Individual Risk Assessment for Population Living on the Territories Long-Term Polluted by Organochlorine Pesticides
Abstract
:1. Introduction
2. Materials and Methods
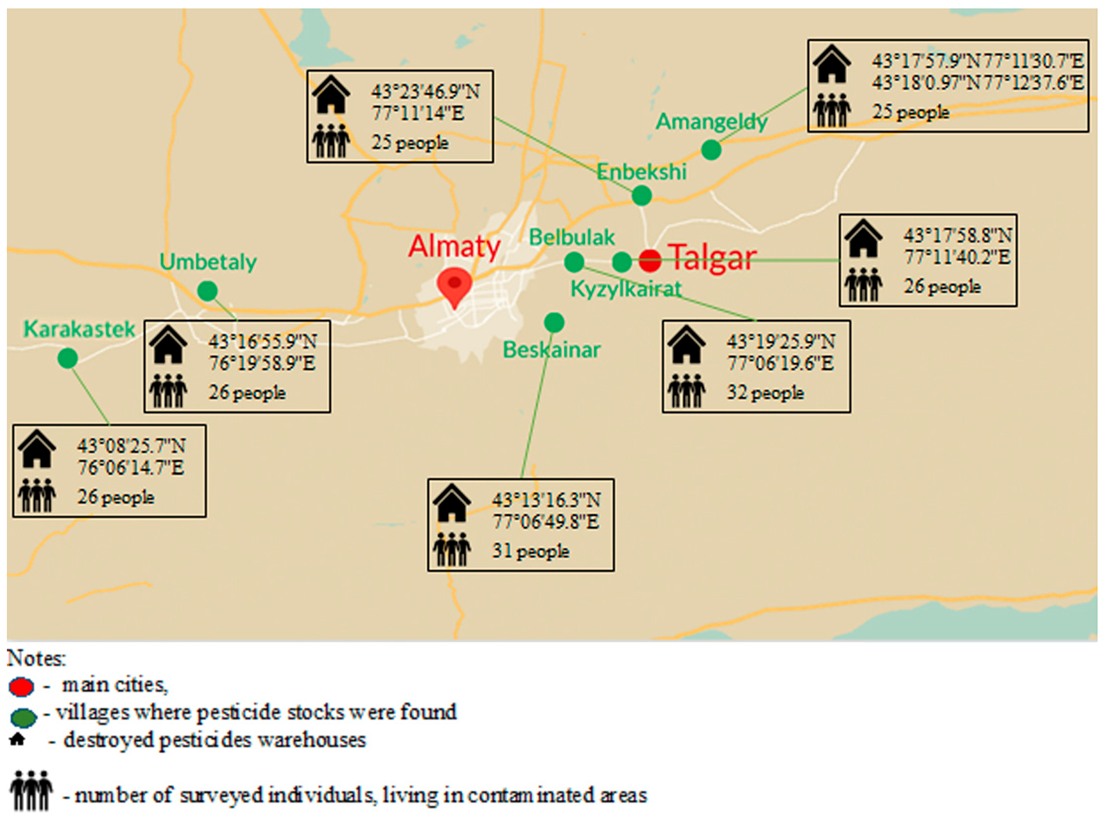
2.1. Objects and Materials
2.2. Questioning
2.3. DNA Isolation
2.4. Genotyping by Multiplex PCR and PCR-RFLP
2.5. Genome-Wide Microarray SNP Genotyping and Bioinformatics Processing
2.6. Statistical Methods
2.6.1. Calculation of Short-Term and Long-Term Risks for Villages
2.6.2. Calculation of Individual Risks
3. Results
3.1. Questioning Results
3.2. Genotyping Results
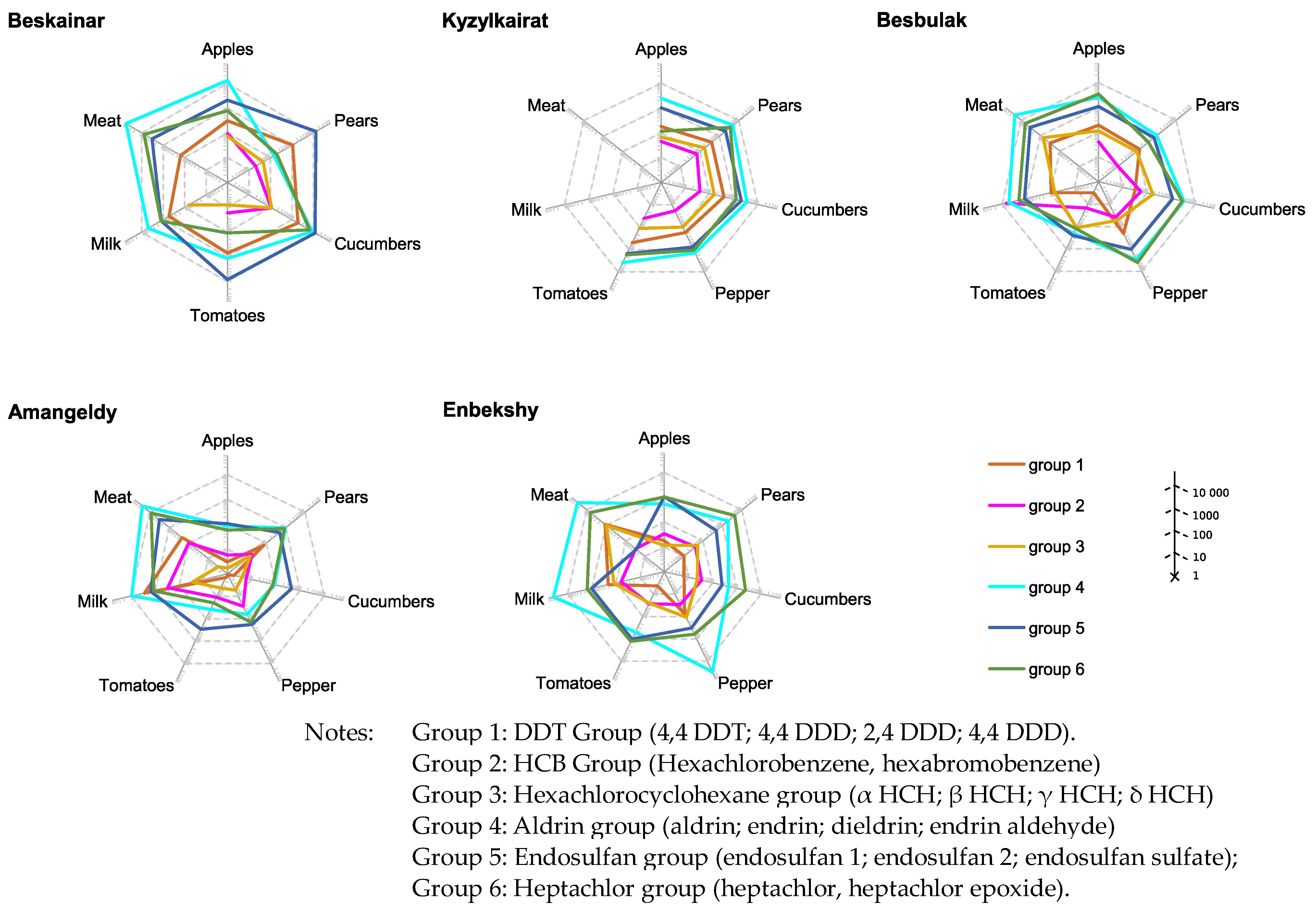
3.3. Analysis of Pesticides Intakes
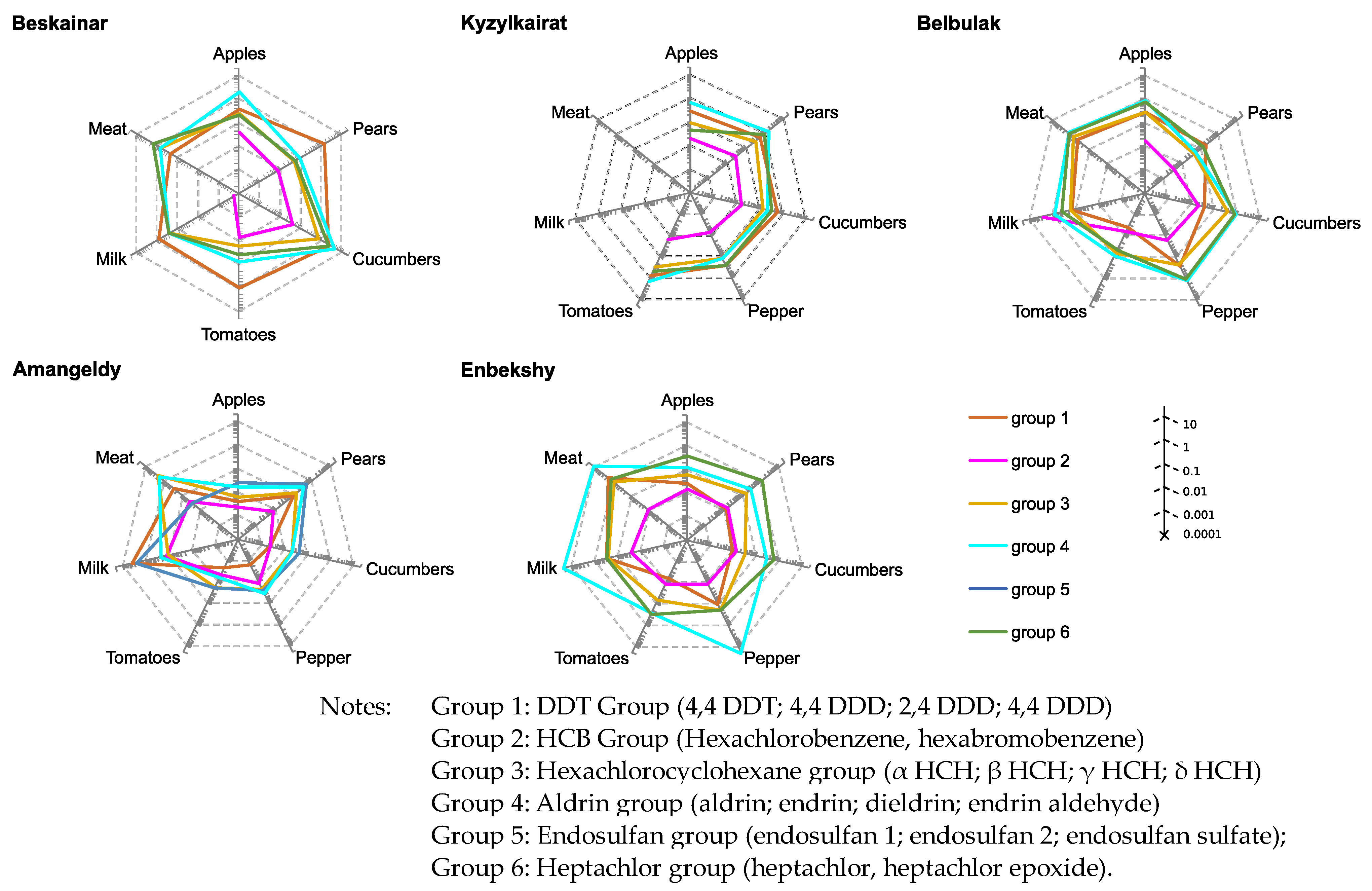
3.4. Assessing of Long-Term and Short-Term Risks
3.5. Assessing of Individual Health Risks
4. Discussion
4.1. Overview of Pesticide Contamination and Calculation of Risks
4.2. Genes Impact in Protection of Human Body from Environmental Influence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedorov, L.A.; Yablokov, A.V. Pesticides—A Toxic Blow to the Biosphere and Man; Nauka: Moscow, Russia, 1999; p. 462. (In Russian) [Google Scholar]
- Rico-Martínez, R.; Alvarado-Flores, J.; Pérez-Legaspi, I.A.; Garza-León, C.V.; Rivera-Dávila, O.L.; Santos-Medrano, D.; Robles-Vargas, G.E.; Carbajal-Hernández, A.L. Chapter 4—Fate and adverse effects of pesticides in the environment. In Pesticides in the Natural Environment, 1st ed.; Singh, P., Singh, S., Sillanpää, M., Eds.; Elsevier: North Andover, MA, USA, 2022; pp. 65–119. [Google Scholar] [CrossRef]
- What Are Pesticides and How Do They Work? NSW EPA. 2013. Available online: http://www.epa.nsw.gov.au/pesticides/pestwhatrhow.htm (accessed on 24 January 2020).
