Cannabis and Other Substance Misuse: Implications and Regulations
Abstract
1. Introduction
2. Fatal Outcomes of Substance Abuse
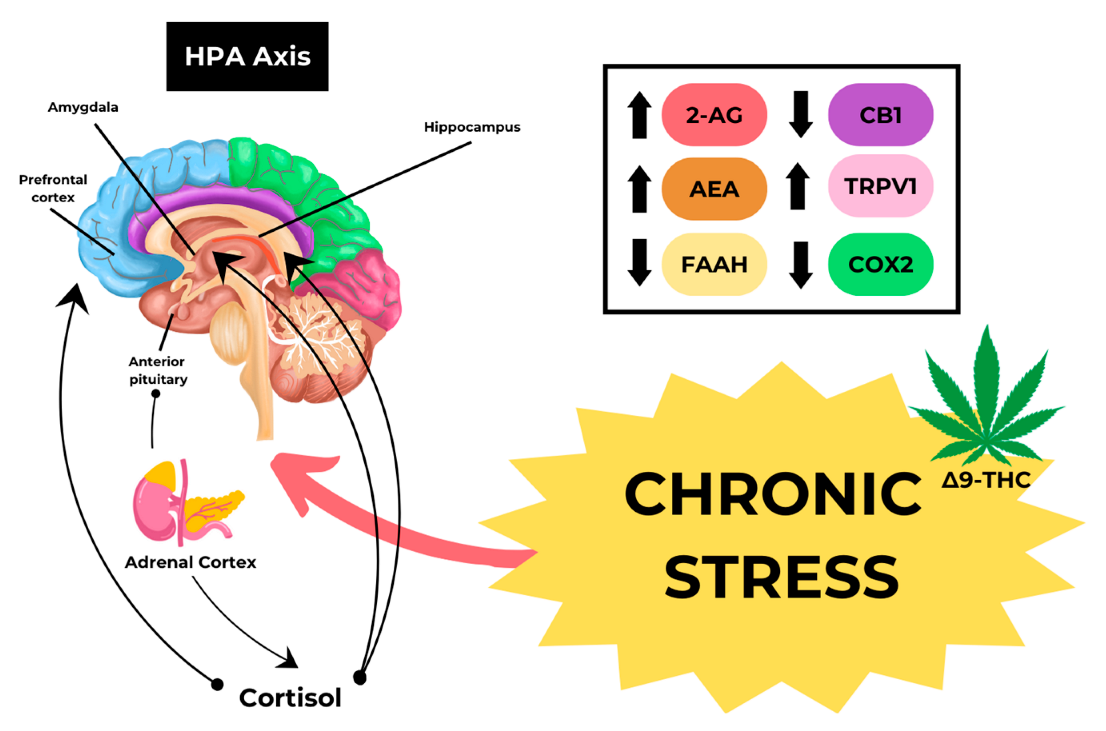
3. Cannabis
4. Amphetamine-Type Stimulant
5. Opioids
6. Cocaine
7. New Psychoactive Substances
8. Hallucinogens
9. Safety and Toxicological Evaluation
10. Regulation
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Lexicon of Alcohol and Drug Terms; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- McLellan, A.T. Substance Misuse and Substance Use Disorders: Why Do They Matter in Healthcare? Trans. Am. Clin. Climatol. Assoc. 2017, 128, 112–130. [Google Scholar] [PubMed]
- United Nations Office of Drugs and Crime. World Drug Report 2022; United Nations: New York, NY, USA, 2022; Volume 1. [Google Scholar]
- Liakoni, E.; Dolder, P.C.; Rentsch, K.M.; Liechti, M.E. Presentations Due to Acute Toxicity of Psychoactive Substances in an Urban Emergency Department in Switzerland: A Case Series. BMC Pharmacol. Toxicol. 2016, 17, 25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rexed, B.; Edmonson, K.; Khan, I.; Samson, R.J. Guidelines for the Control of Narcotic and Psychotropic Substances; World Health Organization: Geneva, Switzerland, 1984. [Google Scholar]
- Nourbakhsh, M.; Miller, A.; Gofton, J.; Jones, G.; Adeagbo, B. Cannabinoid Hyperemesis Syndrome: Reports of Fatal Cases. J. Forensic Sci. 2019, 64, 270–274. [Google Scholar] [CrossRef]
- Behonick, G.; Shanks, K.G.; Firchau, D.J.; Mathur, G.; Lynch, C.F.; Nashelsky, M.; Jaskierny, D.J.; Meroueh, C. Four Postmortem Case Reports with Quantitative Detection of the Synthetic Cannabinoid, 5F-PB-22. J. Anal. Toxicol. 2014, 38, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Takasu, S.; Maebashi, K.; Matsumoto, S.; Murofushi, M.; Sakamoto, K.; Iwadate, K. Fatal Intoxication Due to Transrectal Methamphetamine Overdose: A Case Report. Leg. Med. 2021, 52, 101904. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, I.M.; Nelson, C.L.; Schaber, B.; Hamm, C.E. Antemortem and Postmortem Methamphetamine Blood Concentrations: Three Case Reports. J. Anal. Toxicol. 2013, 37, 386–389. [Google Scholar] [CrossRef]
- Peeters, L.E.J.; Vleut, I.T.; Tan, G.E.; Croes, E.A.; Bethlehem, C. Case Report on Postmortem Fentanyl Measurement after Overdose with More than 67 Fentanyl Patches. Forensic Toxicol. 2022, 40, 199–203. [Google Scholar] [CrossRef]
- Yonemitsu, K.; Sasao, A.; Mishima, S.; Ohtsu, Y.; Nishitani, Y. A Fatal Poisoning Case by Intravenous Injection of “Bath Salts” Containing Acetyl Fentanyl and 4-Methoxy PV8. Forensic Sci. Int. 2016, 267, e6–e9. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Gu, Y.; Ye, Y.; Li, L.; Liu, M.; Yi, X.; Yun, L. Forensic Aspects about Fatal Morphine Intoxication of an Unusual Body Packer: Case Report and Literature Review. Forensic Sci. Int. Rep. 2021, 3, 100207. [Google Scholar] [CrossRef]
- Gioia, S.; Lancia, M.; Bacci, M.; Suadoni, F. Two Fatal Intoxications Due to Tramadol Alone. Am. J. Forensic Med. Pathol. 2017, 38, 345–348. [Google Scholar] [CrossRef]
