Rapid Assessment of Ocular Toxicity from Environmental Contaminants Based on Visually Mediated Zebrafish Behavior Studies
Abstract
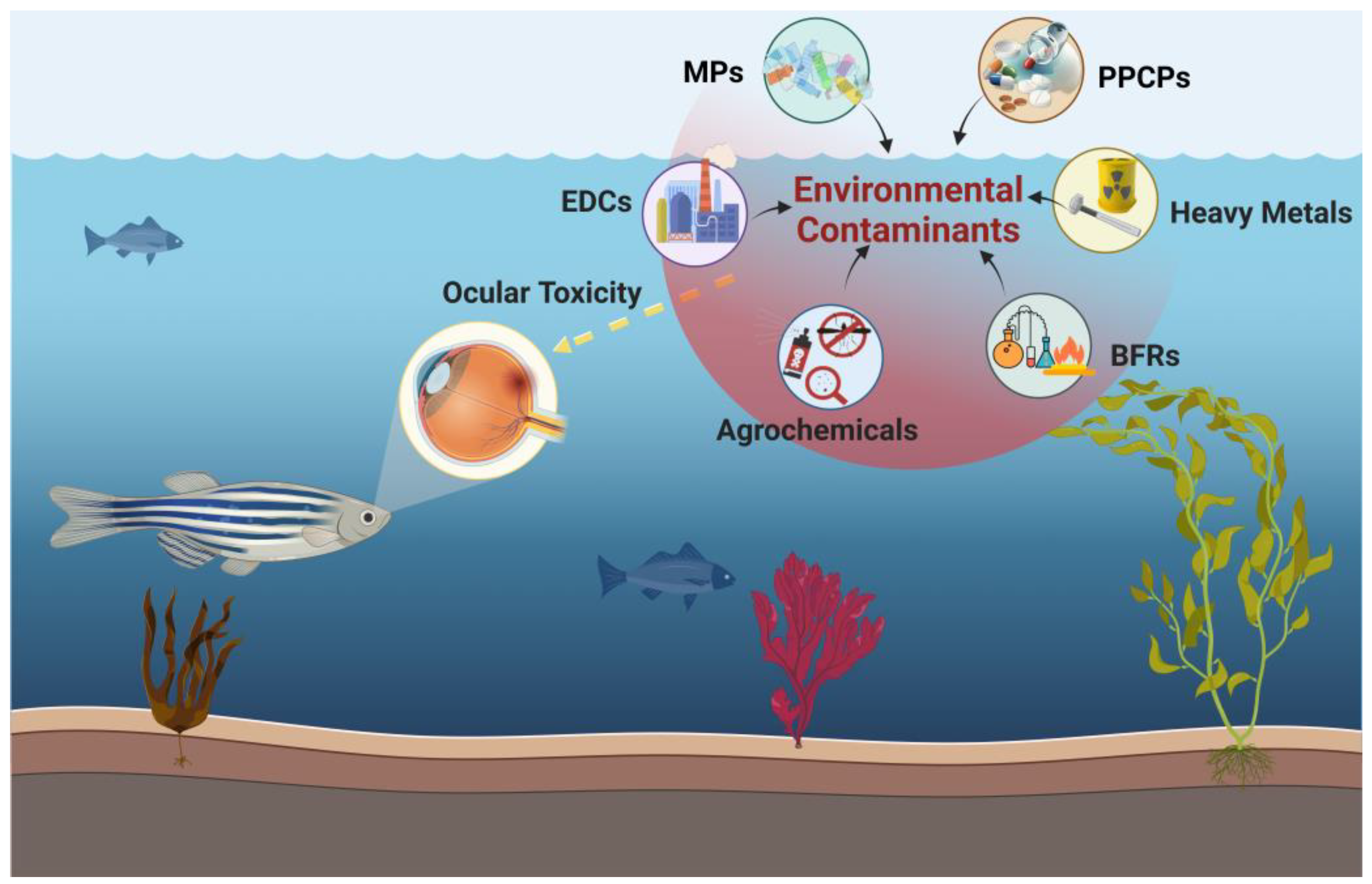
1. Introduction
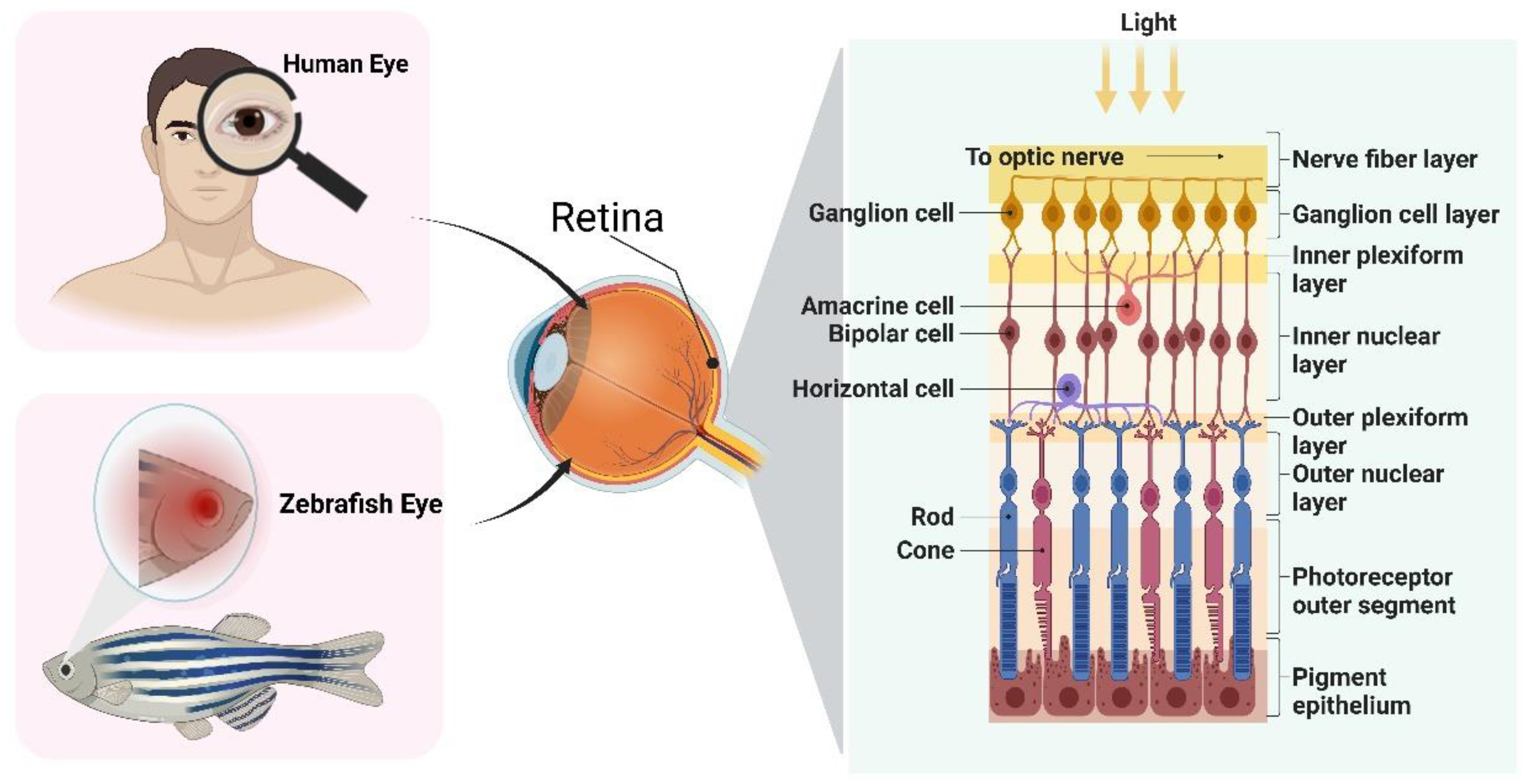
2. The Eye Structure of Zebrafish
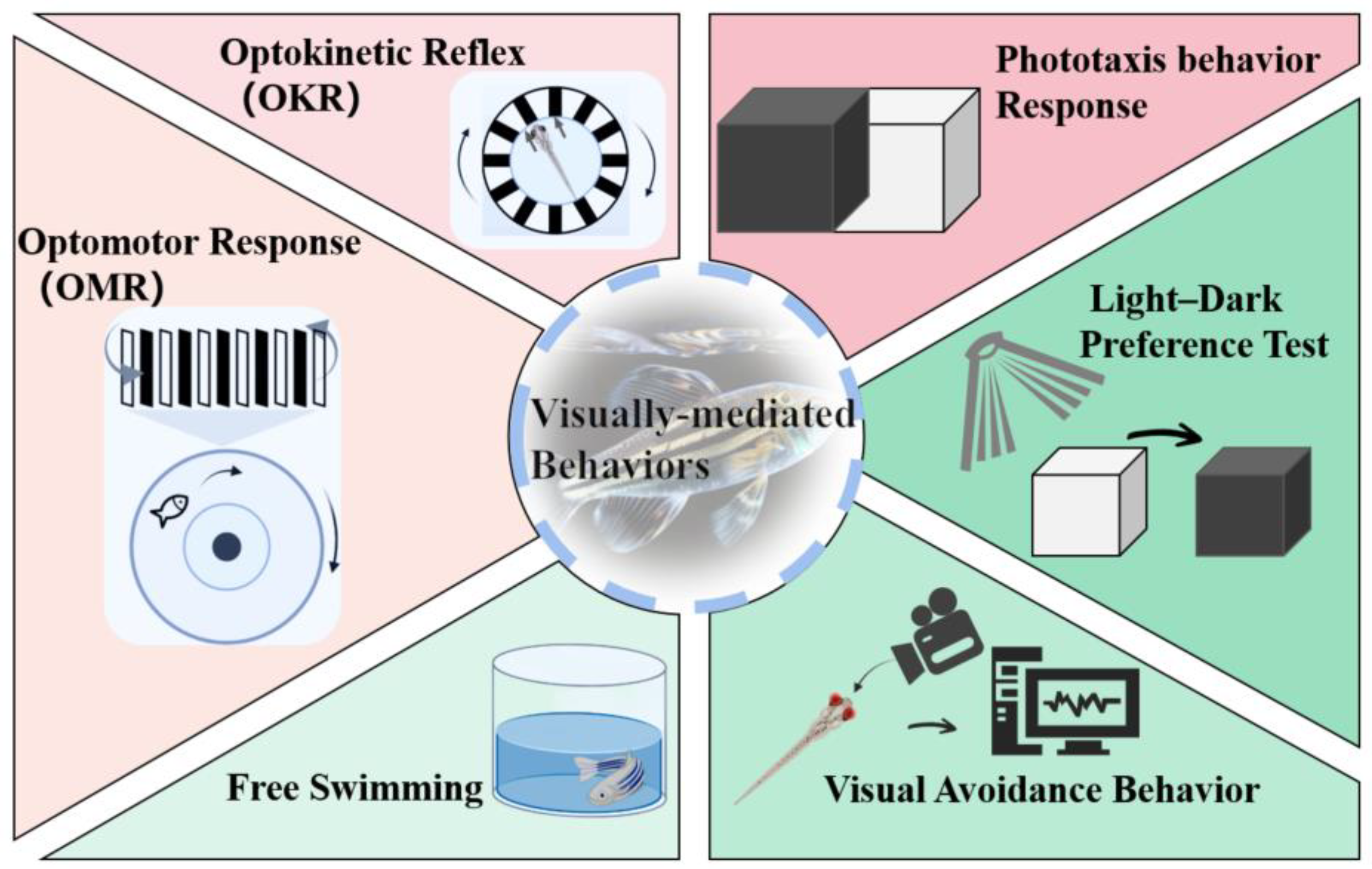
3. Quick Approaches for Assessing Ocular Toxicity
3.1. OKR
| Compounds | Stage | Apparatus | Stripe Pattern | Behavior Evaluation Parameters | Ref. |
|---|---|---|---|---|---|
| BDE-99 | 120 hpf | 35 mm Petri dish containing 3% methylcellulose | Rotational black and white stripes (10 rpm) | Saccadic movements per minute | [16] |
| Crude Oil | 96 hpf | 35 mm Petri dish Containing 3% methylcellulose | Rotating black and white vertical stripes (6 rpm) | [61] | |
| DE-71 | 15 dpf | 35 mm Petri dish containing 6% methylcellulose | A drum lined with 18° vertical black and white stripes. (8.1 rpm) | [63] | |
| BPS | 120 days post-fertilization(dpf) | A specially constructed cylindrical water-filled glass chamber | Rotating drum with black and white stripes (Unstated speed) | Slow phase gain | [56] |
| TPhP | 144 hpf | 35 mm Petri dish containing 6% methylcellulose | Sine-wave grating (with a constant angular velocity of 7.5 per second and a spatial frequency of 0.6 cycles per degree) | Ratio of eye velocity to stimulus velocity | [44] |
| TCC | 144 hpf | Fixed in a glass slide with 1.6% agarose solution | Moving vertical black-white, blue-white, green-white, and red-white stripes (12 cycles/360°, 30°/s) | [62] |
3.2. OMR
| Compounds | Stage | Apparatus | Behavior Evaluation Parameters | Ref. |
|---|---|---|---|---|
| PCB1254 | 7 dpf | Transparent test tanks with black and white stripes are generated using custom-designed raster software. | The proportion of fish that positive swimming, opposite swimming, hesitant swimming, and immobility | [15] |
| BPS | 120 dpf | A round transparent tank with an opaque column in the middle and a rotating drum with black and white stripes outside the tank. (Turn the drum clockwise for 1 min, then counterclockwise) | Concordance ratio (the quotient of male zebrafish stripe tracking time divided by the whole recording time) | [56] |
| Atrazine, diazauron | 7 dpf | Petri dishes over an LCD monitor with moving animations in black and white stripes | Proportion of zebrafish that moved to the end of the dish following the stripes | [66] |
3.3. Phototaxis Behavior Response
| Compounds | Stage | Experiment Duration | Behavior Evaluation Parameters | Ref. | |
|---|---|---|---|---|---|
| Duration in the Dark Chamber | Duration after Removing the Partition | ||||
| TCC | 144 hpf | 10 min | 5 min | Number of larvae swimming from the dark chamber into the illuminated chamber. | [62] |
| BDE-99 | 120 hpf | 5 min | 5 min, 10 min | [16] | |
| Phe | 7 dpf | 2 min | 30 min | [46] | |
| Boscalid | 8 dpf | 10 min | 1 min | [17] | |
| TPhP | 144 hpf | 5 min | 1 min | [44] | |
| DE-71 | 15 dpf | 2 min | 1 min | [63] | |
| BPS | 5 dpf | 15 min | 15 min | Numbers of larvae travelled to the light chamber within 1 min. | [70] |
3.4. Light-Dark Preference Test
| Compounds | Stage and Well Plate | Protocols | Behavior Evaluation Parameters | Ref. |
|---|---|---|---|---|
| Retinoic acid | 120 hpf, 24 well plate (1 fish/well) | Adaptation to 10 min in the dark was followed by a 10 min light (visible light) interval followed by a 10 min dark (infrared light) interval and repeated for 50 min. | Net speed change (or difference) in average swimming speeds between the last minute of 1 light state and the first minute of another light state and the average swimming speed of 10 min intervals for each state (light or dark) | [78] |
| AMI, VEN, SER | 144 hpf, 96-microwell plates | Adaptation to light for 10 min, 16 alternating dark and light periods, each 5 min long, 90 min. | Swimming distance | [80] |
| Prednisolone | 120 hpf, the apparatus consisted of a 6 cm diameter petri dish marked with a grid floor (4.5 mm2; approx. one body length), equally divided into a white and black area. The floor of the dish was further divided into three circular areas (outer, middle and central). | Individual embryos were initially acclimated within a small transparent chamber positioned within the central area for 1 min to explore the tank for 5 min. | The percentage of time spent in each compartment (light vs. dark), the proportion of time within each of the three circular areas within the light compartment, and the total level of activity (total number of lines crossed) while in the white compartment. | [49] |
| PBDE-47 | 4, 5, and 6 dpf, 96-well microplates | Adaptation to the dark took 20 min, and then recording started with a dark period of 10 min, followed by three cycles of alternating 10 min light and dark periods. The total testing time was 70 min. | Distance moved | [77] |
| Atenolol | exposed to atenolol for 7 days, 96-well plates | Alternating 10 min periods of light and dark, beginning with a dark period (i.e., dark photokinesis), for a total of 50 min. | [82] | |
| Linuron | 7 dpf larvae, 96-well plate | [76] | ||
| Esfenvalerate | 5 dpf, 6 dpf, or 7 dpf, 96-well plates | Alternating light-dark periods (10 min light–10 min dark–10 min light–1 min dark) for 50 min. | [75] | |
| F-53B | 120 hpf, 24-well plates (one larva for each well) | Adaptation was performed 30 min before the start of the test, followed by a light (10 min) –dark (10 min) –light (10 min) stimulus. | Swimming speed (mm/s) | [69] |
