Marine Pollution and Advances in Biomonitoring in Cartagena Bay in the Colombian Caribbean
Abstract
1. Introduction
2. Systematic Literature Exploration
3. General Description of Cartagena Bay
4. Environmental and Socioeconomic Dynamics in Cartagena Bay
5. Diversity of Domestic and Industrial Pollution in Cartagena Bay
5.1. Pollution by Hydrocarbons
5.2. Pollution by Pesticides and Persistent Organic Compounds
5.3. Pollution by Trace Metals
5.4. Pollution by Microplastics and Emerging Pollutants
6. Biomonitoring of Pollutants and Impacts on Marine Animals in Cartagena Bay
6.1. Biomonitoring of Organic Pollutants, Per- and Polyfluoroalkyl Substances (PFASs), Polycyclic Aromatic Hydrocarbons (PAHs), and Pesticides
6.2. Biomonitoring of Metals
6.3. Biomarkers and Effects of Pollutants in Marine Organisms
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moberg, F.; Rönnbäck, P. Ecosystem services of the tropical seascape: Interactions, substitutions and restoration. Ocean Coast. Manag. 2003, 46, 27–46. [Google Scholar] [CrossRef]
- Mehvar, S.; Filatova, T.; Dastgheib, A.; Steveninck, E.D.R.V.; Ranasinghe, R. Quantifying Economic Value of Coastal Ecosystem Services: A Review. J. Mar. Sci. Eng. 2018, 6, 5. [Google Scholar] [CrossRef]
- Ali, S.; Darsan, J.; Singh, A.; Wilson, M. Sustainable coastal ecosystem management—An evolving paradigm and its application to Caribbean SIDS. Ocean Coast. Manag. 2018, 163, 173–184. [Google Scholar] [CrossRef]
- Curran, S.; Kumar, A.; Lutz, W.; Williams, M. Interactions between Coastal and Marine Ecosystems and Human Population Systems: Perspectives on How Consumption Mediates This Interaction. AMBIO 2002, 31, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Barbier, E.B. Climate change impacts on rural poverty in low-elevation coastal zones. Estuarine Coast. Shelf Sci. 2015, 165, A1–A13. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, J.; Lu, X.; Su, C.; Zhang, Y.; Wang, C.; Cao, X.; Li, Q.; Su, J.; Ittekkot, V.; et al. Major threats of pollution and climate change to global coastal ecosystems and enhanced management for sustainability. Environ. Pollut. 2018, 239, 670–680. [Google Scholar] [CrossRef]
- Basova, M.; Krasheninnikova, S.; Parrino, V. Intra-Decadal (2012–2021) Dynamics of Spatial Ichthyoplankton Distribution in Sevastopol Bay (Black Sea) Affected by Hydrometeorological Factors. Animals 2022, 12, 3317. [Google Scholar] [CrossRef]
- Shu, Y.; Wang, X.; Huang, Z.; Song, L.; Fei, Z.; Gan, L.; Xu, Y.; Yin, J. Estimating spatiotemporal distribution of wastewater generated by ships in coastal areas. Ocean Coast. Manag. 2022, 222, 106133. [Google Scholar] [CrossRef]
- Afsa, S.; Hamden, K.; Martin, P.A.L.; Ben Mansour, H. Occurrence of 40 pharmaceutically active compounds in hospital and urban wastewaters and their contribution to Mahdia coastal seawater contamination. Environ. Sci. Pollut. Res. 2019, 27, 1941–1955. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Conley, D.J.; Garnier, J.; Doney, S.C.; Marino, R.; Billen, G. Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front. Ecol. Environ. 2011, 9, 18–26. [Google Scholar] [CrossRef]
- Liu, S.; Lou, S.; Kuang, C.; Huang, W.; Chen, W.; Zhang, J.; Zhong, G. Water quality assessment by pollution-index method in the coastal waters of Hebei Province in western Bohai Sea, China. Mar. Pollut. Bull. 2011, 62, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, X.; Hu, Y.; Chen, G.; Xu, J. The influence of anthropogenic activities on heavy metal pollution of estuary sediment from the coastal East China Sea in the past nearly 50 years. Mar. Pollut. Bull. 2022, 181, 113872. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Gallardo, K.; Guerrero-Castilla, A.; Johnson-Restrepo, B.; de la Rosa, J.; Olivero-Verbel, J. Chemical and toxicological characterization of sediments along a Colombian shoreline impacted by coal export terminals. Chemosphere 2015, 138, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Parrino, V.; Costa, G.; Giannetto, A.; De Marco, G.; Cammilleri, G.; Acar, Ü.; Piccione, G.; Fazio, F. Trace elements (Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in Mytilus galloprovincialis and Tapes decussatus from Faro and Ganzirri Lakes (Sicily, Italy): Flow cytometry applied for hemocytes analysis. J. Trace Elem. Med. Biol. 2021, 68, 126870. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.R.; Li, J.; Wang, J.; Schlenk, D.; Gan, J. Occurrence and Probable Sources of Urban-Use Insecticides in Marine Sediments off the Coast of Los Angeles. Environ. Sci. Technol. 2019, 53, 9584–9593. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, R.; León, V.M. Presence and distribution of current-use pesticides in surface marine sediments from a Mediterranean coastal lagoon (SE Spain). Environ. Sci. Pollut. Res. 2017, 24, 8033–8048. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.Z.; Zanardi-Lamardo, E.; Choueri, R.B.; Castro, B. Marine protected areas in Latin America and Caribbean threatened by polycyclic aromatic hydrocarbons. Environ. Pollut. 2021, 269, 116194. [Google Scholar] [CrossRef]
- Menzies, R.; Quinete, N.S.; Gardinali, P.; Seba, D. Baseline occurrence of organochlorine pesticides and other xenobiotics in the marine environment: Caribbean and Pacific collections. Mar. Pollut. Bull. 2013, 70, 289–295. [Google Scholar] [CrossRef]
- Girones, L.; Oliva, A.L.; Marcovecchio, J.E.; Arias, A.H. Spatial Distribution and Ecological Risk Assessment of Residual Organochlorine Pesticides (OCPs) in South American Marine Environments. Curr. Environ. Health Rep. 2020, 7, 147–160. [Google Scholar] [CrossRef]
- Díaz-Mendoza, C.; Mouthon-Bello, J.; Pérez-Herrera, N.L.; Escobar-Díaz, S.M. Plastics and microplastics, effects on marine coastal areas: A review. Environ. Sci. Pollut. Res. 2020, 27, 39913–39922. [Google Scholar] [CrossRef]
- Kanhai, L.D.K.; Asmath, H.; Gobin, J.F. The status of marine debris/litter and plastic pollution in the Caribbean Large Marine Ecosystem (CLME): 1980–2020. Environ. Pollut. 2022, 300, 118919. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Buitrago, N.; Williams, A.; Anfuso, G. Killing the goose with the golden eggs: Litter effects on scenic quality of the Caribbean coast of Colombia. Mar. Pollut. Bull. 2018, 127, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Hidalgo, F.; Alonso, E.; García-Corcoles, M.T.; Vílchez, J.L.; Zafra-Gómez, A. Assessing bioaccumulation potential of personal care, household and industrial products in a marine echinoderm (Holothuria tubulosa). Sci. Total Environ. 2020, 720, 137668. [Google Scholar] [CrossRef] [PubMed]
- Branchet, P.; Arpin-Pont, L.; Piram, A.; Boissery, P.; Wong-Wah-Chung, P.; Doumenq, P. Pharmaceuticals in the marine environment: What are the present challenges in their monitoring? Sci. Total Environ. 2020, 766, 142644. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Castro, V.; Quintana, J.; Rodil, R.; Beiras, R.; Vidal-Liñán, L. Bioaccumulation of organophosphorus flame retardants in the marine mussel Mytilus galloprovincialis. Sci. Total Environ. 2021, 805, 150384. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.K.; Maranho, L.A.; Pereira, C.D.S. Review on the occurrence and biological effects of illicit drugs in aquatic ecosystems. Environ. Sci. Pollut. Res. 2020, 27, 30998–31034. [Google Scholar] [CrossRef]
- David, A.; Fenet, H.; Gomez, E. Alkylphenols in marine environments: Distribution monitoring strategies and detection considerations. Mar. Pollut. Bull. 2009, 58, 953–960. [Google Scholar] [CrossRef]
