Multi-Year Monitoring of the Toxicological Risk of Heavy Metals Related to Fish Consumption by the Population of the Kendari Region (Southeast Sulawesi, Indonesia)
Abstract
1. Introduction
1.1. Background
1.2. Mechanisms, Sources and Effects of Heavy Metal Contamination
1.2.1. Mercury (Hg)
1.2.2. Arsenic (As)
1.2.3. Nickel (Ni)
1.2.4. Cadmium (Cd)
1.2.5. Lead (Pb)
1.3. Objective
2. Materials and Methods
2.1. Study Species
2.2. Sample Selection and Treatment
2.3. Determining Heavy Metal Concentrations
2.4. Calculating the Target Hazard Quotients (THQs)
2.5. Calculating Hazard Indexes (HIs)
3. Results
3.1. Metal Concentrations
3.2. Targeted Risk Quotients (THQs)
3.3. Hazard Indexes (HIs)
3.4. Trophic Level and Hazard Indexes (HIs)
4. Discussion
Heavy Metal Concentrations in the Context of Other Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erdogrul, O.; Erbilir, F. Heavy metals and trace elements in various fish samples from Sir Dam Lake, Kahramanmaras, Turkey. Environ. Monit. Assess. 2007, 130, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, Z.; Khoshnood, R. Health risks evaluation of heavy metal in seafood. Transylv. Rev. Syst. Ecol. Res. 2013, 15, 137–144. [Google Scholar] [CrossRef]
- Tao, Y.; Yuan, Z.; Xiaona, H.; Wei, M. Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu Lake, China. Ecotoxicol. Environ. Saf. 2012, 81, 55–64. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Pan, Y.; Du, E. Liu et al. suspect that Zhu et al. (2015) may have underestimated dissolved organic nitrogen (N) but overestimated total particulate N in wet deposition in China. Sci. Total Environ. 2015, 520, 300–301. [Google Scholar] [CrossRef]
- Smylie, M.; Mcdonough, C.; Reed, L.; Shervette, V. Mercury bioaccumulation in an estuarine predator: Biotic factors, abiotic factors, and assessments of fish health. Environ. Pollut. 2016, 214, 169–176. [Google Scholar] [CrossRef]
- Rahman, M.M.; Naidu, R. Potential Exposure to Arsenic and Other Elements from Rice in Bangladesh: Health Risk Index. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; pp. 333–340. [Google Scholar] [CrossRef]
- Yunus, F.; Khan, S.; Chowdhury, P.; Milton, A.; Hussain, S.; Rahman, M. A Review of Groundwater Arsenic Contamination in Bangladesh: The Millennium Development Goal Era and Beyond. Int. J. Environ. Res. Public Health 2016, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Raessler, M. The Arsenic Contamination of Drinking and Groundwaters in Bangladesh: Featuring Biogeochemical Aspects and Implications on Public Health. Arch. Environ. Contam. Toxicol. 2018, 75, 1–7. [Google Scholar] [CrossRef]
- Palapa, T.M.; Maramis, A.A. Heavy Metals in Water of Stream Near an Amalgamation Tailing Ponds in Talawaan—Tatelu Gold Mining, North Sulawesi, Indonesia. Procedia Chem. 2015, 14, 428–436. [Google Scholar] [CrossRef]
- Shepherd, T.; Rumengan, I.; Sahami, A. Post-depositional behaviour of mercury and arsenic in submarine mine tailings deposited in Buyat Bay, North Sulawesi, Indonesia. Mar. Environ. Res. 2018, 137, 88–97. [Google Scholar] [CrossRef]
- Edinger, E.N.; Azmy, K.; Diegor, W.; Siregar, P.R. Heavy metal contamination from gold mining recorded in Porites lobata skeletons, Buyat-Ratototok district, North Sulawesi, Indonesia. Mar. Pollut. Bull. 2008, 56, 1553–1569. [Google Scholar] [CrossRef]
- Bonnet, X.; Briand, M.J.; Brischouc, F.; Letourneur, Y.; Fauvel, T.; Bustamante, P. Anguilliform fish reveal large-scale contamination by mine trace elements in the coral reefs of New Caledonia. Sci. Total Environ. 2014, 470–471, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Bentley, K.; Soebandrio, A. Arsenic and mercury concentrations in marine fish sourced from local fishermen and fish markets in mine-impacted communities in Ratatotok Subdistrict, North Sulawesi, Indonesia. Mar. Pollut. Bull. 2017, 120, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, R.; Narayanan, M. Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). Int. J. Environ. Sci. Technol. 2008, 5, 179–182. [Google Scholar] [CrossRef]
- Krishna, P.V.; Mounika, M.S.; Sarma, B.A.; Padmaja, B. Health risk assessment of heavy metal accumulation in the food fish, Channa striata from Krishna river, Andhra Pradesh. Int. J. Fish. Aquat. Stud. 2021, 9, 180–184. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, N.; Liu, B.; Zhou, L.; Wang, J.; Wang, C.; Dai, B.; Xiong, W. Metal concentrations and risk assessment in water, sediment and economic fish species with various habitat preferences and trophic guilds from Lake Caizi, Southeast China. Ecotoxicol. Environ. Saf. 2018, 157, 1–8. [Google Scholar] [CrossRef]
- FAO. The Consumption of Fish and Fish Products in the Asia-Pacific Region Based on Household Surveys; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2015; Volume 12, p. 87. Available online: https://www.fao.org/apfic/publications/detail/en/c/396958/ (accessed on 10 April 2018).
- Riani, E.; Cordova, M.R.; Arifin, Z. Heavy metal pollution and its relation to the malformation of green mussels cultured in Muara Kamal waters, Jakarta Bay, Indonesia. Mar. Pollut. Bull. 2018, 133, 664–670. [Google Scholar] [CrossRef]
- Rahman, M.S.; Molla, A.H.; Saha, N.; Rahman, A. Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem. 2012, 483, 1847–1854. [Google Scholar] [CrossRef]
- Cribb, R.; Ford, M. Indonesia as an archipelago: Managing islands, managing the seas. In Indonesia Beyond the Water’s Edge—Managing An Archipelago State; Cribb & Ford, Ed.; Iseas Publishing: Singapore, 2009; p. 248. Available online: http://hdl.handle.net/2123/16146 (accessed on 15 March 2023).