| CP | larvae, 48-well plates | Alternating light-dark periods (10 min light–10 min dark–10 min light–1 min dark) for 50 min. | Swimming speed (mm/min) | [81] |
| BPS | After 120 h of BPS exposure, 24-well plates (1 larvae/well, 2 mL solution/well) | Alternating light-dark periods (1 min light–1 min dark–1 min light–1 min dark) for 4 min. | The traveled distance per minute, 4 min total distances, and average speed. | [79] |
3.5. Free Swimming
| Compounds | Stage and Well Plate | Experimental Plan | Behavior Evaluation Parameters | Ref. |
|---|---|---|---|---|
| Retinoic acid | 120 hpf, 24-well plates (1 fish/well) | Two 20 min swim tests in the light and dark | Swimming speed (mm/s) | [78] |
| The combined exposure of NPs and AVO | Exposure lasted for 144 h of morphologically normal zebrafish larvae, 24-well plates (1 larva/well) | After 10 min of daptation, a 15 min swim test was performed under continuous visible light. | [87] | |
| 120 hpf, 24-well plate (1 larva/well) | [86] |
3.6. Visual Avoidance Behavior
| Compounds | Stage and Well Plate | Visual Stimuli Patterns | Protocols | Behavior Evaluation Parameters | Ref. |
|---|---|---|---|---|---|
| Prednisolone | 144 hpf, six-well plates | Produced by Microsoft PowerPoint, it consists of two black rectangles (0.4–14.6 cm) placed parallel to each other. | Acclimatization was performed on a white screen for 5 min, followed by 5 min of recording before stimulus onset, after which the animation was played for 5 min. | (Pre-animation, during, and post-animation) The amount of time embryos spent in the outer area of the well | [49] |
| PCB-95 | 5 dpf, 6-well plates | Produced by Microsoft PowerPoint, red lines (RGB values were 255, 0, 0) were spaced 1 mm apart and moved from the top to the bottom relative to the dish at a speed of 7 mm every 8 s. | Exposure to a uniform white background without visual stimuli was performed for 10 min, followed by moving the red line for 10 min and then returning to the white background for 10 min. | Swim speed, percentage of larval edge preference, and percentage of larval avoidance | [88] |
| MPs, Cu, and combined exposures of Cu and MPs | 14 dpf, 6-well plates | Produced by Microsoft PowerPoint, static or bouncing two-dimensional red discs (1.35 cm diameter, RGB 255, 0, 0) | Alternating 10 min of a white background and 10 min of a red bouncing ball appeared in the upper half of the well and moved from left-right-left on a 2 cm straight trajectory at 1 cm/s | The time spent in the lower half of the well | [89] |
4. Mechanism of Ocular Toxicity
4.1. Oxidative Stress and Inflammation
4.2. Apoptosis
4.3. Aberrant Gene Expression and Epigenetic Alterations
5. Challenges and Prospects
- (1)
- The direct correlation between ocular toxicity and biological tissue levels, including genes, proteins, tissues, and physiology, cannot be readily determined when using behavioral responses to assess the potential toxicity of environmental contaminants [32]. Therefore, further quantitative investigations employing alternative methodologies are required to establish whether the observed damage to the visual system is directly attributed to the pollutants under scrutiny. In vision science research, the up-regulated or down-regulated trend of key visual pathways can be verified through molecular detection of gene and protein expression related to retinal development and function. Additionally, histological analysis using microscopy and physiological analysis through electroretinograms can serve to corroborate these trends. Further research is necessary to determine whether environmental contaminants have genetic effects on visual system toxicity in zebrafish;
- (2)
- Although many visually mediated behavior testing methods have been applied to zebrafish research, there is currently a lack of unified standards for each method in actual application. Therefore, it is crucial to establish zebrafish behavioral standards under the guidance of vision. For example, in behavioral experiments, it is essential to standardize key experimental parameters such as light intensity, pollutant co-incubation time, and recording time to improve the repeatability of research results across different teams and strengthen the persuasiveness of conclusions based on zebrafish behavioral data. This will promote the construction of zebrafish behavior test standards;
- (3)
- Considering the different routes of environmental pollution entry into zebrafish and mammalian visual systems is important when utilizing zebrafish as a predictive model. There is no doubt that environmental pollutants exist in a variety of environmental substrates, including water, sediments, soil, air, and biota. As a result, both zebrafish and human eyes are inevitably exposed to environmental pollutants, which can cause ocular damage [11,124,125,126]. This commonality indicates the potential shared effects of environmental pollutants on the visual system across different species. Researchers can take advantage of the benefits of zebrafish for rapid evaluation, drug screening, and preliminary studies of mechanisms. Nevertheless, multiple animal models, multiple tissue levels, and multi-angle studies are necessary to further clarify the mechanism of ocular toxicity caused by environmental contaminants.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDE99 | 2,2’,4,4’,5-pentabromodiphenyl ether |
| PBDE-47 | 2,2′,4,4′-tetrabromodiphenyl ether |
| AMI | Amitriptyline |
| AVO | Avobenzone |
