, F.; Pellegrini, M. Atrophy, oxidative switching and ultrastructural defects in skeletal muscle of the ataxia telangiectasia mouse model. J. Cell. Sci. 2019, 132, 223008. [Google Scholar]
- Chen, Z.; Zhou, D.; Zhou, S.; Jia, G. Gender difference in hepatic toxicity of titanium dioxide nanoparticles after subchronic oral exposure in Sprague-Dawley rats. Appl. Toxicol. 2019, 39, 807–819. [Google Scholar]
- Shakeel, M.; Jabeen, F.; Qureshi, N.A.; Fakhr-E-Alam, M. Toxic Effects of Titanium Dioxide Nanoparticles and Titanium Dioxide Bulk Salt in the Liver and Blood of Male Sprague-Dawley Rats Assessed by Different Assays. Biol. Trace Elem. Res. 2016, 173, 405–426. [Google Scholar] [PubMed]
- Hong, F.; Wu, N.; Zhou, Y.; Ji, L.; Chen, T.; Wang, L. Gastric toxicity involving alterations of gastritis-related protein expression in mice following long-term exposure to nano TiO(2). Food Res. Int. 2017, 95, 38–45. [Google Scholar]
- Park, S.; Komatsu, T.; Hayashi, H.; Mori, R.; Shimokawa, I. The Role of Neuropeptide Y in Adipocyte-Macrophage Crosstalk during High Fat Diet-Induced Adipose Inflammation and Liver Steatosis. Biomedicines 2021, 9, 1739. [Google Scholar] [CrossRef]
- Lancha, A.; Rodríguez, A.; Catalán, V.; Becerril, S.; Sainz, N.; Ramírez, B.; Burrell, M.A.; Salvador, J.; Frühbeck, G.; Gómez-Ambrosi, J. Osteopontin deletion prevents the development of obesity and hepatic steatosis via impaired adipose tissue matrix remodeling and reduced inflammation and fibrosis in adipose tissue and liver in mice. PLoS ONE 2014, 9, e98398. [Google Scholar]
- Banerjee, A.; Rose, R.; Johnson, G.A.; Burghardt, R.C.; Ramaiah, S.K. The influence of estrogen on hepatobiliary osteopontin (SPP1) expression in a female rodent model of alcoholic steatohepatitis. Toxicol. Pathol. 2009, 37, 492–501. [Google Scholar]
- Ali, D.; Alarifi, S.; Al-Doaiss, A.; Ali, B.A.; Ahamed, M.; Al-Khedhairy, A.A. Histologic and apoptotic changes induced by titanium dioxide nanoparticles in the livers of rats. Int. J. Nanomed. 2013, 8, 3937–3943. [Google Scholar] [CrossRef]
- Bakour, M.; Hammas, N.; Laaroussi, H.; Ousaaid, D.; Fatemi, H.E.; Aboulghazi, A.; Soulo, N.; Lyoussi, B. Moroccan Bee Bread Improves Biochemical and Histological Changes of the Brain, Liver, and Kidneys Induced by Titanium Dioxide Nanoparticles. Biomed. Res. 2021, 2021, 6632128. [Google Scholar] [CrossRef]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Adams, Lymphocyte recruitment to the liver: Molecular insights into the pathogenesis of liver injury and hepatitis. Toxicology 2008, 254, 136–146. [Google Scholar]
- Wang, Y.; Chen, Z.; Ba, T.; Pu, J.; Chen, T.; Song, Y.; Gu, Y.; Qian, Q.; Xu, Y.; Xiang, K.; et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small 2013, 9, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, J.; Ma, L.; Li, N.; Liu, H.; Wang, J.; Zheng, L.; Liu, C.; Wang, X.; Zhao, X.; et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials 2010, 31, 894–899. [Google Scholar] [CrossRef]
- Peters, R.J.B.; Oomen, A.G.; van Bemmel, G.; van Vliet, L.; Undas, A.K.; Munniks, S.; Bleys, R.L.A.W.; Tromp, P.C.; Brand, W.; van der Lee, M. Silicon dioxide and titanium dioxide particles found in human tissues. Nanotoxicology 2020, 14, 420–432. [Google Scholar] [CrossRef]
- Xie, G.; Wang, C.; Sun, J.; Zhong, G. Tissue distribution and excretion of intravenously administered titanium dioxide nanoparticles. Toxicol. Lett. 2011, 205, 55–61. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15–33. [Google Scholar]
- Shirdare, M.; Jabbari, F.; Salehzadeh, M.; Ziamajidi, N.; Nourian, A.; Heidarisasan, S.; Ghavimishamekh, A.; Azandariani, M.T.; Abbasalipourkabir, R. Curcuma reduces kidney and liver damage induced by titanium dioxide nanoparticles in male Wistar rats. Phytomed 2022, 12, 537–547. [Google Scholar]
- Liu, H.; Ma, L.; Zhao, J.; Liu, J.; Yan, J.; Ruan, J.; Hong, F. Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol. Trace Elem. Res. 2009, 129, 170–180. [Google Scholar]