- UNEP. The Hazardous Chemicals and Waste Conventions; WHO: Geneva, Switzerland; UNEP: Nairobi, Kenya; FAO: Rome, Italy, 2013. Available online: http://www.pops.int/documents/background/hcwc.pdf (accessed on 24 January 2020). (In English)
- Weber, R.; Schlumpf, M.; Nakano, T.; Vijgen, J. The need for better management and control of POPs stockpiles. Environ. Sci. Pollut. Res. 2015, 22, 14385–14390. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Wu, L. Safety impact and farmer awareness of pesticide residues. Food Agric. Immunol. 2010, 21, 191–200. [Google Scholar] [CrossRef]
- Nurzhanova, A.; Kulakow, P.; Rubin, E.; Rakhimbayev, I.; Sedlovskiy, A.; Zhambakin, K.; Kalugin, S.; Kolysheva, E.; Erickson, L. Obsolete Pesticides Pollution and Phytoremediation of Contaminated Soil in Kazakhstan. In Application of Phytotechnologies for Cleanup of Industrial, Agricultural, and Wastewater Contamination; Kulakow, P.A., Pidlisnyuk, V.V., Eds.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2010; pp. 87–111. ISBN 978-90-481-3592-9. [Google Scholar]
- Nurzhanova, A.; Kalugin, S.; Zhambakin, K. Obsolete pesticides and application of colonizing plant species for remediation of contaminated soil in Kazakhstan. Environ. Sci. Pollut. 2013, 20, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Analytical Environmental Agency “Greenwomen”. Overview of the Fulfilment of the Obligations of the Republic of Kazakhstan under the Stockholm Convention on POPs. 2018, pp. 10–17. (In Russian). Available online: http://www.greenwomen.kz/pop1_kz.htm (accessed on 24 January 2020).
