The Accumulation of Toxic Elements (Pb, Hg, Cd, As, and Cu) in Red Swamp Crayfish (Procambarus clarkii) in Qianjiang and the Associated Risks to Human Health
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Treatment and Analysis
2.3. Health Risk Assessment
2.3.1. EDI Calculation
2.3.2. THQ Calculation
2.3.3. CR Calculation
2.4. Estimated Monthly Crayfish Consumption per Person
2.5. Statistical Analysis
3. Results and Discussion
3.1. Concentrations of Toxic Elements in Crayfish Tissues
| Area | Tissue | Metal | Reference | ||||
|---|---|---|---|---|---|---|---|
| Pb | Hg | Cd | As | Cu | |||
| China, Hubei, Qianjiang city | He | 0.228 ± 0.13 | 0.096 ± 13.27 | 4.04 ± 1.9 | 2.046 ± 2.036 | 116.69 ± 140.59 | Present study |
| CV | 0.57 | 0.46 | 0.47 | 1.00 | 1.20 | ||
| Am | 0.139 ± 0.06 | 0.5 ± 0.26 | 0.005 ± 0.008 | 0.7 ± 0.2 | 16.0 ± 12.0 | ||
| CV | 0.43 | 0.52 | 1.70 | 0.29 | 0.75 | ||
| China, Hubei and Hunan | He | 0.46 ± 0.15 | 3.54 ± 0.78 | 3.51 ± 0.83 | [14] | ||
| Am | 0.13 ± 0.04 | 4.59 ± 1.67 | 0.56 ± 0.12 | ||||
| China | Am | <dl-1.01 | 0.003–1.71 | 1.012 | 7.2–157.6 | [48] | |
| China, Hubei | Am | 0–0.05 | 0–0.06 | 0–0.12 | 0–1.27 | 11.11–21.87 | [13] |
| Wc | 0–0.65 | 0–0.05 | 0.05–0.31 | 0.67–4.05 | 38.37–85.62 | ||
| USA, Louisiana | He | 9.15–10.03 | 0.22–0.65 | 0.18–6.79 | 18.46–23.95 | [18] | |
| Am | 2.44–4.49 | 0.06 | 0.15–3.67 | 23.9–34.3 | |||
| USA, California | Am | 0.19 | 1.10 | 0.03 | 0.68 | 44.6 | [62] |
| Spain, Ebro River | Am | 0.41–4.2 | 0.22–3.1 | 12–82.3 | [45] | ||
| Italy, central Italy and Lake Trasimeno | He | 0.49–39.23 | 1.96–73.4 | 3.6–1310.5 | [44] | ||
| Am | 0.5–3.25 | 0.13–5.59 | 12.4–327.3 | ||||
| Italy, south-western Sicily | He | 0.74 ± 2.80 | 0.03 ± 0.04 | 3.76 ± 2.71 | 41.0 ± 38.5 | [63] | |
| Am | 0.18 ± 0.62 | 0.01 ± 0.00 | 1.79 ± 0.8 | 17.3 ± 9.4 | |||
| Italy, Po River Delta | He | <dl-1.11 | <dl-1.26 | 2.58–4.48 | 94.2–686.5 | [60] | |
| Am | <dl-2.43 | <dl | 0.52–0.8 | 30.5–65.3 | |||
| Safety standards (w.w.) | 0.5 | 0.5 | 0.5 | 0.5 | GB2762-2017 | ||
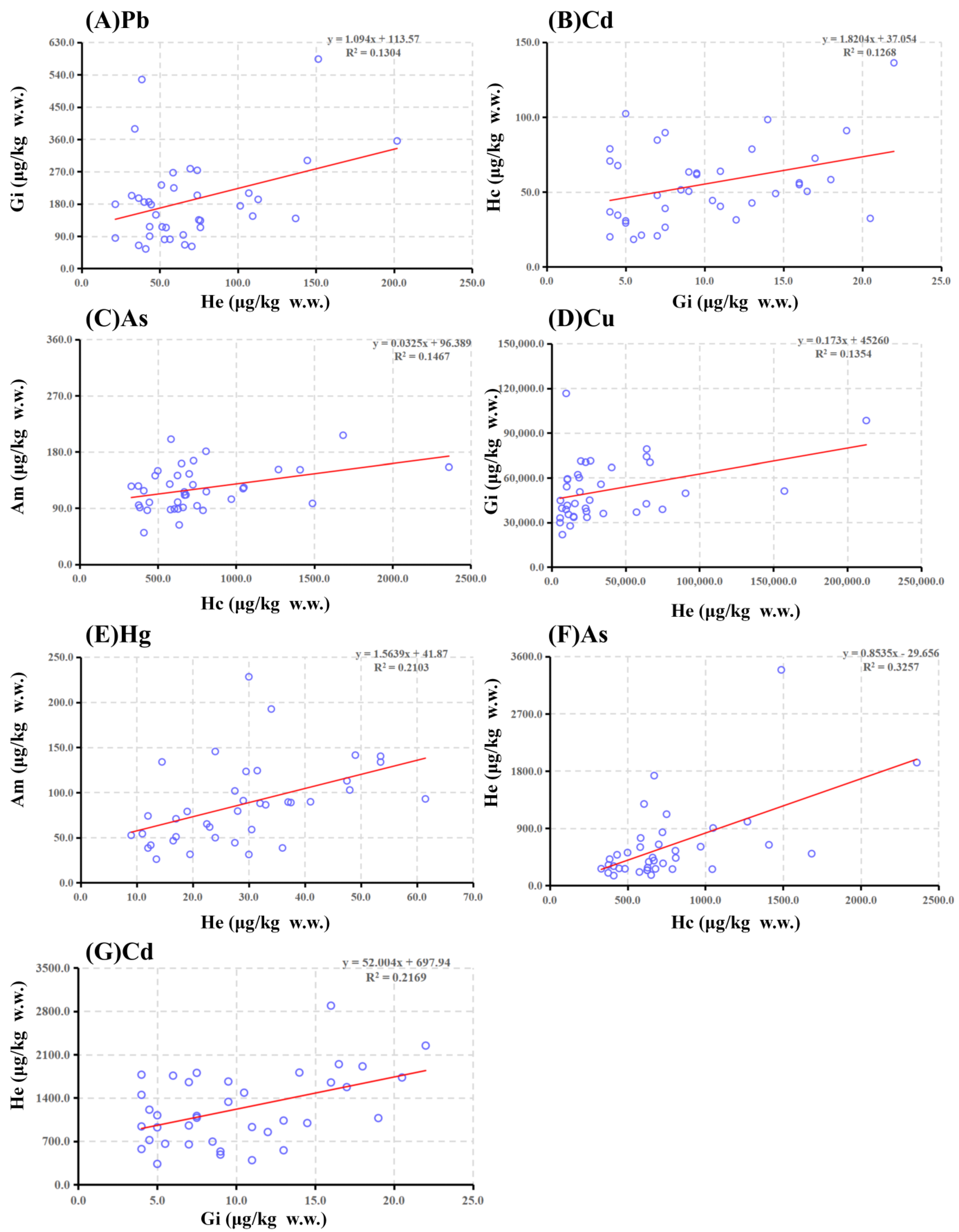
3.2. Correlation Analysis among Five Toxic Elements in Crayfish Four Tissues
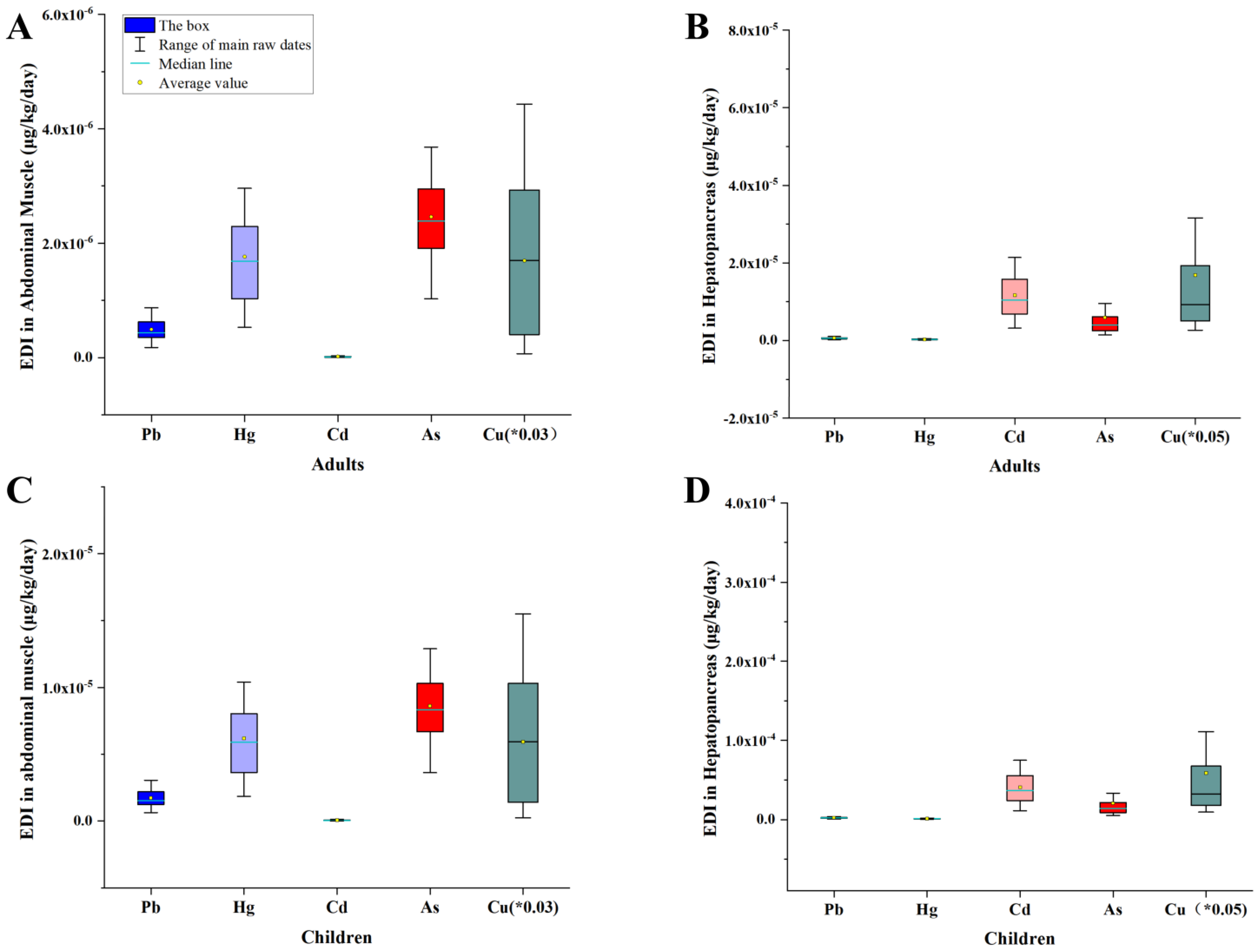
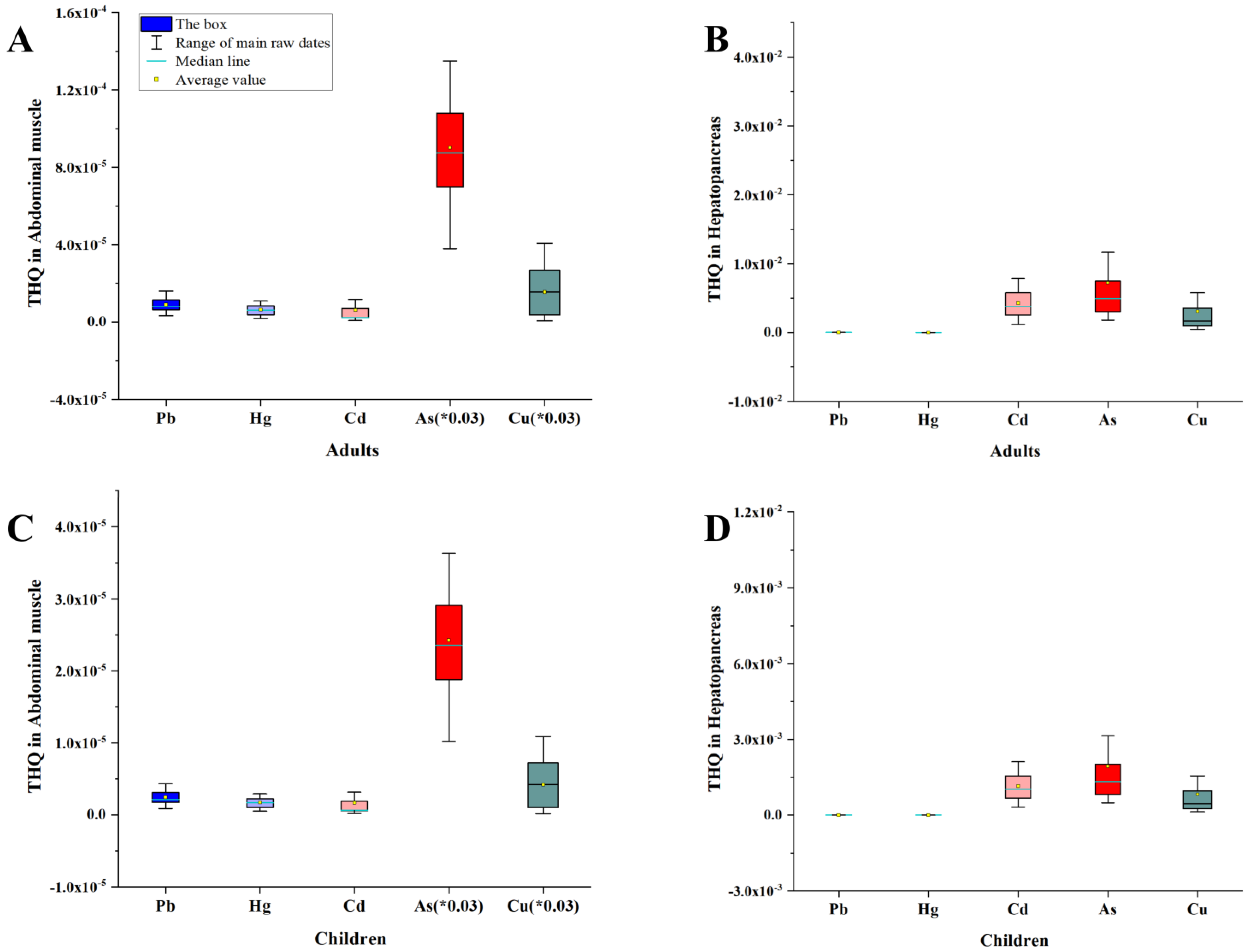
3.3. Risk Assessment for Human Health
