Effects of Nelumbo nucifera Gaertn. Petal Tea Extract on Hepatotoxicity and Oxidative Stress Induced by Mancozeb in Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
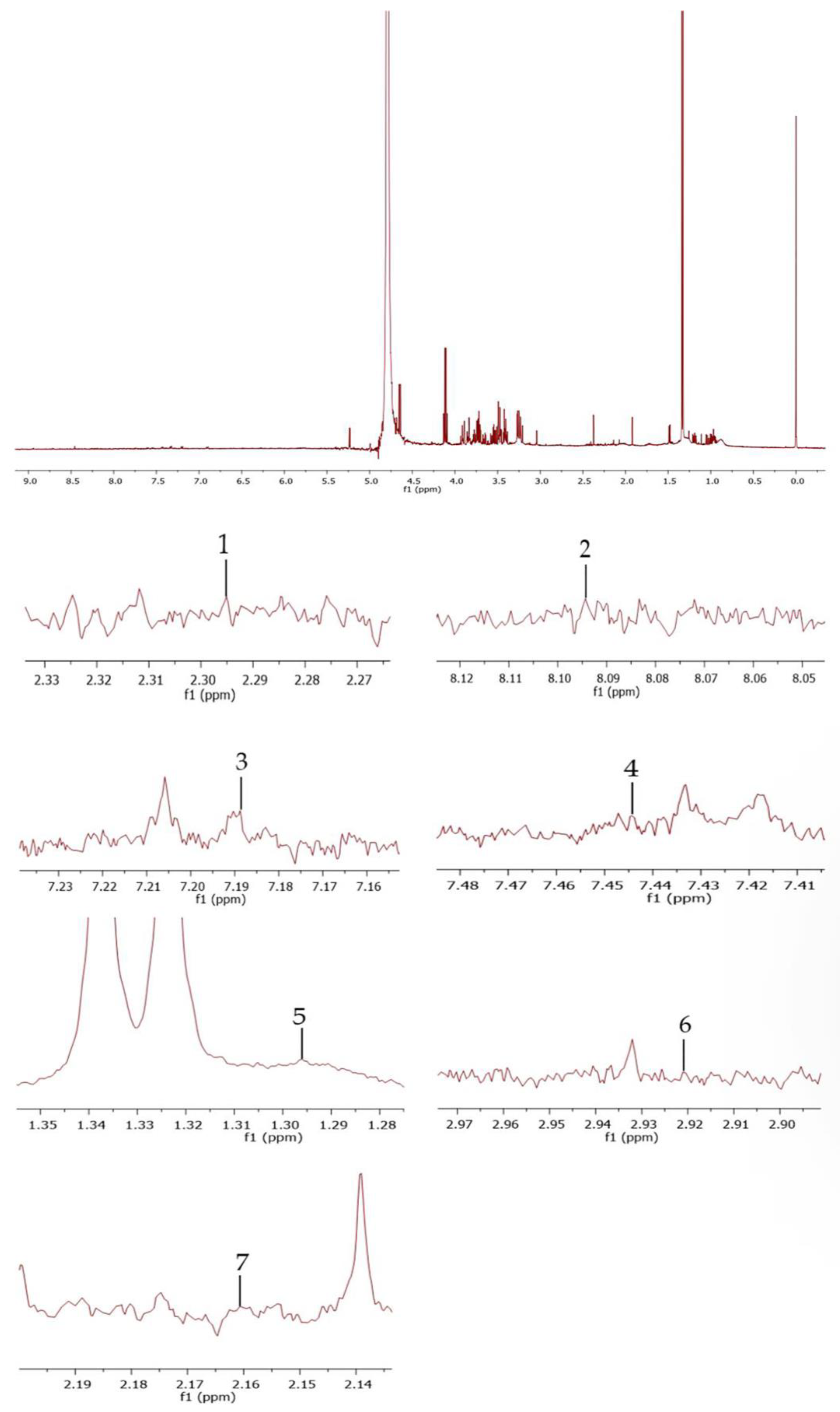
2.2. Plant Collection, Extraction, and Phytochemical Analysis
2.3. Animals and Experimental Design
- -
- Group I (control): rats in this group received 1 mL/day of distilled water.
- -
- Group II, III, and IV (N. nucifera extract): rats were administered N. nucifera extract at doses 0.55, 1.1, and 2.2 mg/kg bw/day, which corresponds to the daily tea consumption in humans (1.1 mg/kg bw/day) [31].
- -
- Group V (olive oil): rats were given 1 mL/day of olive oil as a vehicle group [15].
- -
- Group VI (Mz and olive oil): the rats obtained 500 mg/kg bw/day of Mz; the dose of Mz was chosen due to its ability to induce liver toxicity [32].