- de Jong, W.; Rusli, M.; Bhoelan, S.; Rohde, S.; Rantam, F.A.; Noeryoto, P.A.; Hadi, U.; Gorp, E.C.M.; van Goeijenbier, M. Endemic and emerging acute virus infections in Indonesia: An overview of the past decade and implications for the future. Crit. Rev. Microbiol. 2018, 44, 487–503. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. ESA/P/WP/248. 2017. Available online: https://population.un.org/wpp/publications/files/wpp2017_keyfindings.pdf (accessed on 3 March 2023).
- United Nations, Department of Economic and Social Affairs, Statistics Division. Statistical Yearbook 2018 Edition—Sixty-First Issue. ST/ESA/STAT/SER.S/37. 2018. Available online: https://unstats.un.org/unsd/publications/statistical-yearbook/files/syb61/syb61.pdf (accessed on 25 March 2023).
- FAO. Fishery and Aquaculture Country Profiles. In Country Profile Fact Sheets; (Jakarta, Indonesia); Fisheries and Aquaculture Division: Rome, Italy, 2011; Available online: https://www.fao.org/fishery/en/facp/idn (accessed on 10 March 2023).
- FAO. Fisheries and Aquaculture Department. (Rome, Italy). 2014. Available online: https://www.fao.org/3/i3720e/i3720e.pdf (accessed on 10 April 2018).
- Clay, J.W. World agriculture and the environment: A commodity-by-commodity guide to impacts and practices. Choice Rev. Online 2004, 42, 1539. [Google Scholar] [CrossRef]
- Cramb, R.; McCarthy, J.F.; TEdwards, C. The Oil Palm Complex: Smallholders, Agribusiness and the State in Indonesia and Malaysia. Southeast Asian Econ. 2017, 34, 430–431. [Google Scholar] [CrossRef]
- Rencana Strategis: Kementerian Energi dan Sumber Daya Mineral 2015–2019. 2015. Available online: https://www.esdm.go.id/assets/media/content/content-renstra-sekretariat-jenderal-kesdm-tahun-2015-2019.pdf (accessed on 10 May 2018).
- National Development Planning Agency and Ministry of Public Works. Sulawesi Island: Development Framework and Strategy; Japan International Cooperation Agency Makassar Field Office: Tokyo, Japan, 2008. Available online: https://openjicareport.jica.go.jp/pdf/11881372_02.pdf (accessed on 10 May 2018).
- Muthalib, A.A. Analysis of Economic Growths and Development Gaps Between Cities in Southeast Sulawesi. Int. J. Econ. Financ. Issues 2017, 7, 125–128. Available online: https://www.econjournals.com/index.php/ijefi/article/view/3988 (accessed on 3 March 2023).
- Stergiou, K.I.; Karpouzi, V.S. Feeding habits and trophic levels of Mediterranean fish. Rev. Fish. Biol. Fishes 2002, 11, 217–254. [Google Scholar] [CrossRef]
- Ahmad, N.I.; Noh, M.F.M.; Mahiyuddin, W.R.W.; Jaafar, H.; Ishak, I.; Azmi, W.N.F.W.; Veloo, Y.; Hairi, M.H. Mercury levels of marine fish commonly consumed in Peninsular Malaysia. Environ. Sci. Pollut. Res. 2015, 22, 3672–3686. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, Y.; Nawab, J.; Rahman, Z.; Khan, S.; Idress, M.; Ud din, Z.; Ali, A.; Ahmad, R.; Khan, S.A.; et al. Contamination features, geo-accumulation, enrichments and human health risks of toxic heavy metal(loids) from fish consumption collected along Swat river, Pakistan. Environ. Technol. Innov. 2020, 17, 100554. [Google Scholar] [CrossRef]
- Saha, N.; Mollah, M.Z.I.; Alam, M.F.; Rahman, S.M. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016, 70, 110–118. [Google Scholar] [CrossRef]
- Rainbow, P.S. Biomonitoring of heavy metal availability in the marine environment. Mar. Pollut. Bull. 1995, 31, 183–192. [Google Scholar] [CrossRef]
- El-Moselhy, K.M.; Othman, A.I.; Abd El-Azem, H.; El-Metwally, M.E.A. Bioaccumulation of heavy metals in some tissues of fish in the Red Sea, Egypt. Egypt. J. Basic Appl. Sci. 2014, 1, 97–105. [Google Scholar] [CrossRef]
- Papagiannis, I.; Kagalou, I.; Leonardos, I.; Petridis, D.; Kalfakakou, V. Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece). Environ. Int. 2004, 30, 357–362. [Google Scholar] [CrossRef]
- Mora, S.D.; Fowler, S.W.; Wyse, E.; Azemard, S. Distribution of heavy metals in marine bivalves, fish and coastal sediments in Gulf and Gulf of Oman. Mar. Pollut. Bull. 2004, 49, 410–424. [Google Scholar] [CrossRef]
- Storelli, M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Risk-Based Concentration Table; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- Thouez, J.P. Santé, Maladie et Environnement; Economica Anthropos: Paris, France, 2005; p. 137. [Google Scholar]
- Testud, F. Toxicologie Médicale, Professionnelle et Environnementale, 4th ed.; ESKA: Paris, France, 2012; pp. 175–360. [Google Scholar]
- U.S. Department of Health and Human Services. Toxicological Profile for Nickel. 2007. Available online: https://semspub.epa.gov/work/05/930045.pdf (accessed on 25 May 2018).
