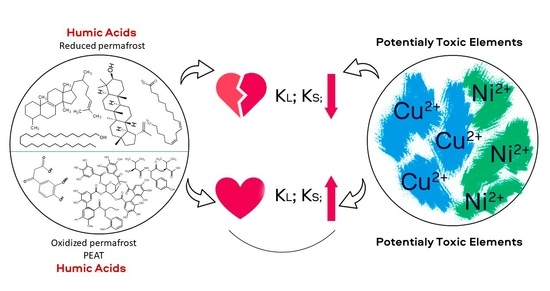
Yedoma Permafrost Releases Organic Matter with Lesser Affinity for Cu2+ and Ni2+ as Compared to Peat from the Non-Permafrost Area: Risk of Rising Toxicity of Potentially Toxic Elements in the Arctic Ocean
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site
2.2. Extraction of Humic Acids
2.3. Characterization of the Initial Samples and Isolated Humic Acids
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Characterization of the Samples of Humic Acids Isolated in this Study
3.2. Effect of pH on Cu2+ and Ni2+ Adsorption on Humic Acids
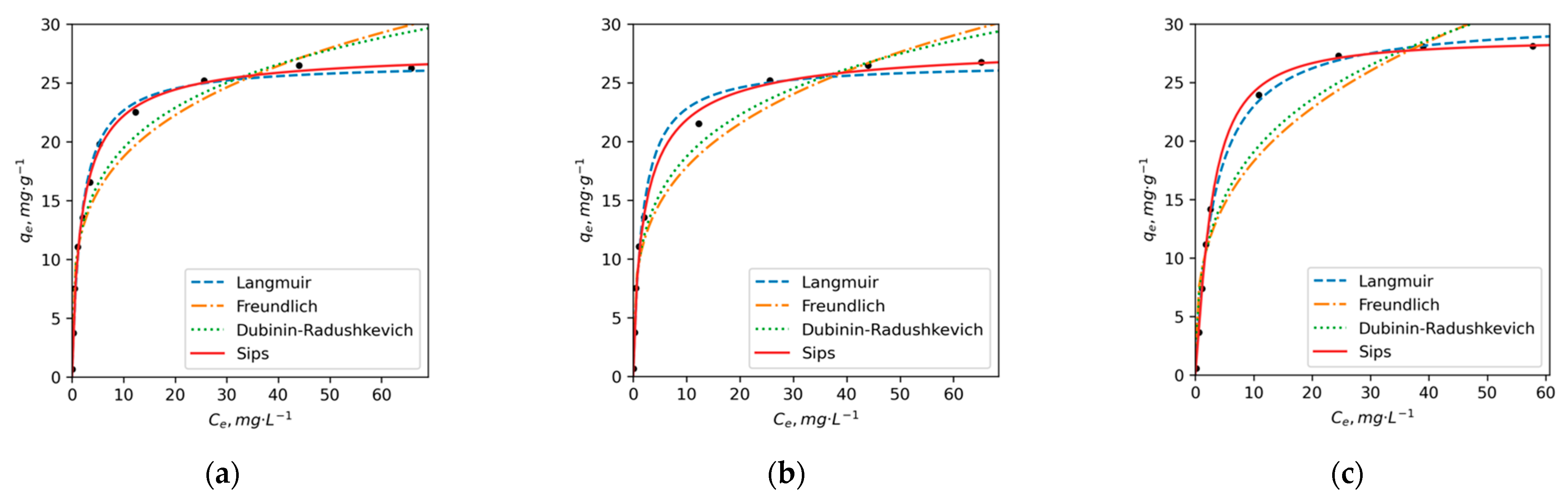
3.3. Adsorption Kinetics and Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, R.T.; Eggert, R.G.; Severson, M.H.; Anderson, C.G. Global Electrification of Vehicles and Intertwined Material Supply Chains of Cobalt, Copper and Nickel. Resour. Conserv. Recycl. 2021, 167, 105198. [Google Scholar] [CrossRef]
- Nakajima, K.; Daigo, I.; Nansai, K.; Matsubae, K.; Takayanagi, W.; Tomita, M.; Matsuno, Y. Global Distribution of Material Consumption: Nickel, Copper, and Iron. Resour. Conserv. Recycl. 2018, 133, 369–374. [Google Scholar] [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable Energy and Geopolitics: A Review. Renew. Sustain. Energy Rev. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Barcan, V. Nature and Origin of Multicomponent Aerial Emissions of the Copper–Nickel Smelter Complex. Environ. Int. 2002, 28, 451–456. [Google Scholar] [CrossRef]
- Boyd, R.; Barnes, S.J.; De Caritat, P.; Chekushin, V.A.; Melezhik, V.A.; Reimann, C.; Zientek, M.L. Emissions from the Copper–Nickel Industry on the Kola Peninsula and at Noril’sk, Russia. Atmos. Environ. 2009, 43, 1474–1480. [Google Scholar] [CrossRef]
- Gong, S.L.; Barrie, L.A. Trends of Heavy Metal Components in the Arctic Aerosols and Their Relationship to the Emissions in the Northern Hemisphere. Sci. Total Environ. 2005, 342, 175–183. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A.; Dinu, M.I.; Kremleva, T.A.; Khoroshavin, V.Y. Water Chemistry of Arctic Lakes under Airborne Contamination of Watersheds. Water 2020, 12, 1659. [Google Scholar] [CrossRef]
- Zhulidov, A.V.; Robarts, R.D.; Pavlov, D.F.; Kämäri, J.; Gurtovaya, T.Y.; Meriläinen, J.J.; Pospelov, I.N. Long-Term Changes of Heavy Metal and Sulphur Concentrations in Ecosystems of the Taymyr Peninsula (Russian Federation) North of the Norilsk Industrial Complex. Environ. Monit. Assess. 2011, 181, 539–553. [Google Scholar] [CrossRef]
- Ji, X.; Abakumov, E.; Antcibor, I.; Tomashunas, V.; Knoblauch, C.; Zubzycki, S.; Pfeiffer, E.M. Influence of Anthropogenic Activities on Metals in Arctic Permafrost: A Characterization of Benchmark Soils on the Yamal and Gydan Peninsulas in Russia. Arch. Environ. Contam. Toxicol. 2019, 76, 540–553. [Google Scholar] [CrossRef]
