Clinical Toxicology of Vitamin D in Pediatrics: A Review and Case Reports
Abstract
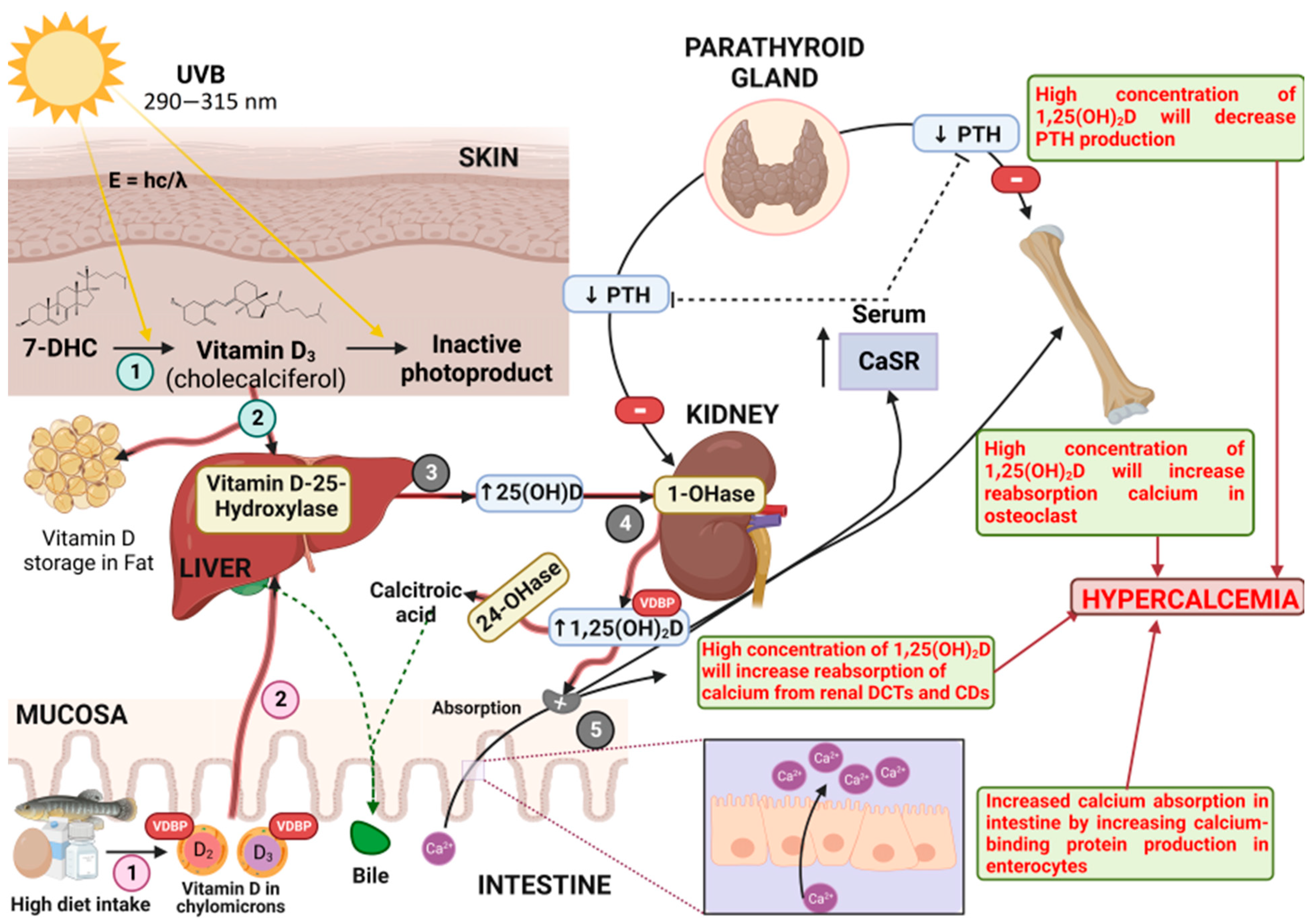
1. Introduction
2. Clinical Features and Adverse Events of Vitamin D
| Total Number of Patients | Age (Years) | Clinical Feature | Treatment | Duration (Weeks) | Adverse Events (AEs) | Refs. |
|---|---|---|---|---|---|---|
| 192 (only 180 completed the study) | 6–16 (mean age 9.8) | Persistent asthma and low vitamin D levels | Vitamin D3 4000 IU/day (n = 96) or placebo (n = 96) and maintained with fluticasone propionate: 176 μg/day (for age 6–11 years) or 220 μg/day (for age 12–16 years) | 48 | There were 36 participants (37.5%) in the vitamin D3 group and 33 (34.4%) in the placebo group who had 1 or more severe exacerbation. Serious AEs included hospitalizations (9 in each group), eosinophilia (1), and severe neutropenia (1). | [37] |
| 63 (only 48 completed the study, and 1 withdrew for AEs) | 8–18 (mean age 14.8) | IBD and baseline 25(OH)D ≥ 20 ng/mL | Arm A received 400 IU of oral vitamin D2/day (n = 32). Arm B received 1000 IU/day in the summer/fall and 2000 IU/day in the winter/spring (n = 31) Participants of arm B were notified via a phone call to change their vitamin D dose on the date such change was due. All participants received daily calcium supplementation: 800 mg elemental calcium (for age < than 11 years) and 1200 mg (for age ≥ 11 years). | 24 | Minor AEs (drowsiness, nausea and vomiting, dryness of mouth, increased thirst, persistent headache, constipation, loss of appetite, increased frequency of urination, bone and muscle pain, etc.) in both groups: 19 (59%) in arm A and 15 (48%) in arm B. More participants in arm A developed a C-reactive protein level of > 1 mg/dL and IL-6 > 3 pg/mL. | [3] |
| 865 (patients at high risk of vitamin D deficiency and children with rickets were excluded) | 1–5 | Asthma based on clinical signs of airflow obstruction and reversibility according to Canadian guidelines; a recent history of asthma exacerbations requiring OCS (≥1 in the past 6 months or ≥2 in the past year, documented in pharmacy and/or medical records); frequent URTIs (≥4 in the past year) and URTIs identified by parents as the main asthma trigger | Intervention group participants received a 2 mL oral bolus of 100,000 IU vitamin D3 (50,000 IU cholecalciferol/mL) at randomization in the fall or early winter, followed by a second 2 mL oral bolus of 100,000 IU vitamin D3 3.5 ± 0.5 months later. Participants also receive a total of five 50 mL coded bottles, containing a 400 IU vitamin D3/mL preparation, to be administered at a dose of 1 mL/day using a dropper, for 7 ± 0.5 months. Each bottle contains 50 daily doses. Placebo group participants received an identical 2 mL placebo bolus and daily dose of 1 mL placebo preparation, with administration timing identical to the intervention group. | 28 | Hypercalciuria and hypercalcemia | [50] |
3. Pharmacodynamics
4. Pharmacokinetics
4.1. Absorption
4.2. Distribution
4.3. Metabolism
4.4. Elimination
5. Toxicokinetics and Case Reports of Vitamin D Toxicity in Pediatrics
6. Toxicodynamics
7. Vitamin D in Pregnancy
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javed, A.; Kullo, I.J.; Balagopal, P.B.; Kumar, S. Effect of vitamin D3 treatment on endothelial function in obese adolescents. Pediatr. Obes. 2016, 11, 279–284. [Google Scholar] [CrossRef]
- Eckard, A.R.; O’Riordan, M.A.; Rosebush, J.C.; Ruff, J.H.; Chahroudi, A.; Labbato, D.; Daniels, J.E.; Uribe-Leitz, M.; Tangpricha, V.; McComsey, G.A. Effects of vitamin D supplementation on bone mineral density and bone markers in HIV-infected youth. J. Acquir. Immune Defic. Syndr. 2017, 76, 539–546. [Google Scholar] [CrossRef]