- Cordy, P.; Veiga, M.M.; Salih, I.; Al-Saadi, S.; Console, S.; Garcia, O.; Mesa, L.A.; Velásquez-López, P.C.; Roeser, M. Mercury contamination from artisanal gold mining in Antioquia, Colombia: The world’s highest per capita mercury pollution. Sci. Total Environ. 2011, 410–411, 154–160. [Google Scholar] [CrossRef]
- Palacios-Torres, Y.; Caballero-Gallardo, K.; Olivero-Verbel, J. Mercury pollution by gold mining in a global biodiversity hotspot, the Choco biogeographic region, Colombia. Chemosphere 2018, 193, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Zulkipli, S.Z.; Tan, C.P.; Seah, Y.G.; Liew, H.J.; Sung, Y.Y.; Ando, M.; Wang, M.; Liang, Y.; McMinn, A.; Mok, W.J. Assessment of mercury contamination and food composition in commercially important marine fishes in the southern South China Sea. Reg. Stud. Mar. Sci. 2023, 58, 102795. [Google Scholar] [CrossRef]
- Li, S.; Zhou, L.; Wang, H.; Liang, Y.; Hu, J.; Chang, J. Feeding habits and habitats preferences affecting mercury bioaccumulation in 37 subtropical fish species from Wujiang River, China. Ecotoxicology 2009, 18, 204–210. [Google Scholar] [CrossRef]
- Khaniki, G.R.J.; Alli, I.; Nowroozi, E.; Nabizadeh, R. Mercury contamination in fish and public health aspects: A review. Pak. J. Nutr. 2005, 4, 276–281. [Google Scholar] [CrossRef]
- Lee, M.R.; Lim, Y.H.; Lee, B.E.; Hong, Y.C. Blood mercury concentrations are associated with decline in liver function in an elderly population: A panel study. Environ. Health. 2017, 16, 17. [Google Scholar] [CrossRef]
- Wilk, A.; Kalisińska, E.; Kosik-Bogacka, D.I.; Romanowski, M.; Rόzański, J.; Ciechanowski, K.; Słojewski, M.; Łanocha-Arendarczyk, N. Cadmium, lead and mercury concentrations in pathologically altered human kidneys. Environ. Geochem. Health 2017, 39, 889–899. [Google Scholar] [CrossRef]
- Pletz, J.; Sánchez-Bayob, F.; Tennekesa, H.A. Dose-response analysis indicating time-dependent neurotoxicity caused by organic and inorganic mercury—Implications for toxic effects in the developing brain. Toxicology 2016, 347–349, 1–5. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Schierl, R.; Nowak, D.; Siebert, U.; William, J.F.; Owi, F.T.; Ir, Y.I. A preliminary study on health effects in villagers exposed to mercury in a small-scale artisanal gold mining area in Indonesia. Environ. Res. 2016, 149, 274–281. [Google Scholar] [CrossRef]
- Williams, M. Arsenic in Mine Waters: An International Study. Environ. Geol. 2001, 40, 12. [Google Scholar] [CrossRef]
- Matschullat, J. Arsenic in the Geosphere—A Review. Sci. Total Environ. 2000, 249, 297–312. [Google Scholar] [CrossRef]
- Bhowmick, S.; Pramanik, S.; Singh, P.; Mondal, P.; Chatterjee, D.; Nriagu, J. Arsenic in groundwater of West Bengal, India: A review of human health risks and assessment of possible intervention options. Sci. Total Environ. 2018, 612, 148–169. [Google Scholar] [CrossRef]
- Pétursdóttir, Á.H.E. Determination of Toxic and Non-Toxic Arsenic Species in Icelandic Fishmeal. Ph.D. Thesis, The University of Iceland, Reykjavík, Iceland, 2010. Available online: https://skemman.is/bitstream/1946/6357/1/MasterThesis-final.pdf (accessed on 3 March 2023).
- ATSDR. 2013. Available online: https://www.atsdr.cdc.gov/spl/resources/2013_atsdr_substance_priority_list.html (accessed on 10 April 2018).
- Mesa Pérez, M.A.; Díaz Rizo, Ó.; García Acosta, H.; Alarcón Santos, O.A.; Tavella, M.J.; Bagué, D.; Sánchez-Pérez, J.M.; Guerrero Domínguez, L.; Hernández Rodríguez, D.; Díaz Almeida, C.M. Heavy metals bioaccumulation and risk estimation in edible freshwater fish from pedroso reservoir (Mayabeque, Cuba). Rev. Int. Contam. Ambient. 2021, 37, 527–537. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals and Metalloids as Micronutrients for Plants and Animals. In Heavy Metals in Soils; Alloway, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 195–209. Available online: https://link.springer.com/book/10.1007/978-94-007-4470-7 (accessed on 3 March 2023).
- Canion, B.; Landsberger, S.; Jacques, C.; Taftazani, A. Trace analysis of Indonesian volcanic ash using thermal and epithermal neutron activation analysis. Nukleonik 2012, 57, 585–589. Available online: https://www.researchgate.net/publication/288666387 (accessed on 3 March 2023).
- Yi, Y.J.; Zhang, S.H. The relationship between fish heavy metals concentrations and fish size in the upper and middle reach of Yangtze River. Procedia Environ. Sci. 2012, 13, 1699–1707. [Google Scholar] [CrossRef]
- Zdrojewicz, Z.; Popowicz, E.; Warniarski, J. Nickel—Role in Human Organism and Toxic Effects. Pollut. Merkur Lek. 2016, 41, 115–118. Available online: https://pubmed.ncbi.nlm.nih.gov/27591452/ (accessed on 2 February 2023).
- Teck Metals Ltd. Cadmium: Fiche de Données de Sécurité (FDS). 2015. Available online: https://docplayer.fr/15282497-Cadmium-fiche-de-donnees-de-securite-fds.html (accessed on 2 February 2023).
- Shalat, S. Toxic Lead Can Stay in the Body for Years after Exposure. 2016. Available online: https://theconversation.com/toxic-lead-can-stay-in-the-body-for-years-after-exposure-53607 (accessed on 2 February 2023).
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Gidlow, D.A. Lead toxicity. Occup. Med. 2015, 65, 348–356. [Google Scholar] [CrossRef]
- Fakhri, Y.; Mohseni-Bandpei, A.; Oliveri Conti, G.; Ferrante, M.; Cristaldi, A.; Jeihooni, A.K.; Karimi Dehkordi, M.; Alinejad, A.; Rasoulzadeh, H.; Mohseni, S.M.; et al. Systematic review and health risk assessment of arsenic and lead in the fished shrimps from the Persian Gulf. Food Chem. Toxicol. 2018, 113, 278–286. [Google Scholar] [CrossRef]
- Breitwieser, M.; Viricel, A.; Churlaud, C.; Guillot, B.; Martin, E.; Stenger, P.-L.; Huet, V.; Fontanaud, A.; Thomas-Guyon, H. First data on three bivalve species exposed to an intra-harbour polymetallic contamination (La Rochelle, France). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 199, 28–37. [Google Scholar] [CrossRef] [PubMed]
- WHO. Health criteria and other supporting information. In Guidelines for Drinking Water Quality 2, 2nd ed.; World Health Organization: Geneva, Switzerland, 1996; Available online: https://apps.who.int/iris/handle/10665/38551 (accessed on 25 March 2023).