3.4. Safety Control Analysis of Crayfish Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO Yearbook. Fishery and Aquaculture Statistics 2019/FAO annuaire. Statistiques des pêches et de l’aquaculture 2019/FAO anuario. In Estadísticas de Pesca y Acuicultura Rome/Roma; FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Liu, F.; Geng, C.; Qu, Y.-K.; Cheng, B.-X.; Zhang, Y.; Wang, A.-M.; Zhang, J.H.; Liu, B.; Tian, H.-Y.; Yang, W.-P.; et al. The feeding of dietary Codonopsis pilosula polysaccharide enhances the immune responses, the expression of immune-related genes and the growth performance of red swamp crayfish (Procambarus clarkii). Fish Shellfish. Immunol. 2020, 103, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Oficialdegui, F.J.; Sánchez, M.I.; Clavero, M. One century away from home: How the red swamp crayfish took over the world. Rev. Fish Biol. Fish. 2020, 30, 121–135. [Google Scholar] [CrossRef]
- Ackefors, H. The positive effects of established crayfish introductions in Europe. In Crayfish in Europe as Alien Species; Routledge: Oxfordshire, UK, 2017; pp. 49–60. [Google Scholar]
- Souty-Grosset, C.; Anastácio, P.M.; Aquiloni, L.; Banha, F.; Choquer, J.; Chucholl, C.; Tricarico, E. The red swamp crayfish Procambarus clarkii in Europe: Impacts on aquatic ecosystems and human well-being. Limnologica 2016, 58, 78–93. [Google Scholar] [CrossRef]
- Richert, J.C.; Sneddon, J. Determination of Inorganics and Organics in Crawfish. Appl. Spectrosc. Rev. 2007, 43, 51–67. [Google Scholar] [CrossRef]
- Yue, G.H.; Li, J.; Bai, Z.; Wang, C.M.; Feng, F. Genetic diversity and population structure of the invasive alien red swamp crayfish. Biol. Invasions 2010, 12, 2697–2706. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, J.; Xu, Y.; Wang, H.; Lu, L.; Song, R.; Zou, J. Epigallocatechin-3-gallate inhibits replication of white spot syndrome virus in the freshwater crayfish Procambarus clarkii. J. Fish Dis. 2021, 45, 445–450. [Google Scholar] [CrossRef]
- Song, S.; Zhu, K.; Han, L.; Sapozhnikova, Y.; Zhang, Z.; Yao, W. Residue Analysis of 60 Pesticides in Red Swamp Crayfish Using QuEChERS with High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 5031–5038. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, Y.; Lu, K.; Xiong, H.; Zhang, Y.; Wei, W. Herbicide atrazine exposure induce oxidative stress, immune dysfunction and WSSV proliferation in red swamp crayfish Procambarus clarkii. Chemosphere 2021, 283, 131227. [Google Scholar] [CrossRef] [PubMed]
- Leigh, C.; Stewart-Koster, B.; Van Sang, N.; Van Truc, L.; Hiep, L.H.; Xoan, V.B.; Tinh, N.T.N.; An, L.T.; Sammut, J.; Burford, M.A. Rice-shrimp ecosystems in the Mekong Delta: Linking water quality, shrimp and their natural food sources. Sci. Total Environ. 2020, 739, 139931. [Google Scholar] [CrossRef] [PubMed]