- Pappa, H.M.; Mitchell, P.D.; Jiang, H.; Kassiff, S.; Filip-Dhima, R.; DiFabio, D.; Quinn, N.; Lawton, R.C.; Bronzwaer, M.E.S.; Koenen, M.; et al. Maintenance of Optimal Vitamin D Status in Children and Adolescents with Inflammatory Bowel Disease: A Randomized Clinical Trial Comparing Two Regimens. J. Clin. Endocrinol. Metab. 2014, 99, 3408–3417. [Google Scholar] [CrossRef] [PubMed]
- Pincikova, T.; Paquin-Proulx, D.; Sandberg, J.K.; Flodström-Tullberg, M.; Hjelte, L. Vitamin D Treatment Modulates Immune Activation in Cystic Fibrosis. Clin. Exp. Immunol. 2017, 189, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Rocchi, L.; Carello, R.; Giampietro, P.G.; Panei, P.; Meglio, P. Serum Vitamin D Levels and Vitamin D Supplementation Do Not Correlate with the Severity of Chronic Eczema in Children. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 41–47. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D: Extraskeletal Health. Endocrinol. Metab. Clin. N. Am. 2010, 39, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Nonclassic Actions of Vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef]
- Yu, J.; Sharma, P.; Girgis, C.M.; Gunton, J.E. Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14434. [Google Scholar] [CrossRef]
- Rajakumar, K.; Moore, C.G.; Khalid, A.T.; Vallejo, A.N.; Virji, M.A.; Holick, M.F.; Greenspan, S.L.; Arslanian, S.; Reis, S.E. Effect of Vitamin D3 Supplementation on Vascular and Metabolic Health of Vitamin D-Deficient Overweight and Obese Children: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2020, 111, 757–768. [Google Scholar] [CrossRef]
- Molinari, C.; Uberti, F.; Grossini, E.; Vacca, G.; Carda, S.; Invernizzi, M.; Cisari, C. 1A,25-Dihydroxycholecalciferol Induces Nitric Oxide Production in Cultured Endothelial Cells. Cell. Physiol. Biochem. 2011, 27, 661–668. [Google Scholar] [CrossRef]
- Öhlund, I.; Lind, T.; Hernell, O.; Silfverdal, S.A.; Liv, P.; Karlsland Åkeson, P. Vitamin D Status and Cardiometabolic Risk Markers in Young Swedish Children: A Double-Blind Randomized Clinical Trial Comparing Different Doses of Vitamin D Supplements. Am. J. Clin. Nutr. 2020, 111, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Cayir, A.; Turan, M.I.; Tan, H. Effect of Vitamin D Therapy in Addition to Amitriptyline on Migraine Attacks in Pediatric Patients. Braz. J. Med. Biol. Res. 2014, 47, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Oberhelman, S.; Thacher, T.D. Handbook of Vitamin D in Human Health: Vitamin D Deficiency in the 21st Century: An Overview; Human Health Handbooks; Watson, R.R., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; Volume 4, ISBN 978-90-8686-210-8. [Google Scholar]
- Özkan, B. Nutritional Rickets. JCRPE J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 137–143. [Google Scholar] [CrossRef]
- Lim, K.; Thadhani, R. Vitamin D toxicity. Braz. J. Nephrol. 2020, 42, 238–244. [Google Scholar] [CrossRef]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin d Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Weydert, J.A. Vitamin D in children’s health. Children 2014, 1, 208–226. [Google Scholar] [CrossRef]
- Steenhoff, A.P.; Schall, J.I.; Samuel, J.; Seme, B.; Marape, M.; Ratshaa, B.; Goercke, I.; Tolle, M.; Nnyepi, M.S.; Mazhani, L.; et al. Vitamin D3 supplementation in Batswana children and adults with HIV: A Pilot Double Blind Randomized Controlled Trial. PLoS ONE 2015, 10, e0117123. [Google Scholar] [CrossRef]