- -
- Group VII, VIII, and IX (N. nucifera extract and Mz): rats were treated with N. nucifera extract 0.55, 1.1, 2.2 mg/kg bw/day followed by 500 mg/kg bw/day of Mz.
2.4. Body Weight, Liver Weight, and Relative Liver Weight Analysis
2.5. Determination of Liver Function
2.6. Liver Histopathological Evaluation
2.7. Oxidative Stress Molecular Markers and Glutathione Levels Measurement
2.8. Statistical Analysis
3. Results
3.1. Effects of N. nucifera Extract on Body Weight
3.2. Effects of N. nucifera Extract on Liver Function Parameters
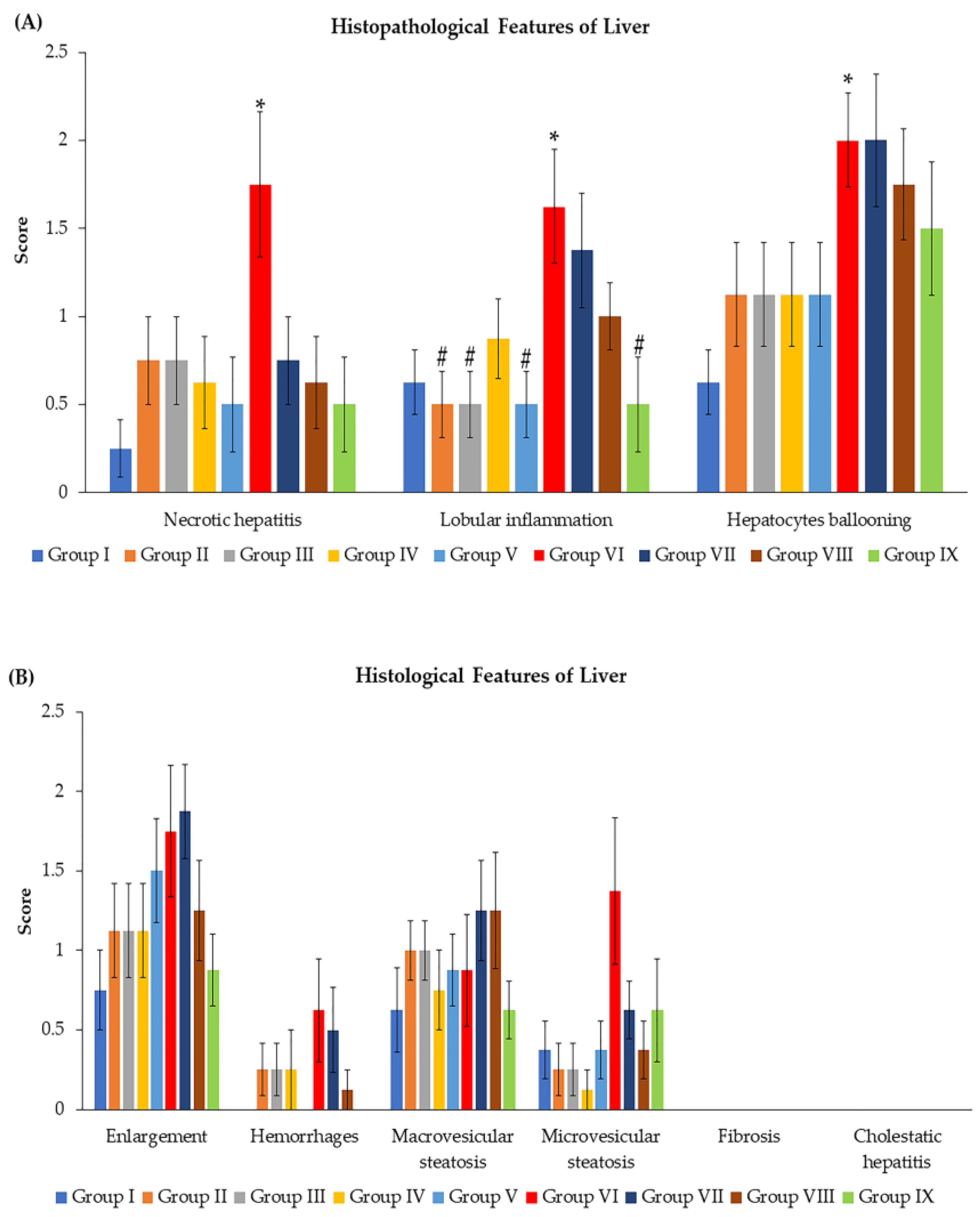
3.3. Effects of N. nucifera Extract on Histopathological Features and Total Score Injury of Livers
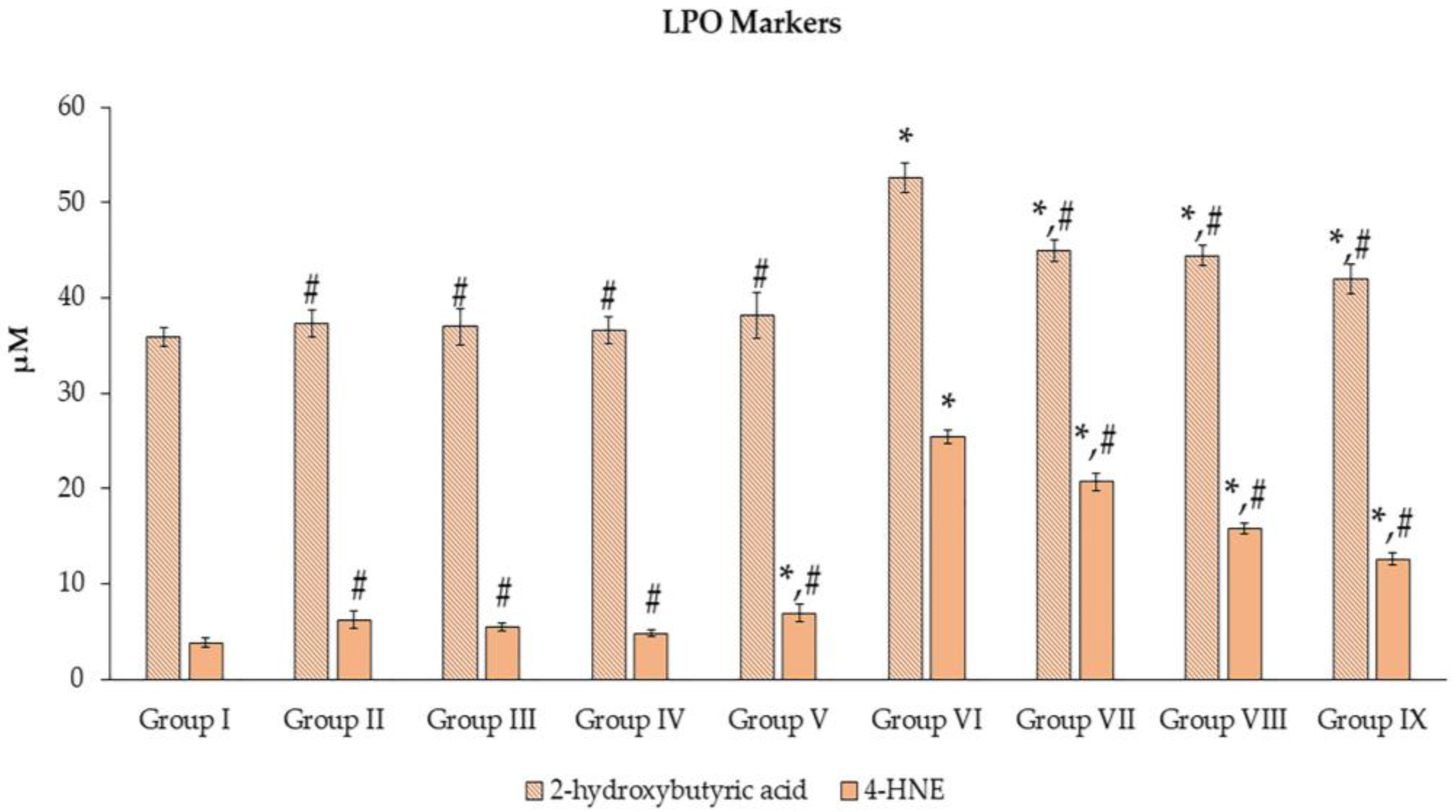
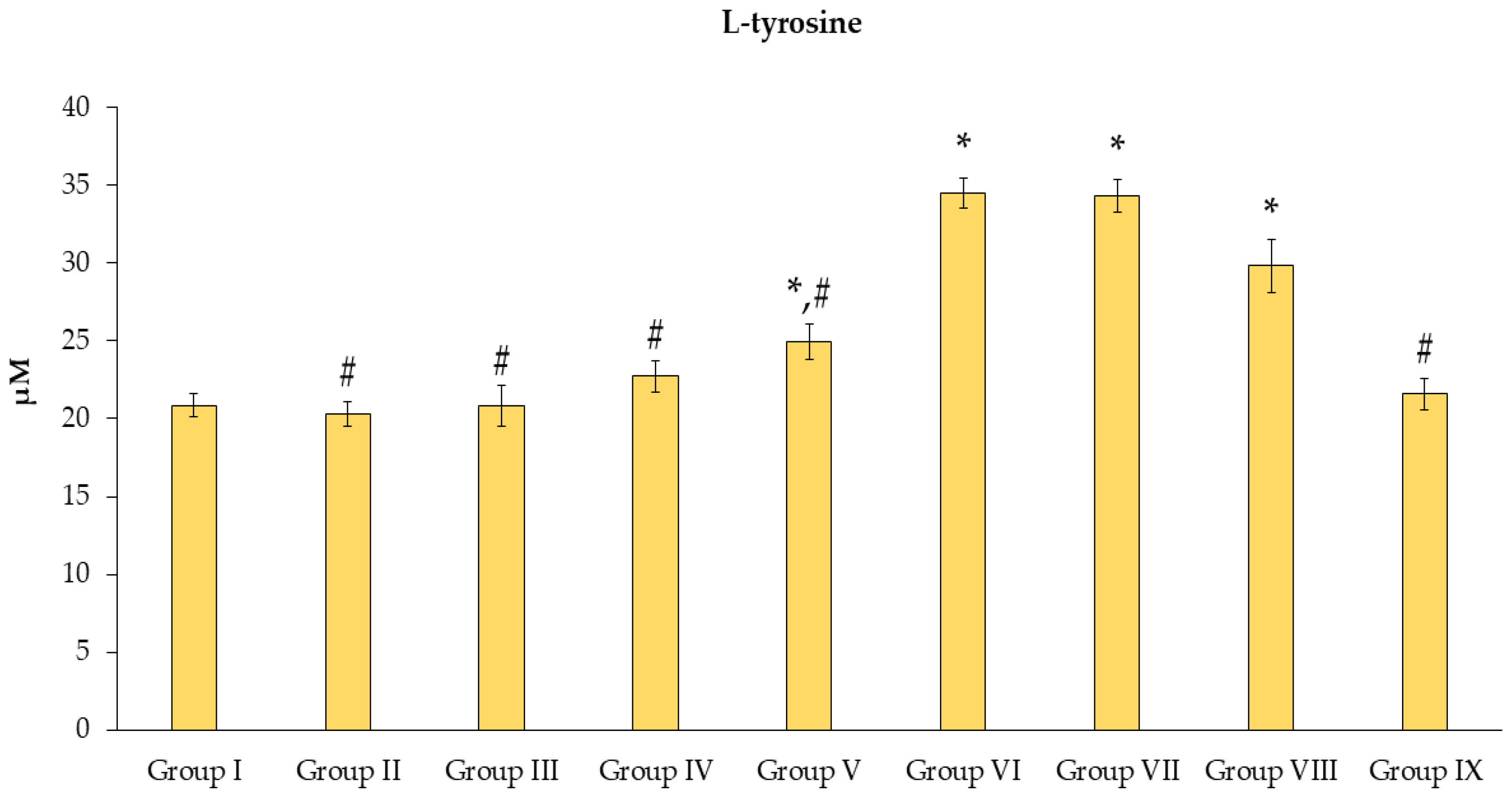
3.4. Effects of N. nucifera Extract on Oxidative Stress Molecular Markers
3.5. Effects of N. nucifera Extract on Glutathione Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panuwet, P.; Siriwong, W.; Prapamontol, T.; Ryan, P.B.; Fiedler, N.; Robson, M.G.; Barr, D.B. Agricultural pesticide management in Thailand: Situation and population health risk. Environ. Sci. Policy 2012, 17, 72–81. [Google Scholar] [CrossRef]
- Costa, L.G.; Aschner, M. Toxicology of Pesticides. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Das, T.K.; Wati, M.R.; Fatima-Shad, K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association with Alzheimer’s Disease. Arch. Neurosci. 2014, 2, e60038. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Sanna, D.; Fadda, A. Role of the Hydroxyl Radical-Generating System in the Estimation of the Antioxidant Activity of Plant Extracts by Electron Paramagnetic Resonance (EPR). Molecules 2022, 27, 4560. [Google Scholar] [CrossRef]
- Kackar, R.; Srivastava, M.K.; Raizada, R.B. Studies on rat thyroid after oral administration of mancozeb: Morphological and biochemical evaluations. J. Appl. Toxicol. 1997, 17, 369–375. [Google Scholar] [CrossRef]
- Iorio, R.; Castellucci, A.; Rossi, G.; Cinque, B.; Cifone, M.G.; Macchiarelli, G.; Cecconi, S. Mancozeb affects mitochondrial activity, redox status and ATP production in mouse granulosa cells. Toxicol. In Vitro 2015, 30, 438–445. [Google Scholar] [CrossRef]
- Yahia, D.; El-Amir, Y.O.; Rushdi, M. Mancozeb fungicide-induced genotoxic effects, metabolic alterations, and histological changes in the colon and liver of Sprague Dawley rats. Toxicol. Ind. Health 2019, 35, 265–276. [Google Scholar] [CrossRef]