- Yifan, L.; Tiaoyan, W.; Shaodong, W.; Xucan, K.; Zhaoman, Z.; Hongyan, L.; Jiaolong, L. Developing integrated rice-animal farming based on climate and farmers choices. Agric. Syst. 2023, 204, 103554. [Google Scholar] [CrossRef]
- Peng, F.; Li, J.; Gong, Z.; Yue, B.; Wang, X.; Manyande, A.; Du, H. Investigation of Bioaccumulation and Human Health Risk Assessment of Heavy Metals in Crayfish (Procambarus clarkii) Farming with a Rice-Crayfish-Based Coculture Breeding Modes. Foods 2022, 11, 261. [Google Scholar] [CrossRef]
- Tan, Y.; Peng, B.; Wu, Y.; Xiong, L.; Sun, J.; Peng, G.; Bai, X. Human health risk assessment of toxic heavy metal and metalloid intake via consumption of red swamp crayfish (Procambarus clarkii) from rice-crayfish co-culture fields in China. Food Control. 2021, 128, 108181. [Google Scholar] [CrossRef]
- Rodriguez-Gil, J.L.; Sauto, J.S.S.; González-Alonso, S.; Sanchez, P.S.; Valcarcel, Y.; Catalá, M. Development of cost-effective strategies for environmental monitoring of irrigated areas in Mediterranean regions: Traditional and new approaches in a changing world. Agric. Ecosyst. Environ. 2013, 181, 41–49. [Google Scholar] [CrossRef]
- Anandkumar, A.; Li, J.; Prabakaran, K.; Jia, Z.X.; Leng, Z.; Nagarajan, R.; Du, D. Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans. J. Food Compos. Anal. 2020, 88, 103449. [Google Scholar] [CrossRef]
- Mo, A.; Huang, Y.; Gu, Z.; Liu, C.; Wang, J.; Yuan, Y. Health risk assessment and bioaccumulation of heavy metals in Procambarus clarkii from six provinces of China. Environ. Sci. Pollut. Res. 2021, 29, 2539–2546. [Google Scholar] [CrossRef]
- Gedik, K.; Kongchum, M.; DeLaune, R.D.; Sonnier, J.J. Distribution of arsenic and other metals in crayfish tissues (Procambarus clarkii) under different production practices. Sci. Total Environ. 2017, 574, 322–331. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, B.; Xie, G.; Zhen, S.; Zhou, Y.; Shao, B.; Zhang, J.; Ji, H.; Wu, Y. Outbreak of Haff Disease caused by consumption of crayfish (Procambarus clarkii), Nanjing, Jiangsu Province, China. Food Control 2015, 59, 690–694. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, G.; Yu, X.; Mao, H.; Xing, C.; Liu, J. Haff Disease after Eating Crayfish in East China. Intern. Med. 2012, 51, 487–489. [Google Scholar] [CrossRef]
- Fischer, P.U.; Weil, G.J. North American paragonimiasis: Epidemiology and diagnostic strategies. Expert Rev. Anti-Infect. Ther. 2015, 13, 779–786. [Google Scholar] [CrossRef]
- Bean, N.H.; Maloney, E.K.; Potter, M.E.; Korazemo, P.; Ray, B.; Taylor, J.P.; Seigler, S.; Snowden, J. Crayfish: A newly recognized vehicle for vibrio infections. Epidemiol. Infect. 1998, 121, 269–273. [Google Scholar] [CrossRef]
