Evaluation of In Vitro Genotoxicity of Polystyrene Nanoparticles in Human Peripheral Blood Mononuclear Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Particle Characterization and Preparation of NPs Suspensions
2.2. Biological Material
2.3. Lymphocyte Isolation
2.4. NPs Uptake Analysis
2.5. Analysis of Cell Viability and Primary DNA Damage (the Comet Assay)
2.6. The Micronucleus Assay
2.7. Statistical Analysis
3. Results
3.1. PS-NPs Characterization
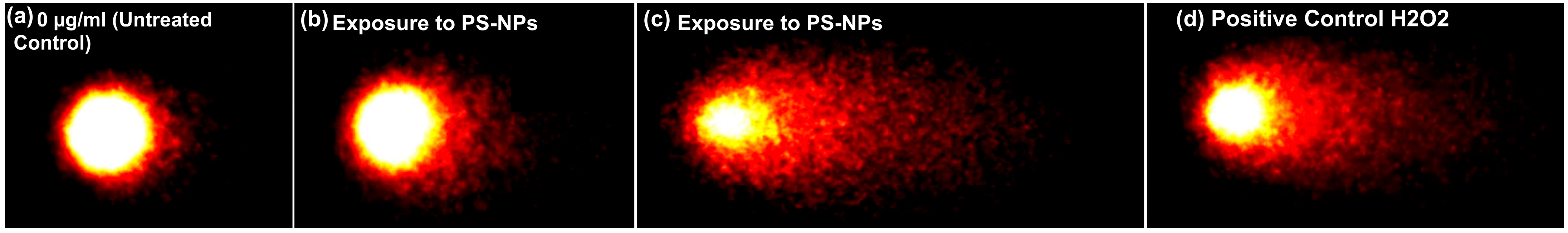
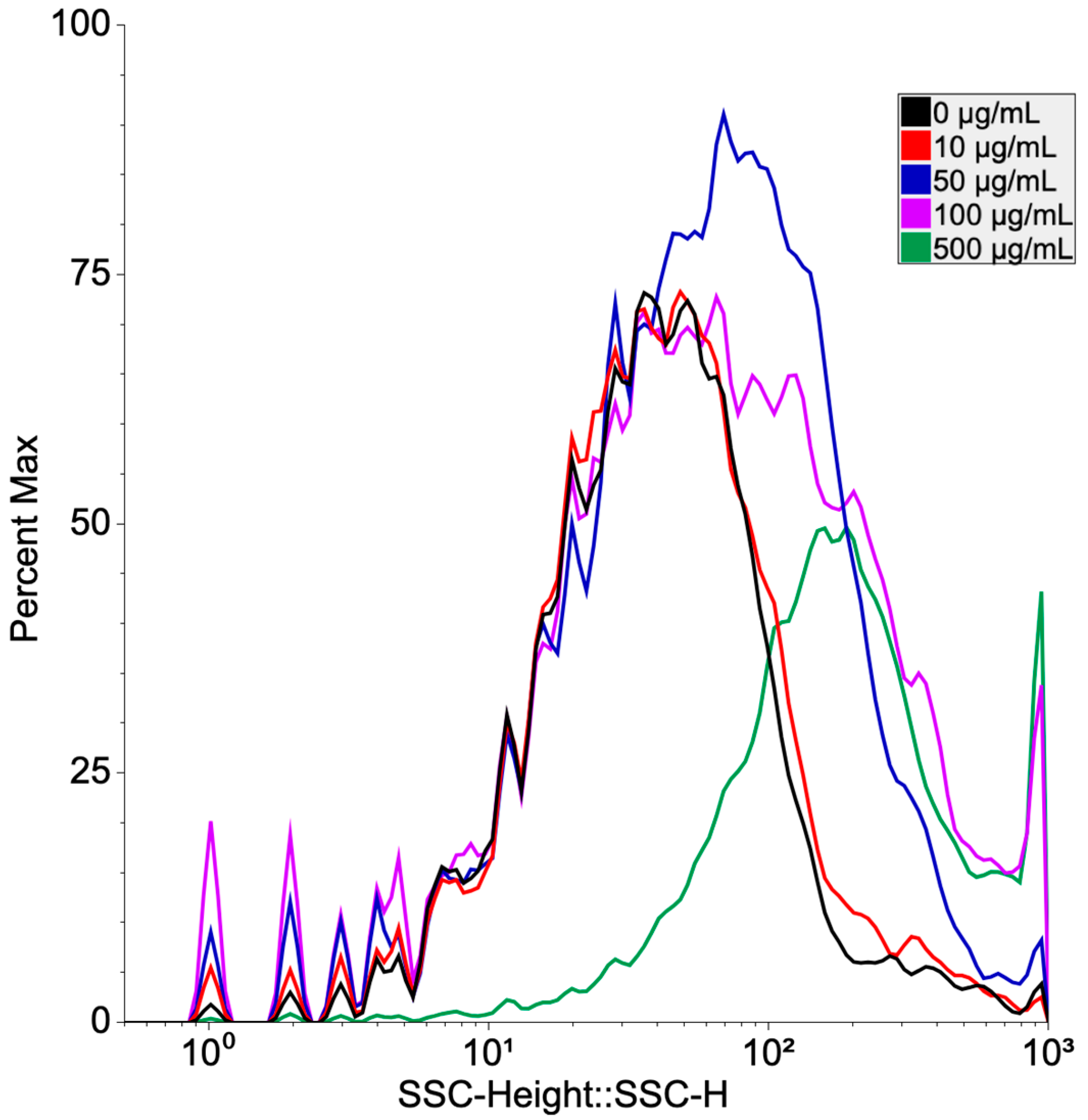
3.2. Uptake Analysis
3.3. Cell Viability and Induction of Primary DNA Damage
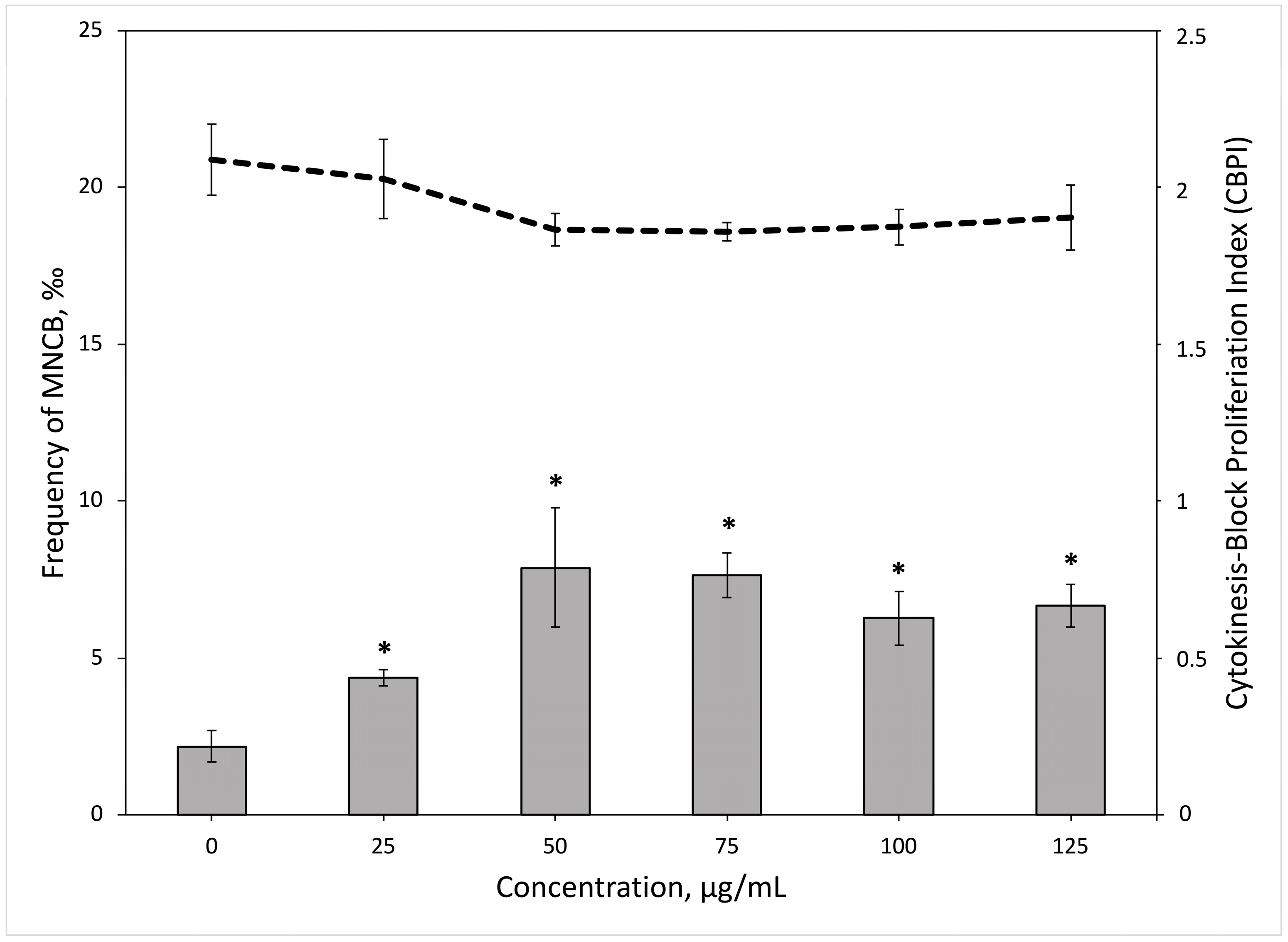
3.4. The Micronucleus Assay
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics—The Facts. Plastics Europe. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 4 March 2023).