- Bainbridge, Z.; Lewis, S.; Bartley, R.; Fabricius, K.; Collier, C.; Waterhouse, J.; Garzon-Garcia, A.; Robson, B.; Burton, J.; Wenger, A.; et al. Fine sediment and particulate organic matter: A review and case study on ridge-to-reef transport, transformations, fates, and impacts on marine ecosystems. Mar. Pollut. Bull. 2018, 135, 1205–1220. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, W.; Song, J.; Qian, Y.; Peng, P. Sorption of organic pollutants by marine sediments: Implication for the role of particulate organic matter. Chemosphere 2006, 65, 2493–2501. [Google Scholar] [CrossRef]
- Peña-Icart, M.; Pereira-Filho, E.R.; Fialho, L.L.; Nóbrega, J.A.; Alonso-Hernández, C.; Bolaños-Alvarez, Y.; Pomares-Alfonso, M.S. Combining contamination indexes, sediment quality guidelines and multivariate data analysis for metal pollution assessment in marine sediments of Cienfuegos Bay, Cuba. Chemosphere 2017, 168, 1267–1276. [Google Scholar] [CrossRef]
- Aslam, S.N.; Venzi, M.S.; Venkatraman, V.; Mikkelsen, Ø. Chemical assessment of marine sediments in vicinity of Norwegian fish farms—A pilot study. Sci. Total Environ. 2020, 732, 139130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Tam, N.F.-Y. Natural attenuation of contaminated marine sediments from an old floating dock–Part I: Spatial and temporal changes of organic and inorganic pollutants. Sci. Total Environ. 2012, 420, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wu, S.; Zhang, C.; Xu, J.; Christie, P.; Zhang, J.; Cao, Y. The role of antibiotics in mercury methylation in marine sediments. J. Hazard. Mater. 2018, 360, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leignel, V.; Pillot, L.; Gerpe, M.S.; Caurant, F. Assessment of Knowledge on Metal Trace Element Concentrations and Metallothionein Biomarkers in Cetaceans. Toxics 2023, 11, 454. [Google Scholar] [CrossRef]
- Leipe, T.; Kersten, M.; Heise, S.; Pohl, C.; Witt, G.; Liehr, G.; Zettler, M.; Tauber, F. Ecotoxicity assessment of natural attenuation effects at a historical dumping site in the western Baltic Sea. Mar. Pollut. Bull. 2005, 50, 446–459. [Google Scholar] [CrossRef]
- Traina, A.; Ausili, A.; Bonsignore, M.; Fattorini, D.; Gherardi, S.; Gorbi, S.; Quinci, E.; Romano, E.; Manta, D.S.; Tranchida, G.; et al. Organochlorines and Polycyclic Aromatic Hydrocarbons as fingerprint of exposure pathways from marine sediments to biota. Mar. Pollut. Bull. 2021, 170, 112676. [Google Scholar] [CrossRef]
- Au, D. The application of histo-cytopathological biomarkers in marine pollution monitoring: A review. Mar. Pollut. Bull. 2004, 48, 817–834. [Google Scholar] [CrossRef]
- Aguzzi, J.; Chatzievangelou, D.; Francescangeli, M.; Marini, S.; Bonofiglio, F.; del Rio, J.; Danovaro, R. The Hierarchic Treatment of Marine Ecological Information from Spatial Networks of Benthic Platforms. Sensors 2020, 20, 1751. [Google Scholar] [CrossRef]
- Hazen, E.L.; Abrahms, B.; Brodie, S.; Carroll, G.; Jacox, M.G.; Savoca, M.S.; Scales, K.L.; Sydeman, W.J.; Bograd, S.J. Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 2019, 17, 565–574. [Google Scholar] [CrossRef]
- Mghili, B.; De-La-Torre, G.E.; Analla, M.; Aksissou, M. Marine macroinvertebrates fouled in marine anthropogenic litter in the Moroccan Mediterranean. Mar. Pollut. Bull. 2022, 185, 114266. [Google Scholar] [CrossRef]
- Al, M.A.; Akhtar, A.; Kamal, A.H.M.; AftabUddin, S.; Islam, S.; Sharifuzzaman, S. Assessment of benthic macroinvertebrates as potential bioindicators of anthropogenic disturbance in southeast Bangladesh coast. Mar. Pollut. Bull. 2022, 184, 114217. [Google Scholar] [CrossRef]
- Youssef, M.; El-Sorogy, A.; Al-Kahtany, K.; Saleh, M. Benthic Foraminifera as Bio-indicators of Coastal Marine Environmental Contamination in the Red Sea-Gulf of Aqaba, Saudi Arabia. Bull. Environ. Contam. Toxicol. 2021, 106, 1033–1043. [Google Scholar] [CrossRef]
- Torres, M.A.; Barros, M.P.; Campos, S.C.G.; Pinto, E.; Rajamani, S.; Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, T.; Danowska, B. Marine environment status assessment based on macrophytobenthic plants as bio-indicators of heavy metals pollution. Mar. Pollut. Bull. 2017, 118, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Schuab, J.M.; Quirino, W.P.; de Paula, M.S.; Milagres, M.R.; Motta, D.G.; Zamprogno, G.C.; Otegui, M.B.P.; Ocaris, E.R.Y.; da Costa, M.B. Abundance of microplastic in different coastal areas using Phragmatopoma caudata (Kroyer in Morch, 1863) (Polychaeta: Sabelariidae) as an indicator. Sci. Total Environ. 2023, 880, 163219. [Google Scholar] [CrossRef]
- Navon, G.; Kaplan, A.; Avisar, D.; Shenkar, N. Assessing pharmaceutical contamination along the Mediterranean and Red Sea coasts of Israel: Ascidians (Chordata, Ascidiacea) as bioindicators. Mar. Pollut. Bull. 2020, 160, 111510. [Google Scholar] [CrossRef]
- Parra-Luna, M.; Pozo, L.M.; Hidalgo, F.; Zafra-Gómez, A. Common sea urchin (Paracentrotus lividus) and sea cucumber of the genus Holothuria as bioindicators of pollution in the study of chemical contaminants in aquatic media. A revision. Ecol. Indic. 2020, 113, 106185. [Google Scholar] [CrossRef]
- Alves, M.B.; Emerenciano, A.K.; Bordon, I.C.A.d.C.; Silva, J.R.M.C.; Fávaro, D.I.T.; Borges, J.C.S.; Borges, R.M.; e Pinto, J.M.; Rezende, K.F.O.; Dzik, L.M. Biomonitoring Assessment of Toxic and Trace Elements in Sterechinus neumayeri Sea Urchins from the Comandante Ferraz Station in Antarctica. Bull. Environ. Contam. Toxicol. 2021, 107, 11–19. [Google Scholar] [CrossRef]
- Yusof, A.M.; Yanta, N.F.; Wood, A.K.H. The use of bivalves as bio-indicators in the assessment of marine pollution along a coastal area. J. Radioanal. Nucl. Chem. 2004, 259, 119–127. [Google Scholar] [CrossRef]
- Moncaleano-Niño, A.M.; Gómez-Cubillos, M.C.; Luna-Acosta, A.; Villamil, L.; Casseres-Ruiz, S.; Ahrens, M.J. Monitoring metallothionein-like protein concentrations and cholinesterase activity in tropical cup oysters as biomarkers of exposure to metals and pesticides in the southern Caribbean, Colombia. Environ. Sci. Pollut. Res. 2021, 29, 25157–25183. [Google Scholar] [CrossRef]
- Aguirre-Rubí, J.R.; Ortiz-Zarragoitia, M.; Izagirre, U.; Etxebarria, N.; Espinoza, F.; Marigómez, I. Prospective biomonitor and sentinel bivalve species for pollution monitoring and ecosystem health disturbance assessment in mangrove–lined Nicaraguan coasts. Sci. Total Environ. 2018, 649, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Romero-Murillo, P.; Espejo, W.; Barra, R.; Orrego, R. Embryo–larvae and juvenile toxicity of Pb and Cd in Northern Chilean scallop Argopecten purpuratus. Environ. Monit. Assess. 2017, 190, 16. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, A.H.; Quiroga, M.L.R.; Liberoff, A.L.; Van der Molen, S. Marine pollution effects on the southern surf crab Ovalipes trimaculatus (Crustacea: Brachyura: Polybiidae) in Patagonia Argentina. Mar. Pollut. Bull. 2015, 91, 524–529. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, N.; Yin, D.; Maltby, L.; Wood, R.M.; Yu, H. Evaluation of sensitivity and specificity of two crustacean biochemical biomarkers. Environ. Toxicol. Chem. 2000, 19, 2085–2092. [Google Scholar] [CrossRef]
- El-Kahawy, R.; El-Shafeiy, M.; Helal, S.; Aboul-Ela, N.; El-Wahab, M.A. Benthic ostracods (crustacean) as a nearshore pollution bio-monitor: Examples from the Red Sea Coast of Egypt. Environ. Sci. Pollut. Res. 2021, 28, 31975–31993. [Google Scholar] [CrossRef]