- Nurzhanova, A.A.; Inelova, Z.A.; Djansugurova, L.B.; Nesterova, S.G.; Mit, N.V.; Zhubanova, A.A.; Zhapbasov, R.Z.; Baizhanov, M.K.; Kapysheva, U.N.; Bakhtiyarova, S.K.; et al. The problem of unutilized and banned pesticides in Kazakhstan. News Natl. Acad. Sci. Repub. Kazakhstan. Ser. Biol. Med. 2018, 4, 86–96. [Google Scholar]
- Landrigan, P.J.; Benbrook, C. GMOs, Herbicides, and Public Health. N. Engl. J. Med. 2015, 373, 693–695. [Google Scholar] [CrossRef]
- Bassil, K.L.; Vakil, C.; Sanborn, M.; Cole, D.C.; Kaur, J.S.; Kerr, K.J. Cancer health effects of pesticides: Systematic review. Can. Fam. Physician 2007, 53, 1704–1711. [Google Scholar]
- Dich, J.; Zahm, S.H.; Hanberg, A.; Adami, H.O. Pesticides and cancer. Cancer Causes Control. 1997, 8, 420–443. [Google Scholar] [CrossRef]
- Cocco, P.; Satta, G.; Dubois, S.; Pili, C.; Pilleri, M.; Zucca, M.; ‘t Mannetje, A.M.; Becker, N.; Benavente, Y.; de Sanjosé, S.; et al. Lymphoma risk and occupational exposure to pesticides: Results of the Epilymph study. Occup. Environ. Med. 2013, 70, 91–98. [Google Scholar] [CrossRef]
- Carbonell, E.; Puig, M.; Xamena, N.; Creus, A.; Marcos, R. Sister chromatid exchange in lymphocytes of agricultural workers exposed to pesticides. Mutagenesis 1990, 5, 403–405. [Google Scholar] [CrossRef]
- Rupa, D.S.; Reddy, P.P.; Sreemannarayana, K.; Reddi, O.S. Frequency of sister chromatid exchange in peripheral lymphocytes of male pesticide applicators. Environ. Mol. Mutagen. 1991, 18, 136–138. [Google Scholar] [CrossRef]
- Bolt, H.M.; Foth, H.; Hengstler, J.G.; Degen, G.H. Carcinogenicity categorization of chemicals-new-aspects to be considered in a European perspective. Toxicol. Lett. 2004, 151, 29–41. [Google Scholar] [CrossRef]
- Gómez-Arroyo, S.; Díaz-Sánchez, Y.; Meneses-Pérez, M.A.; Villalobos-Pietrini, R.; De León-Rodríguez, J. Cytogenetic Biomonitoring in a Mexican Floriculture Worker Group Exposed to Pesticide. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2000, 466, 117–124. [Google Scholar] [CrossRef]
- Wong, R.H.; Chang, S.Y.; Ho, S.W.; Huang, P.L.; Liu, Y.J.; Chen, Y.C.; Yeh, Y.H.; Lee, H.S. Polymorphisms in metabolic GSTP1 and DNA-repair XRCC1 genes with an increased risk of DNA damage in pesticide-exposed fruit growers. Mutat. Res. 2008, 654, 168–175. [Google Scholar] [CrossRef]
- Guo, S.J.; Zhou, Y.T.; Liu, W.Y.; Zuo, Q.N.; Li, X.H. The polymorphism of XRCC1 and coronary artery disease risk: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1559–1567. [Google Scholar]
- Teodoro, M.; Briguglio, G.; Fenga, C.; Costa, C. Genetic polymorphisms as determinants of pesticide toxicity: Recent advances. Toxicol. Rep. 2019, 6, 564–570. [Google Scholar] [CrossRef]
- Voisin, E.M.; Ruthsatz, M.; Collins, J.M.; Hoyle, P.C. Extrapolation of animal toxicity to humans: Interspecies comparisons in drug development. Regul. Toxicol. Pharmacol. 1990, 12, 107–116. [Google Scholar] [CrossRef]
- Adams, V.H.; McAtee, M.J.; Johnson, M.S. Implementation of the basic hazard index screening for health risks associated with simultaneous exposure to multiple chemicals using a standardized target organ and systems framework. Integr. Environ. Assess. Manag. 2017, 13, 852–860. [Google Scholar] [CrossRef]
- Goumenou, M.; Tsatsakis, A. Proposing new approaches for the risk characterisation of single chemicals and chemical mixtures: The source related Hazard Quotient (HQS) and Hazard Index (HIS) and the adversity specific Hazard Index (HIA). Toxicol. Rep. 2019, 6, 632–636. [Google Scholar] [CrossRef]
- Gad Alla, S.A.; Loutfy, N.M.; Shendy, A.H.; Ahmed, M.T. Hazard index; a tool for a long-term risk assessment of pesticide residues in some commodities; a pilot study. Regul. Toxicol. Pharmacol. 2015, 73, 985–991. [Google Scholar] [CrossRef]
- Burns, D.M. Epidemiology of smoking-induced cardiovascular disease. Prog. Cardiovasc. Dis. 2003, 46, 11–29. [Google Scholar] [CrossRef]
- Klatsky, A.L. Alcohol and hypertension. Clin. Chim. Acta 1996, 246, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liang, X.; Chen, L.; Zuo, L.; Chen, K.; Wei, Y.; Chen, S.; Hao, G. Associations Between Household Pesticide Exposure; Smoking and Hypertension. Front. Public Health 2022, 10, 754643. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Gunnell, D.; von Meyer, L.; Eyer, P. Relationship between blood alcohol concentration on admission and outcome in dimethoate organophosphorus self-poisoning. Br. J. Clin. Pharmacol. 2009, 68, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Koide, S.; Kugiyama, K.; Sugiyama, S.; Nakamura, S.; Fukushima, H.; Honda, O.; Yoshimura, M.; Ogawa, H. Association of polymorphism in glutamate-cysteine ligase catalytic subunit gene with coronary vasomotor dysfunction and myocardial infarction. J. Am. Coll. Cardiol. 2003, 41, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kugiyama, K.; Sugiyama, S.; Miyamoto, S.; Koide, S.; Fukushima, H.; Honda, O.; Yoshimura, M.; Ogawa, H. Polymorphism in the 5′-flanking region of human glutamate-cysteine ligase modifier subunit gene is associated with myocardial infarction. Circulation 2002, 105, 2968–2973. [Google Scholar] [CrossRef]