- Karnaeva, A.; Kulikova, O.; Mazlova, E.; Buryak, A. Aged Diesel and Heavy Metal Pollution in the Arctic Tundra (Yamal Peninsula, Russia). Sci. Total Environ. 2021, 792, 148471. [Google Scholar] [CrossRef]
- Law, K.S.; Stohl, A. Arctic Air Pollution: Origins and Impacts. Science 2007, 315, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, A.A.; Ponomareva, T.Y. Atmospheric Transport of Anthropogenic Impurities to the Russian Arctic (1986–2010). Atmos. Ocean. Opt. 2012, 25, 414–422. [Google Scholar] [CrossRef]
- Dauvalter, V.A.; Kashulin, N.A. Assessment of the Ecological State of the Arctic Freshwater System Based on Concentrations of Heavy Metals in the Bottom Sediments. Geochem. Int. 2018, 56, 842–856. [Google Scholar] [CrossRef]
- Vasilevich, R.; Vasilevich, M.; Lodygin, E.; Abakumov, E. Geochemical Characteristics of the Vertical Distribution of Heavy Metals in the Hummocky Peatlands of the Cryolithozone. Int. J. Environ. Res. Public 2023, 20, 3847. [Google Scholar] [CrossRef]
- Martínez Cortizas, A.; García-Rodeja Gayoso, E.; Weiss, D. Peat Bog Archives of Atmospheric Metal Deposition. Sci. Total Environ. 2002, 292, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Linnik, P.N. Complexation as the Most Important Factor in the Fate and Transport of Heavy Metals in the Dnieper Water Bodies. Anal. Bioanal. Chem. 2003, 376, 405–412. [Google Scholar] [CrossRef]
- Miranda, L.S.; Ayoko, G.A.; Egodawatta, P.; Goonetilleke, A. Adsorption-Desorption Behavior of Heavy Metals in Aquatic Environments: Influence of Sediment, Water and Metal Ionic Properties. J. Hazard. Mater. 2022, 421, 126743. [Google Scholar] [CrossRef]
- Chotpantarat, S.; Chunhacherdchai, L.; Wikiniyadhanee, R.; Tongcumpou, C. Effects of Humic Acid Amendment on the Mobility of Heavy Metals (Co, Cu, Cr, Mn, Ni, Pb, and Zn) in Gold Mine Tailings in Thailand. Arab. J. Geosci. 2015, 8, 7589–7600. [Google Scholar] [CrossRef]
- Caporale, A.G.; Violante, A. Chemical Processes Affecting the Mobility of Heavy Metals and Metalloids in Soil Environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Neagoe, A.; Iordache, V.; Fărcăşanu, I.C. The Role of Organic Matter in the Mobility of Metals in Contaminated Catchments. In Bio-Geo Interactions in Metal-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2012; pp. 297–325. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Manasypov, R.M.; Kopysov, S.G.; Krickov, I.V.; Shirokova, L.S.; Loiko, S.V.; Lim, A.G.; Kolesnichenko, L.G.; Vorobyev, S.N.; Kirpotin, S.N. Impact of Permafrost Thaw and Climate Warming on Riverine Export Fluxes of Carbon, Nutrients and Metals in Western Siberia. Water 2020, 12, 1817. [Google Scholar] [CrossRef]