- Plastic Pollution|Definition, Sources, Effects, Solutions, & Facts|Britannica. Available online: https://www.britannica.com/science/plastic-pollution (accessed on 4 March 2023).
- Kik, K.; Bukowska, B.; Sicińska, P. Polystyrene Nanoparticles: Sources, Occurrence in the Environment, Distribution in Tissues, Accumulation and Toxicity to Various Organisms. Environ. Pollut. 2020, 262, 114297. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Shri, B.; Ramasamy, S.; Palanisamy, S. A Review on Occurrence, Characteristics, Toxicology and Treatment of Nanoplastic Waste in the Environment. Env. Sci. Pollut. Res. 2021, 43258–43273. [Google Scholar]
- World Health Organization. Microplastics in Drinking Water; License: CC BY-NC-SA 3.0 IGO, G.; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Holzinger, R. Nanoplastics Measurements in Northern and Southern Polar Ice. Environ. Res. 2022, 208, 112741. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics and Nanoplastics: Emerging Contaminants in Food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef]
- Oliveri, G.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro- and Nano-Plastics in Edible Fruit and Vegetables. The First Diet Risks Assessment for the General Population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Kutralam-muniasamy, G.; Pérez-guevara, F.; Elizalde-martínez, I.; Shruti, V.C. Science of the Total Environment Branded Milks—Are They Immune from Microplastics Contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef]
- Walkinshaw, C.; Lindeque, P.K.; Thompson, R.; Tolhurst, T.; Cole, M. Ecotoxicology and Environmental Safety Microplastics and Seafood: Lower Trophic Organisms at Highest Risk of Contamination. Ecotoxicol. Environ. Saf. 2020, 190, 110066. [Google Scholar] [CrossRef]
- Chen, G.; Feng, Q.; Wang, J. Mini-Review of Microplastics in the Atmosphere and Their Risks to Humans. Sci. Total Environ. 2018, 703, 135504. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Salvia, R.; Rico, L.G.; Bradford, J.A.; Ward, M.D.; Olszowy, M.W.; Martínez, C.; Madrid-Aris, Á.D.; Grífols, J.R.; Ancochea, Á.; Gomez-Muñoz, L.; et al. Fast-Screening Flow Cytometry Method for Detecting Nanoplastics in Human Peripheral Blood. MethodsX 2023, 10, 102057. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene Nanoplastics Penetrate across the Blood-Brain Barrier and Induce Activation of Microglia in the Brain of Mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Cortés, C.; Domenech, J.; Salazar, M.; Pastor, S.; Marcos, R.; Hernández, A. Nanoplastics as a Potential Environmental Health Factor: Effects of Polystyrene Nanoparticles on Human Intestinal Epithelial Caco-2 Cells. Environ. Sci. Nano 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Hesler, M.; Aengenheister, L.; Ellinger, B.; Drexel, R.; Straskraba, S.; Jost, C.; Wagner, S.; Meier, F.; Von Briesen, H.; Büchel, C.; et al. Toxicology In Vitro Multi-Endpoint Toxicological Assessment of Polystyrene Nano- and Microparticles in Different Biological Models In Vitro. Toxicol. In Vitro 2019, 61, 104610. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological Effects, Including Oxidative Stress and Genotoxic Damage, of Polystyrene Nanoparticles in Different Human Hematopoietic Cell Lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.; Vecchiotti, G.; Colafarina, S.; Zarivi, O.; Aloisi, M.; Arrizza, L.; Chichiricc, G.; Di Carlo, P. In Vitro Genotoxicity of Polystyrene Nanoparticles on the Human Fibroblast Hs27 Cell Line. Nanomaterials 2019, 9, 1299. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro-and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef]