- Ullah, A.K.M.A.; Maksud, M.A.; Khan, S.R.; Lutfa, L.N.; Quraishi, S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017, 4, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Statistik. Badan Statistik Indonesia; BPS—Statistics Indonesia Publisher: Jakarta, Indonesia, 2013; ISSN 0126-2912. Available online: https://www.bps.go.id/publication/2013/05/01/c15e0fccfd3d035e6746a3b4/statistik-indonesia-2013.html (accessed on 15 May 2018).
- Antoine, J.M.R.; Hoo Fung, L.A.; Grant, C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.J.; Killough, G.G.; Rohwer, P.S. Composite hazard index for assessing limiting exposures to environmental pollutants: Formulation and derivation. Environ. Sci. Technol. 1978, 12, 799–802. [Google Scholar] [CrossRef]
- Adams, V.H.; McAtee, M.J.; Johnson, M.S. Implementation of the basic hazard index screening for health risks associated with simultaneous exposure to multiple chemicals using a standardized target organ and systems framework. Integr. Environ. Assess. Manag. 2017, 13, 852–860. [Google Scholar] [CrossRef]
- De Witte, B.; Coleman, B.; Bekaert, K.; Boitsov, S.; Botelho, M.J.; Castro-Jiménez, J.; Duffy, C.; Habedank, F.; McGovern, E.; Parmentier, K.; et al. Threshold values on environmental chemical contaminants in seafood in the European Economic Area. Food Control 2022, 138, 108978. [Google Scholar] [CrossRef]
- Alina, M.; Azrina, A.; Mohd Yunus, A.S.; Mohd Zakiuddin, S.; Mohd Izuan Effendi, H.; Muhammad Rizal, R. Heavy Metals (Mercury, Arsenic, Cadmium, Plumbum) in Selected Marine Fish and Shellfish along the Straits of Malacca. Int. Food Res. J. 2012, 19, 135–140. Available online: http://www.ifrj.upm.edu.my/19%20(01)%202011/(18)IFRJ-2010-235%20Alina.pdf (accessed on 3 January 2022).
- Rameshkumar, S.; Prabhakaran, P.; Radhakrishnan, K.; Rajaram, R. Accumulation of Heavy Metals in Some Marine Fisheries Resources Collected from Gulf of Mannar Marine Biosphere Reserve, Southeast Coast of India. Proc. Zool. Soc. 2016, 71, 294–298. [Google Scholar] [CrossRef]
- Kouba, A.; Buřič, M.; Kozák, P. Bioaccumulation and efects of heavy metals in crayfsh: A review. Water Air Soil Pollut. 2010, 211, 5–16. [Google Scholar] [CrossRef]
- Balzani, P.; Kouba, A.; Tricarico, E.; Kourantidou, M.; Haubrock, P.J. Metal accumulation in relation to size and body condition in an all-alien species community. Environ. Sci. Pollut. Res. 2021, 29, 25848–25857. [Google Scholar] [CrossRef]
- Nhiwatiwa, T.; Barson, M.; Harrison, A.P.; Utete, B.; Cooper, R.G. Metal concentrations in water, sediment and sharptooth catfish Clarias gariepinus from three peri-urban rivers in the upper Manyame catchment, Zimbabwe. Afr. J. Aquat. Sci. 2011, 36, 243–252. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, C.; Quan, W.; Chen, X.; Niu, J.; Shen, Z. Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Mar. Pollut. Bull. 2012, 64, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Uysal, K.; Köse, E.; Bülbül, M.; Dönmez, M.; Erdoğan, Y.; Koyun, M.; Ömeroğlu, Ç.; Özmal, F. The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey). Environ. Monit. Assess. 2008, 157, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Jakimska, A.; Konieczka, P.; Skóra, P.; Namieśnik, J. Bioaccumulation of Metals in Tissues of Marine Animals, Part II: Metal Concentrations in Animal Tissues. Pol. J. Environ. Stud. 2011, 20, 1127–1146. Available online: http://www.pjoes.com/pdf-88660-22519?filename=Bioaccumulation%20of%20Metals.pdf (accessed on 3 January 2022).
- Hossain, M.B.; Tanjin, F.; Rahman, M.S.; Yu, J.; Akhter, S.; Noman, M.A.; Sun, J. Metals Bioaccumulation in 15 Commonly Consumed Fishes from the Lower Meghna River and Adjacent Areas of Bangladesh and Associated Human Health Hazards. Toxics 2022, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- FAO. Chanos chanos. In Cultured Aquatic Species Fact Sheets; Nelson, L., Marygrace, C.Q., Valerio, C., Michael, Eds.; FAO: Rome, Italy, 2009; Available online: https://www.fao.org/fishery/docs/DOCUMENT/aquaculture/CulturedSpecies/file/en/en_milkfish.htm (accessed on 10 May 2018).