- Dougherty, K.A.; Schall, J.I.; Bertolaso, C.; Smith-Whitley, K.; Stallings, V.A. Vitamin D supplementation improves health-related quality of life and physical performance in children with sickle cell disease and in healthy children. J. Pediatr. Health Care 2020, 34, 424–434. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. D Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef]
- Wagner, C.L.; Greer, F.R.; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008, 122, 1142–1152. [Google Scholar] [CrossRef]
- Herdea, A.; Ionescu, A.; Dragomirescu, M.C.; Ulici, A. Vitamin D—A Risk factor for bone fractures in children: A population-based prospective case–control randomized cross-sectional study. Int. J. Environ. Res. Public. Health 2023, 20, 3300. [Google Scholar] [CrossRef]
- De Vincentis, S.; Russo, A.; Milazzo, M.; Lonardo, A.; De Santis, M.C.; Rochira, V.; Simoni, M.; Madeo, B. How much vitamin D is too much? A case report and review of the literature. Endocr. Metab. Immune Disord-Drug Targets 2020, 21, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. JAMA-J. Am. Med. Assoc. 2019, 322, 736–745. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef]
- Reid, B.; Pierre-Olivier Girodet, J.S.B.; Abdel-Gadir, A.; Zheng, K.; Wechsler, M.E.; Bacharier, L.B.; Kunselman, S.J.; King, T.S.; Israel, E.; Castro, M.; et al. Vitamin D3 treatment of vitamin D-insufficient asthmatic patients does not alter immune cell function. J. Allergy Clin. Immunol. 2016, 138, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Schall, J.I.; Rutstein, R.M.; Leonard, M.B.; Zemel, B.S.; Stallings, V.A. The impact of vitamin D3 supplementation on muscle function among HIV-infected children and young adults: A randomized controlled trial. J. Musculoskelet. Neuronal Interact. 2015, 15, 145–153. [Google Scholar] [PubMed]
- Jullien, S. Vitamin D Prophylaxis in Infancy. BMC Pediatr. 2021, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Berry, A.; Thoene, M.; Wagner, J.; Lyden, E.; Jones, G.; Kaufmann, M.; Van Ormer, M.; Hanson, C. Randomized Trial of Two Doses of Vitamin D3 in Preterm Infants <32 Weeks: Dose Impact on Achieving Desired Serum 25(OH)D3 in a NICU Population. PLoS ONE 2017, 12, e0185950. [Google Scholar] [CrossRef]
- Girgis, C.M.; Mokbel, N.; Cha, K.M.; Houweling, P.J.; Abboud, M.; Fraser, D.R.; Mason, R.S.; Clifton-Bligh, R.J.; Gunton, J.E. The Vitamin D Receptor (VDR) Is Expressed in Skeletal Muscle of Male Mice and Modulates 25-Hydroxyvitamin D (25OHD) Uptake in Myofibers. Endocrinology 2014, 155, 3227–3237. [Google Scholar] [CrossRef]
- Girgis, C.M.; Brennan-Speranza, T.C. Vitamin D and Skeletal Muscle: Current Concepts from Preclinical Studies. JBMR Plus 2021, 5, e10575. [Google Scholar] [CrossRef]
- Şişmanlar, T.; Aslan, A.T.; Gülbahar, Ö.; Özkan, S. The Effect of Vitamin D on Lower Respiratory Tract Infections in Children. Turk. Pediatr. Ars. 2016, 51, 94–99. [Google Scholar] [CrossRef]
- Mohammadzadeh, I.; Darvish, S.; Qujeq, D.; Hajiahmadi, M.; Vaghari-Tabari, M. Association of Serum 25-OH Vitamin D3 with Serum IgE and the Pediatric Asthma Severity Score in Patients with Pediatric Asthma. Allergy Asthma Proc. 2020, 41, 126–133. [Google Scholar] [CrossRef]
- Stefanidis, C.; Martineau, A.R.; Nwokoro, C.; Griffiths, C.J.; Bush, A. Vitamin D for Secondary Prevention of Acute Wheeze Attacks in Preschool and School-Age Children. Thorax 2019, 25, 977–985. [Google Scholar] [CrossRef]
- Tachimoto, H.; Mezawa, H.; Segawa, T.; Akiyama, N.; Ida, H.; Urashima, M. Improved Control of Childhood Asthma with Low-Dose, Short-Term Vitamin D Supplementation: A Randomized, Double-Blind, Placebo-Controlled Trial. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 1001–1009. [Google Scholar] [CrossRef]