| BPS | Bisphenol S |
| BFRs | Brominated flame retardants |
| CAT | Catalase |
| CP | Cyclophosphamide |
| EDCs | Endocrine disrupting chemicals |
| GCL | Ganglion cell layer |
| hpf | Hours post-fertilization |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| MDA | Malondialdehyde |
| MPs | Microplastics |
| NPs | Nanoplastics |
| OKR | Optokinetic reflex |
| OMR | Optomotor response |
| ONL | Outer nuclear layer |
| OPL | Outer plexiform layer |
| PPCPs | Pharmaceutical and personal care products |
| Phe | Phenanthrene polycyclic aromatic hydrocarbon |
| PBDE | Polybrominated diphenyl ether |
| PAHs | Polycyclic aromatic hydrocarbons |
| ROS | Reactive oxygen species |
| RGC | Retinal ganglion cells |
| RPE | Retinal pigment epithelium |
| SER | Sertraline |
| SOD | Superoxide dismutase |
| TCC | Triclocarban |
| TPhP | Triphenyl phosphate |
| UV | Ultraviolet |
| VEN | Venlafaxine |
References
- Plaisancié, J.; Ceroni, F.; Holt, R.; Zazo Seco, C.; Calvas, P.; Chassaing, N.; Ragge, N.K. Genetics of Anophthalmia and Microphthalmia. Part 1: Non-Syndromic Anophthalmia/Microphthalmia. Hum. Genet. 2019, 138, 799–830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gong, L.; Li, Y.; Xu, H.; Gu, Z.; Zhao, Y. Safety Assessment of Nanomaterials to Eyes: An Important but Neglected Issue. Adv. Sci. 2019, 6, 1802289. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Trends in Prevalence of Blindness and Distance and near Vision Impairment over 30 Years: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef] [PubMed]
- Zona, A.; Iavarone, I.; Buzzoni, C.; Conti, S.; Santoro, M.; Fazzo, L.; Pasetto, R.; Pirastu, R.; Bruno, C.; Ancona, C.; et al. SENTIERI: Epidemiological Study of Residents in National Priority Contaminated Sites. Fifth Report. Epidemiol. Prev. 2019, 43, 1–208. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, S.; Gorini, F.; Santoro, M.; Pierini, A.; Minichilli, F.; Bianchi, F. Environmental and Individual Exposure and the Risk of Congenital Anomalies: A Review of Recent Epidemiological Evidence. Epidemiol. Prev. 2018, 42, 1–34. [Google Scholar] [PubMed]
- Dolk, H.; Vrijheid, M. The Impact of Environmental Pollution on Congenital Anomalies. Br. Med. Bull. 2003, 68, 25–45. [Google Scholar] [CrossRef]
- Pellacani, C.; Tagliaferri, S.; Caglieri, A.; Goldoni, M.; Giordano, G.; Mutti, A.; Costa, L.G. Synergistic Interactions between PBDEs and PCBs in Human Neuroblastoma Cells. Environ. Toxicol. 2014, 29, 418–427. [Google Scholar] [CrossRef]
- Asante, K.A.; Adu-Kumi, S.; Nakahiro, K.; Takahashi, S.; Isobe, T.; Sudaryanto, A.; Devanathan, G.; Clarke, E.; Ansa-Asare, O.D.; Dapaah-Siakwan, S.; et al. Human Exposure to PCBs, PBDEs and HBCDs in Ghana: Temporal Variation, Sources of Exposure and Estimation of Daily Intakes by Infants. Environ. Int. 2011, 37, 921–928. [Google Scholar] [CrossRef]
- Polevoy, C.; Arbuckle, T.E.; Oulhote, Y.; Lanphear, B.P.; Cockell, K.A.; Muckle, G.; Saint-Amour, D. Prenatal Exposure to Legacy Contaminants and Visual Acuity in Canadian Infants: A Maternal-Infant Research on Environmental Chemicals Study (MIREC-ID). Environ. Health 2020, 19, 14. [Google Scholar] [CrossRef]
- Wigle, D.T.; Arbuckle, T.E.; Turner, M.C.; Bérubé, A.; Yang, Q.; Liu, S.; Krewski, D. Epidemiologic Evidence of Relationships between Reproductive and Child Health Outcomes and Environmental Chemical Contaminants. J. Toxicol. Environ. Health B Crit. Rev. 2008, 11, 373–517. [Google Scholar] [CrossRef]
- Chen, L. Visual System: An Understudied Target of Aquatic Toxicology. Aquat. Toxicol. 2020, 225, 105542. [Google Scholar] [CrossRef] [PubMed]
- Harada, M. Minamata Disease: Methylmercury Poisoning in Japan Caused by Environmental Pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Fillion, M.; Lemire, M.; Philibert, A.; Frenette, B.; Weiler, H.A.; Deguire, J.R.; Guimarães, J.R.D.; Larribe, F.; Barbosa, F.; Mergler, D. Toxic Risks and Nutritional Benefits of Traditional Diet on near Visual Contrast Sensitivity and Color Vision in the Brazilian Amazon. Neurotoxicology 2013, 37, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Altmann, L.; Sveinsson, K.; Krämer, U.; Weishoff-Houben, M.; Turfeld, M.; Winneke, G.; Wiegand, H. Visual Functions in 6-Year-Old Children in Relation to Lead and Mercury Levels. Neurotoxicol. Teratol. 1998, 20, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hong, Q.; Yang, L.; Zhang, M.; Guo, X.; Chi, X.; Tong, M. PCB1254 Exposure Contributes to the Abnormalities of Optomotor Responses and Influence of the Photoreceptor Cell Development in Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2015, 118, 133–138. [Google Scholar] [CrossRef][Green Version]
- Wei, S.; Chen, F.; Xu, T.; Cao, M.; Yang, X.; Zhang, B.; Guo, X.; Yin, D. BDE-99 Disrupts the Photoreceptor Patterning of Zebrafish Larvae via Transcription Factor Six7. Environ. Sci. Technol. 2022, 56, 5673–5683. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Wang, Z.; Magnuson, J.T.; Volz, D.C.; Schlenk, D.; Jiang, J.; Wang, C. Environmentally Relevant Concentrations of Boscalid Exposure Affects the Neurobehavioral Response of Zebrafish by Disrupting Visual and Nervous Systems. J. Hazard. Mater. 2021, 404, 124083. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Bilotta, J.; Saszik, S. The Zebrafish as a Model Visual System. Int. J. Dev. Neurosci. 2001, 19, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, J.; Wu, W.; Fang, Y.; Liu, F.; Yang, Q.; Hu, X.; Gu, X.; He, Z.; Sun, D.; et al. Effect of Aerobic Exercise as a Treatment on Type 2 Diabetes Mellitus with Depression-like Behavior Zebrafish. Life Sci. 2022, 300, 120578. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Liu, F.; Fang, Y.; Wang, L.; Chen, H.; Yang, Q.; Dong, H.; Jin, L.; Wu, W.; Sun, D. Improvement in Zebrafish with Diabetes and Alzheimer’s Disease Treated with Pasteurized Akkermansia Muciniphila. Microbiol. Spectr. 2023, 11, e0084923. [Google Scholar] [CrossRef]