- Timilsina, G.R.; Shrestha, A. Transport sector CO2 emissions growth in Asia: Underlying factors and policy options. Energy Policy 2009, 37, 4523–4539. [Google Scholar] [CrossRef]
- Marimuthu, K.; Thilaga, M.; Kathiresan, S.; Xavier, R.; Mas, R.H.M.H. Effect of different cooking methods on proximate and mineral composition of striped snakehead fish (Channa striatus, Bloch). J. Food Sci. Technol. 2012, 49, 373–377. [Google Scholar] [CrossRef]
- Farias, L.A.; Favaro, D.I.; Santos, J.O.; Vasconcellos, M.B.; Pessôa, A.; Aguiar, J.P.L.; Yuyama, L. Cooking process evaluation on mercury content in fish. Acta Amaz. 2010, 40, 741–748. [Google Scholar] [CrossRef]
- He, M.; Ke, C.H.; Wang, W.X. Effects of cooking and subcellular distribution on the bioaccessibility of trace elements in two marine fish species. J. Agric. Food Chem. 2010, 58, 3517–3523. [Google Scholar] [CrossRef]
- Ersoy, B. Effect of cooking methods on the heavy metal concentrations of the Africa Catfish (Clarias gariepinus). J. Food Biochem. 2011, 35, 351–356. [Google Scholar] [CrossRef]
- Chien, L.C.; Hung, T.C.; Choang, K.Y.; Yeh, C.Y.; Meng, P.J.; Shieh, M.J.; Han, B.C. Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci. Total Environ. 2002, 285, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Alturiqi, A.S.; Albedair, L.A. Evaluation of some heavy metals in certain fish, meat and meat products in Saudi Arabian markets. Egypt. J. Aquat. Res. 2012, 38, 45–49. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Jokšas, K.; Galkus, A.; Stakeniene, R. Heavy metal contamination of the Curonian Lagoon bottom sediments (Lithuanian waters area). Baltica 2016, 29, 107–120. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Human health risk assessment due to dietary intake of heavy metals through rice in the mining areas of Singhbhum Copper Belt, India. Environ. Sci. Pollut. Res. 2017, 24, 14945–14956. [Google Scholar] [CrossRef]
- Dung, L.Q.; Tanaka, K.; Dung, L.V.; Fui, S.Y.; Lachs, L.; Kadir, S.T.S.A.; Sano, Y.; Shirai, K. Biomagnifcation of total mercury in the mangrove lagoon foodweb in east coast of Peninsula, Malaysia. Reg. Stud. Mar. Sci. 2017, 16, 49–55. [Google Scholar] [CrossRef]
- Akagi, H.; Castillo, E.S.; Cortes-Maramba, N.; Trinidad Francisco-Rivera, A.; Timbang, T.D. Health assessment for mercury exposure among schoolchildren residing near a gold processing and refining plant in Apokon, Tagum, Davao del Norte, Philippines. Sci. Total Environ. 2000, 259, 31–43. [Google Scholar] [CrossRef]
- Le, D.Q.; Satyanarayana, B.; Fui, S.Y.; Shirai, K. Mercury Bioaccumulation in Tropical Mangrove Wetland Fishes: Evaluating Potential Risk to Coastal Wildlife. Biol. Trace Elem. Res. 2008, 186, 538–545. [Google Scholar] [CrossRef]
- Metian, M.; Warnau, M.; Chouvelon, T.; Pedraza, F.; Rodriguez y Baena, A.; Bustamante, P. Trace element bioaccumulation in reef fish from New Caledonia: Influence of trophic groups and risk assessment for consumers. Mar. Environ. Res. 2013, 87–88, 26–36. [Google Scholar] [CrossRef]