- Plaa, G.L.; Hook, J.B. Target organ toxicity: Liver and kidney. Environ. Health Perspect. 1976, 15, 1. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Williams, C.D.; McGill, M.R.; Jaeschke, H. Liver Toxicity. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Soldatow, V.Y.; LeCluyse, E.L.; Griffith, L.G.; Rusyn, I. In vitro models for liver toxicity testing. Toxicol. Res. 2013, 2, 23–39. [Google Scholar] [CrossRef]
- Kackar, R.; Srivastava, M.K.; Raizada, R.B. Assessment of toxicological effects of mancozeb in male rats after chronic exposure. Indian J. Exp. Biol. 1999, 37, 553–559. [Google Scholar]
- Gök, E.; Deveci, E. Histopathological, immunohistochemical and biochemical alterations in liver tissue after fungicide-mancozeb exposures in Wistar albino rats. Acta Cir. Bras. 2022, 37, e370404. [Google Scholar] [CrossRef]
- Mohammadi-Sardoo, M.; Mandegary, A.; Nabiuni, M.; Nematollahi-Mahani, S.-N.; Amirheidari, B. Mancozeb induces testicular dysfunction through oxidative stress and apoptosis: Protective role of N-acetylcysteine antioxidant. Toxicol. Ind. Health 2018, 34, 798–811. [Google Scholar] [CrossRef]
- Aprioku, J.S.; Amamina, A.M.; Nnabuenyi, P.A. Mancozeb-induced hepatotoxicity: Protective role of curcumin in rat animal model. Toxicol. Res. 2023, 12, 107–116. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Xiao, L.-L.; Zhang, F.; Zhao, Y.-L.; Zhang, L.-J.; Xie, Z.-Y.; Huang, K.-Z.; Ouyang, X.-X.; Wu, X.-X.; Xu, X.-W.; Li, L.-J. Using advanced oxidation protein products and ischaemia-modified albumin to monitor oxidative stress levels in patients with drug-induced liver injury. Sci. Rep. 2020, 10, 18128. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical Screening, Antioxidant and Sperm Viability of Nelumbo nucifera Petal Extracts. Plants 2021, 10, 1375. [Google Scholar] [CrossRef]
- Chang, C.L.; Lin, C.S.; Lai, G.H. Phytochemical Characteristics, Free Radical Scavenging Activities, and Neuroprotection of Five Medicinal Plant Extracts. Evid. Based Complement. Altern. Med. 2012, 2012, 984295. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Koziara, Z.; Baranowska, M.; Bartoszek, A.; Namieśnik, J. Comparison of Redox Properties of Flavonoid Aglycones and Corresponding Glycosides and Their Mixtures in the Cellular Model. Proceedings 2019, 11, 25. [Google Scholar] [CrossRef]