- Ašmonaitė, G.; Boyer, S.; de Souza, K.B.; Wassmur, B.; Sturve, J. Behavioural Toxicity Assessment of Silver Ions and Nanoparticles on Zebrafish Using a Locomotion Profiling Approach. Aquat. Toxicol. 2016, 173, 143–153. [Google Scholar] [CrossRef]
- Legradi, J.; el Abdellaoui, N.; van Pomeren, M.; Legler, J. Comparability of Behavioural Assays Using Zebrafish Larvae to Assess Neurotoxicity. Environ. Sci. Pollut. Res. Int. 2015, 22, 16277–16289. [Google Scholar] [CrossRef]
- Shi, Q.; Tsui, M.M.P.; Hu, C.; Lam, J.C.W.; Zhou, B.; Chen, L. Acute Exposure to Triphenyl Phosphate (TPhP) Disturbs Ocular Development and Muscular Organization in Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2019, 179, 119–126. [Google Scholar] [CrossRef]
- Sloman, K.A.; McNeil, P.L. Using Physiology and Behaviour to Understand the Responses of Fish Early Life Stages to Toxicants. J. Fish Biol. 2012, 81, 2175–2198. [Google Scholar] [CrossRef]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The Zebrafish Eye—A Paradigm for Investigating Human Ocular Genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Liu, D.; Liu, T.; Xing, L. Neurotoxicity of Nanoparticles: Insight from Studies in Zebrafish. Ecotoxicol. Environ. Saf. 2022, 242, 113896. [Google Scholar] [CrossRef]
- Shen, C.; Zuo, Z. Zebrafish (Danio Rerio) as an Excellent Vertebrate Model for the Development, Reproductive, Cardiovascular, and Neural and Ocular Development Toxicity Study of Hazardous Chemicals. Environ. Sci. Pollut. Res. Int. 2020, 27, 43599–43614. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Dunn, C.; Ramos, M.F. Zebrafish as an Animal Model for Ocular Toxicity Testing: A Review of Ocular Anatomy and Functional Assays. Toxicol. Pathol. 2021, 49, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Zhang, W.; Ma, J.; Xia, Y.; Yu, H.; Du, J.; Fang, Y.; Wang, L.; Zhang, K.; Jin, L.; et al. Advances in the Utilization of Zebrafish for Assessing and Understanding the Mechanisms of Nano-/Microparticles Toxicity in Water. Toxics 2023, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Deeti, S.; O’Farrell, S.; Kennedy, B.N. Early Safety Assessment of Human Oculotoxic Drugs Using the Zebrafish Visualmotor Response. J. Pharmacol. Toxicol. Methods 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Salvatore, L.; Stella, J.; Geathers, J.S.; Weber, S.R.; Grillo, M.A.; Barber, A.J.; Sundstrom, J.M.; Grillo, S.L. Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina. Cells 2021, 10, 633. [Google Scholar] [CrossRef]
- Noel, N.C.L.; Allison, W.T.; MacDonald, I.M.; Hocking, J.C. Zebrafish and Inherited Photoreceptor Disease: Models and Insights. Prog. Retin. Eye Res. 2022, 91, 101096. [Google Scholar] [CrossRef]
- Yoshimatsu, T.; Schröder, C.; Nevala, N.E.; Berens, P.; Baden, T. Fovea-like Photoreceptor Specializations Underlie Single UV Cone Driven Prey-Capture Behavior in Zebrafish. Neuron 2020, 107, 320–337.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Cai, S.J.; Cui, J.L.; Chen, Y.; Tang, X.; Li, Y.H. Correlation between Photoreceptor Injury-Regeneration and Behavior in a Zebrafish Model. Neural Regen. Res. 2017, 12, 795–803. [Google Scholar] [CrossRef]
- Hong, Y.; Luo, Y. Zebrafish Model in Ophthalmology to Study Disease Mechanism and Drug Discovery. Pharmaceuticals 2021, 14, 716. [Google Scholar] [CrossRef]
- Blanco-Sánchez, B.; Clément, A.; Phillips, J.B.; Westerfield, M. Zebrafish Models of Human Eye and Inner Ear Diseases. Methods Cell Biol. 2017, 138, 415–467. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller Cells in the Healthy and Diseased Retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Neuhauss, S.C.F. Biochemistry and Physiology of Zebrafish Photoreceptors. Pflug. Arch. 2021, 473, 1569–1585. [Google Scholar] [CrossRef]
- Harsing, L.G.; Szénási, G.; Zelles, T.; Köles, L. Purinergic–Glycinergic Interaction in Neurodegenerative and Neuroinflammatory Disorders of the Retina. Int. J. Mol. Sci. 2021, 22, 6209. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Z.; Chen, L.; Fu, J.; Han, J.; Hu, B.; Zhou, B. Optical Toxicity of Triphenyl Phosphate in Zebrafish Larvae. Aquat. Toxicol. 2019, 210, 139–147. [Google Scholar] [CrossRef]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a Better Understanding of Human Eye Disease Insights from the Zebrafish, Danio Rerio. Prog. Mol. Biol. Transl. Sci. 2011, 100, 287–330. [Google Scholar] [CrossRef]
- Huang, L.; Wang, C.; Zhang, Y.; Wu, M.; Zuo, Z. Phenanthrene Causes Ocular Developmental Toxicity in Zebrafish Embryos and the Possible Mechanisms Involved. J. Hazard. Mater. 2013, 261, 172–180. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, Q.; Chen, J.; Ding, L.; Rao, F.; Lv, J.; Xie, B.; Xiang, S.; Yu, H.; Chen, X.; et al. Image-Guided Optical Coherence Tomography to Assess Structural Changes in Rodent Retinas. J. Vis. Exp. 2023, 192, e64783. [Google Scholar] [CrossRef]
- Conedera, F.M.; Arendt, P.; Trepp, C.; Tschopp, M.; Enzmann, V. Müller Glia Cell Activation in a Laser-Induced Retinal Degeneration and Regeneration Model in Zebrafish. J. Vis. Exp. 2017, 128, 56249. [Google Scholar] [CrossRef]