- Sarma, D.K.; Dubey, R.; Samarth, R.M.; Shubham, S.; Chowdhury, P.; Kumawat, M.; Verma, V.; Tiwari, R.R.; Kumar, M. The Biological Effects of Polystyrene Nanoplastics on Human Peripheral Blood Lymphocytes. Nanomaterials 2022, 12, 1632. [Google Scholar] [CrossRef]
- Ballesteros, S.; Domenech, J.; Barguilla, I.; Cortés, C.; Marcos, R.; Hernández, A. Genotoxic and Immunomodulatory Effects in Human White Blood Cells after Ex Vivo Exposure to Polystyrene Nanoplastics. Environ. Sci. Nano 2020, 7, 3431–3446. [Google Scholar] [CrossRef]
- Kik, K.; Bukowska, B.; Krokosz, A.; Sicińska, P. Oxidative Properties of Polystyrene Nanoparticles with Different Diameters in Human Peripheral Blood Mononuclear Cells (In Vitro Study). Int. J. Mol. Sci. 2021, 22, 4406. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, K.; Bukowska, B.; Piwoński, I.; Foksiński, M.; Kisielewska, A.; Zarakowska, E.; Gackowski, D.; Sicińska, P. Polystyrene Nanoparticles: The Mechanism of Their Genotoxicity in Human Peripheral Blood Mononuclear Cells. Nanotoxicology 2022, 16, 791–811. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of Genotoxicity. A Review of In Vitro and In Vivo Studies with Engineered Nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Toyooka, T.; Ibuki, Y. Simple and Easy Method to Evaluate Uptake Potential of Nanoparticles in Mammalian Cells Using a Flow Cytometric Light Scatter Analysis. Environ. Sci. Technol. 2007, 41, 3018–3024. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.C.; Liu, R.; Wu, X. Dual AO/EB Staining to Detect Apoptosis in Osteosarcoma Cells Compared with Flow Cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef]
- Biola, A.; Pallardy, M.; Bre, J. Apoptosis: Identification of Dying Cells. Cell Biol. Toxicol. 1998, 14, 111–120. [Google Scholar]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A Simple Technique for Quantifying Apoptosis in 96-Well Plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar] [CrossRef]
- Mcgahon, A.J.; Martin, S.J.; Bissonnette, R.P.; Mahboubi, A.; Shi, Y.; Mogil, R.J.; Nishioka, W.K.; Green, D.R. The End of the (Cell) Line: Methods for the Study of Apoptosis In Vitro. In Methods in Cell Biology; Schwartz, L.M., Osborne, B.A., Eds.; Academic Press: La Jolla, CA, USA, 1995; Volume 46. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Møller, P.; Azqueta, A.; Boutet-robinet, E.; Koppen, G.; Bonassi, S.; Mili, M.; Gajski, G.; Costa, S.; Teixeira, J.P. Minimum Information for Reporting on the Comet Assay (MIRCA): Recommendations for Describing Comet Assay Procedures and Results. Nat. Protoc. 2020, 15, 3817–3826. [Google Scholar] [CrossRef]
- Beedanagari, S. Genetic Toxicology. In Comprehensive Medicinal Chemistry III; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Waltham, MA, USA, 2017; pp. 195–200. ISBN 9780124095472. [Google Scholar]
- Fenech, M. The In Vitro Micronucleus Technique. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2000, 455, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Sanderson, B.J.S. Adaptations of the In Vitro MN Assay for the Genotoxicity Assessment of Nanomaterials. Mutagenesis 2011, 26, 185–191. [Google Scholar] [CrossRef]