- Kulikova, N.A.; Perminova, I.V. Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies. Molecules 2021, 26, 2706. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.A.; Albishri, H.M. Dynamics and Thermodynamics of Toxic Metals Adsorption onto Soil-Extracted Humic Acid. Chemosphere 2014, 111, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Albers, J.J.; Filip, Z. Metal Binding in Estuarine Humic and Fulvic Acids: FTIR Analysis of Humic Acid-Metal Complexes. Environ. Technol. 1998, 19, 923–931. [Google Scholar] [CrossRef]
- Erhayem, M.; Sohn, M. Stability Studies for Titanium Dioxide Nanoparticles upon Adsorption of Suwannee River Humic and Fulvic Acids and Natural Organic Matter. Sci. Total Environ. 2014, 468–469, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, E.; Arslan, G. Uptake of metal ions on humic acids. Energy Sources Part A Recovery Util. Environ. Eff. 2006, 28, 1099–1112. [Google Scholar] [CrossRef]
- Smilek, J.; Sedláček, P.; Kalina, M.; Klučáková, M. On the Role of Humic Acids’ Carboxyl Groups in the Binding of Charged Organic Compounds. Chemosphere 2015, 138, 503–510. [Google Scholar] [CrossRef]
- Christl, I.; Milne, C.J.; Kinniburgh, D.G.; Kretzschmar, R. Relating Ion Binding by Fulvic and Humic Acids to Chemical Composition and Molecular Size. 2. Metal Binding. Environ. Sci. Technol. 2001, 35, 2512–2517. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic Acids: Structural Properties and Multiple Functionalities for Novel Technological Developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Bigalke, M.; Weyer, S.; Wilcke, W. Copper Isotope Fractionation during Complexation with Insolubilized Humic Acid. Environ. Sci. Technol. 2010, 44, 5496–5502. [Google Scholar] [CrossRef]
- Shi, W.; Lü, C.; He, J.; En, H.; Gao, M.; Zhao, B.; Zhou, B.; Zhou, H.; Liu, H.; Zhang, Y. Nature Differences of Humic Acids Fractions Induced by Extracted Sequence as Explanatory Factors for Binding Characteristics of Heavy Metals. Ecotoxicol. Environ. Saf. 2018, 154, 59–68. [Google Scholar] [CrossRef]
- Martens, J.; Mueller, C.W.; Joshi, P.; Rosinger, C.; Maisch, M.; Kappler, A.; Bonkowski, M.; Schwamborn, G.; Schirrmeister, L.; Rethemeyer, J. Stabilization of mineral-associated organic carbon in Pleistocene permafrost. Nat. Commun. 2023, 14, 2120. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.E.; Shorina, N.; Bulygina, E.; Vorobjeva, T.; Chupakova, A.; Klimov, S.I.; Kellerman, A.M.; Guillemette, F.; Shiklomanov, A.; Podgorski, D.C.; et al. Flux and Seasonality of Dissolved Organic Matter from the Northern Dvina (Severnaya Dvina) River, Russia. J. Geophys. Res. Biogeosci. 2018, 123, 1041–1056. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Bockheim, J.; Canadell, J.G.; Euskirchen, E.; Field, C.B.; Goryachkin, S.V.; Hagemann, S.; Kuhry, P.; Lafleur, P.M.; Lee, H.; et al. Vulnerability of Permafrost Carbon to Climate Change: Implications for the Global Carbon Cycle. Bioscience 2008, 58, 701–714. [Google Scholar] [CrossRef]
