Usage Pattern and Nicotine Delivery during Ad Libitum Consumption of Pod E-Cigarettes and Heated Tobacco Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims and Ethics
2.2. Study Prodcts and Groups
2.3. Participants
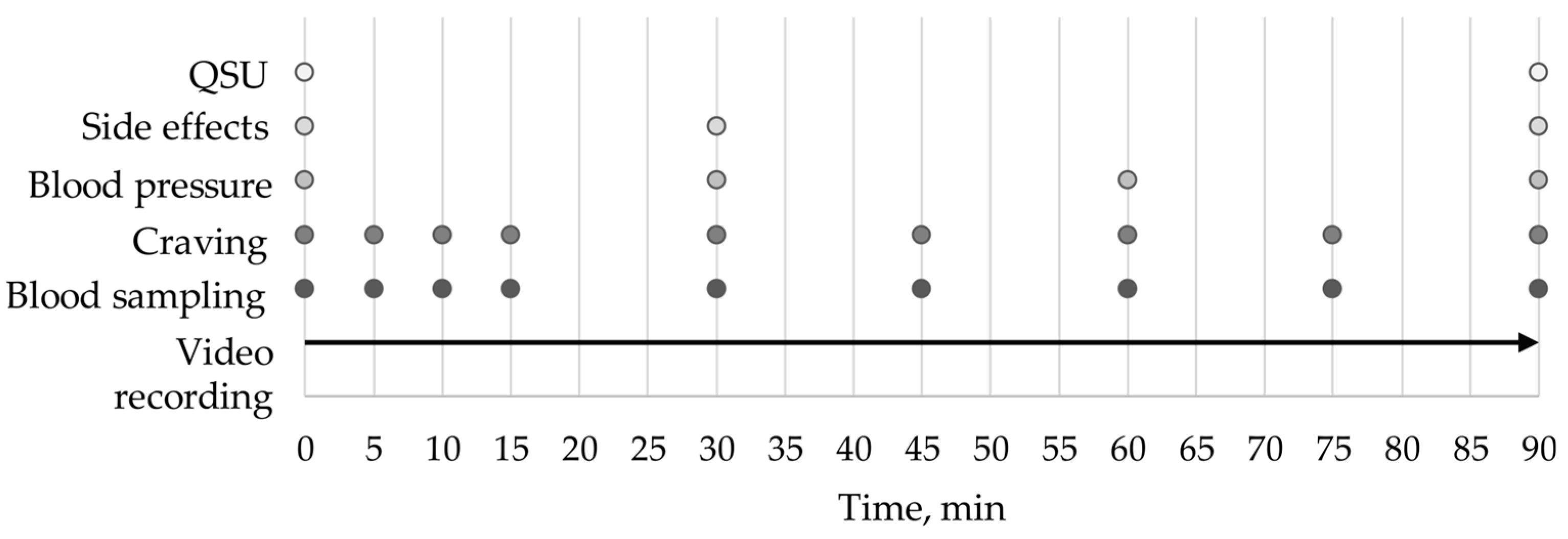
2.4. Study Design and Questionnaires
2.5. Blood Sampling and Determination of Nictoine, Cotinine, and Hydroxycotinine Plasma Levels
2.6. Puffing Behaviour