- Wong FY, K.; Fowler, K.; Desmarchelier, P.M. Vibriosis Due toVibrio mimicusin Australian Freshwater Crayfish. J. Aquat. Anim. Health 1995, 7, 284. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Bin Emran, T.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Alcorlo, P.; Otero, M.; Crehuet, M.; Baltanás, A.; Montes, C. The use of the red swamp crayfish (Procambarus clarkii, Girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci. Total Environ. 2006, 366, 380–390. [Google Scholar] [CrossRef]
- Rodríguez-Estival, J.; Morales-Machuca, C.; Pareja-Carrera, J.; Ortiz-Santaliestra, M.E.; Mateo, R. Food safety risk assessment of metal pollution in crayfish from two historical mining areas: Accounting for bioavailability and cooking extractability. Ecotoxicol. Environ. Saf. 2019, 185, 109682. [Google Scholar] [CrossRef] [PubMed]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.; Kerwath, S.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2015, 96, 32–48. [Google Scholar] [CrossRef]
- Hashimoto, A.; Kambe, T. Mg, Zn and Cu Transport Proteins: A Brief Overview from Physiological and Molecular Perspectives. J. Nutr. Sci. Vitaminol. 2015, 61, S116–S118. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elements Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef]
- Mccarty, K.M.; Hanh, H.T.; Kim, K.-W. Arsenic geochemistry and human health in South East Asia. Rev. Environ. Health 2011, 26, 71–78. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food Chain, and sus-tainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef]
- Nriagu, J.; Becker, C. Volcanic emissions of mercury to the atmosphere: Global and regional inventories. Sci. Total Environ. 2003, 304, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, E.G.; Pacyna, J.M.; Steenhuisen, F.; Wilson, S. Global anthropogenic mercury emission inventory for 2000. Atmos. Environ. 2006, 40, 4048–4063. [Google Scholar] [CrossRef]
- Shui, Y.; Guan, Z.-B.; Liu, G.-F.; Fan, L.-M. Gut microbiota of red swamp crayfish Procambarus clarkii in integrated crayfish-rice cultivation model. AMB Express 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Jitar, O.; Teodosiu, C.; Oros, A.; Plavan, G.; Nicoara, M. Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol. 2015, 32, 369–378. [Google Scholar] [CrossRef]
- Gerhardt, A. Bioindicator species and their use in biomonitoring (Volume 1). In Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO; Eolss Publishers: Oxford, UK, 2002; Available online: http://www.eolss.net/Eolss-sampleAllChapter.aspx (accessed on 24 July 2022).