- McNeil, P.L.; Nebot, C.; Cepeda, A.; Sloman, K.A. Environmental Concentrations of Prednisolone Alter Visually Mediated Responses during Early Life Stages of Zebrafish (Danio Rerio). Environ. Pollut. 2016, 218, 981–987. [Google Scholar] [CrossRef][Green Version]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An Emerging Model to Study Microplastic and Nanoplastic Toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef]
- Chhetri, J.; Jacobson, G.; Gueven, N. Zebrafish—On the Move towards Ophthalmological Research. Eye 2014, 28, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, Z.; Yang, Y.; Jiao, Y.; Qu, J.; Wang, Y.; Zhang, Y. Effects of Common Environmental Endocrine-Disrupting Chemicals on Zebrafish Behavior. Water Res. 2022, 208, 117826. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.J.; Krook, J.; Szaszkiewicz, J.; Burggren, W. Shoaling, Boldness, Anxiety-like Behavior and Locomotion in Zebrafish (Danio Rerio) Are Altered by Acute Benzo[a]Pyrene Exposure. Sci. Total Environ. 2021, 774, 145702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, Q.; Di Paolo, C.; Shao, Y.; Hollert, H.; Seiler, T.B. Behavioral Profile Alterations in Zebrafish Larvae Exposed to Environmentally Relevant Concentrations of Eight Priority Pharmaceuticals. Sci. Total Environ. 2019, 664, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Portugues, R.; Engert, F. The Neural Basis of Visual Behaviors in the Larval Zebrafish. Curr. Opin. Neurobiol. 2009, 19, 644–647. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Wei, P.; Tian, H.; Wang, W.; Ru, S. Long-Term Exposure to Bisphenol S Damages the Visual System and Reduces the Tracking Capability of Male Zebrafish (Danio Rerio). J. Appl. Toxicol. 2018, 38, 248–258. [Google Scholar] [CrossRef]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A Model System for the Study of Eye Genetics. Prog. Retin. Eye Res. 2008, 27, 89–110. [Google Scholar] [CrossRef]
- Easter, S.S.; Nicola, G.N. The Development of Eye Movements in the Zebrafish (Danio Rerio). Dev. Psychobiol. 1997, 31, 267–276. [Google Scholar] [CrossRef]
- Richards, F.M.; Alderton, W.K.; Kimber, G.M.; Liu, Z.; Strang, I.; Redfern, W.S.; Valentin, J.P.; Winter, M.J.; Hutchinson, T.H. Validation of the Use of Zebrafish Larvae in Visual Safety Assessment. J. Pharmacol. Toxicol. Methods 2008, 58, 50–58. [Google Scholar] [CrossRef]
- Tierney, K.B. Behavioural Assessments of Neurotoxic Effects and Neurodegeneration in Zebrafish. Biochim. Biophys. Acta 2011, 1812, 381–389. [Google Scholar] [CrossRef]
- Magnuson, J.T.; Bautista, N.M.; Lucero, J.; Lund, A.K.; Xu, E.G.; Schlenk, D.; Burggren, W.W.; Roberts, A.P. Exposure to Crude Oil Induces Retinal Apoptosis and Impairs Visual Function in Fish. Environ. Sci. Technol. 2020, 54, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Chen, Z.F.; Lin, Z.C.; Liao, X.L.; Zou, T.; Qi, Z.; Cai, Z. Toxic Effects of Triclocarban on Larval Zebrafish: A Focus on Visual Dysfunction. Aquat. Toxicol. 2021, 241, 106013. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Y.; Huang, C.; Hu, B.; Hu, C.; Zhou, B. Acute Exposure to DE-71 Causes Alterations in Visual Behavior in Zebrafish Larvae. Environ. Toxicol. Chem. 2013, 32, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Maaswinkel, H.; Li, L. Spatio-Temporal Frequency Characteristics of the Optomotor Response in Zebrafish. Vis. Res. 2003, 43, 21–30. [Google Scholar] [CrossRef]
- LeFauve, M.K.; Rowe, C.J.; Crowley-Perry, M.; Wiegand, J.L.; Shapiro, A.G.; Connaughton, V.P. Using a Variant of the Optomotor Response as a Visual Defect Detection Assay in Zebrafish. J. Biol. Methods 2021, 8, e144. [Google Scholar] [CrossRef] [PubMed]
- Zaluski, A.B.; Wiprich, M.T.; de Almeida, L.F.; de Azevedo, A.P.; Bonan, C.D.; Vianna, M.R.M. Atrazine and Diuron Effects on Survival, Embryo Development, and Behavior in Larvae and Adult Zebrafish. Front. Pharmacol. 2022, 13, 841826. [Google Scholar] [CrossRef]
- Huang, L.; Zuo, Z.; Zhang, Y.; Wu, M.; Lin, J.J.; Wang, C. Use of Toxicogenomics to Predict the Potential Toxic Effect of Benzo(a)Pyrene on Zebrafish Embryos: Ocular Developmental Toxicity. Chemosphere 2014, 108, 55–61. [Google Scholar] [CrossRef]
- Dehnert, G.K.; Karasov, W.H.; Wolman, M.A. 2,4-Dichlorophenoxyacetic Acid Containing Herbicide Impairs Essential Visually Guided Behaviors of Larval Fish. Aquat. Toxicol. 2019, 209, 1–12. [Google Scholar] [CrossRef]
- Wu, L.; Zeeshan, M.; Dang, Y.; Liang, L.Y.; Gong, Y.C.; Li, Q.Q.; Tan, Y.W.; Fan, Y.Y.; Lin, L.Z.; Zhou, Y.; et al. Environmentally Relevant Concentrations of F-53B Induce Eye Development Disorders-Mediated Locomotor Behavior in Zebrafish Larvae. Chemosphere 2022, 308, 136130. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, S.; Yang, Y.; Zhang, R.; Ru, S.; Zhang, X. Mechanism of Bisphenol S Exposure on Color Sensitivity of Zebrafish Larvae. Environ. Pollut. 2023, 316, 120670. [Google Scholar] [CrossRef]
- Comai, S.; De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; Bedini, A.; Gobbi, G. Dysfunction of Serotonergic Activity and Emotional Responses across the Light-Dark Cycle in Mice Lacking Melatonin MT2 Receptors. J. Pineal Res. 2020, 69, e12653. [Google Scholar] [CrossRef]
- Lahouel, A.; Kebieche, M.; Lakroun, Z.; Rouabhi, R.; Fetoui, H.; Chtourou, Y.; Djamila, Z.; Soulimani, R. Neurobehavioral Deficits and Brain Oxidative Stress Induced by Chronic Low Dose Exposure of Persistent Organic Pollutants Mixture in Adult Female Rat. Environ. Sci. Pollut. Res. Int. 2016, 23, 19030–19040. [Google Scholar] [CrossRef]