- Djangalina, E.D.; Altynova, N.K.; Bakhtiyarova, S.K.; Kapysheva, U.N.; Zhaksymov, B.I.; Shadenova, E.; Baizhanov, M.; Sapargali, O.; Garshin, A.; Seisenbayeva, A.; et al. Comprehensive assessment of unutilized and obsolete pesticides impact on genetic status and health of population of Almaty region. Ecotoxicol. Environ. Saf. 2020, 202, 110905. [Google Scholar] [CrossRef]
- Chamannejadian, A.; Sayyad, G.; Moezzi, A.; Jahangiri, A. Evaluation of estimated daily intake (EDI) of cadmium and lead for rice (Oryza sativa L.) in calcareous soils. Iran. J. Environ. Health Sci. Eng. 2013, 10, 28. [Google Scholar] [CrossRef]
- Lozowicka, B.; Kaczynski, P.; Paritova, C.A.; Kuzembekova, G.B.; Abzhalieva, A.B.; Sarsembayeva, N.B.; Alihan, K. Pesticide residues in grain from Kazakhstan and potential health risks associated with exposure to detected pesticides. Food Chem. Toxicol. 2014, 64, 238–248. [Google Scholar] [CrossRef]
- Mebdoua, S.; Lazali, M.; Ounane, S.M.; Tellah, S.; Nabi, F.; Ounane, G. Evaluation of pesticide residues in fruits and vegetables from Algeria. Food Addit. Contam. Part B Surveill. 2017, 10, 91–98. [Google Scholar] [CrossRef]
- Szpyrka, E.; Kurdziel, A.; Matyaszek, A.; Podbielska, M.; Rupar, J.; Słowik-Borowiec, M. Evaluation of pesticide residues in fruits and vegetables from the region of south-eastern Poland. Food Control 2015, 48, 137–142. [Google Scholar] [CrossRef]
- Yohannes, Y.B.; Ikenaka, Y.; Saengtienchai, A.; Watanabe, K.P.; Nakayama, S.M.; Ishizuka, M. Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley lake—Lake Ziway; Ethiopia. Ecotoxicol. Environ. Saf. 2014, 106, 95–101. [Google Scholar] [CrossRef]
- Akoto, O.; Oppong-Otoo, J.; Osei-Fosu, P. Carcinogenic and non-carcinogenic risk of organochlorine pesticide residues in processed cereal-based complementary foods for infants and young children in Ghana. Chemosphere 2015, 32, 193–199. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Beglaryan, M.; Stepanyan, S.; Asmaryan, S.; Hovsepyan, A.; Merendino, N. Carcinogenic and non-carcinogenic risk assessment of trace elements and POPs in honey from Shirak and Syunik regions of Armenia. Chemosphere 2020, 239, 124809. [Google Scholar] [CrossRef]
- Adeleye, А.O.; Sosan, M.B.; Oyekunle, J.A.O. Dietary exposure assessment of organochlorine pesticides in two commonly grown leafy vegetables in South-western Nigeri. Heliyon 2020, 5, e01895. [Google Scholar] [CrossRef]
- Kim, M.J.; Marchand, P.; Henegar, C.; Antignac, J.P.; Alili, R.; Poitou, C.; Bouillot, J.L.; Basdevant, A.; Le Bizec, B.; Barouki, R.; et al. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ. Health Perspect. 2011, 119, 377–383. [Google Scholar] [CrossRef]
- Kim, M.J.; Pelloux, V.; Guyot, E.; Tordjman, J.; Bui, L.C.; Chevallier, A.; Forest, C.; Benelli, C.; Clément, K.; Barouki, R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ. Health Perspect. 2012, 120, 508–514. [Google Scholar] [CrossRef]
- La Merrill, M.; Emond, C.; Kim, M.J.; Antignac, J.P.; Le Bizec, B.; Clément, K.; Birnbaum, L.S.; Barouki, R. Toxicological function of adipose tissue: Focus on persistent organic pollutants. Environ. Health Perspect. 2013, 121, 162–169. [Google Scholar] [CrossRef]
- Au, W.W.; Salama, S.A.; Sierra Torres, C.H. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ. Health Perspect. 2003, 111, 1843–1850. [Google Scholar] [CrossRef]
- Matullo, G.; Palli, D.; Peluso, M.; Guarrera, S.; Carturan, S.; Celentano, E.; Krogh, V.; Munnia, A.; Tumino, R.; Polidoro, S.; et al. XRCC1; XRCC3; XPD gene polymorphisms; smoking and 32P-DNA adducts in a sample of healthy subjects. Carcinogenesis 2001, 22, 1437–1445. [Google Scholar] [CrossRef]
- Ivashchenko, T.E.; Sideleva, O.G.; Petrova, M.A. Genetic factors of predisposition to bronchial asthma. Genetics 2001, 37, 107–111. (In Russian) [Google Scholar]