- Manickavasagam, S.; Sudhan, C.; Bharathi; Aanand, S. Bioindicators in Aquatic Environment and Their Signifi-cance. J. Aquac. Trop. 2019, 34, 73. [Google Scholar] [CrossRef]
- Luczyńska, J.; Paszczyk, B.; Łuczyński, M.J. Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol. Environ. Saf. 2018, 153, 60–67. [Google Scholar] [CrossRef]
- Nyeste, K.; Dobrocsi, P.; Czeglédi, I.; Czédli, H.; Harangi, S.; Baranyai, E.; Simon, E.; Nagy, S.A.; Antal, L. Age and diet-specific trace element accumulation patterns in different tissues of chub (Squalius cephalus): Juveniles are useful bioindicators of recent pollution. Ecol. Indic. 2019, 101, 1–10. [Google Scholar] [CrossRef]
- Goretti, E.; Pallottini, M.; Ricciarini, M.; Selvaggi, R.; Cappelletti, D. Heavy metals bioaccumulation in selected tissues of red swamp crayfish: An easy tool for monitoring environmental contamination levels. Sci. Total Environ. 2016, 559, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Serrano, A.; Alcaraz, C.; Ibáñez, C.; Trobajo, R.; Barata, C. Procambarus clarkii as a bioindicator of heavy metal pollution sources in the lower Ebro River and Delta. Ecotoxicol. Environ. Saf. 2010, 73, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Ariano, A.; Scivicco, M.; D’ambola, M.; Velotto, S.; Andreini, R.; Bertini, S.; Zaccaroni, A.; Severino, L. Heavy Metals in the Muscle and Hepatopancreas of Red Swamp Crayfish (Procambarus clarkii) in Campania (Italy). Animals 2021, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Zhang, H.; Cao, J.; Ge, T.; Gao, J.; Fang, Y.; Ye, W.; Fang, T.; Shi, Y.; et al. Trace elements in red swamp crayfish (Procambarus clarkii) in China: Spatiotemporal variation and human health implications. Sci. Total Environ. 2023, 857, 159749. [Google Scholar] [CrossRef]
- Peng, Q.; Nunes, L.M.; Greenfield, B.K.; Dang, F.; Zhong, H. Are Chinese consumers at risk due to exposure to metals in crayfish? A bioaccessibility-adjusted probabilistic risk assessment. Environ. Int. 2016, 88, 261–268. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Y.; Xu, N.; Wang, Q.; Sun, X. Occurrence, partition, and risk of four adjacent transition metals in seawater, sediments and demersal fish from the Pearl River Estuary, South China Sea. Mar. Pollut. Bull. 2022, 184, 114159. [Google Scholar] [CrossRef]
- Baki, M.A.; Hossain, M.; Akter, J.; Quraishi, S.B.; Shojib, F.H.; Ullah, A.A.; Khan, F. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf. 2018, 159, 153–163. [Google Scholar] [CrossRef]
- FSANZ. (Food Standards Australia and New Zealand) Australia New Zealand Food Standards Code, Contaminants and Natural Toxicants, Standard 1.4.1. 2013. Available online: http://www.legislation.gov.au/Details/F2013C00140/ (accessed on 16 October 2022).
- Naseri, M.; Vazirzadeh, A.; Kazemi, R.; Zaheri, F. Concentration of some heavy metals in rice types available in Shiraz market and human health risk assessment. Food Chem. 2015, 175, 243–248. [Google Scholar] [CrossRef]
- Javed, M.; Usmani, N. Accumulation of heavy metals and human health risk assessment via the consumption of freshwater fish Mastacembelus armatus inhabiting, thermal power plant effluent loaded canal. Springerplus 2016, 5, 776. [Google Scholar] [CrossRef]
- Chien, L.-C.; Hung, T.-C.; Choang, K.-Y.; Yeh, C.-Y.; Meng, P.-J.; Shieh, M.-J.; Han, B.-C. Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci. Total Environ. 2002, 285, 177–185. [Google Scholar] [CrossRef]
- USEPA. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories. In Risk Assessment and Fish Consumption Limits, 3rd ed.; Office of Science and Technology, United States Environmental Protection Agency: Washington, DC, USA, 2000; Volume 2, EPA 823-B-00-008. [Google Scholar]
- USEPA. Risk Based Screening Table. Composite Table: Summary Tab 0615. 2015. Available online: http:/www2.epa.gov/risk/riskbasedscreeningtablegenerictables (accessed on 23 October 2022).