- Soegianto, A. Trace Metal Concentrations in Shrimp and Fish Collected from Gresik Coastal Waters, Indonesia. Sci. Asia 2007, 33, 235–238. [Google Scholar] [CrossRef]
- Ling, M.P.; Wu, C.C.; Yang, K.R.; Hsu, H.T. Differential accumulation of trace elements in ventral and dorsal muscle tissues in tilapia and milkfish with different feeding habits from the same cultured fishery pond. Ecotoxicol. Environ. Saf. 2013, 89, 222–230. [Google Scholar] [CrossRef]
- Takarina, N.D.; Adiwibowo, A.; Sunardi; Wardhana, W.; Pin, T.G. Bioconcentration of Lead (Pb) in Milkfish (Chanos chanos, Forsk) Related to the Water Quality in Aquaculture Ponds of Marunda, North Jakarta, Indonesia. Int. J. Sci. Res. Publ. 2013, 2, 188–192. [Google Scholar] [CrossRef]
- Solidum, J.M.; De MJ, D.; Abdulla AR, D.; Evangelista, J.H. Quantitative Analysis of Lead, Cadmium and Chromium found in Selected Fish marketed in Metro Manila, Philippines. Int. J. Environ. Sci. Dev. 2013, 207–211. [Google Scholar] [CrossRef]
- Shadrack, R.S.; Gereva, S.; Pickering, T.; Ferreira, M. Seasonality, abundance and spawning season of milkfish Chanos chanos (Forsskål, 1775) at Teouma Bay, Vanuatu. Mar. Policy 2021, 130, 104587. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture: Sustainability in Action; FAO: Rome, Italy, 2020; p. 206. [Google Scholar] [CrossRef]

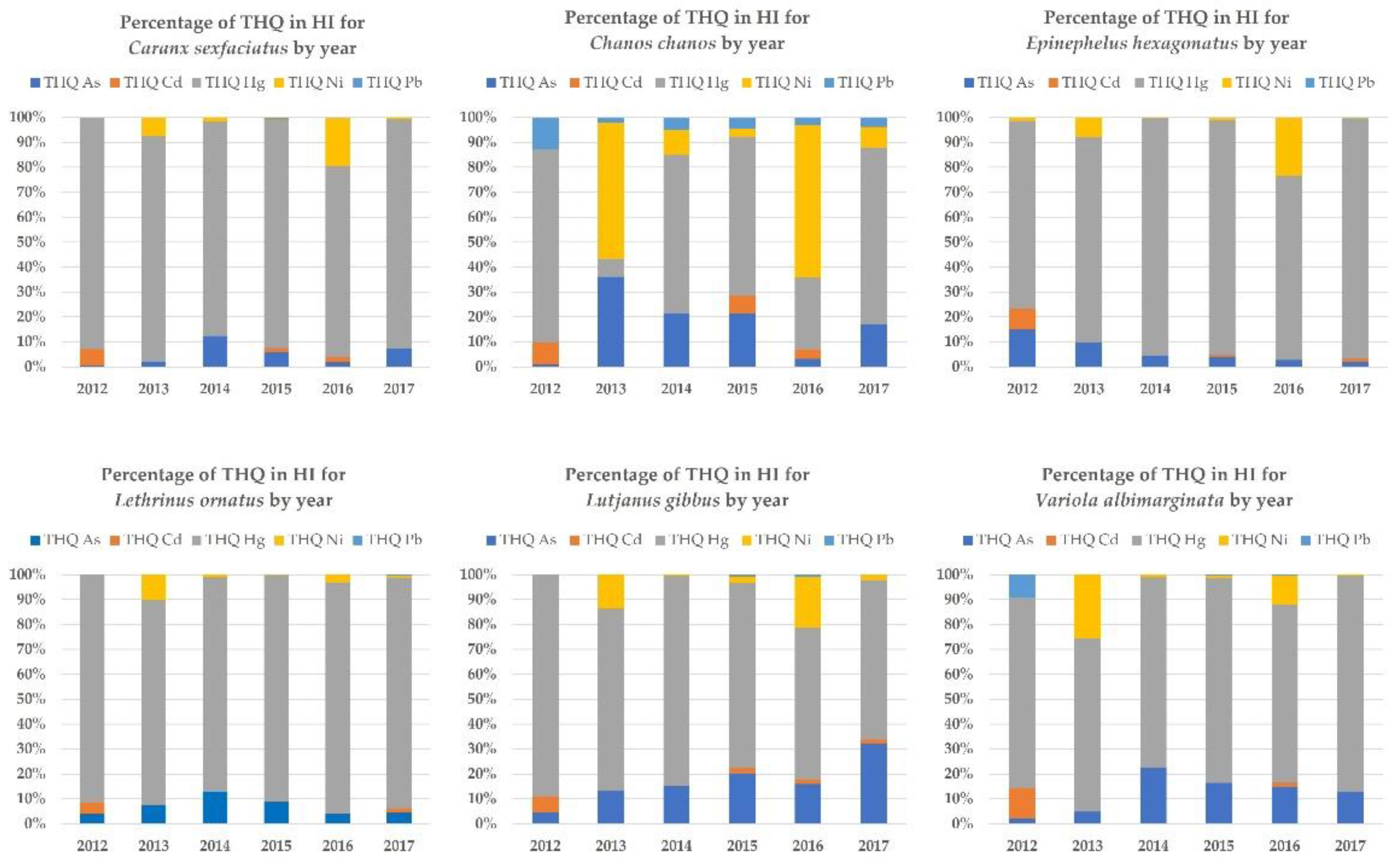
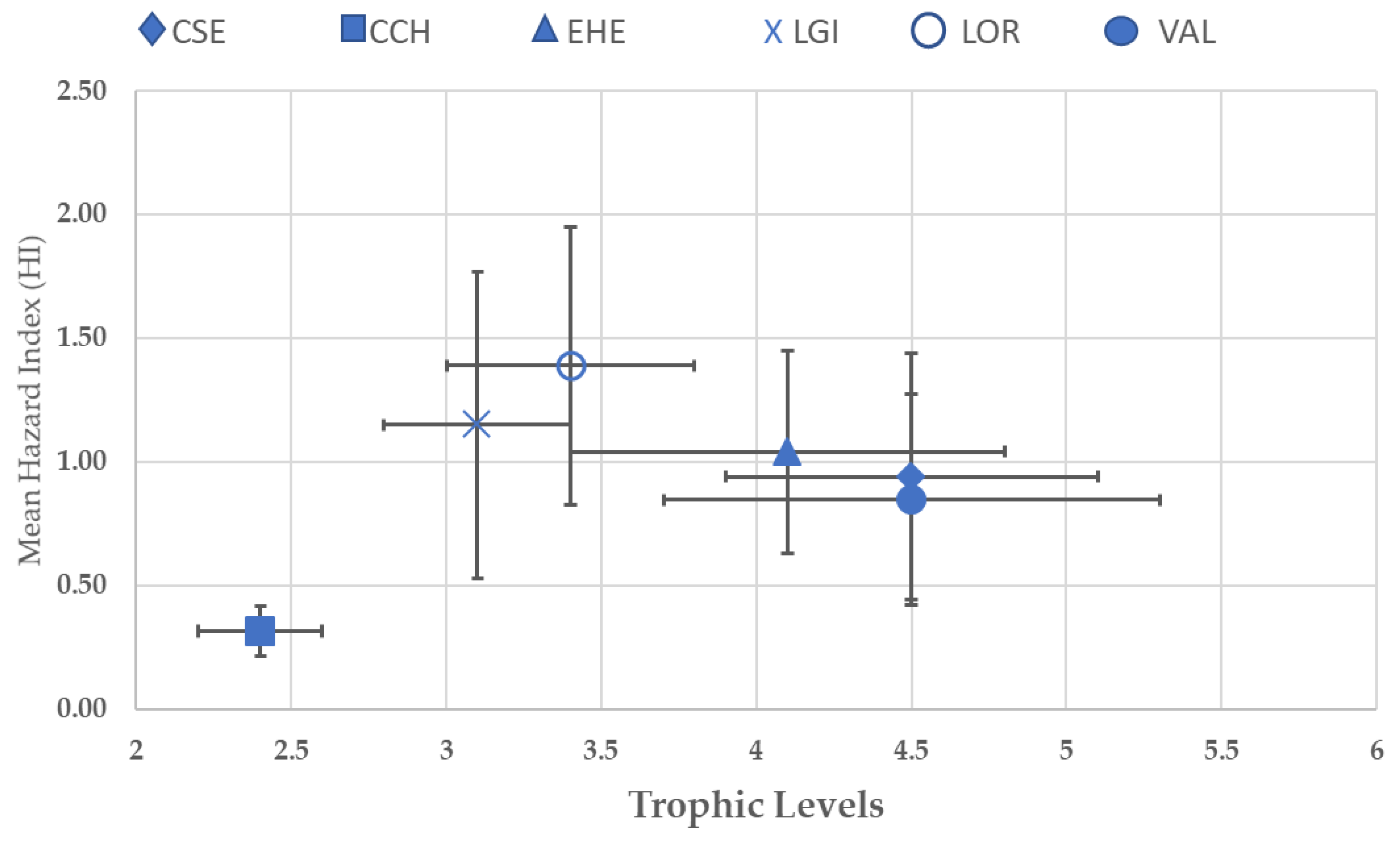
| Species | Common Name | Habitats | Trophic Level | Feeding Habits |
|---|---|---|---|---|
| Caranx sexfasciatus | Bigeye trevally | Pelagic, oceanic and coastal waters, coral reefs | 4.5 ± 0.6 | Fishes, crustaceans |
| Chanos chanos | Milkfish | Benthopelagic, coastal waters | 2.4 ± 0.2 | Plankton |
| Epinephelus hexagonatus | Starspotted grouper | Epibenthic, coastal waters | 4.1 ± 0.7 | Fishes, crustaceans |
| Lethrinus ornatus | Ornate emperor | Demersal, various habitats (sandy, sea-grass meadows, coral reefs) | 3.4 ± 0.4 | Fishes, crustaceans, mollusks, annelids |
| Lutjanus gibbus | Humpback red snapper | Benthopelagic in coral reefs | 3.1 ± 0.3 | Fishes, crustaceans, cephalopods, echinoderms. |
| Variola albimarginata | White-edged lyretail | Benthopelagic in coral reefs | 4.5 ± 0.8 | Fishes |
| Year | Hg | As | Ni | Cd | Pb |
|---|---|---|---|---|---|
| Caranx sexfasciatus | |||||
| 2012 | 0.279 ± 0.072 a | 0.32 ± 0.01 a | ND (0.02) | 0.04 ± 0.02 b | ND (0.03) |
| 2013 | 0.421 ± 0.082 a | 3.49 ± 0.24 bcd | 0.24 ± 0.18 a | ND (0.02) | ND (0.02) |
| 2014 | 0.126 ± 0.032 a | 1.57 ± 0.55 ab | 0.09 ± 0.09 a | ND (0.02) | ND (0.02) |
| 2015 | 0.318 ± 0.002 a | 4.09 ± 0.58 cd | 0.05 ± 0.03 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| 2016 | 0.601 ± 0.476 a | 2.76 ± 0.45 ac | 6.00 ± 6.25 a | 0.03 ± 0.04 a | 0.01 ± 0.00 a |
| 2017 | 0.484 ± 0.306 a | 5.63 ± 1.89 d | 0.11 ± 0.06 a | ND (0.01) | ND (0.01) |
| Chanos chanos | |||||
| 2012 | 0.139 ± 0.033 c | 0.33 ± 0.01 a | ND (0.02) | 0.03 ± 0.00 b | 0.18 ± 0.14 a |
| 2013 | 0.011 ± 0.001 a | 16.20 ± 7.50 b | 0.81 ± 0.23 a | ND (0.02) | 0.05 ± 0.07 a |
| 2014 | 0.055 ± 0.008 ab | 2.82 ± 1.27 a | 0.22 ± 0.03 a | ND (0.02) | 0.03 ± 0.01 a |
| 2015 | 0.052 ± 0.003 ab | 3.47 ± 0.73 a | 0.11 ± 0.07 a | 0.01 ± 0.00 a | 0.03 ± 0.01 a |