- Malihi, Z.; Wu, Z.; Lawes, C.M.M.; Scragg, R. Adverse events from large dose vitamin D supplementation taken for one year or longer. J. Steroid Biochem. Mol. Biol. 2019, 188, 29–37. [Google Scholar] [CrossRef]
- Forno, E.; Bacharier, L.B.; Phipatanakul, W.; Guilbert, T.W.; Cabana, M.D.; Ross, K.; Covar, R.; Gern, J.E.; Rosser, F.J.; Blatter, J.; et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: The VDKA randomized clinical trial. JAMA-J. Am. Med. Assoc. 2020, 324, 752–760. [Google Scholar] [CrossRef]
- Masetti, R.; Vendemini, F.; Zama, D.; Biagi, C.; Gasperini, P.; Pession, A. All-trans retinoic acid in the treatment of pediatric acute promyelocytic leukemia. Expert. Rev. Anticancer Ther. 2012, 12, 1191–1204. [Google Scholar] [CrossRef]
- Souto Filho, J.T.D.; de Andrade, A.S.; Ribeiro, F.M.; Alves, P.d.A.S.; Simonini, V.R.F. Impact of vitamin D deficiency on increased blood eosinophil counts. Hematol. Oncol. Stem Cell Ther. 2018, 11, 25–29. [Google Scholar] [CrossRef]
- Gupta, V.; Kumar, V.; Singh, S.K. Low vitamin D levels are associated with an adverse clinical outcome in febrile neutropenia. J. Pediatr. Hematol. Oncol. 2016, 38, 202–204. [Google Scholar] [CrossRef]
- Kimberg, D.V.; Baerg, R.D.; Gershon, E.; Graudusius, R.T. Effect of cortisone treatment on the active transport of calcium by the small intestine. J. Clin. Investig. 1971, 50, 1309–1321. [Google Scholar] [CrossRef]
- Cutolo, M.; Paolino, S.; Sulli, A.; Smith, V.; Pizzorni, C.; Seriolo, B. Vitamin D, steroid hormones, and autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 39–46. [Google Scholar] [CrossRef]
- Ghaly, S.; Lawrance, I. The role of vitamin D in gastrointestinal inflammation. Expert. Rev. Gastroenterol. Hepatol. 2014, 8, 909–923. [Google Scholar] [CrossRef]
- Jalili, M.; Vahedi, H.; Poustchi, H.; Hekmatdoost, A. Effects of vitamin D supplementation in patients with irritable bowel syndrome: A randomized, double-blind, placebo-controlled clinical trial. Int. J. Prev. Med. 2019, 10, 16. [Google Scholar] [CrossRef]
- Letavernier, E.; Daudon, M. Vitamin D, hypercalciuria and kidney stones. Nutrients 2018, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Tebben, P.J.; Singh, R.J.; Kumar, R. Vitamin D-mediated hypercalcemia: Mechanisms, diagnosis, and treatment. Endocr. Rev. 2016, 37, 521–547. [Google Scholar] [CrossRef]
- Edvardsson, V.; Elidottir, H.; Indridason, O.S.; Palsson, R. High incidence of kidney stones in Icelandic children. Pediatr. Nephrol. 2005, 20, 940–944. [Google Scholar] [CrossRef]
- Walker, M.D.; Shane, E. Hypercalcemia: A review. JAMA J. Am. Med. Assoc. 2022, 328, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Stokes, V.J.; Nielsen, M.F.; Hannan, F.M.; Thakker, R.V. Hypercalcemic disorders in children. J. Bone Miner. Res. 2017, 32, 2157–2170. [Google Scholar] [CrossRef]
- Jensen, M.E.; Ducharme, F.M.; Alos, N.; Mailhot, G.; Mâsse, B.; White, J.H.; Sadatsafavi, M.; Khamessan, A.; Tse, S.M.; Alizadehfar, R.; et al. Vitamin D in the prevention of exacerbations of asthma in preschoolers (DIVA): Protocol for a multicentre randomised placebo-controlled triple-blind trial. BMJ Open 2019, 9, e033075. [Google Scholar] [CrossRef] [PubMed]
- Fassio, A.; Gatti, D.; Rossini, M.; Benini, C.; Fracassi, E.; Bertoldo, E.; Viapiana, O.; Milleri, S.; Gatti, M.; Adami, G. Pharmacodynamics of oral cholecalciferol in healthy individuals with vitamin D deficiency: A randomized open-label study. Nutrients 2021, 13, 2293. [Google Scholar] [CrossRef]