- Karam, Q.; Guermazi, W.; Subrahmanyam, M.N.V.; Al-Enezi, Y.; Ali, M.; Leignel, V.; Annabi-Trabelsi, N. Portunus pelagicus (Linnaeus, 1758) as a Sentinel Species to Assess Trace Metal Occurrence: A Case Study of Kuwait Waters (Northwestern Arabian Gulf). Toxics 2023, 11, 426. [Google Scholar] [CrossRef]
- D’costa, A.; Shyama, S.; Kumar, M.P. Bioaccumulation of trace metals and total petroleum and genotoxicity responses in an edible fish population as indicators of marine pollution. Ecotoxicol. Environ. Saf. 2017, 142, 22–28. [Google Scholar] [CrossRef]
- Bertel-Sevilla, A.; Alzate, J.F.; Olivero-Verbel, J. De novo assembly and characterization of the liver transcriptome of Mugil incilis (lisa) using next generation sequencing. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Caballero-Gallardo, K.; Torres-Fuentes, N. Assessment of mercury in muscle of fish from Cartagena Bay, a tropical estuary at the north of Colombia. Int. J. Environ. Health Res. 2009, 19, 343–355. [Google Scholar] [CrossRef]
- Häder, D.-P.; Banaszak, A.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef]
- Fanning, L.; Mahon, R.; McConney, P. Focusing on Living Marine Resource Governance: The Caribbean Large Marine Ecosystem and Adjacent Areas Project. Coast. Manag. 2009, 37, 219–234. [Google Scholar] [CrossRef]
- Muñoz, J.M.B. Progress of coastal management in Latin America and the Caribbean. Ocean Coast. Manag. 2019, 184, 105009. [Google Scholar] [CrossRef]
- Schuhmann, P.W.; Mahon, R. The valuation of marine ecosystem goods and services in the Caribbean: A literature review and framework for future valuation efforts. Ecosyst. Serv. 2015, 11, 56–66. [Google Scholar] [CrossRef]
- Manrique-Rodríguez, N.; Agudelo, C.; Sanjuan-Muñoz, A.; Manrique-Rodríguez, N.; Agudelo, C.; Sanjuan-Muñoz, A. Comunidad de Octocorales Gorgonáceos Del Arrecife de Coral de Varadero En El Caribe Colombiano: Diversidad y Distribución Espacial. Boletín Investig. Mar. y Costeras-INVEMAR 2019, 48, 55–64. [Google Scholar] [CrossRef]
- Blanco-Libreros, J.F.; Ramírez-Ruiz, K. Threatened Mangroves in the Anthropocene: Habitat Fragmentation in Urban Coastalscapes of Pelliciera spp. (Tetrameristaceae) in Northern South America. Front. Mar. Sci. 2021, 8, 670354. [Google Scholar] [CrossRef]
- Díaz, J.M.; Gómez López, D.I. Cambios Históricos En La Distribución Y Abundancia De Praderas De Pastos Marinos En La Bahía De Cartagena Y Areas Aledañas (Colombia). Bull. Mar. Coast. Res. 2016, 32. [Google Scholar] [CrossRef]
- Daza, D.A.V.; Moreno, H.S.; Portz, L.; Manzolli, R.P.; Bolívar-Anillo, H.J.; Anfuso, G. Mangrove Forests Evolution and Threats in the Caribbean Sea of Colombia. Water 2020, 12, 1113. [Google Scholar] [CrossRef]
- Torregroza, E.; De Cartagena, U.; Hernández, M.; Barraza, D.; Gómez, A.; Borja, F.; De Huelva, U. Unidades ecológicas para una gestión ecosistémica en el distrito Cartagena de Indias (Colombia). Rev. UDCA Actual. Divulg. Científica 2014, 17, 205–215. [Google Scholar] [CrossRef]
- Montoya-Rojas, G.A.; García, M.A.; Bello-Escobar, S.; Singh, K.P. Analysis of the interrelations between biogeographic systems and the dynamics of the Port-Waterfront Cities: Cartagena de Indias, Colombia. Ocean Coast. Manag. 2019, 185, 105055. [Google Scholar] [CrossRef]
- Ibáñez, M. Mangrove Restoration: Cartagena, Colombia, Coastal Oil Spill Case Study. In Proceedings of the International Oil Spill Conference, IOSC, Santafé de Bogotá D.C., Colombia, 1st February 1995; pp. 4565–4568. [Google Scholar]
- Garay-Tinoco, J.A. Vigilancia de la contaminación por petróleo en el Caribe colombiano (Punta Canoas-Barbacoas). Boletín Científico CIOH 1987, 7, 101–117. [Google Scholar] [CrossRef]
- Cowgill, U.; Gowland, R.; Ramirez, C.; Fernandez, V. The history of a chlorpyrifos spill: Cartagena, Colombia. Environ. Int. 1991, 17, 61–71. [Google Scholar] [CrossRef]
- Alonso, D.; Pineda, P.; Olivero, J.; González, H.; Campos, N. Mercury levels in muscle of two fish species and sediments from the Cartagena Bay and the Ciénaga Grande de Santa Marta, Colombia. Environ. Pollut. 2000, 109, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Rendon, S.; Vanegas, T.; Tigreros, P.C. Contaminación en la Bahía de Cartagena por agua de lastre de los buques. Boletín Científico CIOH 2003, 21, 91–100. [Google Scholar] [CrossRef]
- Suárez Castaño, R. (Ed.) ANLA Reporte de Análisis Regional de La Bahía de Cartagena y Canal Del Dique; Autoridad Nacional de Licencias Ambientales: Bogota, Colombia, 2021. [Google Scholar]
- Tuchkovenko, Y.S.; Lonin, S.A.; Calero, L.A. Modelo de eutroficación de la Bahía de Cartagena y su aplicación práctica. Boletín Científico CIOH 2002, 20, 28–44. [Google Scholar] [CrossRef]
- Garay-Tinoco, J.A.; Ospina, L.N.G. Influencia de los aportes de materia orgánica externa y autóctona en el decrecimiento de los niveles de oxígeno disuelto en la Bahía de Cartagena, Colombia. Boletín Científico CIOH 1998, 18, 1–13. [Google Scholar] [CrossRef]
- Urbano, R.J. Estado Actual de La Bahía de Cartagena v/s Contaminación. Bol. Cient. 1992, 10, 3–12. [Google Scholar] [CrossRef]
- Aldana-Domínguez, J.; Montes, C.; Martinez, M.; Medina, N.; Hahn, J.; Duque, M. Biodiversity and Ecosystem Services Knowledge in the Colombian Caribbean. Trop. Conserv. Sci. 2017, 10. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Alcala-Orozco, M.; Barraza-Quiroz, D.; De la Rosa, J.; Olivero-Verbel, J. Environmental risks associated with trace elements in sediments from Cartagena Bay, an industrialized site at the Caribbean. Chemosphere 2019, 242, 125173. [Google Scholar] [CrossRef]
- Manjarres-Suarez, A.; de la Rosa, J.; Gonzalez-Montes, A.; Galvis-Ballesteros, J.; Olivero-Verbel, J. Trace elements, peripheral blood film, and gene expression status in adolescents living near an industrial area in the Colombian Caribbean Coastline. J. Expo. Sci. Environ. Epidemiol. 2021, 32, 146–155. [Google Scholar] [CrossRef]
- Manjarres-Suarez, A.; Olivero-Verbel, J. Hematological parameters and hair mercury levels in adolescents from the Colombian Caribbean. Environ. Sci. Pollut. Res. 2020, 27, 14216–14227. [Google Scholar] [CrossRef]
- Parga-Lozano, C.; Marrugo-González, A.; Fernández-Maestre, R. Hydrocarbon contamination in Cartagena Bay, Colombia. Mar. Pollut. Bull. 2002, 44, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Serna, Y.; Correa-Metrio, A.; Kenney, W.F.; Curtis, J.H.; Velez, M.I.; Brenner, M.; Hoyos, N.; Restrepo, J.C.; Cordero-Oviedo, C.; Buck, D.; et al. Post-colonial pollution of the Bay of Cartagena, Colombia. J. Paleolimnol. 2019, 63, 21–35. [Google Scholar] [CrossRef]
- Villalobos, D.B.; Mendoza, D.D.C.; Pájaro, C.M.; Herrera, R.F.; Lambis-Miranda, H.A. Spatial perspective of hexavalent chromium concentration in superficial waters of the Ciénaga de las Quintas Mangrove Swamp, Cartagena de Indias, Colombia. Lakes Reserv. Res. Manag. 2018, 23, 287–296. [Google Scholar] [CrossRef]
- Tosic, M.; Martins, F.; Lonin, S.; Izquierdo, A.; Restrepo, J.D. Hydrodynamic modelling of a polluted tropical bay: Assessment of anthropogenic impacts on freshwater runoff and estuarine water renewal. J. Environ. Manag. 2019, 236, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Mouthon-Bello, J.A.; Quiñones-Bolaños, E.; Ortiz-Corrales, J.E.; Mouthon-Barraza, N.; Hernández-Fuentes, M.D.; Caraballo-Meza, A.C. Spatial variability study of rainfall in Cartagena de Indias, Colombia. J. Water Land Dev. 2022, 55, 138–149. [Google Scholar] [CrossRef]
- Molares, R. Clasificación e identificación de las componentes de marea del Caribe colombiano. Boletín Científico CIOH 2004, 22, 105–114. [Google Scholar] [CrossRef]