- Moni, C.; Lerch, T.Z.; Knoth de Zarruk, K.; Strand, L.T.; Forte, C.; Certini, G.; Rasse, D.P. Temperature Response of Soil Organ-ic Matter Mineralisation in Arctic Soil Profiles. Soil Biol. Biochem. 2015, 88, 236–246. [Google Scholar] [CrossRef]
- Pengerud, A.; Dignac, M.F.; Certini, G.; Strand, L.T.; Forte, C.; Rasse, D.P. Soil Organic Matter Molecular Composition and State of Decomposition in Three Locations of the European Arctic. Biogeochemistry 2017, 135, 277–292. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Vogel, J.G.; Crummer, K.G.; Lee, H.; Sickman, J.O.; Osterkamp, T.E. The Effect of Permafrost Thaw on Old Carbon Release and Net Carbon Exchange from Tundra. Nature 2009, 459, 556–559. [Google Scholar] [CrossRef]
- Mann, P.J.; Eglinton, T.I.; McIntyre, C.P.; Zimov, N.; Davydova, A.; Vonk, J.E.; Holmes, R.M.; Spencer, R.G.M. Utilization of Ancient Permafrost Carbon in Headwaters of Arctic Fluvial Networks. Nat. Commun. 2015, 6, 7856. [Google Scholar] [CrossRef]
- Vonk, J.E.; Sánchez-García, L.; Van Dongen, B.E.; Alling, V.; Kosmach, D.; Charkin, A.; Semiletov, I.P.; Dudarev, O.V.; Shakhova, N.; Roos, P.; et al. Activation of Old Carbon by Erosion of Coastal and Subsea Permafrost in Arctic Siberia. Nature 2012, 489, 137–140. [Google Scholar] [CrossRef]
- Zherebker, A.; Podgorski, D.C.; Kholodov, V.A.; Orlov, A.A.; Yaroslavtseva, N.V.; Kharybin, O.; Kholodov, A.; Spector, V.; Spencer, R.G.M.; Nikolaev, E.; et al. The Molecular Composition of Humic Substances Isolated from Yedoma Permafrost and Alas Cores in the Eastern Siberian Arctic as Measured by Ultrahigh Resolution Mass Spectrometry. J. Geophys. Res. Biogeosci. 2019, 124, 2432–2445. [Google Scholar] [CrossRef]
- Swift, R.S. Organic Matter Characterization. In Methods of Soil Analysis, Part 3: Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 1011–1069. [Google Scholar] [CrossRef]
- Khreptugova, A.N.; Mikhnevich, T.A.; Molodykh, A.A.; Melnikova, S.V.; Konstantinov, A.I.; Rukhovich, G.D.; Volikov, A.B.; Perminova, I.V. Comparative Studies on Sorption Recovery and Molecular Selectivity of Bondesil PPL versus Bond Elut PPL Sorbents with Regard to Fulvic Acids. Water 2021, 13, 3553. [Google Scholar] [CrossRef]