- Montoya Ramírez, J.P.; Peña-Gutiérrez, A.; Sánchez-Barrios, D.N.; Méndez-Bonilla, A.F.; Escobar-Pérez, J.L.; Hernandez-Rubio, M.A. Suicide Pact in a Cocaine Related Death: A Case Report. J. Forensic Sci. Toxicol. 2019, 2, 1010. [Google Scholar]
- Morentin, B.; Ballesteros, J.; Callado, L.F.; Meana, J.J. Recent Cocaine Use Is a Significant Risk Factor for Sudden Cardiovascular Death in 15-49-Year-Old Subjects: A Forensic Case-Control Study. Addiction 2014, 109, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, P.; Tokarczyk, B.; Stanaszek, R.; Slopianka, M. Fatal Mephedrone Intoxication—A Case Report. J. Anal. Toxicol. 2013, 37, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Anzillotti, L.; Calò, L.; Banchini, A.; Schirripa, M.L.; Marezza, F.; Cecchi, R. Mephedrone and Chemsex: A Case Report. Leg. Med. 2020, 42, 101640. [Google Scholar] [CrossRef]
- Zawadzki, M.; Nowak, K.; Szpot, P. Fatal Intoxication with N-Ethylpentylone: A Case Report and Method for Determining N-Ethylpentylone in Biological Material. Forensic Toxicol. 2020, 38, 255–263. [Google Scholar] [CrossRef]
- Politi, C.; Gabbin, A.; Cecchetto, G.; Montisci, M.; Viel, G.; Pascali, J.P. A Case Study on MDMA. Two Fatal Cases Involving Young Adults. Aust. J. Forensic Sci. 2021, 55, 12–22. [Google Scholar] [CrossRef]
- Lang, J.; Dettmeyer, R.; Henn, V.; Birngruber, C.G.; Veit, F. Fatal Ecstasy-Induced Malignant Hyperthermia with Rhabdomyolysis. A Case Report. Rom. J. Leg. Med. 2016, 24, 212–215. [Google Scholar] [CrossRef]
- Shafi, H.; Imran, M.; Usman, H.F.; Sarwar, M.; Tahir, M.A.; Naveed, R.; Ashiq, M.Z.; Tahir, A.M. Deaths Due to Abuse of Dextromethorphan Sold Over-the-Counter in Pakistan. Egypt. J. Forensic Sci. 2016, 6, 280–283. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Noble, M.J.; Hedberg, K.; Hendrickson, R.G. Acute Cannabis Toxicity. Clin. Toxicol. 2019, 57, 735–742. [Google Scholar] [CrossRef]
- Grimsrud, M.M.; Brekke, M.; Syse, V.L.; Vallersnes, O.M. Acute Poisoning Related to the Recreational Use of Prescription Drugs: An Observational Study from Oslo, Norway. BMC Emerg. Med. 2019, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Reece, A.S.; Norman, A.; Hulse, G.K. Cannabis Exposure as an Interactive Cardiovascular Risk Factor and Accelerant of Organismal Ageing: A Longitudinal Study. BMJ Open 2016, 6, e011891. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.F.; Nappe, T.M. Cannabinoid Toxicity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482175/ (accessed on 7 June 2023).
- Cha, J.M.; Kozarek, R.A.; Lin, O.S. Case of Cannabinoid Hyperemesis Syndrome with Long-Term Follow-Up. World J. Clin. Cases 2014, 2, 930. [Google Scholar] [CrossRef]
- Archie, S.R.; Cucullo, L. Harmful Effects of Smoking Cannabis: A Cerebrovascular and Neurological Perspective. Front. Pharmacol. 2019, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.R.; Spurling, B.C.; Anawal, S. Marijuana Toxicity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430823/ (accessed on 7 June 2023).