| 2016 | 0.051 ± 0.014 ab | 1.14 ± 0.11 a | 4.33 ± 1.76 b | 0.01 ± 0.00 a | 0.04 ± 0.01 a |
| 2017 | 0.095 ± 0.009 bc | 4.54 ± 0.28 a | 0.45 ± 0.07 a | ND (0.01) | 0.04 ± 0.01 a |
| Epinephelus hexagonatus | |||||
| 2012 | 0.242 ± 0.095 ab | 5.10 ± 5.68 a | ND (0.02) | 0.07 ± 0.07 a | ND (0.03) |
| 2013 | 0.359 ± 0.120 ab | 14.91 ± 8.65 b | 0.39 ± 0.27 a | ND (0.02) | ND (0.02) |
| 2014 | 0.547 ± 0.292 ab | 9.03 ± 3.26 ab | 0.10 ± 0.04 a | ND (0.02) | ND (0.02) |
| 2015 | 0.620 ± 0.178 b | 5.27 ± 0.73 a | 0.09 ± 0.07 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| 2016 | 0.112 ± 0.018 a | 1.27 ± 1.42 a | 2.56 ± 1.42 b | ND (0.01) | ND (0.03) |
| 2017 | 0.312 ± 0.078 ab | 1.88 ± 0.24 a | 0.06 ± 0.02 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Lethrinus ornatus | |||||
| 2012 | 0.601 ± 0.046 bc | 5.19 ± 3.47 a | ND (0.02) | 0.06 ± 0.04 b | ND (0.03) |
| 2013 | 0.360 ± 0.107 ab | 7.28 ± 7.99 ac | 0.43 ± 0.08 a | ND (0.02) | ND (0.02) |
| 2014 | 0.358 ± 0.128 ab | 19.47 ± 6.41 c | 0.27 ± 0.35 a | ND (0.02) | ND (0.02) |
| 2015 | 0.872 ± 0.069 d | 16.75 ± 1.49 bc | 0.08 ± 0.00 a | ND (0.01) | 0.01 ± 0.00 a |
| 2016 | 0.718 ± 0.065 cd | 6.01 ± 2.16 ab | 0.97 ± 0.15 b | ND (0.01) | ND (0.01) |
| 2017 | 0.351 ± 0.100 a | 3.03 ± 0.57 a | 0.10 ± 0.03 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Lutjanus gibbus | |||||
| 2012 | 0410 ± 0.067 a | 4.16 ± 2.70 a | ND (0.02) | 0.06 ± 0.01 b | ND (0.03) |
| 2013 | 0.433 ± 0.135 ab | 22.53 ± 10.47 ab | 0.50 ± 0.65 a | ND (0.02) | ND (0.02) |
| 2014 | 0.829 ± 0.278 b | 39.10 ± 21.39 b | 0.09 ± 0.05 a | ND (0.02) | ND (0.02) |
| 2015 | 0.189 ± 0.008 a | 10.27 ± 1.98 a | 0.26 ± 0.13 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| 2016 | 0.210 ± 0.021 a | 10.92 ± 4.14 a | 2.81 ± 3.14 a | 0.01 ± 0.00 a | 0.02 ± 0.00 a |
| 2017 | 0.386 ± 0.162 a | 21.52 ± 3.89 ab | 0.30 ± 0.41 a | 0.01 ± 0.00 a | ND (0.01) |
| Variola albimarginata | |||||
| 2012 | 0.148 ± 0.011 a | 0.86 ± 0.71 a | LD (0.02) | 0.05 ± 0.02 b | 0.14 ± 0.20 a |
| 2013 | 0.477 ± 0.280 b | 7.94 ± 7.43 a | 1.35 ± 1.00 ab | ND (0.02) | ND (0.02) |
| 2014 | 0.163 ± 0.018 a | 7.47 ± 3.26 a | 0.07 ± 0.02 a | ND (0.02) | ND (0.02) |
| 2015 | 0.229 ± 0.036 ab | 9.15 ± 6.01 a | 0.09 ± 0.04 a | ND (0.01) | 0.01 ± 0.00 a |
| 2016 | 0.261 ± 0.075 ab | 10.93 ± 7.76 a | 1.69 ± 1.09 b | 0.01 ± 0.00 a | 0.02 ± 0.00 a |
| 2017 | 0.214 ± 0.030 ab | 11.40 ± 5.73 a | 0.11 ± 0.04 a | ND (0.01) | ND (0.01) |
| Years | CSE | CCH | EHE | LOR | LGI | VAL |
|---|---|---|---|---|---|---|
| 2012 | 0.70 ± 0.16 a | 0.42 ± 0.11 b | 0.70 ± 0.17 ab | 1.53 ± 0.11 b | 1.07 ± 0.18 b | 0.45 ± 0.09 a |
| 2013 | 1.08 ± 0.13 ab | 0.36 ± 0.30 ab | 1.10 ± 0.13 ac | 1.02 ± 0.19 a | 1.37 ± 0.25 b | 1.59 ± 0.33 d |
| 2014 | 0.34 ± 0.08 a | 0.20 ± 0.04 a | 1.34 ± 0.62 bc | 0.97 ± 0.26 a | 2.29 ± 0.52 c | 0.50 ± 0.08 ab |
| 2015 | 0.81 ± 0.07 a | 0.19 ± 0.02 a | 1.52 ± 0.38 c | 2.23 ± 0.22 c | 0.59 ± 0.04 a | 0.65 ± 0.05 ab |
| 2016 | 1.82 ± 1.02 b | 0.41 ± 0.10 b | 0.42 ± 0.07 a | 1.80 ± 0.18 b | 0.80 ± 0.20 ab | 0.85 ± 0.26 bc |