- Hertkorn, N.; Permin, A.; Perminova, I.; Kovalevskii, D.; Yudov, M.; Petrosyan, V.; Kettrup, A. Comparative Analysis of Partial Structures of a Peat Humic and Fulvic Acid Using One- and Two-Dimensional Nuclear Magnetic Resonance Spectroscopy. J. Environ. Qual. 2002, 31, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Roper, W.R.; Robarge, W.P.; Osmond, D.L.; Heitman, J.L. Comparing Four Methods of Measuring Soil Organic Matter in North Carolina Soils. Soil Sci. Soc. Am. J. 2019, 83, 466–474. [Google Scholar] [CrossRef]
- Soil and Waste pH, EPA Method 9045D, Revision 4. 2004; 5. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/9045d.pdf (accessed on 14 April 2023).
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. Stockholm Kongl. Svenska Vetenskaps-Akad. Handlinger 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The Kinetics of Sorption of Basic Dyes from Aqueous Solution by Sphagnum Moss Peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Langmuir, I. THE Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich Isotherm Model at the Solid/Solution Interface: A Theoretical Analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Volikov, A.B.; Sobolev, N.A.; Khreptugova, A.N.; Perminova, I.V. Static and Dynamic Sorption of DOM on Bond Elute PPL and Bondesil PPL Sorbents: Physical-Chemical Characteristics. Sep. Sci. Technol. 2022, 58, 642–653. [Google Scholar] [CrossRef]
- Shahmohammadi-Kalalagh, S.; Babazadeh, H. Isotherms for the Sorption of Zinc and Copper onto Kaolinite: Comparison of Various Error Functions. Int. J. Environ. Sci. Technol. 2014, 11, 111–118. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive Adsorption of Heavy Metals onto Sesame Straw Biochar in Aqueous Solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Gonzalez, R.D. Modeling Adsorption of Copper(II), Cadmium(II) and Lead(II) on Purified Humic Acid. Langmuir 2000, 16, 3902–3909. [Google Scholar] [CrossRef]
- Wang, D.; Zang, S.; Wu, X.; Ma, D.; Li, M.; Chen, Q.; Liu, X.; Zhang, N. Soil Organic Carbon Stabilization in Permafrost Peatlands. Saudi J. Biol. Sci. 2021, 28, 7037–7045. [Google Scholar] [CrossRef]
- Perminova, I.V.; Grechishcheva, N.Y.; Petrosyan, V.S. Relationships between Structure and Binding Affinity of Humic Substances for Polycyclic Aromatic Hydrocarbons: Relevance of Molecular Descriptors. Environ. Sci. Technol. 1999, 33, 3781–3787. [Google Scholar] [CrossRef]
- Vasilevich, R.; Lodygin, E.; Beznosikov, V.; Abakumov, E. Molecular Composition of Raw Peat and Humic Substances from Permafrost Peat Soils of European Northeast Russia as Climate Change Markers. Sci. Total Environ. 2018, 615, 1229–1238. [Google Scholar] [CrossRef]
- Chung, T.L.; Chen, J.S.; Chiu, C.Y.; Tian, G. 13C-NMR Spectroscopy Studies of Humic Substances in Subtropical Perhumid Montane Forest Soil. J. For. Res. 2011, 17, 458–467. [Google Scholar] [CrossRef]