- Taioli, E.; Gaspari, L.; Benhamou, S.; Boffetta, P.; Brockmoller, J.; Butkiewicz, D.; Cascorbi, I.; Clapper, M.L.; Dolzan, V.; Haugen, A.; et al. Polymorphisms in CYP1A1; GSTM1; GSTT1 and lung cancer below the age of 45 years. Int. J. Epidemiol. 2003, 32, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wołejko, E.; Wydro, U.; Butarewicz, A. The impact of pesticides on oxidative stress level in human organism and their activity as an endocrine disruptor. J. Environ. Sci. Health B 2017, 52, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martínez, M.A.; Dai, M.; Chen, D.; Ares, I.; Romero, A.; Castellano, V.; Martínez, M.; Rodríguez, J.L.; Martínez-Larrañaga, M.R.; et al. Permethrin-induced oxidative stress and toxicity and metabolism. A review. Environ. Res. 2016, 149, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Semren, T.Ž.; Žunec, S.; Pizent, A. Oxidative stress in triazine pesticide toxicity: A review of the main biomarker findings. Arh. Hig. Rada Toksikol. 2018, 69, 109–125. [Google Scholar] [CrossRef]
- Tang-Péronard, J.L.; Heitmann, B.L.; Andersen, H.R.; Steuerwald, U.; Grandjean, P.; Weihe, P.; Jensen, T.K. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: A prospective cohort study of 656 children from the Faroe Islands. Am. J. Clin. Nutr. 2014, 99, 5–13. [Google Scholar] [CrossRef]
- Valvi, D.; Mendez, M.A.; Martinez, D.; Grimalt, J.O.; Torrent, M.; Sunyer, J.; Vrijheid, M. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: A prospective birth cohort study. Environ. Health Perspect. 2012, 120, 451–457. [Google Scholar] [CrossRef]
- Donaldson, S.G.; Van Oostdam, J.; Tikhonov, C.; Feeley, M.; Armstrong, B.; Ayotte, P.; Boucher, O.; Bowers, W.; Chan, L.; Dallaire, F.; et al. Environmental contaminants and human health in the Canadian Arctic. Sci. Total. Environ. 2010, 408, 5165–5234. [Google Scholar] [CrossRef]
- Muckle, G.; Ayotte, P.; Dewailly, E.E.; Jacobson, S.W.; Ja-cobson, J.L. Prenatal exposure of the northern Québec Inuit infants to environmental contaminants. Environ. Health Perspect. 2001, 109, 1291–1299. [Google Scholar] [CrossRef]
- Tvermosegaard, M.; Dahl-Petersen, I.K.; Nielsen, N.O.; Bjerregaard, P.; Jørgensen, M.E. Cardiovascular Disease Susceptibility and Resistance in Circumpolar Inuit Populations. Can. J. Cardiol. 2015, 31, 1116–1123. [Google Scholar] [CrossRef]
- Krieger, R.I.; Krieger, W.C. Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 819–835. ISBN 978-0-12-426260-7. [Google Scholar] [CrossRef]
- Maurice, C.; Kaczmarczyk, M.; Côté, N.; Tremblay, Y.; Kimmins, S.; Bailey, J.L. Prenatal exposure to an environmentally relevant mixture of Canadian Arctic contaminants decreases male reproductive function in an aging rat model. J. Dev. Orig. Health Dis. 2018, 9, 511–518. [Google Scholar] [CrossRef]
- Eriksson, P.; Johansson, U.; Ahlbom, J.; Fredriksson, A. Neonatal exposure to DDT induces increased susceptibility to pyrethroid (bioallethrin) exposure at adult age—Changes in cholinergic muscarinic receptor and behavioural variables. Toxicology 1993, 77, 21–30. [Google Scholar] [CrossRef]
- Eriksson, P. Developmental neurotoxicity of environmental agents in the neonate. NeuroToxicology 1997, 18, 719–726. [Google Scholar]
- Liu, J.H.; Chou, C.Y.; Liu, Y.L.; Liao, P.Y.; Lin, P.W.; Lin, H.H.; Yang, Y.F. Acid-base interpretation can be the predictor of outcome among patients with acute organophosphate poisoning before hospitalization. Am. J. Emerg. Med. 2008, 26, 24–30. [Google Scholar] [CrossRef]
- Yu, J.R.; Hou, Y.C.; Fu, J.F.; Wang, I.K.; Chan, M.J.; Chen, C.Y.; Weng, C.H.; Huang, W.H.; Yang, H.Y.; Hsu, C.W.; et al. Outcomes of elderly patients with organophosphate intoxication. Sci. Rep. 2021, 11, 11615. [Google Scholar] [CrossRef]
- Kasner, E.J.; Keralis, J.M.; Mehler, L.; Beckman, J.; Bonnar-Prado, J.; Lee, S.J.; Diebolt-Brown, B.; Mulay, P.; Lackovic, M.; Waltz, J.; et al. Gender differences in acute pesticide-related illnesses and injuries among farmworkers in the United States, 1998–2007. Am. J. Ind. Med. 2012, 55, 571–583. [Google Scholar] [CrossRef]
- Sidorova, I.S.; Unanyan, A.L.; Evtina, I.P.; Zaletaev, D.V. Method for Prediction of Cervical Cancer in Benign and Pre-Cancer Cervical Processes in Females of Reproductive Age. RU2466392C2, 15 February 2011. [Google Scholar]
- Conforti-Froes, N.; El-Zein, R.; Abdel-Rahman, S.Z.; Zwishenberger, J.B.; Au, W.W. Predisposing genes and increased chromosome aberration in lung cancer cigarette smokers. Mutation Res. 1995, 335, 171–184. [Google Scholar]
- Maurice, C.; Dalvai, M.; Lambrot, R.; Deschênes, A.; Scott-Boyer, M.-P.; McGraw, S.; Chan, D.; Côté, N.; Ziv-Gal, A.; Flaws, J.A.; et al. Early-Life Exposure to Environmental Contaminants Perturbs the Sperm Epigenome and Induces Negative Pregnancy Outcomes for Three Generations via the Paternal Lineage. Epigenomes 2021, 5, 10. [Google Scholar] [CrossRef]