| 2017 | 0.89 ± 0.21 a | 0.31 ± 0.02 ab | 1.17 ± 0.65 ac | 0.78 ± 0.17 a | 0.78 ± 0.08 ab | 1.04 ± 0.38 c |
| Heavy Metals (HMs) | Species | Study Area | Concentration HMs (mg kg−1 DW) | References |
|---|---|---|---|---|
| Mercury (Hg) | Caranx sexfasciatus | Kendari, Indonesia | 0.370 | This study |
| Malaysia | 0.290 | [33] | ||
| Caranx ignobilis | Malaysia | 0.210 | [97] | |
| Chanos chanos | Kendari, Indonesia | 0.139 | This study | |
| Philippines | 0.013 | [98] | ||
| Epinephelus hexagonatus | Kendari, Indonesia | 0.620 | This study | |
| Epinephelus sexfaciatus | Straits of Malacca | 0.015 | [77] | |
| Epinephelus quoyanus | India | 0.237 | [94] | |
| Lethrinus ornatus | Kendari, Indonesia | 0.872 | This study | |
| Lethrinus lentjan | India | 0.212 | [94] | |
| Malaysia | 0.048 | [99] | ||
| Lutjanus gibbus | Kendari, Indonesia | 0.829 | This study | |
| Malaysia | 0.436 | [33] | ||
| Lutjanus argentimeculatus | Straits of Malacca | 0.007 | [77] | |
| Arsenic (As) | Lethrinus ornatus | Kendari, Indonesia | 19.47 | This study |
| Flores, Indonesia | 8.54 | Unpublished study, 2014 | ||
| Lethrinus laticaudis | New Calidonia | 16.70 | [100] | |
| Lethrinus argentimeculatus | New Calidonia | 7.70 | [100] | |
| Straits of Malacca | 1.55 | [77] | ||
| Lutjanus gibbus | Kendari, Indonesia | 39.10 | This study | |
| Flores, Indonesia | 10.87 | Unpublished study, 2014 | ||
| Taiwan | 9.04 | [101] | ||
| Cadmium (Cd) | Lethrinus ornatus | Kendari, Indonesia | 0.060 | This study |
| Lethrinus rubrioperculatus | Hainan, China | 0.006 | [4] | |
| Lethrinus lentjan | India | 0.130 | [94] | |
| Lead (Pb) | Chanos chanos | Kendari, Indonesia | 0.050 | This study |
| Jakarta, Indonesia | 1.447 | [102] | ||
| Philippines | 0.164 | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputri, M.; Yusnaini, Y.; Sara, L.; Widowati, I.; Guyot, T.; Fichet, D.; Radenac, G. Multi-Year Monitoring of the Toxicological Risk of Heavy Metals Related to Fish Consumption by the Population of the Kendari Region (Southeast Sulawesi, Indonesia). Toxics 2023, 11, 592. https://doi.org/10.3390/toxics11070592
Saputri M, Yusnaini Y, Sara L, Widowati I, Guyot T, Fichet D, Radenac G. Multi-Year Monitoring of the Toxicological Risk of Heavy Metals Related to Fish Consumption by the Population of the Kendari Region (Southeast Sulawesi, Indonesia). Toxics. 2023; 11(7):592. https://doi.org/10.3390/toxics11070592
Chicago/Turabian StyleSaputri, Mimie, Yusnaini Yusnaini, La Sara, Ita Widowati, Thierry Guyot, Denis Fichet, and Gilles Radenac. 2023. "Multi-Year Monitoring of the Toxicological Risk of Heavy Metals Related to Fish Consumption by the Population of the Kendari Region (Southeast Sulawesi, Indonesia)" Toxics 11, no. 7: 592. https://doi.org/10.3390/toxics11070592
APA StyleSaputri, M., Yusnaini, Y., Sara, L., Widowati, I., Guyot, T., Fichet, D., & Radenac, G. (2023). Multi-Year Monitoring of the Toxicological Risk of Heavy Metals Related to Fish Consumption by the Population of the Kendari Region (Southeast Sulawesi, Indonesia). Toxics, 11(7), 592. https://doi.org/10.3390/toxics11070592