- Fiencke, C.; Marushchak, M.E.; Sanders, T.; Wegner, R.; Beer, C. Microbiogeochemical Traits to Identify Nitrogen Hotspots in Permafrost Regions. Nitrogen 2022, 3, 458–501. [Google Scholar] [CrossRef]
- Hodgkins, S.B.; Tfaily, M.M.; McCalley, C.K.; Logan, T.A.; Crill, P.M.; Saleska, S.R.; Rich, V.I.; Chanton, J.P. Changes in Peat Chemistry Associated with Permafrost Thaw Increase Greenhouse Gas Production. Proc. Natl. Acad. Sci. USA 2014, 111, 5819–5824. [Google Scholar] [CrossRef]
- Manninen, S.; Zverev, V.; Kozlov, M.V. Foliar Stable Isotope Ratios of Carbon and Nitrogen in Boreal Forest Plants Exposed to Long-Term Pollution from the Nickel-Copper Smelter at Monchegorsk, Russia. Environ. Sci. Pollut. Res. 2022, 29, 48880–48892. [Google Scholar] [CrossRef] [PubMed]
- Norseth, T. Environmental Pollution around Nickel Smelters in the Kola Peninsula (Russia). Sci. Total Environ. 1994, 148, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Streets, D.G.; Zhang, Q.; Wang, S.; Carmichael, G.R.; Cheng, Y.F.; Wei, C.; Chin, M.; Diehl, T.; Tan, Q. Sulfur Dioxide Emissions in China and Sulfur Trends in East Asia since 2000. Atmos. Chem. Phys. 2010, 10, 6311–6331. [Google Scholar] [CrossRef]
- Cape, J.N.; Fowler, D.; Davison, A. Ecological Effects of Sulfur Dioxide, Fluorides, and Minor Air Pollutants: Recent Trends and Research Needs. Environ. Int. 2003, 29, 201–211. [Google Scholar] [CrossRef]
- Nelson, W.O.; Campbell, P.G.C. The Effects of Acidification on the Geochemistry of Al, Cd, Pb and Hg in Freshwater Environments: A Literature Review. Environ. Pollut. 1991, 71, 91–130. [Google Scholar] [CrossRef]
- Lobbes, J.M.; Fitznar, H.P.; Kattner, G. Biogeochemical Characteristics of Dissolved and Particulate Organic Matter in Russian Rivers Entering the Arctic Ocean. Geochim. Cosmochim. Acta 2000, 64, 2973–2983. [Google Scholar] [CrossRef]
- Moiseenko, T. Acidification and Critical Loads in Surface Waters: Kola, Northern Russia. Ambio 1994, 23, 418–424. [Google Scholar]
- Yang, B.; Tong, X.; Deng, Z.; Lv, X. The Adsorption of Cu Species onto Pyrite Surface and Its Effect on Pyrite Flotation. J. Chem. 2016, 2016, 4627929. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, M.R. Effects of pH on Isotherms Modeling for Cu(II) Ions Adsorption Using Maple Wood Sawdust. Chem. Eng. J. 2009, 149, 273–280. [Google Scholar] [CrossRef]
- Fang, L.; Li, W.; Chen, H.; Xiao, F.; Huang, L.; Holm, P.E.; Hansen, H.C.B.; Wang, D. Synergistic Effect of Humic and Fulvic Acids on Ni Removal by the Calcined Mg/Al Layered Double Hydroxide. RSC Adv. 2015, 5, 18866–18874. [Google Scholar] [CrossRef]