- Orejarena-Rondón, A.F.; Otero-Díaz, L.J.; Restrepo, L.J.C.; Ramos De la Hoz, I.M.; Marriaga-Rocha, L. Methodology for determining the mean and extreme sea level regimes (astronomical and meteorological tides) considering scarce records in microtidal zones: Colombian Caribbean case. Dyna 2018, 85, 274–283. [Google Scholar] [CrossRef]
- Cuervo, G.V. Paleogeografía del canal y delta del Dique, Colombia. Cuad. De Geogr. Rev. Colomb. De Geogr. 2021, 30, 239–256. [Google Scholar] [CrossRef]
- Lonin, S.; Parra, C.; Andrade, C.; Thomas, Y.-F. Patrones de la pluma turbia del canal del Dique en la bahía de Cartagena. Boletín Científico CIOH 2004, 22, 77–89. [Google Scholar] [CrossRef]
- Tirado-Muñoz, O.; Tirado-Ballestas, I.; Valdelamar-Villegas, J.C.; Castro-Angulo, I. Annual Behavior of Cu, Pb, Cr and Total Hg in Superficial Waters from Dique Channel during 2006-2010, Cartagena, Colombia. J. Waste Water Treat. Anal. 2017, 8, 3. [Google Scholar] [CrossRef]
- Restrepo, J.D.; Escobar, R.; Tosic, M. Fluvial fluxes from the Magdalena River into Cartagena Bay, Caribbean Colombia: Trends, future scenarios, and connections with upstream human impacts. Geomorphology 2018, 302, 92–105. [Google Scholar] [CrossRef]
- Andrade, C.A.; Thomas, Y.F. Geometría de los depósitos cuaternarios y actuales de la Bahía de Cartagena de Indias, Colombia. Bol. Científico CIOH 2012, 30, 117–131. [Google Scholar] [CrossRef]
- Alvarado, C.; Velasco, S.; Leones-cerpa, J.; Sánchez-, E.; Ojeda, K.A. Socioeconomic and Environmental Analysis Based on Water Sustainability Index in the Juan Angola Creek (Cartagena Colombia). Chem. Eng. Trans. 2023, 98, 153–158. [Google Scholar] [CrossRef]
- Richerzhagen, C.; de Francisco, J.C.R.; Weinsheimer, F.; Döhnert, A.; Kleiner, L.; Mayer, M.; Morawietz, J.; Philipp, E. Ecosystem-Based Adaptation Projects, More than just Adaptation: Analysis of Social Benefits and Costs in Colombia. Int. J. Environ. Res. Public Health 2019, 16, 4248. [Google Scholar] [CrossRef]
- Hobday, A.J.; Cochrane, K.; Downey-Breedt, N.; Howard, J.; Aswani, S.; Byfield, V.; Duggan, G.; Duna, E.; Dutra, L.X.C.; Frusher, S.D.; et al. Planning adaptation to climate change in fast-warming marine regions with seafood-dependent coastal communities. Rev. Fish Biol. Fish. 2016, 26, 249–264. [Google Scholar] [CrossRef]
- Grima, N.; Singh, S.J. The self-(in)sufficiency of the Caribbean: Ecosystem services potential Index (ESPI) as a measure for sustainability. Ecosyst. Serv. 2020, 42, 101087. [Google Scholar] [CrossRef]
- Gilman, E. Guidelines for coastal and marine site-planning and examples of planning and management intervention tools. Ocean Coast. Manag. 2002, 45, 377–404. [Google Scholar] [CrossRef]
- Lewison, R.L.; Rudd, M.A.; Al-Hayek, W.; Baldwin, C.; Beger, M.; Lieske, S.N.; Jones, C.; Satumanatpan, S.; Junchompoo, C.; Hines, E. How the DPSIR framework can be used for structuring problems and facilitating empirical research in coastal systems. Environ. Sci. Policy 2016, 56, 110–119. [Google Scholar] [CrossRef]
- Dzoga, M.; Simatele, D.M.; Munga, C.; Yonge, S. Application of the DPSIR Framework to Coastal and Marine Fisheries Management in Kenya. Ocean Sci. J. 2020, 55, 193–201. [Google Scholar] [CrossRef]
- DANE, Demografía y Población. Available online: https://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion (accessed on 13 May 2023).
- Valdelamar Villegas, J.C.; Andrade-Quintero, K.; Díaz-Mendoza, C.; Manjarrez-Paba, G. Temporal Space Behavior of Three Environmental Quality Determinants from Touristic Beaches in Cartagena, Colombia. Coast. Res. Libr. 2018, 24, 845–858. [Google Scholar] [CrossRef]
- IDEAM; PNUD; MADS; DNP; CANCILLERÍA. Tercera Comunicación Nacional de Colombia a La Convención Marco de Las Naciones Unidas Sobre Cambio Climático (CMNUCC); IDEAM: Bogotá, Colombia, 2017; ISBN 978-958-8971-73-5. [Google Scholar]
- Nevermann, H.; Gomez, J.N.B.; Fröhle, P.; Shokri, N. Land loss implications of sea level rise along the coastline of Colombia under different climate change scenarios. Clim. Risk Manag. 2023, 39, 100470. [Google Scholar] [CrossRef]
- Mendoza, C.D.; Valdelamar, J.C.; Jimenez, J.J.; Avila, G.R. Solid Waste Characterization, Fats and Oils in Two Tourist Resorts Cartagena Colombia. J. Environ. Prot. 2013, 4, 1–4. [Google Scholar] [CrossRef]
- Villegas, F.F.V. Modernización urbana y exclusión social en Cartagena de Indias, una mirada desde la prensa local. Territorios 2017, 36, 159–188. [Google Scholar] [CrossRef]
- Wainer, L.S.; Vale, L.J. Wealthier-but-poorer: The complex sociology of homeownership at peripheral housing in Cartagena, Colombia. Habitat Int. 2021, 114, 102388. [Google Scholar] [CrossRef]
- Bernal, G.; Osorio, A.; Urrego, L.; Peláez, D.; Molina, E.; Zea, S.; Montoya, R.; Villegas, N. Occurrence of energetic extreme oceanic events in the Colombian Caribbean coasts and some approaches to assess their impact on ecosystems. J. Mar. Syst. 2016, 164, 85–100. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.G.; Anfuso, G.; Williams, A.T. Coastal erosion along the Caribbean coast of Colombia: Magnitudes, causes and management. Ocean Coast. Manag. 2015, 114, 129–144. [Google Scholar] [CrossRef]
- Restrepo, J.D. Applicability of LOICZ catchment–coast continuum in a major Caribbean basin: The Magdalena River, Colombia. Estuarine, Coast. Shelf Sci. 2008, 77, 214–229. [Google Scholar] [CrossRef]
- Vergara Arrieta, J.J.; Carbal Herrera, A.E. Costos Sociales y Ambientales de La Doble Calzada Vía Al Mar Cartagena-Barranquilla Tramo 1. Espacios 2017, 38, 12. [Google Scholar]
- Correa Ayram, C.A.; Etter, A.; Díaz-Timoté, J.; Rodríguez Buriticá, S.; Ramírez, W.; Corzo, G. Spatiotemporal evaluation of the human footprint in Colombia: Four decades of anthropic impact in highly biodiverse ecosystems. Ecol. Indic. 2020, 117, 106630. [Google Scholar] [CrossRef]
- Cuartas, A.G.; Suarez, K.L. Evaluación de contaminación microbiologica antropogénica en agua de mar de la bahía de Cartagena-Bolívar durante abril a julio de 2019. Bol. Cient. CIOH 2020, 39, 41–52. [Google Scholar] [CrossRef]
- Duarte-Restrepo, E.; Noguera-Oviedo, K.; Butryn, D.; Wallace, J.S.; Aga, D.S.; Jaramillo-Colorado, B.E. Spatial distribution of pesticides, organochlorine compounds, PBDEs, and metals in surface marine sediments from Cartagena Bay, Colombia. Environ. Sci. Pollut. Res. 2020, 28, 14632–14653. [Google Scholar] [CrossRef] [PubMed]
- Manjarrez-Paba, G.; Blanco Herrera, J.I.; Arrunategui, B.P.G. Environmental and Health Risk by the Presence of Parasites in the Sand of Cartagena Beaches. Coast. Res. Libr. 2018, 24, 831–844. [Google Scholar] [CrossRef]
- Acosta-Coley, I.; Olivero-Verbel, J. Microplastic resin pellets on an urban tropical beach in Colombia. Environ. Monit. Assess. 2015, 187, 435. [Google Scholar] [CrossRef] [PubMed]
- Escobar, R.; Luna-Acosta, A.; Caballero, S. DNA barcoding, fisheries and communities: What do we have? Science and local knowledge to improve resource management in partnership with communities in the Colombian Caribbean. Mar. Policy 2018, 99, 407–413. [Google Scholar] [CrossRef]
- Ospina-Arango, J.; Pardo-Rodríguez, F.; Álvarez-León, R. Madurez Gonadal de La Ictiofauna Presente En La Bahía de Cartagena, Caribe Colombiano. Boletín Científico del Mus. Hist. Nat. 2008, 12, 117–140. [Google Scholar]
- Alcaldía Distrital de Cartagena de Indias; Establecimiento Público Ambiental de Cartagena, E. Formulación y Adopción Del Plan Integral de Gestión Del Cambio Climático Del Distrito de Cartagena de Indias. Available online: https://plan4c.cartagena.gov.co/ (accessed on 13 May 2023).