- Hwang, K.S.; Son, Y.; Kim, S.S.; Shin, D.S.; Lim, S.H.; Yang, J.Y.; Jeong, H.N.; Lee, B.H.; Bae, M.A. Size-Dependent Effects of Polystyrene Nanoparticles (PS-NPs) on Behaviors and Endogenous Neurochemicals in Zebrafish Larvae. Int. J. Mol. Sci. 2022, 23, 10682. [Google Scholar] [CrossRef]
- Tang, Y.; Mi, P.; Li, M.; Zhang, S.; Li, J.; Feng, X. Environmental Level of the Antidepressant Venlafaxine Induces Behavioral Disorders through Cortisol in Zebrafish Larvae (Danio Rerio). Neurotoxicol Teratol. 2021, 83, 106942. [Google Scholar] [CrossRef]
- Wang, X.H.; Souders, C.L.; Xavier, P.; Li, X.Y.; Yan, B.; Martyniuk, C.J. The Pyrethroid Esfenvalerate Induces Hypoactivity and Decreases Dopamine Transporter Expression in Embryonic/Larval Zebrafish (Danio Rerio). Chemosphere 2020, 243, 125416. [Google Scholar] [CrossRef]
- Maharaj, S.; El Ahmadie, N.; Rheingold, S.; El Chehouri, J.; Yang, L.; Souders, C.L.; Martyniuk, C.J. Sub-Lethal Toxicity Assessment of the Phenylurea Herbicide Linuron in Developing Zebrafish (Danio Rerio) Embryo/Larvae. Neurotoxicol. Teratol. 2020, 81, 106917. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, T.; Yin, D.Q. Locomotor Activity Changes on Zebrafish Larvae with Different 2,2′,4,4′-Tetrabromodiphenyl Ether (PBDE-47) Embryonic Exposure Modes. Chemosphere 2014, 94, 53–61. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Du, C.; Li, C.; Huang, C.; Dong, Q. Characterization of Retinoic Acid-Induced Neurobehavioral Effects in Developing Zebrafish. Environ. Toxicol. Chem. 2014, 33, 431–437. [Google Scholar] [CrossRef]
- Wei, S.; Qiu, L.; Ru, S.; Yang, Y.; Wang, J.; Zhang, X. Bisphenol S Disrupts Opsins Gene Expression and Impairs the Light-Sensing Function via Antagonizing TH-TRβ Signaling Pathway in Zebrafish Larvae. Food Chem. Toxicol. 2023, 172, 113588. [Google Scholar] [CrossRef]
- Sehonova, P.; Hodkovicova, N.; Urbanova, M.; Örn, S.; Blahova, J.; Svobodova, Z.; Faldyna, M.; Chloupek, P.; Briedikova, K.; Carlsson, G. Effects of Antidepressants with Different Modes of Action on Early Life Stages of Fish and Amphibians. Environ. Pollut. 2019, 254, 112999. [Google Scholar] [CrossRef]
- Li, D.; Sun, W.; Chen, H.; Lei, H.; Li, X.; Liu, H.; Huang, G.-Y.; Shi, W.J.; Ying, G.G.; Luo, Y.; et al. Cyclophosphamide Affects Eye Development and Locomotion in Zebrafish (Danio Rerio). Sci. Total Environ. 2022, 805, 150460. [Google Scholar] [CrossRef] [PubMed]
- Ivantsova, E.; Konig, I.; Souders, C.L.; McNabney, D.; Simmons, D.D.B.; Martyniuk, C.J. Lipidomic, Metabolomic, and Behavior Responses of Zebrafish (Danio Rerio) Exposed to Environmental Levels of the Beta Blocker Atenolol. Sci. Total Environ. 2023, 866, 161272. [Google Scholar] [CrossRef] [PubMed]
- Saint-Amant, L.; Drapeau, P. Time Course of the Development of Motor Behaviors in the Zebrafish Embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Alex, S.A.; Seenivasan, R.; Chandrasekaran, N.; Mukherjee, A. Interactive Effects of Micro/Nanoplastics and Nanomaterials/Pharmaceuticals: Their Ecotoxicological Consequences in the Aquatic Systems. Aquat. Toxicol. 2021, 232, 105747. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Baptista Neto, J.A.; da Fonseca, E.M. Nanoplastics in Aquatic Systems—Are They More Hazardous than Microplastics? Environ. Pollut. 2021, 272, 115950. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ling, X.; Yan, Z.; Wu, D.; Liu, J.; Lu, G. Effects of Nanoplastics and Butyl Methoxydibenzoylmethane on Early Zebrafish Embryos Identified by Single-Cell RNA Sequencing. Environ. Sci. Technol. 2021, 55, 1885–1896. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Li, N.; Jiang, S. Avobenzone and Nanoplastics Affect the Development of Zebrafish Nervous System and Retinal System and Inhibit Their Locomotor Behavior. Sci. Total Environ. 2022, 806, 150681. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Thorn, R.J.; Seto, R.; Creton, R.; Bridges, W.C.; Chapman, S.C.; Lee, C.M. Embryonic Exposure to 2,2′,3,5′,6-Pentachlorobiphenyl (PCB-95) Causes Developmental Malformations in Zebrafish. Environ. Toxicol. Chem. 2020, 39, 162–170. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Matos, C.; Bellas, J.; Monteiro, S.M.; Félix, L. Microplastics Alone or Co-Exposed with Copper Induce Neurotoxicity and Behavioral Alterations on Zebrafish Larvae after a Subchronic Exposure. Aquat. Toxicol. 2021, 235, 105814. [Google Scholar] [CrossRef]
- Alhasani, R.H.; Zhou, X.; Biswas, L.; Li, X.; Reilly, J.; Zeng, Z.; Shu, X. Gypenosides Attenuate Retinal Degeneration in a Zebrafish Retinitis Pigmentosa Model. Exp. Eye Res. 2020, 201, 108291. [Google Scholar] [CrossRef]
- Yang, H.; Gu, X.; Chen, H.; Zeng, Q.; Mao, Z.; Jin, M.; Li, H.; Ge, Y.; Zha, J.; Martyniuk, C.J. Transcriptome Profiling Reveals Toxicity Mechanisms Following Sertraline Exposure in the Brain of Juvenile Zebrafish (Danio Rerio). Ecotoxicol. Environ. Saf. 2022, 242, 113936. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Hong, Q.; Qin, D.; Kou, C.Z.; Zhang, C.M.; Guo, M.; Guo, X.R.; Chi, X.; Tong, M.L. Effects of Embryonic Exposure to Polychlorinated Biphenyls on Zebrafish (Danio Rerio) Retinal Development. J. Appl. Toxicol. 2012, 32, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Vimalkumar, K.; Seethappan, S.; Pugazhendhi, A. Fate of Triclocarban (TCC) in Aquatic and Terrestrial Systems and Human Exposure. Chemosphere 2019, 230, 201–209. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, S.; Shi, X.; Luo, C.; Huang, W.; Lin, H.; Peng, J.; Tan, W.; Wu, K. Neurodevelopmental Toxicity of Organophosphate Flame Retardant Triphenyl Phosphate (TPhP) on Zebrafish (Danio Rerio) at Different Life Stages. Environ. Int. 2023, 172, 107745. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, W.; Chen, J.; Zhan, J.; Pan, C.; Lei, X.; Wu, M. Growth Inhibition and Coordinated Physiological Regulation of Zebrafish (Danio Rerio) Embryos upon Sublethal Exposure to Antidepressant Amitriptyline. Aquat. Toxicol. 2014, 151, 68–76. [Google Scholar] [CrossRef]