- Lodygin, E.D.; Alekseev, I.I.; Vasilevich, R.S.; Abakumov, E.V. Complexation of Lead and Cadmium Ions with Humic Acids from Arctic Peat Soils. Environ. Res. 2020, 191, 110058. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, Q.; Dai, Y.; Zhu, N.; Dang, Z.; Li, P.; Wu, J.; Wang, X. Adsorption of Cu(II), Cd(II) and Cr(III) Ions from Aqueous Solutions on Humic Acid Modified Ca-Montmorillonite. Geoderma 2011, 164, 215–219. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption Isotherms, Kinetics, Thermodynamics and Desorption Studies of 2,4,6-Trichlorophenol on Oil Palm Empty Fruit Bunch-Based Activated Carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Aguareles, M.; Barrabés, E.; Myers, T.; Valverde, A. Mathematical Analysis of a Sips-Based Model for Column Adsorption. Phys. D 2023, 448, 133690. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead Removal from Aqueous Solution by Natural and Pretreated Clinoptilolite: Adsorption Equilibrium and Kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef]
- Pagano, T.; Bida, M.; Kenny, J.E. Trends in Levels of Allochthonous Dissolved Organic Carbon in Natural Water: A Review of Potential Mechanisms under a Changing Climate. Water 2014, 6, 2862–2897. [Google Scholar] [CrossRef]
- Dinu, M.I. Element Specification in Small Lakes of the Kola Peninsula: Geochemical and Technogenic Factors. Environ. Res. Lett. 2021, 16, 065005. [Google Scholar] [CrossRef]
- Chabani, M.; Amrane, A.; Bensmaili, A. Kinetic Modelling of the Adsorption of Nitrates by Ion Exchange Resin. Chem. Eng. J. 2006, 125, 111–117. [Google Scholar] [CrossRef]


| Sample | pH | OC (g/kg) | TN (g/kg) | MC (%) |
|---|---|---|---|---|
| PE | 3.52 ± 0.08 | 478 ± 2 | 12.2 ± 0.4 | 84 ± 7 |
| DY1 | 7.54 ± 0.10 | 22.1 ± 0.7 | 2.4 ± 0.1 | 28 ± 2 |
| DY2 | 7.60 ± 0.06 | 11.1 ± 0.3 | 1.3 ± 0.1 | 22 ± 3 |
| Conditions for All Line Registrations | |
|---|---|
| RF power (kW) | 1.40 |
| Plasma flow (L/min) | 18.0 |
| Axial flow (L/min) | 1.50 |
| Nebulizer flow (L/min) | 1.00 |
| Replicate read time (s) | 20 |
| Instrument stabilization delay (s) | 15 |
| Replicates | 4 |
| Sample uptake delay (s) | 25 |
| Pump rate (rpm) | 12 |
| Sample | CHn | CH3O | CHnO | OCO | Car | CarO | COO | C=O | ΣOx | Car/Calk | H/C | O/C | C/N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE | 18.0 | 7.1 | 23.1 | 9.9 | 23.8 | 9.1 | 11.5 | 4.6 | 25.2 | 18.0 | 1.07 ± 0.01 | 0.46 ± 0.01 | 17.2 ± 0.1 |
| DY1 | 12.9 | 5.3 | 27.2 | 8.9 | 24.3 | 9.0 | 12.4 | 5.3 | 26.7 | 12.9 | 1.09 ± 0.02 | 0.48 ± 0.01 | 17.1 ± 0.1 |
| DY2 | 19.1 | 6.6 | 26.9 | 8.3 | 24.1 | 6.6 | 11.2 | 3.8 | 21.6 | 19.1 | 1.18 ± 0.01 | 0.41 ± 0.01 | 13.9 ± 0.1 |
| Cu | Ni | ||||||
|---|---|---|---|---|---|---|---|
| Sample | PE | DY2 | DY1 | PE | DY2 | DY1 | |
| PFO | k1 | 0.35 | 0.38 | 0.37 | 0.45 | 0.47 | 0.52 |
| r2 | 0.72 | 0.62 | 0.59 | 0.99 | 0.91 | 0.89 | |