- Ayaz Memon, A.; Naz, L.; Shabbir, S.; Khan, Z. The Hepato-Renal Protective Effect of Nelumbo nucifera Gaertn Seeds against Carbon Tetra Chloride Toxicity in Rats. Int. J. Pure Appl. Biosci. 2019, 7, 15–24. [Google Scholar] [CrossRef]
- Du, H.; You, J.-S.; Zhao, X.; Park, J.-Y.; Kim, S.-H.; Chang, K.J. Antiobesity and hypolipidemic effects of lotus leaf hot water extract with taurine supplementation in rats fed a high fat diet. J. Biomed. Sci. 2010, 17 (Suppl. S1), S42. [Google Scholar] [CrossRef]
- Li, C.; He, Y.; Yang, Y.; Gou, Y.; Li, S.; Wang, R.; Zeng, S.; Zhao, X. Antioxidant and Inflammatory Effects of Nelumbo nucifera Gaertn. Leaves. Oxidative Med. Cell Longev. 2021, 2021, 8375961. [Google Scholar] [CrossRef]
- Guo, F.; Yang, X.; Li, X.; Feng, R.; Guan, C.; Wang, Y.; Li, Y.; Sun, C. Nuciferine Prevents Hepatic Steatosis and Injury Induced by a High-Fat Diet in Hamsters. PLoS ONE 2013, 8, e63770. [Google Scholar] [CrossRef]
- Lee, D.-B.; Kim, D.-H.; Je, J.-Y. Antioxidant and Cytoprotective Effects of Lotus (Nelumbo nucifera) Leaves Phenolic Fraction. Prev. Nutr. Food Sci. 2015, 20, 22–28. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Chellappan, D.R.; Joseph, J.; Ravindhran, D.; Shabi, M.M.; Uthrapathy, S.; Rajamanickam, V.G.; Dubey, G.P. Antioxidant activity of Nelumbo nucifera (Gaertn) flowers in isolated perfused rat kidney. Rev. Bras. Farm. 2009, 19, 224–229. [Google Scholar] [CrossRef]
- Laoung-On, J.; Sudwan, P. Effect of Moringa oleiferra leaf tea on sperm concentration and sperm viability. In Proceedings of the 36th International Conference of the Microscopy Society of Thailand, Bangkok, Thailand, 26–29 March 2019; Volume 36, pp. 207–211. [Google Scholar]
- Laoung-On, J.; Saenphet, K.; Jaikang, C.; Sudwan, P. Effect of Moringa oleifera Lam. Leaf Tea on Sexual Behavior and Reproductive Function in Male Rats. Plants 2021, 10, 2019. [Google Scholar] [CrossRef]
- Farag, A. Hemato—Biochemical Responses under Stress of Mancozeb Fungicide (75% WP) in Male Albino Rat. Int. J. Adv. Res. Biol. Sci. 2017, 4, 116–127. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Loomba, R.; Anstee, Q.M.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018, 68, 349–360. [Google Scholar] [CrossRef]
- Miura, T.; Sugimoto, N.; Bhavaraju, S.; Yamazaki, T.; Nishizaki, Y.; Liu, Y.; Bzhelyansky, A.; Amezcua, C.; Ray, J.; Zailer, E.; et al. Collaborative Study to Validate Purity Determination by 1H Quantitative NMR Spectroscopy by Using Internal Calibration Methodology. Chem. Pharm. Bull. 2020, 68, 868–878. [Google Scholar] [CrossRef]