- Hazelhoff, M.H.; Bulacio, R.P.; Torres, A.M. Renal tubular response to titanium dioxide nanoparticles exposure. Drug Chem. Toxicol. 2023, 46, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.Z.; Li, J.C.; Zhu, B.W.; Huang, X.R.; Wang, H.L.; Jia, J.; Zhong, X.; Liu, J.; Wang, L.; Lan, H.Y. Neuropeptide Y protects kidney from acute kidney injury by inactivating M1 macrophages via the Y1R-NF-κB-Mincle-dependent mechanism. Int. J. Biol. Sci. 2023, 19, 531–536. [Google Scholar] [CrossRef] [PubMed]


| Parameters/ Characterization Method | Results |
|---|---|
| Shape/TEM | Spherical, primary size 60–40 nm and irregular length about 60 nm, with about 40 nm width |
| Size distribution/SEM | Average diameter 70 nm–1.2 μm with a pick from 60 to 90 nm |
| Z-Average/DLS | 604 ± 24 nm |
| Polydispersity Index/DLS | 0.211 ± 0.035 |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| 0 mg/kg | 1 mg/kg | 2 mg/kg | 0 mg/kg | 1 mg/kg | 2 mg/kg | |
| Body weight TD6 (g; M ± SD) | 275.90 ± 13.57 | 274.36 ± 14.57 | 277.18 ± 8.96 | 236.46 ± 33.63 | 198.96 ± 6.67 * | 197.78 ± 2.72 * |
| Food consumption (g/day; M ± SD) | 23.66 ± 1.85 | 26.37 ± 1.71 | 16.98 ± 1.00 * | 18.98 ± 1.50 | 16.54 ± 1.08 * | 16.46 ± 0.97 * |
| Liver absolute weight (g; M ± SD) | 13.31 ± 1.14 | 12.79 ± 0.61 | 13.07 ± 0.58 | 8.53 ± 0.50 | 8.76 ± 0.26 | 8.78 ± 0.48 |
| Liver relative weight (×100; M ± SD) | 4.82 ± 0.23 | 4.82 ± 0.16 | 4.91 ± 0.09 | 4.16 ± 0.29 | 4.48 ± 0.20 | 4.47 ± 0.21 |
| Kidneys absolute weight (g; M ± SD) | 1.88 ± 0.07 | 1.94 ± 0.12 | 1.89 ± 0.03 | 1.40 ± 0.05 | 1.34 ± 0.09 | 1.40 ± 0.08 |
| Kidneys relative weight (×100; M ± SD) | 0.69 ± 0.01 | 0.74 ± 0.02 * | 0.75 ± 0.04 * | 0.66 ± 0.03 | 0.71 ± 0.06 | 0.78 ± 0.04 * |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| 0 mg/kg | 1 mg/kg | 2 mg/kg | 0 mg/kg | 1 mg/kg | 2 mg/kg | |
| Liver | ||||||
| Hepatocyte vacuolization/steatosis | 0/5 | 2/5 | 3/5 * | 0/5 | 4/5 * | 3/5 * |
| Intralobular lymphoid infiltration | 0/5 §§ | 4/5 * | 5/5 ** | 0/5 | 5/5 ** | 2/5 |
| Congestion in central vein with enlargement of sinusoids | 0/5 §§ | 4/5 * | 5/5 ** | 0/5 §§ | 2/5 | 5/5 ** |
| Focal intralobular necrosis in the middle zone of liver lobule | 0/5 | 0/5 | 0/5 | 0/5 § | 1/5 | 4/5 * |
| Kidney | ||||||
| Tubule dilatation | 1/5 | 0/5 | 1/5 | 0/5 §§ | 3/5 * | 5/5 * |
| Vascular congestion and dilatation | 1/5 | 5/5 * | 4/5 (p = 0.0537) | 0/5 § | 2/5 | 4/5 * |
| Glomerular area (μm2) | 7773.7 ± 753.2 | 7235.6 ± 111.1 | 6584.2 ± 395.6 * | 6236.4 ± 382.4 | 5849.3 ± 598.3 | 6086.2 ± 353.7 |
| Glomerular diameter (μm) | 93.6 ± 4.6 | 91.4 ± 3.2 | 84.1 ± 3.1* | 87.6 ± 5.3 | 79.3 ± 6.0 | 82.3 ± 2.5 |
| Glomerular volume (μm3) | 965,817.4 ± 40,089.0 | 854,634.5 ± 19,851.1 | 751,084.6 ± 66,154.5 * | 692,813.0 ± 63,609.5 | 630,580.6 ± 97,728.9 | 667,862.4 ± 58,424.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassinari, R.; Tammaro, A.; Martinelli, A.; Valeri, M.; Maranghi, F. Sex-Specific Effects of Short-Term Oral Administration of Food-Grade Titanium Dioxide Nanoparticles in the Liver and Kidneys of Adult Rats. Toxics 2023, 11, 776. https://doi.org/10.3390/toxics11090776
Tassinari R, Tammaro A, Martinelli A, Valeri M, Maranghi F. Sex-Specific Effects of Short-Term Oral Administration of Food-Grade Titanium Dioxide Nanoparticles in the Liver and Kidneys of Adult Rats. Toxics. 2023; 11(9):776. https://doi.org/10.3390/toxics11090776
Chicago/Turabian StyleTassinari, Roberta, Alessia Tammaro, Andrea Martinelli, Mauro Valeri, and Francesca Maranghi. 2023. "Sex-Specific Effects of Short-Term Oral Administration of Food-Grade Titanium Dioxide Nanoparticles in the Liver and Kidneys of Adult Rats" Toxics 11, no. 9: 776. https://doi.org/10.3390/toxics11090776
APA StyleTassinari, R., Tammaro, A., Martinelli, A., Valeri, M., & Maranghi, F. (2023). Sex-Specific Effects of Short-Term Oral Administration of Food-Grade Titanium Dioxide Nanoparticles in the Liver and Kidneys of Adult Rats. Toxics, 11(9), 776. https://doi.org/10.3390/toxics11090776