- Peng, Q.; Greenfield, B.K.; Dang, F.; Zhong, H. Human exposure to methylmercury from crayfish (Procambarus clarkii) in China. Environ. Geochem. Health 2015, 38, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Xu, T.; Li, R.; Johnson, D.; Ren, D.; Liu, H.; Xi, Y.; Huang, Y. Heavy metal accumulation and health risk assessment of crayfish collected from cultivated and uncultivated ponds in the Middle Reach of Yangtze River. Sci. Total Environ. 2020, 739, 139963. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, M.; Bagatto, G.; Zia, S. The crayfish as a “biological indicator” of aquatic contamination by heavy metals. Water Res. 1990, 24, 1069. [Google Scholar] [CrossRef]
- Mistri, M.; Munari, C.; Pagnoni, A.; Chenet, T.; Pasti, L.; Cavazzini, A. Accumulation of trace metals in crayfish tissues: Is Procambarus clarkii a vector of pollutants in Po Delta inland waters? Eur. Zool. J. 2020, 87, 46–57. [Google Scholar] [CrossRef]
- Colovic, M.B.; Vasic, V.M.; Djuric, D.M.; Krstić, D.Z. Sulphur-containing Amino Acids: Protective Role Against Free Radicals and Heavy Metals. Curr. Med. Chem. 2018, 25, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Hothem, R.L.; Bergen, D.R.; Bauer, M.L.; Crayon, J.J.; Meckstroth, A.M. Mercury and Trace Elements in Crayfish from Northern California. Bull. Environ. Contam. Toxicol. 2007, 79, 628–632. [Google Scholar] [CrossRef]
- Bellante, A.; Maccarone, V.; Buscaino, G.; Buffa, G.; Filiciotto, F.; Traina, A.; Del Core, M.; Mazzola, S.; Sprovieri, M. Trace element concentrations in red swamp crayfish (Procambarus clarkii) and surface sediments in Lake Preola and Gorghi Tondi natural reserve, SW Sicily. Environ. Monit. Assess. 2015, 187, 1–18. [Google Scholar] [CrossRef]
- Irnidayant, Y. Toxicity and Traces of Hg, Pb and Cd in the Hepatopancreas, Gills and Muscles of Perna viridis from Jakarta Bay, Indonesia. Pak. J. Biol. Sci. 2015, 18, 94–98. [Google Scholar] [CrossRef]
- Ponzoni, S. Manganese tissue accumulation and tyrosine hydroxylase immunostaining response in the Neotropical freshwater crab, Dilocarcinus pagei, exposed to manganese. Invertebr. Neurosci. 2017, 17, 439. [Google Scholar] [CrossRef]
- Kuklina, I.; Kouba, A.; Buřič, M.; Horká, I.; Ďuriš, Z.; Kozák, P. Accumulation of Heavy Metals in Crayfish and Fish from Selected Czech Reservoirs. BioMed Res. Int. 2014, 2014, 306103. [Google Scholar] [CrossRef]
- Peng, Q.; Greenfield, B.K.; Dang, F.; Gong, Y.; Bu, W.; Zhong, H. Mechanistic understanding of low methylmercury bioaccessibility from crayfish (Procambarus clarkii) muscle tissue. Sci. Total Environ. 2017, 603–604, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Song, Z.; Liang, H.; Zhong, S.; Yu, Y.; Liu, T.; Sha, H.; He, L.; Gan, J. Mercury Induced Tissue Damage, Redox Metabolism, Ion Transport, Apoptosis, and Intestinal Microbiota Change in Red Swamp Crayfish (Procambarus clarkii): Application of Multi-Omics Analysis in Risk Assessment of Hg. Antioxidants 2022, 11, 1944. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Huertas, D.; Soto, D.X.; Grimalt, J.O.; Catalan, J.; Riva, M.C.; Barata, C. Contaminant accumulation and multi-biomarker responses in field collected zebra mussels (Dreissena polymorpha) and crayfish (Procambarus clarkii), to evaluate toxicological effects of industrial hazardous dumps in the Ebro river (NE Spain). Chemosphere 2010, 78, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Al Kaddissi, S.; Legeay, A.; Elia, A.C.; Gonzalez, P.; Floriani, M.; Cavalie, I.; Massabuau, J.-C.; Gilbin, R.; Simon, O. Mitochondrial gene expression, antioxidant responses, and histopathology after cadmium exposure. Environ. Toxicol. 2012, 29, 893–907. [Google Scholar] [CrossRef]