- Wang, H.; Meng, Z.; Liu, F.; Zhou, L.; Su, M.; Meng, Y.; Zhang, S.; Liao, X.; Cao, Z.; Lu, H. Characterization of Boscalid-Induced Oxidative Stress and Neurodevelopmental Toxicity in Zebrafish Embryos. Chemosphere 2020, 238, 124753. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, Tissue Distribution, and Toxicity of Polystyrene Nanoparticles in Developing Zebrafish (Danio Rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.-H.; Liang, S.-T.; Chen, J.-R.; Chen, K.H.-C.; Hsiao, C.-D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, T.; Hu, Z.; Li, Y.; Yuan, H.; Zhao, K.; Zhang, H.; Liu, C. Effects of Triclocarban on Oxidative Stress and Innate Immune Response in Zebrafish Embryos. Chemosphere 2018, 210, 93–101. [Google Scholar] [CrossRef]
- Xu, H.; Dong, X.; Zhang, Z.; Yang, M.; Wu, X.; Liu, H.; Lao, Q.; Li, C. Assessment of Immunotoxicity of Dibutyl Phthalate Using Live Zebrafish Embryos. Fish Shellfish. Immunol. 2015, 45, 286–292. [Google Scholar] [CrossRef]
- Yang, H.; Lai, H.; Huang, J.; Sun, L.; Mennigen, J.A.; Wang, Q.; Liu, Y.; Jin, Y.; Tu, W. Polystyrene Microplastics Decrease F–53B Bioaccumulation but Induce Inflammatory Stress in Larval Zebrafish. Chemosphere 2020, 255, 127040. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Jiang, Y.; Zhang, S.; Hong, Q.; Guo, X.; Chi, X.; Tong, M. Expression and Significance of MiR—20b in Retinal Photoreceptor Cells Exposed to PCB1254. Aging 2019, 11, 8969–8981. [Google Scholar] [CrossRef]
- Xu, T.; Chen, L.; Hu, C.; Zhou, B. Effects of Acute Exposure to Polybrominated Diphenyl Ethers on Retinoid Signaling in Zebrafish Larvae. Environ. Toxicol. Pharmacol. 2013, 35, 13–20. [Google Scholar] [CrossRef]
- Roy, N.M.; Carneiro, B.; Ochs, J. Glyphosate Induces Neurotoxicity in Zebrafish. Environ. Toxicol. Pharmacol. 2016, 42, 45–54. [Google Scholar] [CrossRef]
- Caioni, G.; Merola, C.; Bertolucci, C.; Lucon-Xiccato, T.; Savaşçı, B.B.; Massimi, M.; Colasante, M.; Fioravanti, G.; Cacciola, N.A.; Ippoliti, R.; et al. Early-Life Exposure to Environmentally Relevant Concentrations of Triclocarban Impairs Ocular Development in Zebrafish Larvae. Chemosphere 2023, 324, 138348. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; De la Vieja, A.; de Alba Gonzalez, M.; Esteban Lopez, M.; Castaño Calvo, A.; Cañas Portilla, A.I. Toxicity of Nanoplastics for Zebrafish Embryos, What We Know and Where to Go Next. Sci. Total Environ. 2021, 797, 149125. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, J.; Yin, D.; Zhao, Q.; Dong, B. High-Throughput RNA Sequencing Reveals the Effects of 2,2′,4,4′-Tetrabromodiphenyl Ether on Retina and Bone Development of Zebrafish Larvae. BMC Genom. 2015, 16, 23. [Google Scholar] [CrossRef]
- Palacio-Cortés, A.M.; Horton, A.A.; Newbold, L.; Spurgeon, D.; Lahive, E.; Pereira, M.G.; Grassi, M.T.; Moura, M.O.; Disner, G.R.; Cestari, M.M.; et al. Accumulation of Nylon Microplastics and Polybrominated Diphenyl Ethers and Effects on Gut Microbial Community of Chironomus Sancticaroli. Sci. Total Environ. 2022, 832, 155089. [Google Scholar] [CrossRef]
- PubChem 2,2′,4,4′,5-Pentabromodiphenyl Ether. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/36159 (accessed on 28 July 2023).
- PubChem Hazardous Substances Data Bank (HSDB): 2166. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/2166#section=LogP (accessed on 28 July 2023).
- PubChem Hazardous Substances Data Bank (HSDB): 7836. Available online: https://pubchem.ncbi.nlm.nih.gov/source/hsdb/7836 (accessed on 28 July 2023).
- PubChem Concept 53623. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Polychlorinated-Biphenyls (accessed on 28 July 2023).
- PubChem 4,4′-Sulfonyldiphenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6626 (accessed on 28 July 2023).
- PubChem Triclocarban. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7547 (accessed on 28 July 2023).
- Wang, S.; Huang, J.; Yang, Y.; Hui, Y.; Ge, Y.; Larssen, T.; Yu, G.; Deng, S.; Wang, B.; Harman, C. First Report of a Chinese PFOS Alternative Overlooked for 30 Years: Its Toxicity, Persistence, and Presence in the Environment. Environ. Sci. Technol. 2013, 47, 10163–10170. [Google Scholar] [CrossRef]
- PubChem Triphenyl Phosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8289 (accessed on 28 July 2023).
- PubChem Boscalid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/213013 (accessed on 28 July 2023).
- PubChem Amitriptyline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2160 (accessed on 28 July 2023).
- PubChem Venlafaxine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5656 (accessed on 28 July 2023).
- PubChem Sertraline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/68617 (accessed on 28 July 2023).
- PubChem Avobenzone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/51040 (accessed on 28 July 2023).