- Bellamy, S.E.; Loor, B.; Gutierrez-Castillo, M. A Case of Cannabinoid Hyperemesis Syndrome and Acute Kidney Injury: A Review of the Literature. Cureus 2023, 15, e34350. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Selvam, B.; Das, A.; Shukla, D. Mechanistic Origin of Partial Agonism of Tetrahydrocannabinol for Cannabinoid Receptors. J. Biol. Chem. 2022, 298, 101764. [Google Scholar] [CrossRef] [PubMed]
- Jacotte-Simancas, A.; Fucich, E.A.; Stielper, Z.F.; Molina, P.E. Traumatic Brain Injury and the Misuse of Alcohol, Opioids, and Cannabis. Int. Rev. Neurobiol. 2021, 157, 195–243. [Google Scholar] [CrossRef]
- Ghuran, A.; Nolan, J. Recreational Drug Misuse: Issues for the Cardiologist. Heart 2000, 83, 627–633. [Google Scholar] [CrossRef]
- Rock, K.L.; Englund, A.; Morley, S.; Rice, K.; Copeland, C.S. Can Cannabis Kill? Characteristics of Deaths Following Cannabis Use in England (1998–2020). J. Psychopharmacol. 2022, 36, 1362–1370. [Google Scholar] [CrossRef]
- United Nations Office of Drugs and Crime. World Drug Report 2022; United Nations: New York, NY, USA, 2022; Volume 4. [Google Scholar]
- Winek, C.L.; Wahba, W.W.; Winek, C.L.; Balzer, T.W. Drug and Chemical Blood-Level Data 2001. Forensic Sci. Int. 2001, 122, 107–123. [Google Scholar] [CrossRef]
- Molina, N.M.; Jejurikar, S.G. Toxicological Findings in a Fatal Ingestion of Methamphetamine. J. Anal. Toxicol. 1999, 23, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.J.; Han, E. A Commentary on the Effects of Methamphetamine and the Status of Methamphetamine Abuse among Youths in South Korea, Japan, and China. Forensic Sci. Int. 2018, 286, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Liakoni, E.; Yates, C.; Dines, A.M.; Dargan, P.I.; Heyerdahl, F.; Hovda, K.E.; Wood, D.M.; Eyer, F.; Liechti, M.E. Acute Recreational Drug Toxicity: Comparison of Self-Reports and Results of Immunoassay and Additional Analytical Methods in a Multicenter European Case Series. Medicine 2018, 97, e9784. [Google Scholar] [CrossRef]
- Wahba, M.A.; Alshehri, B.M.; Hefny, M.M.; Al Dagrer, R.A.; Al-Malki, S.D.S. Incidence and Profile of Acute Intoxication among Adult Population in Najran, Saudi Arabia: A Retrospective Study. Sci. Prog. 2021, 104, 00368504211011339. [Google Scholar] [CrossRef]
- Weng, T.I.; Chen, H.Y.; Chin, L.W.; Chou, H.H.; Wu, M.H.; Chen, G.Y.; Chen, J.Y.; Shih, C.P.; Lin, C.C.; Fang, C.C. Comparison of Clinical Characteristics between Meth/Amphetamine and Synthetic Cathinone Users Presented to the Emergency Department. Clin. Toxicol. 2022, 60, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Kaland, M.E.; Klein-Schwartz, W. Comparison of Lisdexamfetamine and Dextroamphetamine Exposures Reported to U.S. Poison Centers. Clin. Toxicol. 2015, 53, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Pathan, H.; Williams, J. Basic Opioid Pharmacology: An Update. Br. J. Pain. 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Kaplan, L.A.; Khan, S.; Petersen, M.; Sauce, E.; Causey, C.D.; Cornett, E.M.; Imani, F.; Moradi Moghadam, O.; Kaye, A.M.; et al. Full Opioid Agonists and Tramadol: Pharmacological and Clinical Considerations. Anesthesiol. Pain. Med. 2021, 11, e119156. [Google Scholar] [CrossRef]
- Baertsch, N.A.; Bush, N.E.; Burgraff, N.J.; Ramirez, J.-M. Dual Mechanisms of Opioid-Induced Respiratory Depression in the Inspiratory Rhythm-Generating Network. Elife 2021, 10, e67523. [Google Scholar] [CrossRef]
- Yoo, I.S.; Lee, S.H.; Suk, S.-H. A Case of Fentanyl Intoxication and Delayed Hypoxic Leukoencephalopathy Caused by Incidental Use of Fentanyl Patch in a Healthy Elderly Man. J. Neurocritical Care 2015, 8, 35–38. [Google Scholar] [CrossRef]
- Woodall, K.L.; Martin, T.L.; McLellan, B.A. Oral Abuse of Fentanyl Patches (Duragesic®): Seven Case Reports. J. Forensic Sci. 2008, 53, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Nakhaee, S.; Hoyte, C.; Dart, R.C.; Askari, M.; Lamarine, R.J.; Mehrpour, O. A Review on Tramadol Toxicity: Mechanism of Action, Clinical Presentation, and Treatment. Forensic Toxicol. 2021, 39, 293–310. [Google Scholar] [CrossRef]
- De Decker, K.; Cordonnier, J.; Jacobs, W.; Coucke, V.; Schepens, P.; Jorens, P.G. Fatal Intoxication Due to Tramadol Alone: Case Report and Review of the Literature. Forensic Sci. Int. 2008, 175, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Eagerton, D.; Goodbar, N.; Dansby, M.; Abel, S.; Bell, W. Morphine Overdose in a 6 ½ Week-Old Infant: A Case Report. Austin J. Pharmacol. Ther. 2014, 2, 4. [Google Scholar]