- Galván-Borja, D.; Olivero-Verbel, J.; Barrios-García, L. Occurrence of Ascocotyle (Phagicola) longa Ransom, 1920 (Digenea: Heterophyidae) in Mugil incilis from Cartagena Bay, Colombia. Veter. Parasitol. 2010, 168, 31–35. [Google Scholar] [CrossRef]
- Johnson-Restrepo, B.; Olivero-Verbel, J.; Lu, S.; Guette-Fernández, J.; Baldiris-Avila, R.; O’Byrne-Hoyos, I.; Aldous, K.M.; Addink, R.; Kannan, K. Polycyclic aromatic hydrocarbons and their hydroxylated metabolites in fish bile and sediments from coastal waters of Colombia. Environ. Pollut. 2008, 151, 452–459. [Google Scholar] [CrossRef]
- Jaramillo, B.; Marrugo, M.P.; Duarte, E. Monitoreo de residuos de pesticidas organoclorados en camaron (penaeus vannamei) del area costera de la bahía Cartagena (Colombia). Biotecnol. En El Sect. Agropecu. Y Agroind. 2010, 8, 66–71. [Google Scholar]
- Olivero-Verbel, J.; Johnson-Restrepo, B.; Baldiris-Avila, R.; Güette-Fernández, J.; Magallanes-Carreazo, E.; Vanegas-Ramírez, L.; Kunihiko, N. Human and crab exposure to mercury in the Caribbean coastal shoreline of Colombia: Impact from an abandoned chlor-alkali plant. Environ. Int. 2008, 34, 476–482. [Google Scholar] [CrossRef]
- Barrios, R.A. Diagnóstico preeliminar ambiental de playas de Cartagena de Indias, Caribe colombiano. Tek. Rev. Científica 2017, 17, 38–46. [Google Scholar] [CrossRef]
- Páez, M.L.C.; Venegas, T.; Gavilán, M.; Morris, L.F.; Tous, G. Dinámica planctónica, microbiológica y fisicoquímica en cuatro muelles de la Bahía de Cartagena y buques de tráfico internacional. Bol. Cien. CIOH 2005, 23, 46–59. [Google Scholar] [CrossRef]
- Baldiris, I.; Acosta, J.C.; Martinez, C.E.; Sanchez, J.; Castro, I.; Severiche, C. Multivariate Analysis of Surface Water Quality of the Bay of Cartagena (Colombia) Period 2001-2017. Int. J. Chem. Tech Res. 2017, 10, 421–432. [Google Scholar]
- Restrepo, J.D.; Tosic, M. BASIC Interacciones Entre Cuenca, Mar y Comunidades; Universidad EAFIT: Medellín, Colombia, 2017. [Google Scholar]
- Díaz, M.M.A. El Canal Del Dique y Su Subregion: Una Economía Basada En La Riqueza Hidrica; Banco de la República: Cartagena de Indias, Colombia, 2006. [Google Scholar]
- UNESCO World Heritage Centre. Decision 44 COM 7B.167 Port, Fortresses and Group of Monuments, Cartagena (Colombia) (C 285); UNESCO World Heritage Centre: Paris, France, 2021. [Google Scholar]
- Malagón, J. Impacto Económico y Social Del Puerto de Cartagena Informe Final. Fedesarrollo 2014, 1–79. Available online: http://hdl.handle.net/11445/707 (accessed on 15 July 2023).
- Ministerio de Comercio Industria y Turismo Resultados Para El Turismo 2018. Mincomercio 2019, 1–19. Available online: https://www.mincit.gov.co/prensa/noticias/turismo/el-turismo-obtuvo-resultados-historicos-en-2018 (accessed on 10 March 2023).
- DANE. Cuentas Nacionales Departamentales: PIB Por Departamento. Available online: https://www.dane.gov.co/index.php/estadisticas-por-tema/cuentas-nacionales/cuentas-nacionales-departamentales (accessed on 14 May 2023).
- Tosic, M.; Restrepo, J.D.; Lonin, S.; Izquierdo, A.; Martins, F. Water and sediment quality in Cartagena Bay, Colombia: Seasonal variability and potential impacts of pollution. Estuarine, Coast. Shelf Sci. 2019, 216, 187–203. [Google Scholar] [CrossRef]
- Jaramillo-Colorado, B.E.; Arroyo-Salgado, B.; Ruiz-Garcés, L.C. Organochlorine pesticides and parasites in Mugil incilis collected in Cartagena Bay, Colombia. Environ. Sci. Pollut. Res. 2015, 22, 17475–17485. [Google Scholar] [CrossRef]
- Manjarrez-Paba, G.; Castro, I.; Utria, L. Bioacumulación de Cadmio En Ostras de La Bahía de Cartagena. Rev. Ing. Univ. Medellín 2008, 7, 11–20. [Google Scholar]
- Olivero-Verbel, J.; Agudelo-Frias, D.; Caballero-Gallardo, K. Morphometric parameters and total mercury in eggs of snowy egret (Egretta thula) from Cartagena Bay and Totumo Marsh, north of Colombia. Mar. Pollut. Bull. 2013, 69, 105–109. [Google Scholar] [CrossRef]
- Buchman, M.F. Screening Quick Reference Tables (SQuiRTs); United States, National Oceanic and Atmospheric Administration. 2008. Available online: https://repository.library.noaa.gov/view/noaa/9327 (accessed on 15 July 2023).
- Müller, G. Schwermetalle in Den Sedimenten Des Rheins—Veranderungen Seit 1971. Umsch. Wissensch. Techn. 1979, 79, 778–783. [Google Scholar]
- Jahan, S.; Strezov, V. Comparison of pollution indices for the assessment of heavy metals in the sediments of seaports of NSW, Australia. Mar. Pollut. Bull. 2018, 128, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Birch, G. Determination of sediment metal background concentrations and enrichment in marine environments—A critical review. Sci. Total. Environ. 2017, 580, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Birch, G. A review and critical assessment of sedimentary metal indices used in determining the magnitude of anthropogenic change in coastal environments. Sci. Total Environ. 2023, 854, 158129. [Google Scholar] [CrossRef] [PubMed]
- Truskewycz, A.; Gundry, T.D.; Khudur, L.S.; Kolobaric, A.; Taha, M.; Aburto-Medina, A.; Ball, A.S.; Shahsavari, E. Petroleum Hydrocarbon Contamination in Terrestrial Ecosystems—Fate and Microbial Responses. Molecules 2019, 24, 3400. [Google Scholar] [CrossRef] [PubMed]
- Manzetti, S. Polycyclic Aromatic Hydrocarbons in the Environment: Environmental Fate and Transformation. Polycycl. Aromat. Compd. 2013, 33, 311–330. [Google Scholar] [CrossRef]
- Catalán, F.S.; Mancebo, G.M.; Hernandez, J.R.; Páez, J.P.M.; Restrepo, B.G.J. Caracterización fisicoquímica, determinación de mercurio total e hidrocarburos disueltos y dispersos en aguas y sedimentos de la bahía de cartagena, Colombia. Bol. Cient. CIOH 2020, 39, 41–50. [Google Scholar] [CrossRef]
- Espinosa-Díaz, L.F.; Sánchez-Cabeza, J.-A.; Sericano, J.L.; Parra, J.P.; Ibarra-Gutierrez, K.P.; Garay-Tinoco, J.A.; Betancourt-Portela, J.M.; Alonso-Hernández, C.; Ruiz-Fernández, A.C.; Quejido-Cabezas, A.; et al. Sedimentary record of the impact of management actions on pollution of Cartagena bay, Colombia. Mar. Pollut. Bull. 2021, 172, 112807. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Corada-Fernández, C.; Lara-Martín, P.A.; Juan-García, A. Emerging contaminants and priority substances in marine sediments from Cartagena Bay and the Grand Marsh of Santa Marta (Ramsar site), Colombia. Environ. Monit. Assess. 2021, 193, 1–18. [Google Scholar] [CrossRef]
- Gallego, J.L.; Olivero-Verbel, J. Cytogenetic toxicity from pesticide and trace element mixtures in soils used for conventional and organic crops of Allium cepa L. Environ. Pollut. 2021, 276, 116558. [Google Scholar] [CrossRef]
- Lesmes-Fabian, C.; García-Santos, G.; Leuenberger, F.; Nuyttens, D.; Binder, C.R. Dermal exposure assessment of pesticide use: The case of sprayers in potato farms in the Colombian highlands. Sci. Total Environ. 2012, 430, 202–208. [Google Scholar] [CrossRef]
- Espana, V.A.A.; Pinilla, A.R.R.; Bardos, P.; Naidu, R. Contaminated land in Colombia: A critical review of current status and future approach for the management of contaminated sites. Sci. Total Environ. 2018, 618, 199–209. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat/es/ (accessed on 2 March 2020).