- Van Berlo, D.; Woutersen, M.; Muller, A.; Pronk, M.; Vriend, J.; Hakkert, B. 10% Body weight (gain) change as criterion for the maximum tolerated dose: A critical analysis. Regul. Toxicol. Pharmacol. 2022, 134, 105235. [Google Scholar] [CrossRef]
- Perera, T.; Ranasinghe, S.; Alles, N.; Waduge, R. Effect of fluoride on major organs with the different time of exposure in rats. Environ. Health Prev. Med. 2018, 23, 17. [Google Scholar] [CrossRef]
- Rajput, M.A.; Zehra, T.; Zafar, S.; Kumar, G.; Ali, F.; Aslam, N. Toxicological evaluation of Nelumbo nucifera fruit ethanol extract. Int. J. Pharm. Res. Allied Sci. 2020, 9, 87–96. [Google Scholar]
- Sayed, M.A. The protective effect of Zn-Metallothionein (Zn-Mt) on the toxicity of diazenon, mancozeb and their mixture on rats. J. Egypt. Soc. Toxicol. 2023, 36, 35–41. [Google Scholar]
- Seshoka, M.; van Zijl, M.; Aneck-Hahn, N.; Barnhoorn, I. Endocrine-disrupting activity of the fungicide mancozeb used in the Vhembe District of South Africa. Afr. J. Aquat. Sci. 2021, 46, 100–109. [Google Scholar] [CrossRef]
- Marino, D.J. Age-Specific Absolute and Relative Organ Weight Distributions For B6C3F1 Mice. J. Toxicol. Environ. Health Part A 2012, 75, 76–99. [Google Scholar] [CrossRef]
- Kasarala, G.; Tillmann, H.L. Standard liver tests. Clin. Liver Dis. 2016, 8, 13–18. [Google Scholar] [CrossRef]
- Yahia, E.; Aiche, M.A.; Chouabia, A.; Boulakoud, M.S. Biochemical and hematological changes following long term exposure to mancozeb. Adv. Biol. Res. 2015, 6, 83–86. [Google Scholar]
- Sudwan, P.; Saenphet, K.; Saenphet, S.; Suwansirikul, S. Effect of Kaempferia parviflora Wall. ex. Baker on sexual activity of male rats and its toxicity. Southeast Asian J. Trop. Med. Public Health 2006, 3737 (Suppl. S3), 210–215. [Google Scholar]
- Suarez Uribe, N.D.; Pezzini, M.F.; Agnol, J.D.; Marroni, N.; Benitez, S.; Benedetti, D.; Da Silva, J.; Cerski, C.T.; Dallegrave, E.; Macedo, S.; et al. Liver toxicity and DNA damage from exposure to the pesticide Mancozeb. J. Clin. Exp. Gastroenterol. 2022, 1, 27–34. [Google Scholar]
- Festjens, N.; Vanden Berghe, T.; Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: Signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta 2006, 1757, 1371–1387. [Google Scholar] [CrossRef]