- Brunton, L.L.; Hilal-Dandan, R.; Knollmann, B.C. The Pharmacological Basis of Therapeutics, 13th ed.; Mc Graw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. HIV Clin. Trials 2000, 1, 58–99. [Google Scholar]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D binding protein: A historic overview. Front. Endocrinol. 2019, 10, 910. [Google Scholar] [CrossRef]
- Lee, J.Y.; So, T.Y.; Thackray, J. A review on vitamin D deficiency treatment in pediatric patients. J. Pediatr. Pharmacol. Ther. 2013, 18, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391. [Google Scholar] [CrossRef]
- Bacchetta, J.; Edouard, T.; Laverny, G.; Bernardor, J.; Bertholet-Thomas, A.; Castanet, M.; Garnier, C.; Gennero, I.; Harambat, J.; Lapillonne, A.; et al. Vitamin D and calcium intakes in general pediatric populations: A French expert consensus paper. Arch. Pédiatr. 2022, 29, 312–325. [Google Scholar] [CrossRef]
- Mendel, C.M. The free hormone hypothesis: A physiologically based mathematical model. Endocr. Rev. 1989, 10, 232–274. [Google Scholar] [CrossRef]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 132–137. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Rowling, M.J.; Kemmis, C.M.; Taffany, D.A.; Welsh, J. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J. Nutr. 2006, 136, 2754–2759. [Google Scholar] [CrossRef]
- Cheng, J.B.; Motola, D.L.; Mangelsdorf, D.J.; Russell, D.W. De-orphanization of cytochrome P450 2R1. J. Biol. Chem. 2003, 278, 38084–38093. [Google Scholar] [CrossRef] [PubMed]
- Kämpe, A.; Enlund-Cerullo, M.; Valkama, S.; Holmlund-Suila, E.; Rosendahl, J.; Hauta-Alus, H.; Pekkinen, M.; Andersson, S.; Mäkitie, O. Genetic variation in GC and CYP2R1 affects 25-hydroxyvitamin D concentration and skeletal parameters: A genomewide association study in 24-month-old Finnish children. PLoS Genet. 2019, 15, e1008530. [Google Scholar] [CrossRef]
- Fraser, D.R.; Kodicek, E. Unique biosynthesis by kidney of a biologically active vitamin D metabolite. Nature 1970, 228, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Kottler, M.L.; Schlingmann, K.P. Genetic diseases of vitamin D metabolizing enzymes. Endocrinol. Metab. Clin. N. Am. 2017, 46, 1095–1117. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-Mediated Metabolism of Vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef]
- Bailie, G.R.; Johnson, C.A. Comparative review of the pharmacokinetics of vitamin D analogues. Semin. Dial. 2002, 15, 352–357. [Google Scholar] [CrossRef]
- Makris, K.; Sempos, C.; Cavalier, E. The measurement of vitamin D metabolites: Part I-Metabolism of vitamin D and the measurement of 25-hydroxyvitamin D. Hormones 2020, 19, 81–96. [Google Scholar] [CrossRef]
- Al-Zohily, B.; Al-Menhali, A.; Gariballa, S.; Haq, A.; Shah, I. Epimers of vitamin D: A review. Int. J. Mol. Sci. 2020, 21, 470. [Google Scholar] [CrossRef]
- Krishnan, K.; White, P. Toxicologic Pathology, 3rd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Ashauer, R.; Escher, B.I. Advantages of toxicokinetic and toxicodynamic modelling in aquatic ecotoxicology and risk assessment. J. Environ. Monit. 2010, 12, 2056–2061. [Google Scholar] [CrossRef]
- Coecke, S.; Pelkonen, O.; Leite, S.B.; Bernauer, U.; Bessems, J.G.M.; Bois, F.Y.; Gundert-Remy, U.; Loizou, G.; Testai, E.; Zaldívar, J.M. Toxicokinetics as a key to the integrated toxicity risk assessment based primarily on non-animal approaches. Toxicol. Vitr. 2013, 27, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Konwick, B.J.; Garrison, A.W.; Black, M.C.; Avants, J.K.; Fisk, A.T. Bioaccumulation, biotransformation, and metabolite formation of fipronil and chiral legacy pesticides in rainbow trout. Environ. Sci. Technol. 2006, 40, 2930–2936. [Google Scholar] [CrossRef]