- Shui, Y.; Xie, J.; Zhou, Y.; Li, J.; Gan, J. Molecular characterization of p38 MAPK and tissue-specific expression under cadmium stress in red swamp crayfish (Procambarus clarkii). Sci. Total Environ. 2020, 720, 137325. [Google Scholar] [CrossRef]
- A Jara, E.; Winter, C.K. Dietary exposure to total and inorganic arsenic in the United States, 2006–2008. Int. J. Food Contam. 2014, 1, 3. [Google Scholar] [CrossRef]
- Zhou, Q.; Gu, Y.; Yue, X.; Mao, G.; Wang, Y.; Su, H.; Xu, J.; Shi, H.; Zou, B.; Zhao, J.; et al. Combined toxicity and underlying mechanisms of a mixture of eight heavy metals. Mol. Med. Rep. 2016, 15, 859–866. [Google Scholar] [CrossRef]
- Neuberger, J.S.; Mulhall, M.; Pomatto, M.C.; Sheverbush, J.; Hassanein, R.S. Health problems in Galena, Kansas: A heavy metal mining Superfund site. Sci. Total Environ. 1990, 94, 261–272. [Google Scholar] [CrossRef]
- Klinova, S.V.; Minigalieva, I.A.; Privalova, L.I.; Valamina, I.E.; Makeyev, O.H.; Shuman, E.A.; Korotkov, A.A.; Panov, V.G.; Sutunkova, M.P.; Ryabova, J.V.; et al. Further verification of some postulates of the combined toxicity theory: New animal experimental data on separate and joint adverse effects of lead and cadmium. Food Chem. Toxicol. 2019, 136, 110971. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Yang, D.; Lei, B.; Zhang, X.; Zhang, X. Evaluation of human health risks posed by carcinogenic and non-carcinogenic multiple contaminants associated with consumption of fish from Taihu Lake, China. Food Chem. Toxicol. 2014, 69, 86–93. [Google Scholar] [CrossRef]

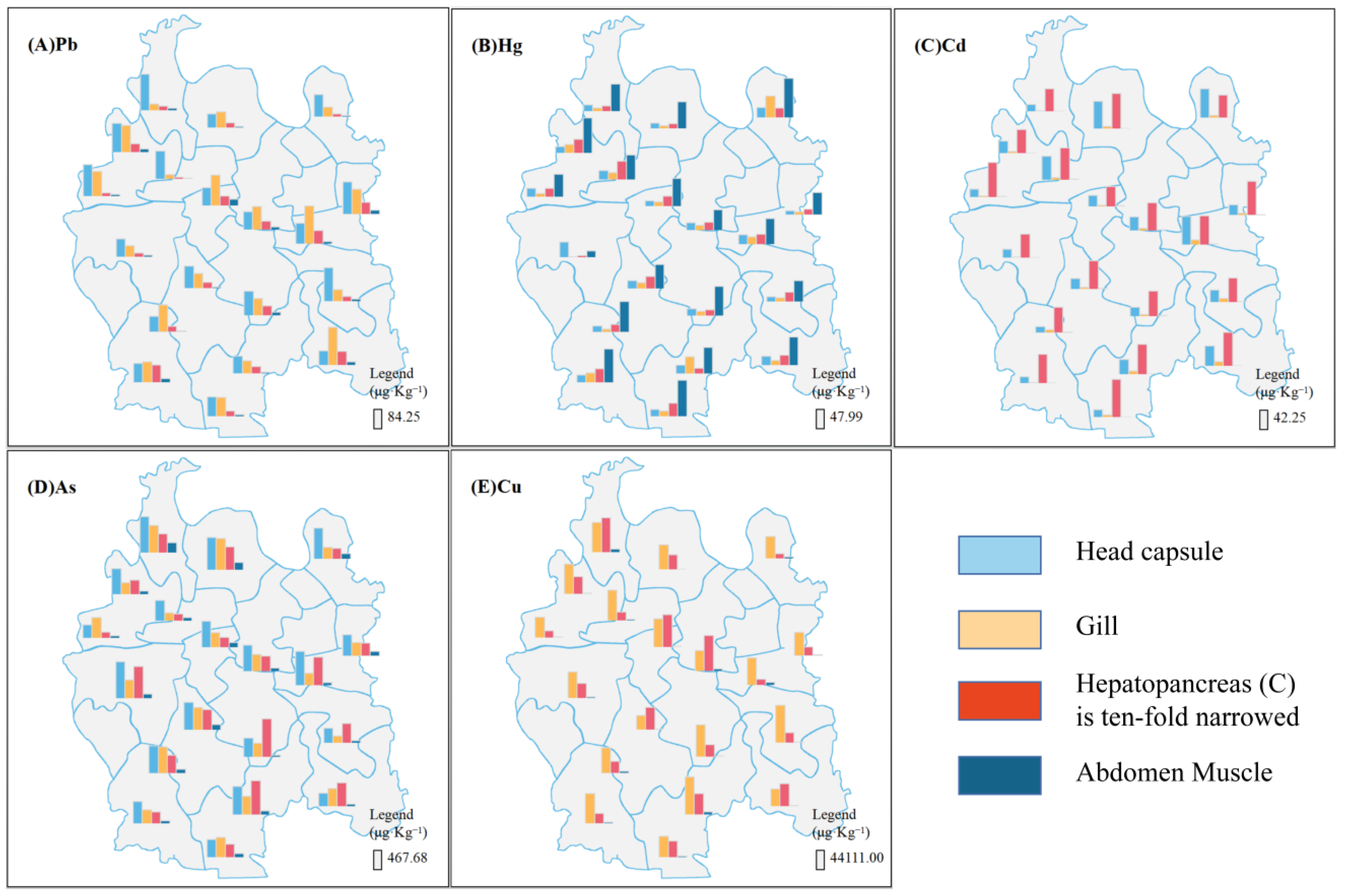
| Pb | Hg | Cd | As | Cu | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gi | He | Am | Gi | He | Am | Gi | He | Am | Gi | He | Am | He | Am | |
| Hc | −0.167 | −0.041 | 0.177 | −0.073 | 0.178 | −0.006 | 0.356 * | 0.201 | 0.076 | 0.158 | 0.571 ** | 0.383 * | ||
| Gi | 0.361 * | 0.291 | −0.091 | 0.031 | 0.466 ** | 0.101 | 0.171 | 0.205 | 0.368 * | −0.073 | ||||
| He | 0.305 | 0.459 ** | 0.193 | 0.008 | 0.119 | |||||||||
| Tissue | Metal | ||||||
|---|---|---|---|---|---|---|---|
| Pb | Hg | Cd | As | Cu | |||
| RfD | 0.02 | 0.1 | 0.001 | 3 × 10−4 | 0.04 | ||
| CSF | 8.5 × 10−3 | 1.5 | |||||
| PTDI | 3.6 × 10−3 | 0.83 × 10−3 | 2.14 × 10−3 | ||||
| EDI | Adults | He | 6.58 × 10−7 | 2.76 × 10−7 | 5.47 × 10−6 | 1.77 × 10−7 | 1.88 × 10−4 |
| Am | 2.87 × 10−7 | 1.77 × 10−6 | 6.27 × 10−9 | 7.39 × 10−8 | 8.74 × 10−5 | ||
| He + Am | 9.45 × 10−7 | 2.05 × 10−6 | 5.47 × 10−6 | 2.51 × 10−7 | 2.75 × 10−4 | ||
| Children | He | 3.37 × 10−6 | 9.65 × 10−7 | 3.70 × 10−5 | 6.19 × 10−7 | 1.89 × 10−4 | |
| Am | 2.42 × 10−6 | 6.18 × 10−6 | 7.11 × 10−9 | 2.59 × 10−7 | 2.34 × 10−5 | ||
| He + Am | 5.79 × 10−6 | 7.15 × 10−6 | 3.70 × 10−5 | 8.78 × 10−7 | 2.53 × 10−4 | ||