- Siegel, S.; Ellsworth, D.W. Pavlovian Conditioning and Death from Apparent Overdose of Medically Prescribed Morphine: A Case Report. Bull. Psychon. Soc. 1986, 24, 278–280. [Google Scholar] [CrossRef]
- Wright, J.A.; Baselt, R.C.; Hine, C.H. Blood Codeine Concentrations in Fatalities Associated with Codeine. Clin. Toxicol. 1975, 8, 457–463. [Google Scholar] [CrossRef]
- Tormey, W.P. Oxycodone in Palliative Care—Art and Empathy Still Have a Place. J. Palliat. Care 2017, 32, 40–42. [Google Scholar] [CrossRef]
- Molina, D.K.; Hargrove, V.M. What Is the Lethal Concentration of Hydrocodone?: A Comparison of Postmortem Hydrocodone Concentrations in Lethal and Incidental Intoxications. Am. J. Forensic Med. Pathol. 2011, 32, 108–111. [Google Scholar] [CrossRef]
- Gill, J.R.; Lin, P.T.; Nelson, L. Reliability of Postmortem Fentanyl Concentrations in Determining the Cause of Death. J. Med. Toxicol. 2013, 9, 34–41. [Google Scholar] [CrossRef]
- Taylor, K.P.; Singh, K.; Goyal, A. Fentanyl Transdermal. In Papich Handbook of Veterinary Drugs; Elsevier: Amsterdam, The Netherlands, 2023; pp. 363–365. [Google Scholar] [CrossRef]
- Rop, P.P.; Fornaris, M.; Salmon, T.; Burle, J.; Bresson, M. Concentrations of Heroin, 06-Monoacetylmorphine, and Morphine in a Lethal Case Following an Oral Heroin Overdose. J. Anal. Toxicol. 1997, 21, 232–235. [Google Scholar] [CrossRef][Green Version]
- Dupuy, G.; Cavalcanti, L.; Bourgogne, E.; Brichant-Petitjean, C.; Gomberoff, L.; Bloch, V.; Bellivier, F.; Lépine, J.P.; Laprévote, O.; Vorspan, F. Are Empty Methadone Bottles Empty? An Analytic Study. Harm Reduct. J. 2014, 11, 1–4. [Google Scholar] [CrossRef][Green Version]
- Mégarbane, B.; Hreiche, R.; Pirnay, S.; Marie, N.; Baud, F.J. Does High-Dose Buprenorphine Cause Respiratory Depression? Possible Mechanisms and Therapeutic Consequences. Toxicol. Rev. 2006, 25, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Meatherall, R.; Lee, C.; Phillips, S. Accidental Death from Hydromorphone Ingestion. J. Forensic Sci. 2011, 56, S271–S274. [Google Scholar] [CrossRef] [PubMed]
- Vongpatanasin, W.; Taylor, J.A.; Victor, R.G. Effects of Cocaine on Heart Rate Variability in Healthy Subjects. Am. J. Cardiol. 2004, 93, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Heesch, C.M.; Wilhelm, C.R.; Ristich, J.; Adnane, J.; Bontempo, F.A.; Wagner, W.R. Cocaine Activates Platelets and Increases the Formation of Circulating Platelet Containing Microaggregates in Humans. Heart 2000, 83, 688–695. [Google Scholar] [CrossRef]
- Esposito, M.; Liberto, A.; Zuccarello, P.; Ministeri, F.; Licciardello, G.; Barbera, N.; Sessa, F.; Salerno, M. Heart Rupture as an Acute Complication of Cocaine Abuse: A Case Report. Leg. Med. 2022, 58, 102084. [Google Scholar] [CrossRef]
- Pomara, C.; Villani, A.; D’Errico, S.; Riezzo, I.; Turillazzi, E.; Fineschi, V. Acute Myocarditis Mimicking Acute Myocardial Infarction: A Clinical Nightmare with Forensic Implications. Int. J. Cardiol. 2006, 112, 119–121. [Google Scholar] [CrossRef]
- Siegel, A.J.; Mendelson, J.H.; Sholar, M.B.; McDonald, J.C.; Lewandrowski, K.B.; Lewandrowski, E.L.; Lipinska, I.; Ridker, P.M.; Tofler, G.H. Effect of Cocaine Usage on C-Reactive Protein, von Willebrand Factor, and Fibrinogen. Am. J. Cardiol. 2002, 89, 1133–1135. [Google Scholar] [CrossRef]
- Mo, W.; Singh, A.K.; Arruda, J.A.L.; Dunea, G. Role of Nitric Oxide in Cocaine-Induced Acute Hypertension. Am. J. Hypertens. 1998, 11, 708–714. [Google Scholar] [CrossRef]
- Wilbert-Lampen, U.; Seliger, C.; Zilker, T.; Arendt, R.M. Cocaine Increases the Endothelial Release of Immunoreactive Endothelin and Its Concentrations in Human Plasma and Urine. Circulation 1998, 98, 385–390. [Google Scholar] [CrossRef]
- Moliterno, D.J.; Lange, R.A.; Gerard, R.D.; Willard, J.E.; Lackner, C.; Hillis, L.D. Influence of Intranasal Cocaine on Plasma Constituents Associated with Endogenous Thrombosis and Thrombolysis. Am. J. Med. 1994, 96, 492–496. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office of Drugs and Crime New Psychoactive Substances (NPS). Available online: https://www.unodc.org/wdr2013/en/nps.html (accessed on 7 June 2023).