- Lismer, A.; Shao, X.; Dumargne, M.C.; Lafleur, C.; Lambrot, R.; Chan, D.; Toft, G.; Bonde, J.P.; MacFarlane, A.J.; Bornman, R.; et al. Exposure of Greenlandic Inuit and South African VhaVenda men to the persistent DDT metabolite is associated with an altered sperm epigenome at regions implicated in paternal epigenetic transmission and developmental disease—A cross-sectional study. bioRxiv 2022. [Google Scholar] [CrossRef]
- De Oliveira, A.; de Souza, M.R.; Benedetti, D.; Scotti, A.S.; Piazza, L.S.; Garcia, A.H.; Dias, J.F.; Niekraszewicz, L.B.; Duarte, A.; Bauer, D.; et al. Investigation of pesticide exposure by genotoxicological, biochemical, genetic polymorphic and in silico analysis. Ecotoxicol. Environ. Saf. 2019, 179, 135–142. [Google Scholar] [CrossRef]
- Van der Plaat, D.A.; de Jong, K.; de Vries, M.; van Diemen, C.C.; Nedeljković, I.; Amin, N.; Kromhout, H. Biobank-based Integrative Omics Study Consortium; Vermeulen, R.; Postma, D.S.; et al. Occupational exposure to pesticides is associated with differential DNA methylation. Occup. Environ. Med. 2018, 75, 427–435. [Google Scholar] [CrossRef]
- Marutescu, L.; Chifiriuc, M.C. Molecular mechanisms of pesticides toxicity. In New Pesticides and Soil Sensors; Academic Press: Cambridge, MA, USA, 2017; pp. 393–435. [Google Scholar]
- Boada, L.D.; Henríquez-Hernández, L.A.; Zumbado, M.; Almeida-González, M.; Álvarez-León, E.E.; Navarro, P.; Luzardo, O.P. Organochlorine Pesticides Exposure and Bladder Cancer: Evaluation from a Gene-Environment Perspective in a Hospital-Based Case-Control Study in the Canary Islands. J. Agromed. 2016, 21, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Sharma, T.; Gupta, S.; Banerjee, B. CYP1A1 expression and organochlorine pesticides level in the etiology of bladder cancer in North Indian population. Hum. Exp. Toxicol. 2018, 37, 817–826. [Google Scholar] [CrossRef]
- Kumar, V.; Banerjee, B.D.; Datta, S.K.; Yadav, C.S.; Singh, S.; Ahmed, R.S.; Gupta, S. Association of CYP1A1; CYP1B1 and CYP17 gene polymorphisms and organochlorine pesticides with benign prostatic hyperplasia. Chemosphere 2014, 108, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Dorji, P.W.; Tshering, G.; Na-Bangchang, K. CYP2C9; CYP2C19; CYP2D6 and CYP3A5 polymorphisms in South-East and East Asian populations: A systematic review. J. Clin. Pharm. Ther. 2019, 44, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.K.; Honkisz, E.; Zięba-Przybylska, D.; Milewicz, T.; Kajta, M. Effects of two isomers of DDT and their metabolite DDE on CYP1A1 and AhR function in human placental cells. Pharmacol. Rep. 2011, 63, 1460–1468. [Google Scholar] [CrossRef]
- Darney, K.; Lautz, L.S.; Béchaux, C.; Wiecek, W.; Testai, E.; Amzal, B.; Dorne, J.L.C.M. Human variability in polymorphic CYP2D6 metabolism: Implications for the risk assessment of chemicals in food and emerging designer drugs. Environ. Int. 2021, 156, 106760. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Vashisht, K.; Singh, P.; Banerjee, B.D.; Rautela, R.S.; Grover, S.S.; Rawat, D.S.; Pasha, S.T.; Jain, S.K.; et al. Role of genetic polymorphisms of CYP1A1, CYP3A5, CYP2C9, CYP2D6, and PON1 in the modulation of DNA damage in workers occupationally exposed to organophosphate pesticides. Toxicol. Appl. Pharmacol. 2011, 15, 84–92. [Google Scholar] [CrossRef]
- Anwarullah; Aslam, M.; Badshah, M.; Abbasi, R.; Sultan, A.; Khan, K.; Ahmad, N.; von Engelhardt, J. Further evidence for the association of CYP2D6*4 gene polymorphism with Parkinson’s disease: A case control study. Genes Environ. 2017, 39, 18. [Google Scholar] [CrossRef]
- Lemaire, G.; de Sousa, G.; Rahmani, R. A PXR reporter gene assay in a stable cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem. Pharmacol. 2004, 15, 2347–2358. [Google Scholar] [CrossRef]
- Perovani, I.S.; Mariano Bucci, J.L.; Carrão, D.B.; Santos Barbetta, M.F.; Moreira da Silva, R.; Lopes, N.P.; Moraes de Oliveira, A.R. Enantioselective inhibition of human CYP2C19 by the chiral pesticide ethofumesate: Prediction of pesticide-drug interactions in humans. Chem. Biol. Interact. 2021, 345, 109552. [Google Scholar] [CrossRef]
- Trist, B.G.; Genoud, S.; Roudeau, S.; Rookyard, A.; Abdeen, A.; Cottam, V.; Hare, D.J.; White, M.; Altvater, J.; Fifita, J.A.; et al. Altered SOD1 maturation and post-translational modification in amyotrophic lateral sclerosis spinal cord. Brain 2022, 145, 3108–3130. [Google Scholar] [CrossRef]
- Vakonaki, E.; Androutsopoulos, V.P.; Liesivuori, J.; Tsatsakis, A.M.; Spandidos, D.A. Pesticides and oncogenic modulation. Toxicology 2013, 307, 42–45. [Google Scholar] [CrossRef]
- Almusafri, F.; Elamin, H.E.; Khalaf, T.E.; Ali, A.; Ben-Omran, T.; El-Hattab, A.W. Clinical and molecular characterization of 6 children with glutamate-cysteine ligase deficiency causing hemolytic anemia. Blood Cells Mol. Dis. 2017, 65, 73–77. [Google Scholar] [CrossRef]