- Inoue, Y.; Kaizaki-Mitsumoto, A.; Numazawa, S. Toxicokinetic evaluation during intoxication of psychotropic drugs using brain microdialysis in mice. J. Toxicol. Sci. 2022, 47, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Davies, J.S. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br. J. Clin. Pharmacol. 2018, 84, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R. Hypercalcemia due to hypervitaminosis D: Report of seven patients. J. Trop. Pediatr. 2009, 55, 396–398. [Google Scholar] [CrossRef]
- Häusler, D.; Torke, S.; Weber, M.S. High-dose vitamin D-mediated hypercalcemia as a potential risk factor in central nervous system demyelinating disease. Front. Immunol. 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D toxicity—A Clinical perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]
- Asif, A.; Farooq, N. Vitamin D Toxicity; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Farnaghi, F.; Hassanian-Moghaddam, H.; Zamani, N.; Gholami, N.; Gachkar, L.; Hosseini Yazdi, M. Vitamin D toxicity in a pediatric toxicological referral center: A cross-sectional study from Iran. BMC Pediatr. 2020, 20, 4–8. [Google Scholar] [CrossRef]
- Bilbao, N.A. Vitamin D toxicity in young breastfed infants: Report of 2 cases. Glob. Pediatr. Health 2017, 4, 2333794X17731695. [Google Scholar] [CrossRef]
- Rajakumar, K.; Reis, E.C.; Holick, M.F. Dosing error with over-the-counter vitamin D supplement. Clin. Pediatr. 2013, 52, 82–85. [Google Scholar] [CrossRef]
- Gerhard, C.; Jaffey, J.A. Persistent increase in serum 25-hydroxyvitamin D concentration in a dog following cholecalciferol intoxication. Front. Vet. Sci. 2020, 6, 472. [Google Scholar] [CrossRef]
- Gérard, A.O.; Fresse, A.; Gast, M.; Merino, D.; Gourdan, P.; Laurain, A.; Margaritis, I.; Gauci, P.-A.; Huret, F.; Parassol, N.; et al. Case report: Severe hypercalcemia following vitamin D intoxication in an infant, the underestimated danger of dietary supplements. Front. Pediatr. 2022, 10, 816965. [Google Scholar] [CrossRef]
- Ranatunga, K.; Jayasekera, K. Iatrogenic hypercalcemia induced acute pancreatitis following verapamil overdose: A case report. Anuradhapura Med. J. 2022, 16, 35. [Google Scholar] [CrossRef]
- Wijaya, I.; Oehadian, A.; Sumantri, R. Hypercalcemia of malignancy: Clinical characteristics and treatment outcome. Maj. Kedokt. Bdg. 2014, 46, 111–117. [Google Scholar] [CrossRef]
- Clines, G.A.; Guise, T.A. Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocr. Relat. Cancer 2005, 12, 549–583. [Google Scholar] [CrossRef]
- Stewart, A.F. Hypercalcemia associated with cancer. N. Engl. J. Med. 2005, 352, 373–379. [Google Scholar] [CrossRef]
- Makras, P.; Papapoulos, S. Medical treatment of hypercalcaemia. Hormones 2009, 8, 83–95. [Google Scholar] [CrossRef]
- Maier, J.D.; Levine, S.N. Hypercalcemia in the Intensive Care Unit: A review of pathophysiology, diagnosis, and modern therapy. J. Intensive Care Med. 2015, 30, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Carrick, A.I.; Costner, H.B. Rapid fire: Hypercalcemia. Emerg. Med. Clin. N. Am. 2018, 36, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Machado, V.; Proença, L.; Delgado, A.S.; Mendes, J.J. Vitamin D deficiency and oral health: A comprehensive review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L. The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef]
- Ashok, T.; Palyam, V.; Azam, A.T.; Odeyinka, O.; Alhashimi, R.; Thoota, S.; Sange, I. Relationship between vitamin D and thyroid: An Enigma. Cureus 2022, 14, e21069. [Google Scholar] [CrossRef]