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New Psychoactive Substances: A Review and Updates. Ther. Adv. Psychopharmacol. 2020, 10, 204512532096719. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.-A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; de la Torre, R.; Farré, M. Human Pharmacology of Mephedrone in Comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704–2713. [Google Scholar] [CrossRef]
- Wood, D.M.; Davies, S.; Puchnarewicz, M.; Button, J.; Archer, R.; Ovaska, H.; Ramsey, J.; Lee, T.; Holt, D.W.; Dargan, P.I. Recreational Use of Mephedrone (4-Methylmethcathinone, 4-MMC) with Associated Sympathomimetic Toxicity. J. Med. Toxicol. 2010, 6, 327–330. [Google Scholar] [CrossRef]
- Gerace, E.; Petrarulo, M.; Bison, F.; Salomone, A.; Vincenti, M. Toxicological Findings in a Fatal Multidrug Intoxication Involving Mephedrone. Forensic Sci. Int. 2014, 243, 68–73. [Google Scholar] [CrossRef]
- Martínez-Clemente, J.; López-Arnau, R.; Abad, S.; Pubill, D.; Escubedo, E.; Camarasa, J. Dose and Time-Dependent Selective Neurotoxicity Induced by Mephedrone in Mice. PLoS ONE 2014, 9, e99002. [Google Scholar] [CrossRef] [PubMed]
- Won, S.; Hong, R.A.; Shohet, R.V.; Seto, T.B.; Parikh, N.I. Methamphetamine-Associated Cardiomyopathy. Clin. Cardiol. 2013, 36, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Buzhdygan, T.P.; Rodrigues, C.R.; McGary, H.M.; Khan, J.A.; Andrews, A.M.; Rawls, S.M.; Ramirez, S.H. The Psychoactive Drug of Abuse Mephedrone Differentially Disrupts Blood-Brain Barrier Properties. J. Neuroinflamm. 2021, 18, 63. [Google Scholar] [CrossRef]
- Costa, J.L.; Cunha, K.F.; Lanaro, R.; Cunha, R.L.; Walther, D.; Baumann, M.H. Analytical Quantification, Intoxication Case Series, and Pharmacological Mechanism of Action for N-ethylnorpentylone (N-ethylpentylone or Ephylone). Drug Test. Anal. 2019, 11, 461–471. [Google Scholar] [CrossRef]
- Thirakul, P.; Hair, L.S.; Bergen, K.L.; Pearson, J.M. Clinical Presentation, Autopsy Results and Toxicology Findings in an Acute N-Ethylpentylone Fatality. J. Anal. Toxicol. 2017, 41, 342–346. [Google Scholar] [CrossRef][Green Version]
- Ikeji, C.; Sittambalam, C.D.; Camire, L.M.; Weisman, D.S. Fatal Intoxication with N-Ethylpentylone: A Case Report. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Sessa, B.; Higbed, L.; Nutt, D. A Review of 3,4-Methylenedioxymethamphetamine (MDMA)-Assisted Psychotherapy. Front. Psychiatry 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tran, H.T.N.; Saber, Y.H.; Hall, F.S. High Ambient Temperature Increases the Toxicity and Lethality of 3,4-Methylenedioxymethamphetamine and Methcathinone. Pharmacol. Biochem. Behav. 2020, 192, 172912. [Google Scholar] [CrossRef] [PubMed]
- Chyka, P.A.; Erdman, A.R.; Manoguerra, A.S.; Christianson, G.; Booze, L.L.; Nelson, L.S.; Woolf, A.D.; Cobaugh, D.J.; Caravati, E.M.; Scharman, E.J.; et al. Dextromethorphan Poisoning: An Evidence-Based Consensus Guideline for out-of-Hospital Management. Clin. Toxicol. 2007, 45, 662–677. [Google Scholar] [CrossRef] [PubMed]
- Haufroid, V.; Hantson, P. CYP2D6 Genetic Polymorphisms and Their Relevance for Poisoning Due to Amfetamines, Opioid Analgesics and Antidepressants. Clin. Toxicol. 2015, 53, 501–510. [Google Scholar] [CrossRef]
- Dao, C.K.; Nowinski, S.M.; Mills, E.M. The Heat Is on: Molecular Mechanisms of Drug-Induced Hyperthermia. Temperature 2014, 1, 183–191. [Google Scholar] [CrossRef]
- Brown, G.R.; McLaughlin, K.; Vaughn, K. Identifying and Treating Patients with Synthetic Psychoactive Drug Intoxication. J. Am. Acad. Physician Assist. 2018, 31, 1–5. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Drug and Opioid-Involved Overdose Deaths—United States, 2017–2018. Morb. Mortal. Wkly. Rep. 2020, 69, 290–297.