| PSO | k2 | 0.089 | 0.083 | 0.14 | 0.32 | 0.29 | 0.47 |
| r2 | 0.90 | 0.90 | 0.92 | 0.63 | 0.70 | 0.69 | |
| W-M | Cid | 12 | 13 | 8.8 | 7.8 | 8.8 | 7.5 |
| kid | 0.25 | 0.25 | 0.16 | 0.067 | 0.075 | 0.049 | |
| r2 | 0.64 | 0.61 | 0.69 | 0.28 | 0.34 | 0.36 | |
| Sample | Cu | Ni | |||||
|---|---|---|---|---|---|---|---|
| PE | DY2 | DY1 | PE | DY2 | DY1 | ||
| Langmuir | qm, mg/g | 27 | 27 | 30 | 15 | 17 | 19 |
| KL, L/g | 0.56 | 0.58 | 0.30 | 0.52 | 0.28 | 0.17 | |
| r2 | 0.99 | 0.99 | 0.99 | 0.94 | 0.93 | 0.96 | |
| Freundlich | n | 3.84 | 3.67 | 3.10 | 4.3 | 3.5 | 3.0 |
| KF, L/g | 10 | 9.5 | 8.7 | 6.0 | 5.3 | 4.6 | |
| r2 | 0.90 | 0.94 | 0.90 | 0.96 | 0.98 | 0.97 | |
| D-R | qm, mg/g | 64 | 65 | 88 | 31 | 44 | 52 |
| E (KJ/mol) | 15 | 15 | 14 | 16 | 14 | 13 | |
| r2 | 0.94 | 0.96 | 0.93 | 0.97 | 0.99 | 0.99 | |
| Sips | qm, mg/g | 28 | 29 | 29 | 20 | 32 | 27 |
| KS, L/g | 0.55 | 0.52 | 0.29 | 0.41 | 0.19 | 0.17 | |
| n | 0.85 | 0.80 | 1.26 | 0.49 | 0.45 | 0.58 | |
| r2 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolev, N.A.; Larionov, K.S.; Mryasova, D.S.; Khreptugova, A.N.; Volikov, A.B.; Konstantinov, A.I.; Volkov, D.S.; Perminova, I.V. Yedoma Permafrost Releases Organic Matter with Lesser Affinity for Cu2+ and Ni2+ as Compared to Peat from the Non-Permafrost Area: Risk of Rising Toxicity of Potentially Toxic Elements in the Arctic Ocean. Toxics 2023, 11, 483. https://doi.org/10.3390/toxics11060483
Sobolev NA, Larionov KS, Mryasova DS, Khreptugova AN, Volikov AB, Konstantinov AI, Volkov DS, Perminova IV. Yedoma Permafrost Releases Organic Matter with Lesser Affinity for Cu2+ and Ni2+ as Compared to Peat from the Non-Permafrost Area: Risk of Rising Toxicity of Potentially Toxic Elements in the Arctic Ocean. Toxics. 2023; 11(6):483. https://doi.org/10.3390/toxics11060483
Chicago/Turabian StyleSobolev, Nikita A., Konstantin S. Larionov, Darya S. Mryasova, Anna N. Khreptugova, Alexander B. Volikov, Andrey I. Konstantinov, Dmitry S. Volkov, and Irina V. Perminova. 2023. "Yedoma Permafrost Releases Organic Matter with Lesser Affinity for Cu2+ and Ni2+ as Compared to Peat from the Non-Permafrost Area: Risk of Rising Toxicity of Potentially Toxic Elements in the Arctic Ocean" Toxics 11, no. 6: 483. https://doi.org/10.3390/toxics11060483
APA StyleSobolev, N. A., Larionov, K. S., Mryasova, D. S., Khreptugova, A. N., Volikov, A. B., Konstantinov, A. I., Volkov, D. S., & Perminova, I. V. (2023). Yedoma Permafrost Releases Organic Matter with Lesser Affinity for Cu2+ and Ni2+ as Compared to Peat from the Non-Permafrost Area: Risk of Rising Toxicity of Potentially Toxic Elements in the Arctic Ocean. Toxics, 11(6), 483. https://doi.org/10.3390/toxics11060483