- Saxena, R. 1—Microscopic Anatomy, Basic Terms, and Elemental Lesions. In Practical Hepatic Pathology: A Diagnostic Approach, 2nd ed.; Saxena, R., Ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 3–29. [Google Scholar]
- Pirozzi, A.V.A.; Stellavato, A.; La Gatta, A.; Lamberti, M.; Schiraldi, C. Mancozeb, a fungicide routinely used in agriculture, worsens nonalcoholic fatty liver disease in the human HepG2 cell model. Toxicol. Lett. 2016, 249, 1–4. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Hoofnagle, J.H.; Serrano, J.; Knoben, J.E.; Navarro, V.J. LiverTox: A website on drug-induced liver injury. Hepatology 2013, 57, 873–874. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. 2022, 9, 882558. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Szczepski, K.; Poulson, B.G.; Chandra, K.; McKay, R.T.; Dhahri, M.; Alahmari, F.; Jaremko, L.; Lachowicz, J.I.; Jaremko, M. NMR as a “Gold Standard” Method in Drug Design and Discovery. Molecules 2020, 25, 4597. [Google Scholar] [CrossRef]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.-P.; Mitchell, M.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. Alpha-Hydroxybutyrate Is an Early Biomarker of Insulin Resistance and Glucose Intolerance in a Nondiabetic Population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef]
- Podszun, M.C.; Chung, J.-Y.; Ylaya, K.; Kleiner, D.E.; Hewitt, S.M.; Rotman, Y. 4-HNE Immunohistochemistry and Image Analysis for Detection of Lipid Peroxidation in Human Liver Samples Using Vitamin E Treatment in NAFLD as a Proof of Concept. J. Histochem. Cytochem. 2020, 68, 635–643. [Google Scholar] [CrossRef]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Effect of Nelumbo nucifera Petals Extract on Antioxidant Activity and Sperm Quality in Charolais Cattle Sperm Induced by Mancozeb. Plants 2022, 11, 637. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Mitch, W.E. Chapter 90—Chronic Kidney Disease: Pathophysiology and the Influence of Dietary Protein. In Seldin and Giebisch’s The Kidney, 5th ed.; Alpern, R.J., Moe, O.W., Caplan, M., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 3021–3072. [Google Scholar]
- Afolabi, O.K.; Aderibigbe, F.A.; Folarin, D.T.; Arinola, A.; Wusu, A.D. Oxidative stress and inflammation following sub-lethal oral exposure of cypermethrin in rats: Mitigating potential of epicatechin. Heliyon 2019, 5, e02274. [Google Scholar] [CrossRef]