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the Prefrontal Cortex in Addiction: Neuroimaging Findings and Clinical Implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef]
- National Institute of Drugs Abuse Drugs Overdose Death Rates. Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed on 12 August 2023).
- Schiller, E.Y.; Goyal, A.; Mechanic, O.J. Opioid Overdose Continuing Education Activity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Center for Substance Abuse Treatment. A Guide to Substance Abuse Services for Primary Care Clinicians; Treatment Improvement Protocol (TIP) Series, No. 24; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 1997; Chapter 4—Assessment; 2008. [Google Scholar]
- Zhu, Y.; Ouyang, Z.; Du, H.; Wang, M.; Wang, J.; Sun, H.; Kong, L.; Xu, Q.; Ma, H.; Sun, Y. New Opportunities and Challenges of Natural Products Research: When Target Identification Meets Single-Cell Multiomics. Acta Pharm. Sin. B 2022, 12, 4011–4039. [Google Scholar] [CrossRef]
- National Institute of Drug Abuse. Drugs, Brains, and Behavior: The Science of Addiction; National Institute of Drug Abuse: North Bethesda, MD, USA, 2020.
- U.S. Department of Justice Drug Enforcement Administration. Drugs of Abuse: A DEA Resource Guide 2020 Edition; U.S. Department of Justice Drug Enforcement Administration: Springfield, IL, USA, 2020.
- Lopez, M.J.; Tadi, P. Drug Enforcement Administration Drug Scheduling; StatPearls Publishing: Treasure Island FL, USA, 2023. [Google Scholar]
- Preuss, C.V.; Kalava, A.; King, K.C. Prescription of Controlled Substances: Benefits and Risks; StatPearls Publishing: Treasure Island FL, USA, 2023. [Google Scholar]
- Washington Office on Latin America Systems Overload: Drug Laws and Prison in Latin America; Washington Office on Latin America: Washington DC, 2011.
- Hughes, C.; Stevens, A. The Effects of Decriminalization of Drug Use in Portugal. The Buckley Foundation Drug Policy Programme; Portugal, 2007. Available online: https://core.ac.uk/download/pdf/91904.pdf (accessed on 12 August 2023).
- Félix, S.; Portugal, P.; Tavares, A. Discussion Paper Series Going after the Addiction, Not the Addicted: The Impact of Drug Decriminalization in Portugal; IZA—Institute of Labor Economics: Bonn, Germany, 2017. [Google Scholar]
- Substance Abuse and Mental Health Services Administration; Office of the Surgeon. General Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs and Health; US Department of Health and Human Services: Washington, DC, USA, 2016.
- Garibotto, G. Prisons and Drugs in Uruguay. In Systems Overload, Drug Laws and Prisons in Latin America; Metaal, P., Youngers, C., Eds.; Transnational Institute: Amsterdam, The Netherlands; Washington Office on Latin America (WOLA): Washington, DC, USA, 2011; pp. 81–87. [Google Scholar]
- Butler, A.J.; Rehm, J.; Fischer, B. Health Outcomes Associated with Crack-Cocaine Use: Systematic Review and Meta-Analyses. Drug Alcohol. Depend. 2017, 180, 401–416. [Google Scholar] [CrossRef]
- Degenhardt, L.; Hall, W. Extent of Illicit Drug Use and Dependence, and Their Contribution to the Global Burden of Disease. Lancet 2012, 379, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Chandler, R.K.; Fletcher, B.W.; Volkow, N.D. Treating Drug Abuse and Addiction in the Criminal Justice System. JAMA 2009, 301, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Alya Attiah, A. Cognitive Behavioral Therapy Treatment for Drug Addiction. J. Addict. Ther. Res. 2023, 7, 005–007. [Google Scholar] [CrossRef]

| Reference (Country) | Chemical Substance (Route) | Classification | Type of Biological Sample | Dose | Mechanism of Intoxication | Side Effect | |
|---|---|---|---|---|---|---|---|
| Sample Concentration | Lethal Dose | ||||||
| Nourbakhsh et al., 2019 (Canada) [6]. | Tetrahydrocannabinol (Inhalation) | Cannabis | Femoral Blood | Delta-9-THC: 7.7 μg/L Carboxy-THC: 24.9 μg/L | not specified | Chronic overstimulation of CB1 cannabinoid receptor activation bypassing the endocannabinoid system will lead to erratic neurotransmitter modulation that can cause toxicity. | Cyclic attacks of nausea and vomiting |