- Marrugo-Negrete, J.L.; Navarro-Frómeta, A.E.; Urango-Cardenas, I.D. Organochlorine Pesticides in Soils from the Middle and Lower Sinú River Basin (Córdoba, Colombia). Water Air Soil Pollut. 2014, 225, 1–13. [Google Scholar] [CrossRef]
- Cogua, P.; Campos-Campos, N.H.; Duque, G. Concentración de Mercurio Total y Metilmercurio En Sedimento y Seston de La Bahía de Cartagena, Caribe Colombiano. Bol. Investig. Mar. Costeras 2012, 41, 267–285. [Google Scholar]
- Fernandez-Maestre, R.; Johnson-Restrepo, B.; Olivero-Verbel, J. Heavy Metals in Sediments and Fish in the Caribbean Coast of Colombia: Assessing the Environmental Risk. Int. J. Environ. Res. 2018, 12, 289–301. [Google Scholar] [CrossRef]
- Acosta-Coley, I.; Mendez-Cuadro, D.; Rodriguez-Cavallo, E.; de la Rosa, J.; Olivero-Verbel, J. Trace elements in microplastics in Cartagena: A hotspot for plastic pollution at the Caribbean. Mar. Pollut. Bull. 2019, 139, 402–411. [Google Scholar] [CrossRef]
- Acosta-Coley, I.; Duran-Izquierdo, M.; Rodriguez-Cavallo, E.; Mercado-Camargo, J.; Mendez-Cuadro, D.; Olivero-Verbel, J. Quantification of microplastics along the Caribbean Coastline of Colombia: Pollution profile and biological effects on Caenorhabditis elegans. Mar. Pollut. Bull. 2019, 146, 574–583. [Google Scholar] [CrossRef]
- Zeller, D.; Pauly, D. Reconstructing Marine Fisheries Catch Data. Methods Catch Alloc. Sea around Us 2015, 1–43. Available online: www.seaaroundus.org (accessed on 24 February 2023).
- Morales, J.; García-Alzate, C. Ecología trófica y rasgos ecomorfológicos de Triportheus magdalenae (Characiformes: Triportheidae) en el embalse El Guájaro, cuenca baja del río Magdalena, Colombia. Rev. De Biol. Trop. 2018, 66, 1208–1222. [Google Scholar] [CrossRef]
- García, C.B.; Contreras, C.C. Trophic levels of fish species of commercial importance in the Colombian Caribbean. Rev. De Biol. Trop. 2010, 59, 1195–1203. [Google Scholar] [CrossRef]
- Juárez-Camargo, P.G.; Sosa-López, A.; Torres-Rojas, Y.E.; Mendoza-Franco, E.F.; García, S.A. Feeding habits variability of Lutjanus synagris and Lutjanus griseus in the littoral of Campeche, Mexico: An approach of food web trophic interactions between two snapper species. Lat. Am. J. Aquat. Res. 2020, 48, 552–569. [Google Scholar] [CrossRef]
- Sandoval, L.; Mancera-Pineda, J.; Leal-Flórez, J.; Blanco-Libreros, J.; Delgado-Huertas, A. Mangrove carbon sustains artisanal fish and other estuarine consumers in a major mangrove area of the southern Caribbean Sea. Mar. Ecol. Prog. Ser. 2022, 681, 21–35. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Tao, L.; Johnson-Restrepo, B.; Guette-Fernández, J.; Baldiris-Avila, R.; O’Byrne-Hoyos, I.; Kannan, K. Perfluorooctanesulfonate and related fluorochemicals in biological samples from the north coast of Colombia. Environ. Pollut. 2006, 142, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Rubí, J.R.; Luna-Acosta, A.; Etxebarría, N.; Soto, M.; Espinoza, F.; Ahrens, M.J.; Marigómez, I. Chemical contamination assessment in mangrove-lined Caribbean coastal systems using the oyster Crassostrea rhizophorae as biomonitor species. Environ. Sci. Pollut. Res. 2017, 25, 13396–13415. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.H. Selected Bivalves for Monitoring of Heavy Metal Contamination in the Colombian Caribbean. In Metals in Coastal Environments of Latin America; Springer: Berlin, Heidelberg, Germany, 1988; pp. 270–275. [Google Scholar] [CrossRef]
- Sierra-Marquez, L.; Marquez, J.D.S.; De La Rosa, J.; Olivero-Verbel, J. Imposex in Stramonita haemastoma from coastal sites of Cartagena, Colombia. Braz. J. Biol. 2017, 78, 548–555. [Google Scholar] [CrossRef]
- Valdelamar-Villegas, J.; Olivero-Verbel, J. Bioecological Aspects and Heavy Metal Contamination of the Mollusk Donax denticulatus in the Colombian Caribbean Coastline. Bull. Environ. Contam. Toxicol. 2017, 100, 234–239. [Google Scholar] [CrossRef]
- Aguirre-Rubí, J.; Luna-Acosta, A.; Ortiz-Zarragoitia, M.; Zaldibar, B.; Izagirre, U.; Ahrens, M.; Villamil, L.; Marigómez, I. Assessment of ecosystem health disturbance in mangrove-lined Caribbean coastal systems using the oyster Crassostrea rhizophorae as sentinel species. Sci. Total Environ. 2018, 618, 718–735. [Google Scholar] [CrossRef]

| DPSIR Component | Trends |
|---|---|
| Drivers | The population increases 1.16% per year [103]; almost 30% of the inhabitants live in poverty and 5.5% in extreme poverty [97]; concentration of high pollutant industries; weak land use policies and controls; an increase in tourism [75,104]; and a temperature increase of +0.9 to +2.23 °C, a precipitation decrease by 15% to 17%, a rise in the average sea level by +15 to +20 cm, and a 30% increase in the intensity of extreme precipitation to the year 2100, due to climate change scenarios [105,106]. |
| Pressures | Informality and low adaptation of sustainable practices in economic activities; increased solid waste generation and wastewater discharges; increased water demand from tourism and industrial activities [75,104,107,108,109]; permanent sediment and pollutant loads from the Dique Channel [93,94]; the occurrence of extreme events [105,110]; and land use changes related to the loss of productive lands, filling of coasts, occupation of conservation areas, sediment loads, and coastal erosion [65,110,111,112,113,114]. |