- Singh, P.; Kesharwani, R.K.; Keservani, R.K. 24–Antioxidants and Vitamins: Roles in Cellular Function and Metabolism. In Sustained Energy for Enhanced Human Functions and Activity; Bagchi, D., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 385–407. [Google Scholar]
- Lee, J.; Ju, M.; Cho, O.H.; Kim, Y.; Nam, K.T. Tyrosine-Rich Peptides as a Platform for Assembly and Material Synthesis. Adv. Sci. 2018, 6, 1801255. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Sajithlal, G.; Chithra, P.; Chandrakasan, G. The Role of Metal-Catalyzed Oxidation in the Formation of Advanced Glycation End Products: An In Vitro Study on Collagen. Free. Radic. Biol. Med. 1998, 25, 265–269. [Google Scholar] [CrossRef]
- Qais, F.A.; Khan, M.S.; Althubiani, A.S.; Al-Ghamdi, S.B.; Ahmad, I. Chapter 13—Understanding Biochemical and Molecular Mechanism of Complications of Glycation and Its Management by Herbal Medicine. In New Look to Phytomedicine; Ahmad Khan, M.S., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 331–366. [Google Scholar]
- Jung, H.A.; Jung, Y.J.; Yoon, N.Y.; Jeong, D.M.; Bae, H.J.; Kim, D.-W.; Na, D.H.; Choi, J.S. Inhibitory effects of Nelumbo nucifera leaves on rat lens aldose reductase, advanced glycation endproducts formation, and oxidative stress. Food Chem. Toxicol. 2008, 46, 3818–3826. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Kruk, I.; Kładna, A.; Lichszteld, K.; Michalska, T. Scavenging effects of phenolic compounds on reactive oxygen species. Biopolymers 2007, 86, 222–230. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Meng, S.; Chen, X.; Song, C.; Fan, L.; Qiu, L.; Zheng, Y.; Chen, J.; Xu, P. Effect of Chronic Exposure to Pesticide Methomyl on Antioxidant Defense System in Testis of Tilapia (Oreochromis niloticus) and Its Recovery Pattern. Appl. Sci. 2021, 11, 3332. [Google Scholar] [CrossRef]
- Desai, S.N.; Farris, F.F.; Ray, S.D. Lipid Peroxidation. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 89–93. [Google Scholar]
- Castell, J.V.; Gómez-Lechón, M.J.; Ponsoda, X.; Bort, R. 16—In Vitro Investigation of the Molecular Mechanisms of Hepatotoxicity. In In Vitro Methods in Pharmaceutical Research; Castell, J.V., Gómez-Lechón, M.J., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 375–410. [Google Scholar]
- Chen, G.; Zhu, M.; Guo, M. Research advances in traditional and modern use of Nelumbo nucifera: Phytochemicals, health promoting activities and beyond. Crit. Rev. Food Sci. Nutr. 2019, 59, S189–S209. [Google Scholar] [CrossRef]