| Behonick et al., 2014 (Japan) [7] | 5F-PB-22 (Inhalation) | Synthetics Cannabinoids | Iliac Blood | 1.5 ng/mL | not specified | Chronic overstimulation of CB1 cannabinoid receptors leads to visceral congestion, pulmonary oedema, and pulmonary granulomatous inflammation changes. | Convulsions, tachycardia, respiratory failures, severe agitation, unconsciousness, nausea, vomiting, and death |
| Takasu et al., 2021 (Japan) [8] | Methamphetamine (Transrectal) | ATS | Blood | 9.44 μg/mL | 1.4–13 μg/mL | Increasing release of monoamine neurotransmitters such as serotonin, dopamine, and norepinephrine that can lead to Serious neurotoxicity and cardiovascular dysfunction. | Increasing body temperature, respiratory rate, cutaneous vasoconstriction, and blood pressure. |
| McIntyre et al., 2013 (USA) [9] | Methamphetamine (Oral) | ATS | Peripheral blood | 13 μg/mL | 1.4–13 μg/mL | Loss consciousness, agonal breathing, but no heartbeat. | |
| Peeters et al., 2022 (Netherland) [10] | Fentanyl (Patch) | Opioid | Subclavian blood | 57.9 ng/mL | 1.8–81 ng/mL | OIRD originates from medullary preBötzinger complex (preBötC) and is characterized by a significant reduction in the regularity of the inspiratory rhythm. | Respiratory depression, deterioration of the central nervous system, hypothermia, cold, damp skin, floppy muscles, bradycardia, and hypotension |
| Yonemitsu et al., 2016 (Japan) [11] | Acetyl Fentanyl (Intravenous) | Opioid | Femoral vein blood | 153 ng/mL | 1.8–81 ng/mL | ||
| Li et al., 2021 (China) [12] | Morphine (Oral) | Opioid | Blood | 2.18 µg/mL | 0.5 μg/mL | Excessive binding to μ-receptors and inhibition of serotonin reuptake results in decreased CNS function leading to death | CNS depression, tachycardia, seizures, nausea and vomiting. |
| Gioia et al., 2017 (Italy) [13] | Tramadol (Oral) | Opioid | Femoral Blood | 5.8 μg/mL | 1.6 μg/mL | ||
| Montoya-Ramirez et al., 2019 (Colombia) [14] | Cocaine (Oral) | Cocaine | Blood, Vitreous humor Nasal smear | 2.0 mcg/mL | not specified | Cocaine-induced hypertension which leads to acute respiratory failure | Hypertension, hemorrhagic gastropathy, pulmonary edema |
| Morentin et al., 2014 (Spain) [15] | Cocaine (Not reported) | Cocaine | Blood | 1.0 mg/L | not specified | Cocaine-induced cardiovascular disease | Acute cardiovascular syndrome |
| Adamowicz et al., 2013 (Germany) [16] | Mephedrone (Oral) | NPS | Blood | 5.5 mg/mL | >0.15 mg/mL | Promote BBB degradation, resulting in neurotoxic effect The contraction bands’ necrosis and micro-hemorrhages show that the heart is suffering. | Tachycardia and palpitation, hypertension, dilated pupils, hallucination and psychosis. Arrhythmias, vasospasm, atherosclerosis, acute coronary syndrome, sudden cardiac death, and cardiomyopathy |
| Anzillotti et al., 2020 (Italy) [17] | Mephedrone (Intramuscular) | NPS | Urine Bile Blood samples | 2.0 mg/L (urine) 1.1 mg/L (bile) 0.8–1.0 mg/L (blood) | >0.74 mg/L | ||
| Zawadzki et al., 2020 (Poland) [18] | N-ethylpentylone (Not reported) | NPS | Blood Urine | 10.6 μg/mL (blood) 17.6 μg/mL (urine) | not specified | Related to a serotonin syndrome | Psychomotor agitation, confusion, tachycardia, psychosis, inconsistent speech, and cardiac arrest |
| Politi et al., 2021 (Italy) [19] | MDMA (Oral) | Hallucinogen | Blood Urine | 3700 ng/mL (blood) 168,000 ng/mL(urine) | >7.72 μg/mL | Poor metabolizers (abnormal, dysfunctional CYP2D6) | Unconscious, pale and sweaty |
| Lang et al., 2016 (Germany) [20] | Ecstasy (Oral) | Hallucinogen | Femoral blood | 4200 μg/L | 600 μg/L | Induce malignant hyperthermia with rhabdomyolysis. | Sweating and hematoma |
| Shafi et.al., 2016 (Pakistan) [21] | Dextromethorphan (Oral) | Hallucinogen | Whole blood | 7.3–41.7 mg/L | 3.3–9.5 mg/L | Serotonin syndrome | Euphoria, stupor, hyperexcitability, laughing, nystagmus, mydriasis, nausea, vomiting and diaphoresis |
| Opioids | Lethal Dose/s | Reference |
|---|---|---|
| Morphine | 5 µg/mL | D. H. Eagerton et al. [50] |
| Codeine | 0.5 to 1.0 g | James A. Wright et al. [52] |
| Oxycodone | 1200 mg/L | William P. Tormey [53] |
| Hydrocodone | 0.47–0.38 mg/L | Molina et al. [54] |