| THQ | Adults | He | 8.26 × 10−6 | 1.01 × 10−6 | 3.22 × 10−3 | 2.16 × 10−4 | 8.86 × 10−4 |
| Am | 7.89 × 10−6 | 6.47 × 10−6 | 1.17 × 10−5 | 9.02 × 10−5 | 1.04 × 10−3 | ||
| He + Am | 1.62 × 10−5 | 7.48 × 10−6 | 3.23 × 10−3 | 3.06 × 10−4 | 1.93 × 10−3 | ||
| Children | He | 3.56 × 10−6 | 2.72 × 10−7 | 1.55 × 10−3 | 5.82 × 10−5 | 2.31 × 10−4 | |
| Am | 3.48 × 10−6 | 1.74 × 10−6 | 6.18 × 10−7 | 2.43 × 10−5 | 1.92 × 10−5 | ||
| He + Am | 7.04 × 10−6 | 2.01 × 10−7 | 1.55 × 10−3 | 8.25 × 10−5 | 2.50 × 10−4 | ||
| CR | Adults | He | 2.05 × 10−9 | 9.73 × 10−8 | |||
| Am | 1.53 × 10−9 | 4.06 × 10−8 | |||||
| He + Am | 3.58 × 10−9 | 1.38 × 10−7 | |||||
| Children | He | 4.38 × 10−9 | 2.08 × 10−7 | ||||
| Am | 4.13 × 10−10 | 1.09 × 10−8 | |||||
| He + Am | 4.79 × 10−9 | 2.19 × 10−7 | |||||
| CRlimn (Kg/day) | Adults | He | 20.24 | 241.51 | 0.06 | 1.13 | 0.08 |
| Am | 57.78 | 80.55 | 83.21 | 5.78 | 1.01 | ||
| He + Am | 78.02 | 322.06 | 83.27 | 6.91 | 1.09 | ||
| Children | He | 5.78 | 69 | 0.02 | 0.32 | 0.02 | |
| Am | 16.5 | 23.02 | 23.77 | 1.65 | 0.29 | ||
| He + Am | 22.28 | 92.02 | 23.79 | 1.97 | 0.31 | ||
| CRmm (meals/month) | Adults | He | >180 | >180 | 7.94 | 149.56 | 10.58 |
| Am | >180 | >180 | >180 | >180 | 133.6 | ||
| He + Am | >180 | >180 | >180 | >180 | 144.3 | ||
| Children | He | >180 | >180 | 2.65 | 42.35 | 2.65 | |
| Am | >180 | >180 | >180 | >180 | 38.38 | ||
| He + Am | >180 | >180 | >180 | >180 | 41.03 | ||
| CRlimc (Kg/day) | Adults | He | 1.19 | 0.03 | |||
| Am | 3.4 | 0.13 | |||||
| He + Am | 4.59 | 0.16 | |||||
| Children | He | 0.34 | 0.007 | ||||
| Am | 0.97 | 0.036 | |||||
| He + Am | 1.31 | 0.043 | |||||
| CRmm (meals/month) | Adults | He | 157.49 | 3.97 | |||
| Am | >180 | 17.2 | |||||
| He + Am | >180 | 21.17 | |||||
| Children | He | 45 | 0.93 | ||||
| Am | 128.38 | 4.76 | |||||
| He + Am | >180 | 5.69 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Song, Z.; Zhou, Y.; Zhong, S.; Yu, Y.; Liu, T.; Gao, X.; Li, L.; Kong, C.; Wang, X.; et al. The Accumulation of Toxic Elements (Pb, Hg, Cd, As, and Cu) in Red Swamp Crayfish (Procambarus clarkii) in Qianjiang and the Associated Risks to Human Health. Toxics 2023, 11, 635. https://doi.org/10.3390/toxics11070635
Zhang L, Song Z, Zhou Y, Zhong S, Yu Y, Liu T, Gao X, Li L, Kong C, Wang X, et al. The Accumulation of Toxic Elements (Pb, Hg, Cd, As, and Cu) in Red Swamp Crayfish (Procambarus clarkii) in Qianjiang and the Associated Risks to Human Health. Toxics. 2023; 11(7):635. https://doi.org/10.3390/toxics11070635
Chicago/Turabian StyleZhang, Lang, Ziwei Song, Yuntao Zhou, Shan Zhong, Yali Yu, Ting Liu, Xiaoping Gao, Lekang Li, Chiping Kong, Xinna Wang, and et al. 2023. "The Accumulation of Toxic Elements (Pb, Hg, Cd, As, and Cu) in Red Swamp Crayfish (Procambarus clarkii) in Qianjiang and the Associated Risks to Human Health" Toxics 11, no. 7: 635. https://doi.org/10.3390/toxics11070635
APA StyleZhang, L., Song, Z., Zhou, Y., Zhong, S., Yu, Y., Liu, T., Gao, X., Li, L., Kong, C., Wang, X., He, L., & Gan, J. (2023). The Accumulation of Toxic Elements (Pb, Hg, Cd, As, and Cu) in Red Swamp Crayfish (Procambarus clarkii) in Qianjiang and the Associated Risks to Human Health. Toxics, 11(7), 635. https://doi.org/10.3390/toxics11070635