- Peterson, M.E.; Fluegeman, K. Cholecalciferol. Top. Companion Anim. Med. 2013, 28, 24–27. [Google Scholar] [CrossRef]
- Hollis, B.W.; Horst, R.L. The assessment of circulating 25(OH)D and 1,25(OH)2D: Where we are and where we are going. J. Steroid Biochem. Mol. Biol. 2007, 103, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Kalra, S. Vitamin D supplementation in pregnancy. Indian J. Endocrinol. Metab. 2014, 18, 593–596. [Google Scholar] [CrossRef]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2011, 26, 2341–2357. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obstet. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef]
- Ferrari, D.; Lombardi, G.; Banfi, G. Concerning the vitamin D reference range: Pre-analytical and analytical variability of vitamin D measurement. Biochem. Med. 2017, 27, 030501. [Google Scholar] [CrossRef] [PubMed]
| Pediatrics | Dose of Vitamin D Supplementation |
|---|---|
| Age 0 to 12 months breastfed or partially breastfed babies | A dose of 400 IU/day starting from the first few days. |
| Supplementation should be continued unless the infant is weaned to at least 1000 mL of vitamin D-fortified formula or whole milk/day. | |
| All infants ingesting less than 1000 mL of formula milk/day or non-breastfed infants should receive a vitamin D supplement of 400 IU/day. | |
| Age 1 year to 18 years | A dose of 600 IU/day in the absence of sun exposure. |
| Older children who are ingesting less than 1000 mL of vitamin D-fortified formula or whole milk/day should receive a vitamin D supplement of 400–600 IU/day. |
| Information about the Patients (Age in Months) | Results of Examination | Toxicity Management | Refs. |
|---|---|---|---|
| A total of 15 pediatric patients aged 24–60 months (mean age 46.53 ± 10.14 months) with a history of ingestion of more than 1500 IU/day of vitamin D supplements | The mean ingested dose was 8.13 ± 4.54 soft gelatin capsules or 406,700.7 ± 227,400.1 IU vitamin D. One patient had ingested 500,000 IU vitamin D with serum Ca level of 12.5 mg/dL. Eight (53.3%) cases had 25(OH)D levels > 100 ng/mL. The mean serum 25(OH)D levels of the patients was higher than normal: 111.3 ± 113.6 ng/mL (normal 30–100 ng/mL). There was no significant difference between variables in patients with and without a high level of 25(OH)D. | There were 8 patients (53.3%) who were hospitalized and treated with activated charcoal and fluid therapy, discontinued consumption of vitamin D supplements, kept low-calcium and vitamin D diet, took more liquid for at least one month, and were monitored for 25(OH)D levels. | [84] |
| Two infants with an identical presentation. One was a 3.5-month-old Caucasian female and the other a 2.5-month-old Caucasian male. The patients were exclusively breastfed and had received OTC vitamin D supplementation higher than the recommended dose. The female infant was given vitamin D3 2000 IU/day for 2.5 months, while the male infant was given vitamin D3 20,000 IU/day for 1.5 weeks | Physical examination of both patients showed evidence of moderate dehydration. Laboratory analysis of the female infant: Serum Ca: 21 mg/dL (normal 8.8–11.2 mg/dL) 25(OH)D: 644 ng/mL (normal 30–100 ng/mL) PTH: <1 pg/mL (normal 14–72 pg/mL) Laboratory analysis of the male infant: Serum Ca: 15 mg/dL (normal 8.5–10.1 mg/dL) 25(OH)D: 680 ng/mL (normal 30–100 ng/mL) 1,25(OH)2D: 166 pg/mL (normal 15–75 pg/mL) PTH: <7 pg/mL (normal 15–65 pg/mL) | The infants received IV hydration with normal saline and dextrose-containing solution at the PICU where they received furosemide 1 mg/kg/dose every 8 h and prednisone 1 mg/kg/day. The male infant received calcitonin 4 IU/kg × 1 dose. They both exhibited improvement of hypercalcemia after 2 to 3 days of treatment. On discharge, the serum Ca of the female infant was 11 mg/dL and the male infant was 10.8 mg/dL; both were clinically improved. | [85] |