| Fentanyl | 1.8 ng/mL–81 ng/mL | Katherine P. Taylor et al.; James R. Gill et al. [55,56] |
| Heroin | 200 mg | Pok P. Rop et al. [57] |
| Methadone | 1 mg/kg | Gaël Dupuy et al. [58] |
| Buprenorphine | 2–20 ng/mL | Bruno Mégarbane et al. [59] |
| Tramadol | 1.6 μg/mL | Samaneh Nakhaee et al. [48] |
| Hydromorphone | 60 ng ⁄mL | Robert Meatherall et al. [60] |
| Schedule | Definitions | Examples |
|---|---|---|
| Schedule I | High abuse potential, without a recognized medicinal purpose; medications within this schedule may not be prescribed, dispensed, or administered | Heroin, Marijuana (cannabis), and MDMA (“ecstasy”) |
| Schedule II | High abuse potential with severe psychological or physical dependence | Morphine, codeine, fentanyl, pentobarbital |
| Schedule III | Intermediate abuse potential may lead to moderate or low physical dependence or high psychological dependence (i.e., less than Schedule II but more than Schedule IV medications) | Hydrocodone/acetaminophen 5 mg/500 mg or 10 mg/650 mg; codeine in combination with acetaminophen, aspirin, or ibuprofen; ketamine |
| Schedule IV | Low potential for abuse relative to substances in schedule III. | Alprazolam, clonazepam, diazepam, midazolam, phenobarbital, Tramadol. |
| Schedule V | Low potential for abuse in comparison to chemicals in schedule IV and largely consist of preparations with trace amounts of specific opioids. These are usually used for analgesic, antidiarrheal, and antitussive uses. | Robitussin AC, Phenergan with codeine |
| Requirements | Schedule II | Schedule III and IV | Schedule V |
|---|---|---|---|
| Registration | Required | Required | Required |
| Receiving Records | Order Forms (DEA Form-222) | Invoices, Readily Retrievable | Invoices, Readily Retrievable |
| Prescriptions | Written Prescription (See exceptions *) | Written, Oral, or Fax | Written, Oral, Fax, or Over the Counter ** |
| Refills | No | No more than 5 within 6 months | As authorized when prescription is issued |
| Distribution Between Registrants | Order Forms (DEA Form-222) | Invoices | Invoices |
| Security/Storages | Locked Cabinet or other secure storage | Locked Cabinet or other secure storage | Locked Cabinet or other secure storage |
| Theft or Significant Loss | Report and Complete DEA Form 106 | Report and Complete DEA Form 106 | Report and Complete DEA Form 106 |
| Stages | Description |
|---|---|
| Treating Withdrawal | When patients first stop taking the drug, they will experience a variety of physical and emotional symptoms, including restlessness and trouble sleeping. Medication and plans need to be made to reduce these symptoms to make it easier to stop taking the drug. |
| Staying in Treatment | Several medications and mobile applications are used to help the brain gradually adapt to the absence of medication. This medication works slowly and has a calming effect on the patient’s body so they focus on counseling and other psychotherapy they are undergoing. |
| Preventing Relapse | Science has shown that the most typical trigger for relapse is stress cues associated with drug use (such as people, places, things, and moods). To help patients maintain their recovery, researchers have been working on medicines that interfere with these triggers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khairinisa, M.A.; Alfaqeeh, M.; Rafif, S.N.; Muljono, F.O.; Colin, M.N. Cannabis and Other Substance Misuse: Implications and Regulations. Toxics 2023, 11, 756. https://doi.org/10.3390/toxics11090756
Khairinisa MA, Alfaqeeh M, Rafif SN, Muljono FO, Colin MN. Cannabis and Other Substance Misuse: Implications and Regulations. Toxics. 2023; 11(9):756. https://doi.org/10.3390/toxics11090756
Chicago/Turabian StyleKhairinisa, Miski Aghnia, Mohammed Alfaqeeh, Syauqi Nawwar Rafif, Fajar Oktavian Muljono, and Michelle Natasha Colin. 2023. "Cannabis and Other Substance Misuse: Implications and Regulations" Toxics 11, no. 9: 756. https://doi.org/10.3390/toxics11090756
APA StyleKhairinisa, M. A., Alfaqeeh, M., Rafif, S. N., Muljono, F. O., & Colin, M. N. (2023). Cannabis and Other Substance Misuse: Implications and Regulations. Toxics, 11(9), 756. https://doi.org/10.3390/toxics11090756