- Kirsch-volders, M.; Sofuni, T.; Aardema, M.; Albertini, S.; Eastmond, D.; Fenech, M.; Ishidate, M.; Kirchner, S.; Lorge, E.; Morita, T.; et al. Report from the In Vitro Micronucleus Assay Working Group. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 540, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Barnoud, J.; Monticelli, L. Polystyrene Nanoparticles Perturb Lipid Membranes. J. Phys. Chem. Lett. 2014, 5, 241–246. [Google Scholar] [CrossRef]
- Gopinath, P.M.; Saranya, V.; Vijayakumar, S.; Mythili Meera, M.; Ruprekha, S.; Kunal, R.; Pranay, A.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Assessment on Interactive Prospectives of Nanoplastics with Plasma Proteins and the Toxicological Impacts of Virgin, Coronated and Environmentally Released-Nanoplastics. Sci. Rep. 2019, 9, 8860. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Smajdova, L. The Use of Genotoxicity Biomarkers in Molecular Epidemiology: Applications in Environmental, Occupational and Dietary Studies. AIMS Genet. 2017, 4, 166–191. [Google Scholar] [CrossRef]
- Dusinska, M.; Boland, S.; Saunders, M.; Juillerat-Jeanneret, L.; Tran, L.; Pojana, G.; Marcomini, A.; Volkovova, K.; Tulinska, J.; Knudsen, L.E.; et al. Towards an Alternative Testing Strategy for Nanomaterials Used in Nanomedicine: Lessons from NanoTEST. Nanotoxicology 2015, 9, 118–132. [Google Scholar] [CrossRef]
- Landsiedel, R.; Honarvar, N.; Seiffert, S.B.; Oesch, B.; Oesch, F. Genotoxicity Testing of Nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2022, 14, e1833. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Wang, J. Equilibrium, Kinetics and Molecular Dynamic Modeling of Sr2+ Sorption onto Microplastics. J. Hazard. Mater. 2020, 400, 123324. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Liu, X.; Huang, R.; Su, R.; Qi, W.; Che, J.; He, Z. Synergistic Effect of Polystyrene Nanoplastics and Contaminants on the Promotion of Insulin Fibrillation. Ecotoxicol. Environ. Saf. 2021, 214, 112115. [Google Scholar] [CrossRef] [PubMed]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; d’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as Vehicles of Environmental PAHs to Marine Organisms: Combined Chemical and Physical Hazards to the Mediterranean Mussels, Mytilus Galloprovincialis. Front. Mar. Sci. 2018, 5, 103. [Google Scholar] [CrossRef]


| Matrix | Time after Sonication, h | Highest Peak Size, nm | Mean Size, nm (SD) |
|---|---|---|---|
| (a) Distilled H2O | 0 | 71 | 80.4 (22.4) |
| (b) RPMI 1640 | 0 | 79 | 114.9 (45.9) |
| (c) RPMI 1640 | 3 | 105 | 202 (103.1) |
| (d) RPMI 1640 | 24 | 17 | 47.5 (34.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babonaitė, M.; Čepulis, M.; Kazlauskaitė, J.; Lazutka, J.R. Evaluation of In Vitro Genotoxicity of Polystyrene Nanoparticles in Human Peripheral Blood Mononuclear Cells. Toxics 2023, 11, 627. https://doi.org/10.3390/toxics11070627
Babonaitė M, Čepulis M, Kazlauskaitė J, Lazutka JR. Evaluation of In Vitro Genotoxicity of Polystyrene Nanoparticles in Human Peripheral Blood Mononuclear Cells. Toxics. 2023; 11(7):627. https://doi.org/10.3390/toxics11070627
Chicago/Turabian StyleBabonaitė, Milda, Matas Čepulis, Jūratė Kazlauskaitė, and Juozas Rimantas Lazutka. 2023. "Evaluation of In Vitro Genotoxicity of Polystyrene Nanoparticles in Human Peripheral Blood Mononuclear Cells" Toxics 11, no. 7: 627. https://doi.org/10.3390/toxics11070627
APA StyleBabonaitė, M., Čepulis, M., Kazlauskaitė, J., & Lazutka, J. R. (2023). Evaluation of In Vitro Genotoxicity of Polystyrene Nanoparticles in Human Peripheral Blood Mononuclear Cells. Toxics, 11(7), 627. https://doi.org/10.3390/toxics11070627