- Kannan, M.B.; Dodard-Friedman, I.; Blank, V. Stringent Control of NFE2L3 (Nuclear Factor; Erythroid 2-Like 3; NRF3) Protein Degradation by FBW7 (F-box/WD Repeat-containing Protein 7) and Glycogen Synthase Kinase 3 (GSK3). J. Biol. Chem. 2015, 23, 26292–26302. [Google Scholar] [CrossRef]

| Gene | Primers | PCR Conditions | Restriction Endonuclease | Restriction Products (b.p.) |
|---|---|---|---|---|
| GSTP1 Ile105Val | (F) 5′-ACC CCA GGG CTC TAT GGG AA-3′ (R) 5′-TGA GGG CAC AAG AAG CCC CT-3′ | 30 cycles: 94 °C—30 s 55 °C—30 s 72 °C—30 s | Alw26I | Val/Val: 91 + 85; Ile/Val: 176 + 91 + 85; Ile/Ile: 176. |
| XRCC1 Arg399Gln | (F) 5′-CAA GTA CAG CCA GGT CCT AG-3′ (R) 5′-CCT TCC CTC ATC TGG AGT AC-3′ | 40 cycles: 94 °C—15 s 55 °C—30 s 72 °C—45 s | NciI | Arg/Arg: 89 + 59 Arg/Gln: 248 + 159 + 89 Gln/Gln: 248 |
| XRCC1 Arg194Trp | (F) 5′-GCC CCG TCC CAG GTA-3′ (R) 5′-AGC CCC AAG ACC CTT T-3′ | 40 cycles: 94 °C—15 s 57 °C—45 s 72 °C—45 s | PvuII | Arg/Arg: 490 Arg/Trp: 490 + 294 + 196 Trp/Trp: 294 + 196 |
| XRCC3 Met241Trp | (F) 5′-GCC TGG TGG TCA TCG ACT C-3′ (R) 5′-ACA GGG CTC TGG AAG GCA CTG CTC AGC TCA CGC ACC-3′ | 40 cycles: 94 °C—15 s 60 °C—30 s 72 °C—45 s | Nco1 | Trp/Trp: 136 Trp/Met: 136 + 97 + 39 Met/Met: 97 + 39 |
| XPD Lys751Gln | (F) 5′-GCC CGC TCT GGA TTA TAC G-3′ (R) 5′-CTA TCA TCT CCT GGC CCC C-3′ | 38 cycles: 94 °C—45 s 60 °C—45 s 72 °C—60 s | PstI | Lys/Lys: 290 + 146 Gln/Gln: 227 + 146 + 63 Lys/Gln: 290 + 227 + 146+ 63 |
| GSTT1 | (F) 5′-CCT TAC TGG TCC TCA CAT CTC-3′ (R) 5′-TCA CCG GAT CAT GGC CAG CA-3′ | 35 cycles: 94 °C—2 min 59 °C—1 min 72 °C—1 min | _ | +/+;+/−: 480 |
| GSTM1 | (F) 5′-GAA CTC CCT GAA AAG CTA AAG C-3′ (R) 5′-GTT GGG CTC AAA TAT ACG GTG G-3′ | +/+;+/−: 215 | ||
| β-globin | (F) 5′-CAA CTT CAT CCA CGT TCA CC-3′ (R) 5′-GAA GAG CCT AGG ACA GGT AC-3′ | +/+: 268 | ||
| GPX4 | (F) 5′-GAG AAG GAC CTG CCC CAC TA-3′ (R) 5′-GTC ATG AGT GCC GGT GGA AG-3′ | 35 cycles: 95 °C—30 s, 61 °C—30 s, 72 °C—45 s, | StyI | TT: 68 + 28; TC: 96 + 68 + 28; CC: 96. |
| GCLC | (F) 5′-TCG TCC CAA GTC TCA CAG TC-3′ (R) 5′-CGC CCT CCC CGC TGC TCC TC-3′ | 35 cycles: 95 °C—30 s, 61 °C—30 s, 72 °C—45 s, | Tsp45I | CC: 500 + 113; CT: 500 + 302 + 198 + 113; TT: 302 + 198 + 113. |
| GCLM | (F) 5′-CTC AAG GGC AAA GAC TCA-3′ (R) 5′-CCG CCT GGT GAG GTA GAC AC-3′ | 35 cycles: 95 °C—30 s, 58 °C—30 s, 72 °C—45 s, | MspI | CC: 200 + 84 + 45; CT: 200 + 129 + 84 + 45; TT: 200 + 129. |
| Locality | Total Persons | Ethnicity, pers. (%) | Males (%) | Females (%) | Years of Birth (Average Age) |
|---|---|---|---|---|---|
| Kyzylkairat | 32 | Kazakhs—27 (84%) Russians—1 (3%) Others—4 (13%) | 10 (31%) | 22 (69%) | 1947–1999 (48.6 ± 12.8) |
| Belbulak | 26 | Kazakhs—14 (54%) Russians—7 (27%) Others—5 (19%) | 5 (19%) | 21 (81%) | 1942–2004 (49.5 ± 13.6) |
| Beskainar | 31 | Kazakhs—25 (81%) Russians—5 (16%) Others—1 (3%) | 7 (23%) | 24 (77%) | 1950–1989 (52.8 ± 11.8) |
| Amangeldy | 25 | Kazakhs—18 (72%) Russians—4 (16%) Others—3 (12%) | 14 (56%) | 11 (44%) | 1948–1997 (50.6 ± 12.8) |
| Enbekshi | 27 | Kazakhs—21 (78%) Russians—6 (22%) | 8 (30%) | 19 (70%) | 1943–1996 (50.6 ± 13.3) |
| Karakastek | 25 | Kazakhs—24 (96%) Russians—1 (4%) | 4 (16%) | 21 (84%) | 1939–1997 (50.48 ± 3.14) |
| Umbetaly | 25 | Kazakhs—25 (100%) | 10 (40%) | 15 (60%) | 1954–1999 (40.84 ± 2.91) |
| Locality | Meat | Cucumbers | Tomato | Pepper | Apples | Pears | Milk |
|---|---|---|---|---|---|---|---|
| Kyzylkairat | 0.14 ± 0.12 | 0.13 ± 0.16 | 0.18 ± 0.21 | 0.07 ± 0.087 | 0.28 ± 0.33 | 0.11 ± 0.14 | 0.65 ± 0.60 |
| Belbulak | 0.14 ± 0.09 | 0.07 ± 0.06 | 0.11 ± 0.09 | 0.03 ± 0.02 | 0.40 ± 0.30 | 0.15 ± 0.17 | 0.35 ± 0.29 |
| Beskainar | 0.14 ± 0.11 | 0.21 ± 0.17 | 0.46 ± 0.35 | 0.12 ± 0.14 | 0.60 ± 0.50 | 0.67 ± 0.62 | 0.41 ± 0.37 |
| Amangeldy | 0.16 ± 0.11 | 0.32 ± 0.17 | 0.58 ± 0.29 | 0.12 ± 0.12 | 0.62 ± 0.41 | 0.79 ± 0.58 | 0.93 ± 0.43 |
| Enbekshi | 0.05 ± 0.03 | 0.19 ± 0.14 | 0.35 ± 0.21 | 0.02 ± 0.03 | 0.10 ± 0.12 | 0.10 ± 0.13 | 0.51 ± 0.54 |
| Karakastek | 0.32 ± 0.3 | 0.26 ± 0.21 | 0.46 ± 0.33 | 0.22 ± 0.19 | 0.38 ± 0.38 | 0.13 ± 0.16 | 0.40 ± 0.26 |
| Umbetaly | 0.19 ± 0.13 | 0.47 ± 0.36 | 0.53 ± 0.35 | 0.19 ± 0.30 | 0.42 ± 0.43 | 0.31 ± 0.39 | 0.53 ± 0.39 |
| Karakastek | Belbulak | Beskainar | Amangeldy | Enbekshi | Karakastek | Umbetaly | |
|---|---|---|---|---|---|---|---|
| Mean weight for males, kg | 71.9 ± 9.17 | 79.1 ± 12.13 | 79.83 ± 11.21 | 68.09 ± 5.36 | 76 ± 2.67 | 78.67 ± 14.22 | 70.7 ± 11.9 |
| Mean weight for females, kg | 68.54 ± 9.1 | 73.76 ± 14.72 | 70.37 ± 10.78 | 68.45 ± 13.42 | 71.38 ± 10.13 | 69.09 ± 9.1 | 64.93 ± 7.67 |
| t-test | 0.0216 | ||||||