| State | Low environmental quality [115]; degraded ecosystems; the presence of persistent organic pollutants in sediments from different areas of the bay; metals As, Cd, Cr, Cu, Hg, and Pb at levels above the threshold effect level [80,116]; solid waste and the contamination of beaches [104,117,118]; threats to species of interest due to degradation of refuges and breeding areas and overfishing [119,120]; and a high vulnerability to global change, with scenarios directly compromising 27.5% of the population and a risk of flooding in 28% of industries and 35% of public infrastructure [121]. |
| Impacts | Loss of habitat; seagrasses’ reduction by 63% in the last 25 years [66]; decrease in the coral community [64]; loss of mangroves; reduced connectivity between ecosystems [65,86]; alteration in the condition of fish related to increased infection by parasites [122]; bioaccumulation of organic contaminants and metals in different species [59,123,124,125]; increased environmental health threats for surrounding populations [81,82]; and the alteration of the physicochemical and microbiological water quality of the bay [115,126,127,128]. |
| Responses | Education programs; strengthening pollution control and policies; the implementation of climate change adaptation programs; institutional articulation for environmental monitoring; and access to information systems [75,121,129]. |
| PAH Compound (ng g−1) | Study Area | ||
|---|---|---|---|
| Cartagena Bay (2003–2004) | Cartagena Bay (2017–2018) | Santa Marta Bay (2017–2018) | |
| Acenaphthene | 1.6 | 0 | |
| Acenaphthylene | 5.8 | 0 | |
| Anthracene | 37.5 | ||
| Benzo[a]anthracene | 364.0 | 27.8 | 2.7 |
| Benzo[a]pyrene | 156.0 | 143.2 | 0 |
| Benzo[b]flurantene | 526.0 | 38.3 | 3.4 |
| Benzo[g,h,i]perylene | 145.0 | 27.0 | 1.9 |
| Chrysene | 252.0 | ||
| Dibenzo[a,h]anthracene | 138.0 | ||
| Fluoranthene | 68.4 | ||
| Fluorene | 13.8 | 5.4 | 4.4 |
| Indeno(1,2,3,cd)pyrene | 36.3 | ||
| Naphthalene | 2.3 | 1.9 | |
| Phenanthrene | 105.0 | 46.7 | 11.4 |
| Pyrene | 250.0 | 29.0 | 4.7 |
| Reference | [123] | [148] | |
| Metal (µg/g) | Reports of Trace Metals in Sediments (Year of Sampling) | Threshold Effect Level (TEL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2018 | 2015 | 2014–2015 | 2014 | 2012–2013 | 2006 | 1996 | ||
| As | 3.62–20.6 | 4.1–13.1 | 2–8.5 | 7.24 | ||||
| Cd | 0.11–2.1 | 0.2–2.3 | 0.232–0.877 | 0.015–0.057 | 0.13–0.55 | 0.68 | ||
| Cr | 24.1–268.2 | 22.6–137.2 | 5.9–59.8 | 5.1–18.7 | 52.3 | |||
| Cu | 11.5–147.7 | 20.5–429.0 | 3.1–38.6 | 6.8–65 | 18.7 | |||
| Hg | 0.01–0.84 | 0.065–0.30 | 0.02–0.17 | 0.02–0.55 | 0.094–10.29 | 0.13 | ||
| Ni | 11.2–67.1 | 24.6–32.7 | 14.9–23.9 | 3.9–11.3 | 15.9 | |||
| Pb | 3.6–54.4 | 7.7–37.1 | 1.6–14.6 | 1.4–2.0 | 2.7–6.4 | 30.24 | ||
| Sn | 0.1–3.3 | 0.20–0.53 | 0.048 | |||||
| Zn | 46–78 | 28–34 | 124 | |||||
| Sampling sites | 12 | 10 | 8 | 2 | 4 | 5 | 6 | |
| Reference | [80] | [116] | [135] | [155] | [50] | [154] | [73] | [139] |
| Species | Trophic Level | Data Base/Reference |
|---|---|---|
| Triportheus magdalenae | 0.12 | [159] |
| Crassostrea rhizophora | 2.00 | LME |
| Saccostrea sp. | 2.00 | FAO Area |
| Mugil incilis | 2.01 | LME |
| Kyphosus sp. | 2.05 | LME |
| Stramonita haemastoma | 2.10 | FAO Area |
| Mugil cephalus | 2.13 | LME |
| Penaeusvannamei | 2.70 | FAO Area |
| Archosargus rhomboidalis | 2.89 | EEZ |
| Eugerres plumieri | 3.29 | LME |
| Gerres cinereus | 3.47 | LME |
| Elops saurus | 3.49 | LME |
| Bagre marinus | 3.51 | EEZ |
| Chloroscombrus chrysurus | 3.54 | EEZ |
| Dactylopterus volitans | 3.65 | FAO Area |
| Haemulon steindachneri | 3.73 | LME |
| Cathorops mapale | 3.77 | [160] |
| Lutjanus synagris | 3.82 | EEZ |
| Lutjanus cf. griseus | 3.90 | [161] |
| Callinectes sapidus | 4.00 | LME |
| Centropomus undecimalis | 4.17 | EEZ |
| Cynoscion jamaicensis | 4.20 | LME |
| Caranx hipos | 4.23 | [160] |
| Oligoplites saliens | 4.30 | [162] |
| Trichiurus lepturus | 4.42 | EEZ |
| Seriola rivoliana | 4.45 | FAO Area |
| Opisthonema oglinum | 4.50 | EEZ |
| Isognomon alatus | No information | |
| Callinectes bocourti | No information | |
| Sciades herzbergi | No information | |
| Donax denticulatus | No information |
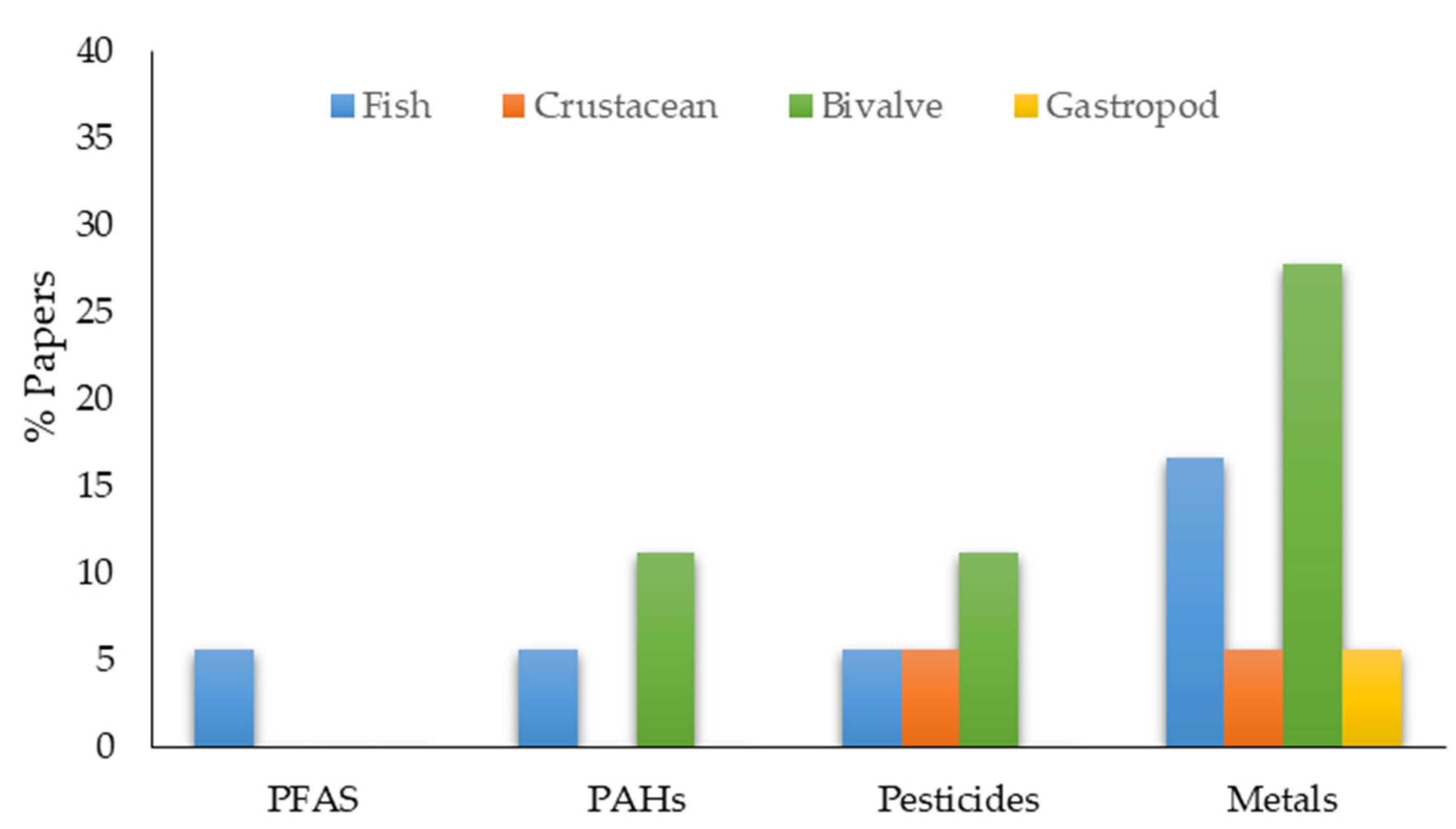
| Sampling Season | Species | Taxonomic Group | Pollutant Concentration (ng/g) | Trophic Level | Reference |
|---|---|---|---|---|---|
| December 2003 | Mugil incilis | Fish | PFOA: 370 ± 65.7 PFHxS: 0.489 ± 0.08 PFOSA: <0.3 | Detritivorous | [163] |
| August 2003 to June 2004 | Mugil incilis | Fish | ∑OH-PAH: 1250 | Detritivorous | [123] |
| January, June, and November 2008 | Penaeus vannamei | Crustacean | Metoxychlor: 94.6–163 Endrinsulfate: 1.6–17.9 BHC: 9.4–15.1 Endrinaldehyde: 3.4–5.6 | Detritivorous | [124] |
| June–November 2009 | Mugil incilis | Fish | β-HCH: 0.00185–0.00638 | Detritivorous | [136] |
| Aldrin: 0.00115–0.00333 | |||||
| 4,4′-DDD: 0.00404–0.00452 | |||||
| γ-HCH: 0.00851 ± 0.002 | |||||
| Heptachlor: 0.00436–0.00725 | |||||
| Endosulfan: 0.00415 ± 0.001 | |||||
| 4,4′-DDE: 0.00401 ± 0.001 | |||||
| Dieldrin: 0.00206 ± 0.000 | |||||
| October 2012 and March 2013 | Crassostrea rhizophora | Bivalve | ΣPAHs: 41.0–1299.5 ΣHMWPAHs: 87.8–986.3 ΣLMWPAHs: 0.8–265.6 Galaxolide (HHCB): 0.4–71.0 Tonalide (AHTN): 0.2–48.7 ΣMusks: 0.4–119.6 ΣPCBs (PCB7): 0.0–29.3 ΣPOPs: 6.1–140.6 | Filter-feeding | [164] |
| October 2012 March 2013 | Saccostrea sp. | Bivalve | HCHs: <LOD 50 DDT: <LOD 2 Chlorpyrifos:<LOD 2 | Filter-feeding | [50] |