| A 3-month-old Asian–American male infant who had been exclusively breastfed. The oral vitamin D supplementation was started on day 5 (400 IU/day), but the parents had made a mistake when administering a new brand of infant vitamin D liquid preparation to a 30-fold overdose of vitamin D (12,000 IU) daily for 20 days | The infant had no history of irritability, constipation, or abnormal movements. Laboratory analysis: Serum Ca: 10.5 mg/dL (normal 8.5–10.1 mg/dL) Phosphorus: 6.4 mg/dL (normal 4.5–6.5 mg/dL) Electrolyte panel, creatinine, and blood urea nitrogen were normal. Serum 25(OH)D: 422 ng/mL (normal 30–100 ng/mL) Serum 1,25(OH)2D: 61 pg/mL (normal 27–71 pg/mL) PTH: <3 pg/mL (normal 15–65 pg/mL) | The parents were asked to stop giving vitamin D to the infants. | [86] |
| A 3-month-old male infant with severe anorexia, vomiting, and weight loss. The infant was born at term, weighing 2.3 kg, and had been exclusively breastfed with no medical problems. The infant was exposed to 40,000–50,000 IU of vitamin D supplement/day, which represents 50-fold the Upper tolerable Level (UL) recommended. | At the time of admission, the infant weighed 4.5 kg with no fever. HR 109 beats/minute, BP 107/85 mmHg, oxygen saturation 99%. Laboratory analysis: Serum Na: 139 mmol/L Serum K: 4.4 mmol/L Alkaline reserve: 21 mmol/L Hb: 9.1 g/dL Leukocytes: 11.11 g/L Platelets: 471 g/L C-reactive protein: 13 mg/L with a negative procalcitonin Serum Ca: 3.08 mmol/L (normal 2.15–2.55 mmol/L) Serum albumin: 41 g/L (normal 34–42 mg/L) PTH: <18 ng/L (normal 18–88 ng/L) Serum 25(OH)D: > 400 ng/mL (normal 30–400 ng/mL) Serum 1,25(OH)2D: 200 pg/mL (normal < 182 pg/mL) Phosphate: 1.8 mmol/L (normal 1.6–2.4 mmol/L) Serum creatinine: 23 μmol/L (normal 15–37 μmol/L) Urea: 2.3 mmol/L (normal 1.8–6.4 mmol/L) Blood gases analysis and liver function tests were within normal range. | The patient was hospitalized, given IV hydration, and examined for immunoglobulin E (IgE) antibodies to cow milk, cranial ultrasonography, brain magnetic resonance imaging, and abdominal ultrasound. Because of a suspicion of urinary infection, IV antibiotic therapy was also given for 3 days. The patient was discharged after 2 weeks with a weight of 4.8 kg and a close monitoring of his serum Ca. | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levita, J.; Wilar, G.; Wahyuni, I.; Bawono, L.C.; Ramadaini, T.; Rohani, R.; Diantini, A. Clinical Toxicology of Vitamin D in Pediatrics: A Review and Case Reports. Toxics 2023, 11, 642. https://doi.org/10.3390/toxics11070642
Levita J, Wilar G, Wahyuni I, Bawono LC, Ramadaini T, Rohani R, Diantini A. Clinical Toxicology of Vitamin D in Pediatrics: A Review and Case Reports. Toxics. 2023; 11(7):642. https://doi.org/10.3390/toxics11070642
Chicago/Turabian StyleLevita, Jutti, Gofarana Wilar, Ika Wahyuni, Lidya Cahyo Bawono, Tiara Ramadaini, Rohani Rohani, and Ajeng Diantini. 2023. "Clinical Toxicology of Vitamin D in Pediatrics: A Review and Case Reports" Toxics 11, no. 7: 642. https://doi.org/10.3390/toxics11070642
APA StyleLevita, J., Wilar, G., Wahyuni, I., Bawono, L. C., Ramadaini, T., Rohani, R., & Diantini, A. (2023). Clinical Toxicology of Vitamin D in Pediatrics: A Review and Case Reports. Toxics, 11(7), 642. https://doi.org/10.3390/toxics11070642