| Name | Gene | Number of Individuals with Recorded Genotypes | Allele Frequencies | χ2 p Value | |||
|---|---|---|---|---|---|---|---|
| AA | AB | BB | A | B | |||
| rs861539 | XRCC3 | 95 | 38 | 18 | 0.755 | 0.245 | 15.445 0.0004 |
| rs1799782 | XRCC1 | 102 | 41 | 8 | 0.811 | 0.189 | 1.940 0.379 |
| rs25487 | XRCC1 | 50 | 63 | 38 | 0.540 | 0.460 | 3.878 0.144 |
| rs13181 | XPD | 88 | 55 | 8 | 0.765 | 0.235 | 0.025 0.988 |
| rs138002121 | SOD1 | 133 | 1 | 17 | 0.884 | 0.116 | 141.398 0 |
| rs1041740 | SOD1 | 59 | 55 | 37 | 0.573 | 0.427 | 9.875 0.007 |
| rs17861084 | CYP1A1 | 134 | 0 | 17 | 0.887 | 0.113 | 151.000 0 |
| rs8192718 | CYP2B6 | 132 | 2 | 17 | 0.881 | 0.119 | 132.532 0 |
| rs186133763 | CYP2D6 | 134 | 0 | 17 | 0.887 | 0.113 | 151.000 0 |
| rs11592737 | CYP2C19 | 115 | 29 | 7 | 0.858 | 0.142 | 6.890 0.032 |
| Deletion | GSTT1 | 128 (+/+, +/−) | 89 (−/−) | 0.360 (+) | 0.640 (−) | ||
| Deletion | GSTM1 | 110 (+/+, +/−) | 110 (−/−) | 0.500 (+) | 0.500 (−) | ||
| rs1138272 | GSTP1 | 115 | 17 | 19 | 0.818 | 0.182 | 58.435 0 |
| rs1695 | GSTP1 | 87 | 50 | 14 | 0.742 | 0.258 | 2.783 0.249 |
| rs1871042 | GSTP1 | 72 | 48 | 31 | 0.636 | 0.364 | 14.854 0.0006 |
| rs2237329 | NFE2L3 | 106 | 23 | 22 | 0.778 | 0.222 | 47.158 0 |
| rs713041 | GPX4 | 21 | 97 | 33 | 0.460 | 0.540 | 12.957 0.002 |
| rs41303970 | GCLM | 117 | 27 | 7 | 0.864 | 0.136 | 8.554 0.014 |
| rs12524550 | GCLC | 131 | 3 | 17 | 0.877 | 0.123 | 124.384 0 |
| rs3799694 | GCLC | 81 | 55 | 15 | 0.718 | 0.282 | 1.494 0.474 |
| rs524553 | GCLC | 126 | 23 | 2 | 0.911 | 0.089 | 0.628 0.730 |
| Villages | DDT | HCB | HCH | Aldrin | Endosulfans | Heptachlor |
|---|---|---|---|---|---|---|
| Kyzylkairat | 0.40 ± 0.7 | 0.008 ± 0.01 | 0.03 ± 0.05 | 0.07 ± 0.11 | 0.14 ± 0.21 | 0.04 ± 0.07 |
| Belbulak | 0.18 ± 0.3 | 0.04 ± 0.08 | 0.06 ± 0.1 | 0.094 ± 0.16 | 0.14 ± 0.20 | 0.07 ± 0.10 |
| Beskainar | 1.26 ± 2.16 | 0.12 ± 0.22 | 0.05 ± 0.08 | 0.27 ± 0.49 | 0.8 ± 1.32 | 0.07 ± 0.12 |
| Amangeldy | 0.26 ± 0.49 | 0.007 ± 0.01 | 0.02 ± 0.04 | 0.14 ± 0.26 | 0.08 ± 0.14 | 0.05 ± 0.10 |
| Enbekshi | 0.21 ± 0.36 | 0.01 ± 0.02 | 0.06 ± 0.09 | 0.2 ± 0.34 | 0.1 ± 0.125 | 0.09 ± 0.12 |
| Karakastek | 0.002 ± 0.003 | 0.001 ± 0.001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| Umbetaly | 0.002 ± 0.002 | 0.001 ± 0.001 | <0.0001 | 0.0001 | <0.0001 | 0.001 ± 0.001 |
| ADI | 0.01 | - | - | 0.0002 | 0.006 | 0.0001 |
| Villages | DDT | HCB | HCH | Aldrin | Endosulfans | Heptachlor |
|---|---|---|---|---|---|---|
| Kyzylkairat | 0.11 ± 0.1 | 0.04 ± 0.03 | 0.02 ± 0.012 | 0.05 ± 0.04 | 0.08 ± 0.06 | 0.003 ± 0.003 |
| Belbulak | 0.04 ± 0.03 | 0.03 ± 0.02 | 0.02 ± 0.007 | 0.04 ± 0.03 | 0.03 ± 0.01 | 0.02 ± 0.009 |
| Beskainar | 0.56 ± 0.3 | 0.02 ± 0.01 | 0.02 ± 0.009 | 0.04 ± 0.02 | 0.36 ± 0.24 | 0.01 ± 0.006 |
| Amangeldy | 0.44 ± 0.2 | 0.004 ± 0.002 | 0.011 ± 0.005 | 0.02 ± 0.006 | 0.03 ± 0.01 | 0.01 ± 0.005 |
| Enbekshi | 0.03 ± 0.03 | 0.001 ± 0.001 | 0.005 ± 0.004 | 0.006 ± 0.005 | 0.02 ± 0.01 | 0.006 ± 0.005 |
| Karakastek | 0.0002 ± 0.0001 | 0.0001 ± 0 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| Umbetaly | 0.0002 ± 0.0001 | 0.0001 ± 0 | <0.0001 | 0.0001 | <0.0001 | <0.0001 |
| ADI | 0.01 | - | - | 0.0002 | 0.006 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garshin, A.; Altynova, N.; Djangalina, E.; Khamdiyeva, O.; Baratzhanova, G.; Tolebaeva, A.; Zhaniyazov, Z.; Khussainova, E.; Cakir-Kiefer, C.; Jurjanz, S.; et al. Individual Risk Assessment for Population Living on the Territories Long-Term Polluted by Organochlorine Pesticides. Toxics 2023, 11, 482. https://doi.org/10.3390/toxics11060482
Garshin A, Altynova N, Djangalina E, Khamdiyeva O, Baratzhanova G, Tolebaeva A, Zhaniyazov Z, Khussainova E, Cakir-Kiefer C, Jurjanz S, et al. Individual Risk Assessment for Population Living on the Territories Long-Term Polluted by Organochlorine Pesticides. Toxics. 2023; 11(6):482. https://doi.org/10.3390/toxics11060482
Chicago/Turabian StyleGarshin, Aleksandr, Nazym Altynova, Erika Djangalina, Ozada Khamdiyeva, Gulminyam Baratzhanova, Anar Tolebaeva, Zhasulan Zhaniyazov, Elmira Khussainova, Céline Cakir-Kiefer, Stefan Jurjanz, and et al. 2023. "Individual Risk Assessment for Population Living on the Territories Long-Term Polluted by Organochlorine Pesticides" Toxics 11, no. 6: 482. https://doi.org/10.3390/toxics11060482
APA StyleGarshin, A., Altynova, N., Djangalina, E., Khamdiyeva, O., Baratzhanova, G., Tolebaeva, A., Zhaniyazov, Z., Khussainova, E., Cakir-Kiefer, C., Jurjanz, S., Delannoy, M., & Djansugurova, L. (2023). Individual Risk Assessment for Population Living on the Territories Long-Term Polluted by Organochlorine Pesticides. Toxics, 11(6), 482. https://doi.org/10.3390/toxics11060482