| Sampling Season | Species | Taxonomic Group | Metal Concentration | Trophic Level | Reference |
|---|---|---|---|---|---|
| November 1980 | Crassotrea rhizophorae Isognomon alatus | Bivalve | Cd: 2.51–15.9 0.80–15.60 Cu: 11.70–23 0.87–4.77 Pb: 1.26–5.13 0.75–3.16 | Filter-feeding | [165] |
| March, May, August, and November 1996 | Mugil incilis Eugerres plumieri | Fish | Hg: 0.007 to 0.166 0.019 to 0.852 | Detritivorous Omnivorous | [73] |
| March–April, May–June July–August 2007 | Not reported | Bivalve | Cd: 4.98 to 21.33 | Filter-feeding | [137] |
| 2004–2005 | Callinectes sapidus Callinectes bocourti | Crustacean | Hg: 0.124 ± 0.011 | Omnivorous | [125] |
| March–July 2006 | Chloroscombrus chrysurus Cynoscion jamaicensis Caranx hipos Elops saurus Lutjanus synagris Centropomus undecimalis Trichiurus lepturus | Fish | Hg: 0.26 ± 0.16 0.11 ± 0.05 0.09 ± 0.03 0.05 ± 0.02 0.08 ± 0.01 0.09 ± 0.04 0.08 ± 0.03 | Carnivorous Second Order | [59] |
| Opisthonema oglinum Dactylopterus volitans Gerres cinereus Eugerres plumieri Haemulon steindachneri Oligoplites saliens Sciades herzbergi | Fish | Hg: 0.11 ± 0.04 0.05 ± 0.02 0.10 ± 0.08 0.04 ± 0.04 0.08 ± 0.04 0.09 ± 0.02 0.11 ± 0.06 | Carnivorous Third Order | ||
| Triportheus magdalenae Archosargus rhomboidalis | Fish | Hg: 0.07 + 0.01 | Omnivorous | ||
| Mugil cephalus Mugil incilis | Fish | Hg: 0.02 ± 0.01 0.03 ± 0.02 | Detritivorous | ||
| 2013 | Stramonita haemastoma | Gastropod | As: 0.158 Cd: 0.02 Cr: 0.056 Cu: 0.880 Ni: <0.01 Pb: 0.695 Sn: 0.126 Zn: 0.479 | Detritivorous | [166] |
| September 2012 and May 2013 | Donax denticulatus | Bivalve | Cd: 0.040 Hg: 0.006 Pb: 0.060 | Filter-feeding | [167] |
| October 2012 and March 2013 | Crassostrea rhizophora | Bivalve | ΣAg, Al, As, Cd, Cr, Cu, Hg, Ni, Pb, Ti, V, and Zn 629.80–2490.53 | Filter-feeding | [164] |
| October 2012 and March 2013 | Saccostrea sp. | Bivalve | As: 5.96–7.62 Cd: 3.43–15.88 Cr: 0.23–9.14 Cu: 38.72–296.68 Hg: 0.04–0.09 Pb: 0.15–0.75 Ni: 0.43–1.61 Sn: 0–1.05 Zn: 488.6–3390.2 | Filter-feeding | [50] |
| June–July 2014 | Kyphosus sp. Seriola rivoliana Lutjanus cf. griseus Mugil incilis Cathorops mapale Bagre marinus. | Fish | Zn: 0.330–3.90 Cd: ND-0.0053 Ni: ND-0.500 Pb: 0.010–0.110 | Carnivorous | [155] |
| Species | Biomarker Level | Method | Inference | Reference |
|---|---|---|---|---|
| Mugil incilis | Morphology | Measurements of total length and weight; condition factor; gill-somatic index; hepato-somatic index; spleen-somatic index | t-test between sampling sites. | [163] |
| Morphology | Measurements of total length and weight; condition factor; hepato-somatic index; bazosomatic index | Correlation of morphometric parameters, parasitic intensity, and concentration of organochlorine pesticides and comparison with histopathological changes | [136] | |
| Histology | Parasitic infection, histopathology recorded by lesions, nonspecific inflammatory changes (infiltration of inflammatory cells and granulomatosis), necrosis, apoptosis, and the presence of melano-macrophage centers (MMCs) | |||
| Molecular | RNA-Seq gene markers of heavy metal exposure, xenobiotic metabolism, nuclear receptor modulation, oxidative stress, DNA damage, inflammation, and lipid metabolism | Gene expression | [58] | |
| 18 Fish species | Morphology | Measurements of total length and weight; condition factor; gill-somatic index; hepato-somatic index; spleen-somatic index | Spearman correlations between T-Hg levels and morphometric indexes | [59] |
| Crassostrea rhizophorae | Histology | Parasitic infection, histopathology with inflammatory response index (IRI), haemocytic infiltration, brown cell aggregates, and disseminated neoplasia | Statistical differences between sampling sites and season. | [168] |
| Morphology | Flesh condition index, shell length, flesh dry weight, shell cavity volume, gamete developmental stage | |||
| Molecular | Total metallothionein proteins, cholinesterase activity (ChE), eserine-resistant cholinesterase (Er-ChE) activity in digestive glands and gills | Statistical differences between sampling sites. | [50] | |
| Stramonita haemastoma | Morphology | Imposex: relative penis length index (RPLI), relative penis size index (RPSI). | Prevalence by sampling sites. | [166] |
| Donax denticulatus | Morphology | Measurements of anteroposterior length, total width, total height, total weight, and tissue biomass | Pearson correlation for Hg, Pb, and Cd (not significative) and distribution of sampling sites according to Principal Components Analysis. | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Murillo, P.; Gallego, J.L.; Leignel, V. Marine Pollution and Advances in Biomonitoring in Cartagena Bay in the Colombian Caribbean. Toxics 2023, 11, 631. https://doi.org/10.3390/toxics11070631
Romero-Murillo P, Gallego JL, Leignel V. Marine Pollution and Advances in Biomonitoring in Cartagena Bay in the Colombian Caribbean. Toxics. 2023; 11(7):631. https://doi.org/10.3390/toxics11070631
Chicago/Turabian StyleRomero-Murillo, Patricia, Jorge L. Gallego, and Vincent Leignel. 2023. "Marine Pollution and Advances in Biomonitoring in Cartagena Bay in the Colombian Caribbean" Toxics 11, no. 7: 631. https://doi.org/10.3390/toxics11070631
APA StyleRomero-Murillo, P., Gallego, J. L., & Leignel, V. (2023). Marine Pollution and Advances in Biomonitoring in Cartagena Bay in the Colombian Caribbean. Toxics, 11(7), 631. https://doi.org/10.3390/toxics11070631