| Group | Body Weight Gain (%) | Liver Weight (g) | Relative Liver Weight (%) |
|---|---|---|---|
| Group I | 79.46 ± 6.16 | 14.38 ± 0.80 | 3.44 ± 0.14 |
| Group II | 75.53 ± 3.55 # | 14.67 ± 0.71 | 3.57 ± 0.07 # |
| Group III | 72.73 ± 1.40 # | 13.82 ± 0.51 | 3.43 ± 0.10 # |
| Group IV | 76.62 ± 2.41# | 14.14 ± 0.42 | 3.51 ± 0.08 # |
| Group V | 87.81 ± 8.75 # | 15.16 ± 0.75 | 3.45 ± 0.15 # |
| Group VI | 56.22 ± 3.39 # | 14.87 ± 0.44 | 4.01 ± 0.10 # |
| Group VII | 55.25 ± 9.62 * | 15.21 ± 1.24 | 4.13 ± 0.12 * |
| Group VIII | 64.60 ± 2.90 | 15.05 ± 0.68 | 3.85 ± 0.11 * |
| Group IX | 56.82 ± 3.80 * | 12.72 ± 0.48 | 3.75 ± 0.14 |
| Group (n = 8) | AST (U/L) | ALT (U/L) |
|---|---|---|
| Group I | 171.37 ± 15.25 | 63.00 ± 10.14 |
| Group II | 220.12 ± 42.06 | 71.50 ± 10.93 |
| Group III | 151.87 ± 14.70 | 52.00 ± 5.16 |
| Group IV | 226.25 ± 64.16 | 54.62 ± 8.98 |
| Group V | 184.37 ± 11.25 | 53.12 ± 6.12 |
| Group VI | 175.37 ± 19.12 | 70.37 ± 11.77 |
| Group VII | 159.50 ± 23.87 | 46.25 ± 8.03 |
| Group VIII | 194.50 ± 38.46 | 68.87 ± 12.14 |
| Group IX | 161.25 ± 19.44 | 58.25 ± 9.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuchniyom, P.; Intui, K.; Laoung-on, J.; Jaikang, C.; Quiggins, R.; Photichai, K.; Sudwan, P. Effects of Nelumbo nucifera Gaertn. Petal Tea Extract on Hepatotoxicity and Oxidative Stress Induced by Mancozeb in Rat Model. Toxics 2023, 11, 480. https://doi.org/10.3390/toxics11060480
Nuchniyom P, Intui K, Laoung-on J, Jaikang C, Quiggins R, Photichai K, Sudwan P. Effects of Nelumbo nucifera Gaertn. Petal Tea Extract on Hepatotoxicity and Oxidative Stress Induced by Mancozeb in Rat Model. Toxics. 2023; 11(6):480. https://doi.org/10.3390/toxics11060480
Chicago/Turabian StyleNuchniyom, Pimchanok, Ketsarin Intui, Jiraporn Laoung-on, Churdsak Jaikang, Ranida Quiggins, Kornravee Photichai, and Paiwan Sudwan. 2023. "Effects of Nelumbo nucifera Gaertn. Petal Tea Extract on Hepatotoxicity and Oxidative Stress Induced by Mancozeb in Rat Model" Toxics 11, no. 6: 480. https://doi.org/10.3390/toxics11060480
APA StyleNuchniyom, P., Intui, K., Laoung-on, J., Jaikang, C., Quiggins, R., Photichai, K., & Sudwan, P. (2023). Effects of Nelumbo nucifera Gaertn. Petal Tea Extract on Hepatotoxicity and Oxidative Stress Induced by Mancozeb in Rat Model. Toxics, 11(6), 480. https://doi.org/10.3390/toxics11060480