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative Investigation of the Mechanisms of Microplastics and Nanoplastics toward Zebrafish Larvae Locomotor Activity. Sci. Total Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Yang, D.L.; Liu, H.; Bi, J.; Bao, Y.B.; Ma, J.Y.; Zheng, Q.X.; Cui, D.L.; Chen, W.; Xiang, P. Effects of TCPP and TCEP Exposure on Human Corneal Epithelial Cells: Oxidative Damage, Cell Cycle Arrest, and Pyroptosis. Chemosphere 2023, 331, 138817. [Google Scholar] [CrossRef] [PubMed]
- Boucher, O.; Burden, M.J.; Muckle, G.; Saint-Amour, D.; Ayotte, P.; Dewailly, É.; Nelson, C.A.; Jacobson, S.W.; Jacobson, J.L. Response Inhibition and Error Monitoring during a Visual Go/No-Go Task in Inuit Children Exposed to Lead, Polychlorinated Biphenyls, and Methylmercury. Environ. Health Perspect. 2012, 120, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Yang, Y.; Zhou, Y.; Huang, W.; Wang, Z.; Zeng, X.Y.; Liu, R.Q.; Yang, B.Y.; Hu, L.W.; Zeng, X.W.; et al. Incidence of Ocular Conditions Associated with Perfluoroalkyl Substances Exposure: Isomers of C8 Health Project in China. Environ. Int. 2020, 137, 105555. [Google Scholar] [CrossRef] [PubMed]

| Categories | Compounds | Water Solubility | Exposure Dose | Exposure Time | Changes in Genes Associated with Visual Development | Ref. |
|---|---|---|---|---|---|---|
| EDCs | PBDE-47 | Insoluble in water | 500 μg/L | / | rx2, opn1sw1, opn1mw1, opn1sw2 ↓; opn1lw1 ↑ | [107,108] |
| DE-71 | water-soluble | 0.32, 3.58, and 31.0 μg/L | from 2 hpf to 15 dpf | zfrho, zfgr1 ↑ (3.58, and 31.0 μg/L) | [63] | |
| BDE-99 | 9e-10 mg/mL | 5, 50 μg/L | from 3 hpf to 120 hpf | opn1sw1, opn1sw2, opn1mw1, opn1mw2, opn1lw2, rho ↓ | [16,109] | |
| Phe | 1.10 mg/L | 3.56, 35.6 and 356 μg/L | from 2 hpf to 72 hpf | Zeb1 ↓, Mitf ↑ | [46,110] | |
| Crude oil | 6.6e-3 mg/L | 12.8, 38.5, 64.2, and 89.8 μg/L | from 4 hpf to 72 hpf | arr3b, gnat2, opn1mw, rgr, rho, rpe65a, sws1 ↓ (38.5, 64.2 and 89.8 μg/L); crx, pde6c, pde6h ↓ (64.2, 89.8 μg/L) | [62,111] | |
| PCB1254 | extremely low | 0.125, 0.25, 0.5 and 1 mg/L | 7 days of continuous exposure | SWS2 ↓ (0.125,0.25,0.5 and 1 mg/L); CRX, RHO, SWS1 ↓ (0.5, 1 mg/L) | [15,112] | |
| BPS | Insoluble in water | 1, 10, 100 and 1000 μg/L | from 2 hpf to 120 dpf | zfrho ↑ (1,10 μg/L); zfblue ↑ (1000 μg/L); zfred, zfgr1 ↑ (1, 10, 100, 1000 μg/L) | [57,113] | |
| 4400 nM | from 2 hpf to 24 hpf | opn1sw1, arr3a, pde6h, gk1b ↑, opn1mw2, opn1lw1, opn1lw2, rho, pde6a ↓ (4 nM); opn1sw1, opn1sw2, opn1mw1, opn1mw2, opn1mw3, opn1lw1, opn1lw2, arr3a, pde6h, gk1b ↓, rho, pde6a ↑ (400 nM) | [79] | |||
| TCC | 0.11 mg/L | 0.20, 40.0 µg/L | from 2 hpf to 6 dpf | opn1sw2, opn1mw1, opn1mw3, opn1lw1, opn1lw2, rho ↓ | [62,114] | |
| F-53B | 200 mg/L | 0.15, 1.5 and 15 μg/L | from 2 hpf to 120 hpf | aldh1a2, cryaa, crybb, crygn, mipa, pax6, rx1, gant1, rho, opn1sw, opn1lw ↓ | [69,115] | |
| BFRs | TPhP | Insoluble in water | 4, 100 μg/L | from 2 hpf to 144 hpf | zfrho, zfred, zfgr1, zfuv, zfblue, rhodopsin ↓ (100 μg/L); retinoschisin 1a ↓ (4 μg/L) | [27,116] |
| 0.1, 1, 10, and 30 μg/L | from 2 to 144 hpf | zfrho, opn1sw1, opn1sw2, opn1mw1, opn1mw2, opn1mw3, opn1mw4, opn1lw1, opn1lw2 ↓ | [44] | |||
| Agricultural chemicals | Boscalid | 4.6 mg/L | 0.3, 0.6, and 1.2 mg/L | from 2 hpf to 4 dpe | opn1sw1, opn1mw1, opn4.1, rho ↑ (0.3 mg/L); opn1sw1, opn1mw1, opn1lw2, opn4xb, opn4.1, rho, rhol ↓ (1.2 mg/L) | [17,117] |
| from 2 hpf to 8 dpe | opn1sw1, opn1lw2, opn4xb, rho ↑ (0.3 mg/L) | [17] | ||||
| PPCPs | AMI | 9.71 mg/L | 0.3, 30 μg/L | to 144 hpf | pax 6 ↓ (30 μg/L) | [80,118] |
| VEN | 267 mg/L | pax 6, otx2 ↓ | [80,119] | |||
| SER | 3.8 mg/mL | 0.1, 10 μg/L | pax 6 ↓, otx2 ↑ | [80,120] | ||
| AVO | 2.2 mg/L | 10 μg/L | from 2 hpf to 144 hpf | six6 ↓; lhx9, pax2, pax6 ↑ | [87,121] | |
| MPs | NPs | Insoluble in water | lhx9, six6 ↓; six3, pax2, pax6 ↑ | [87,122] | ||
| MPs | Insoluble in water | 1 mg/L | from 3 hpf to 1120 hpf | Zfrho ↑ | [108,123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.; Ma, Y.; Ma, J.; Yu, H.; Zhang, K.; Jin, L.; Yang, Q.; Sun, D.; Wu, D. Rapid Assessment of Ocular Toxicity from Environmental Contaminants Based on Visually Mediated Zebrafish Behavior Studies. Toxics 2023, 11, 706. https://doi.org/10.3390/toxics11080706
Yi J, Ma Y, Ma J, Yu H, Zhang K, Jin L, Yang Q, Sun D, Wu D. Rapid Assessment of Ocular Toxicity from Environmental Contaminants Based on Visually Mediated Zebrafish Behavior Studies. Toxics. 2023; 11(8):706. https://doi.org/10.3390/toxics11080706
Chicago/Turabian StyleYi, Jia, Yilei Ma, Jiahui Ma, Haiyang Yu, Kun Zhang, Libo Jin, Qinsi Yang, Da Sun, and Dejun Wu. 2023. "Rapid Assessment of Ocular Toxicity from Environmental Contaminants Based on Visually Mediated Zebrafish Behavior Studies" Toxics 11, no. 8: 706. https://doi.org/10.3390/toxics11080706
APA StyleYi, J., Ma, Y., Ma, J., Yu, H., Zhang, K., Jin, L., Yang, Q., Sun, D., & Wu, D. (2023). Rapid Assessment of Ocular Toxicity from Environmental Contaminants Based on Visually Mediated Zebrafish Behavior Studies. Toxics, 11(8), 706. https://doi.org/10.3390/toxics11080706