2.7. Pharmacokinetic (PK) Parameters and Statistical Analysis
3. Results
3.1. Participants
3.2. Usage Behavior
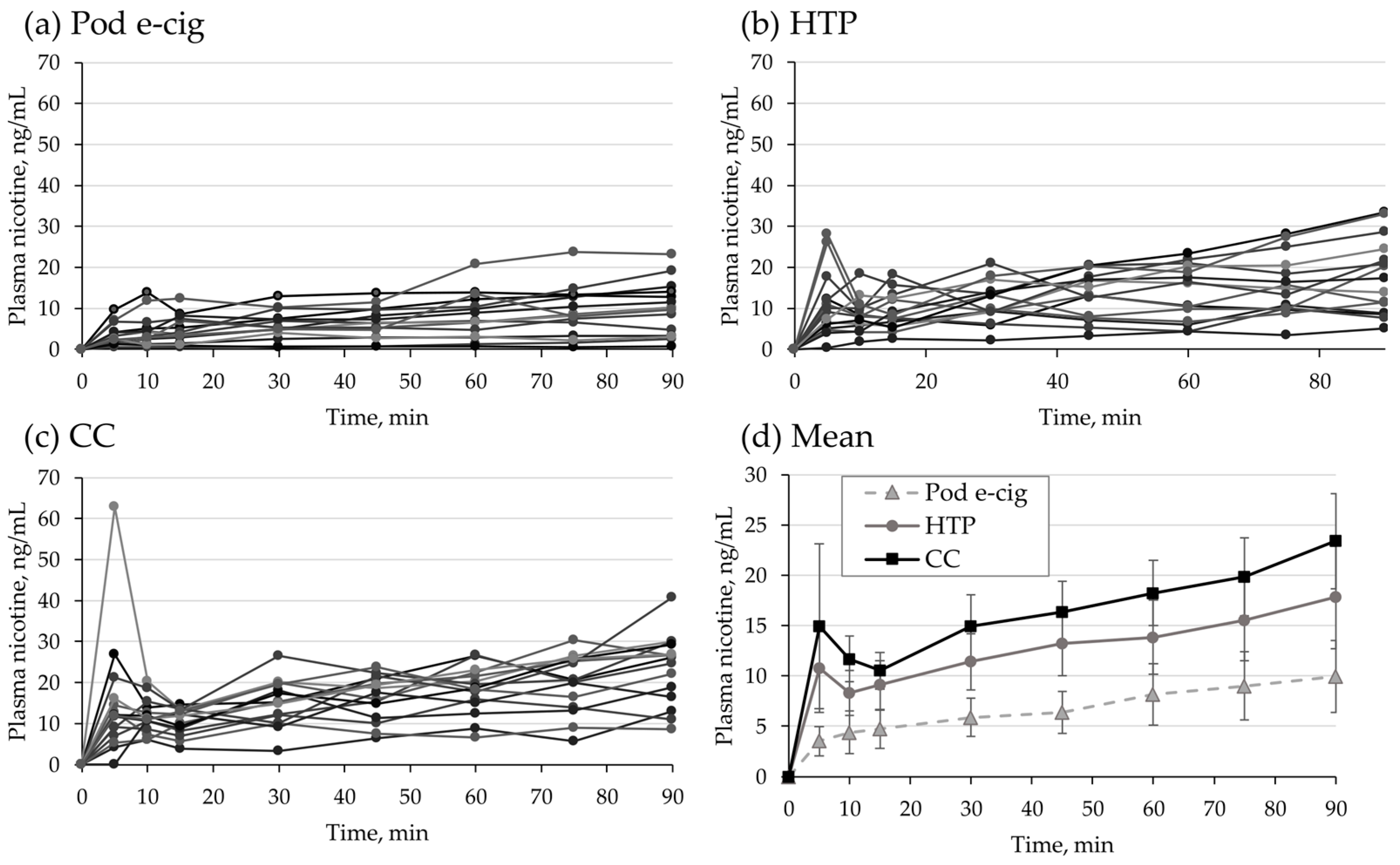
3.3. Nicotine Delivery
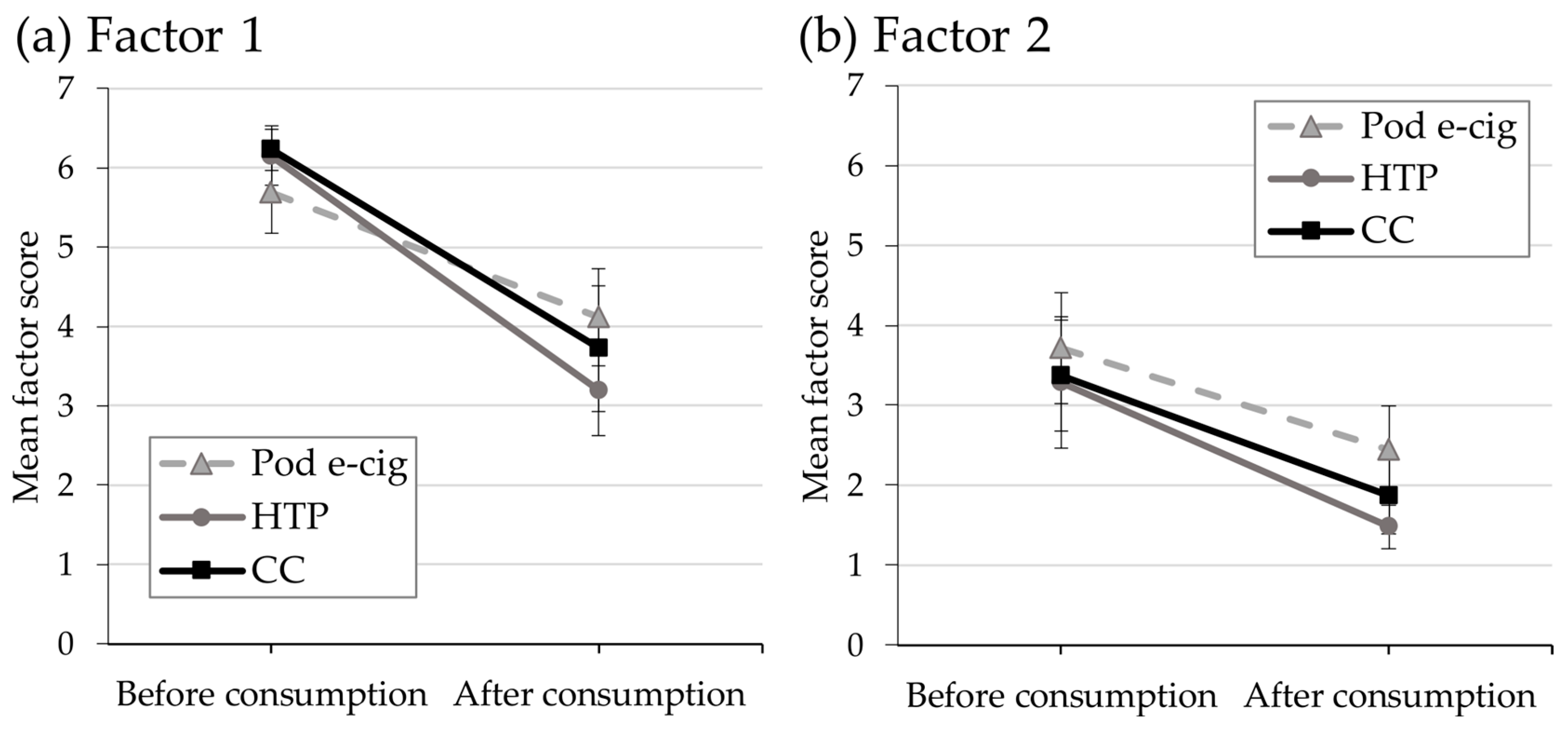
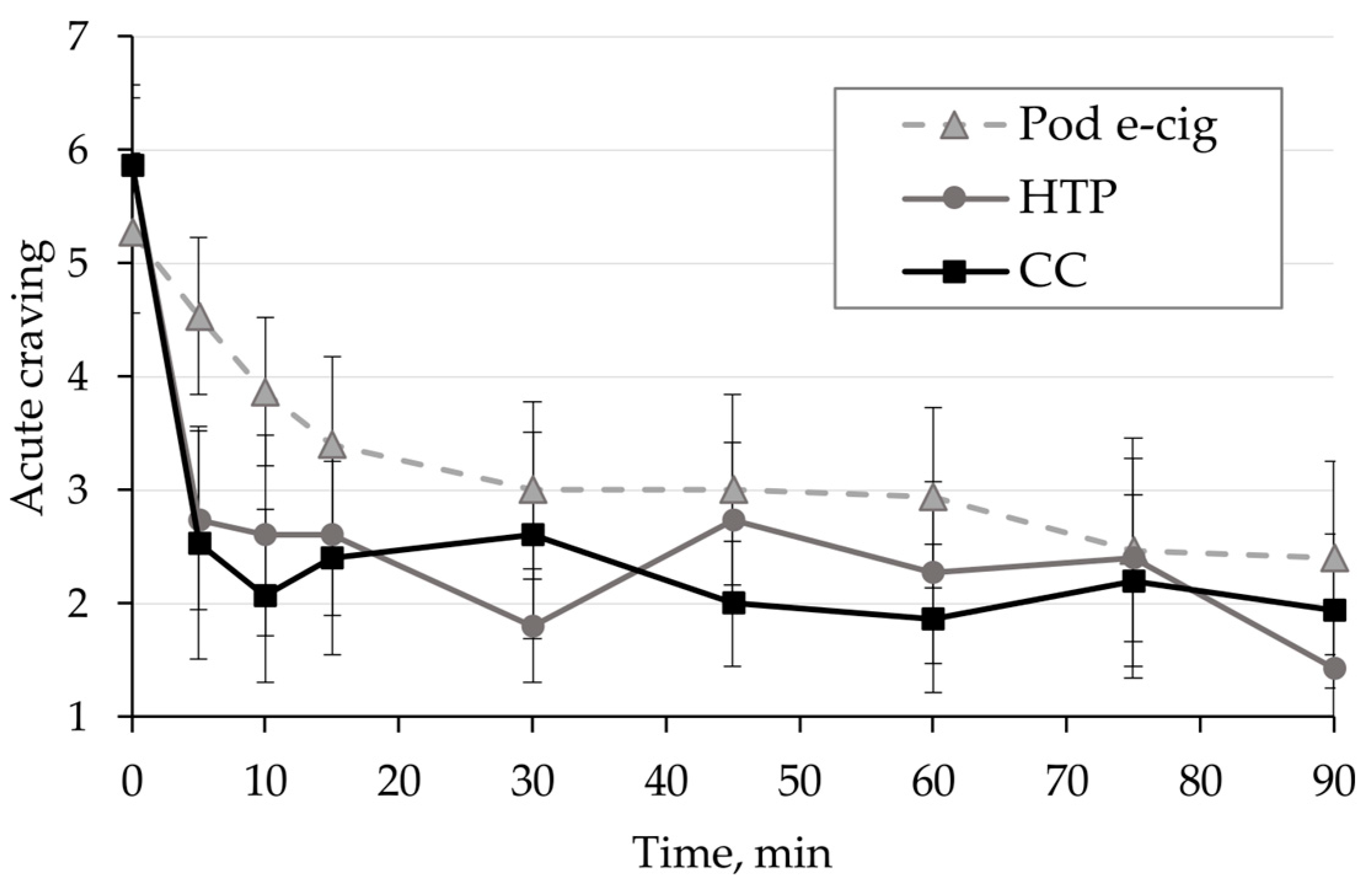
3.4. Relief of Craving and Urges to Use the Product
3.5. Side Effects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AlMulla, A.; Hassan-Yassoub, N.; Fu, D.; El-Awa, F.; Alebshehy, R.; Ismail, M.; Fraser, C.P. Smoking cessation services in the Eastern Mediterranean Region: Highlights and findings from the WHO Report on the Global Tobacco Epidemic 2019. East. Mediterr. Health J. 2020, 26, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A.J.; Shopland, D.R.; Cummings, K.M. The 2014 Surgeon General’s report: Commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am. J. Epidemiol. 2014, 179, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Bartal, M. Health effects of tobacco use and exposure. Monaldi Arch. Chest Dis. 2001, 56, 545–554. [Google Scholar]
- Sherman, C.B. Health effects of cigarette smoking. Clin. Chest Med. 1991, 12, 643–658. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.A. The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Periodontol. 2000 2022, 90, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, M.; Gasparro, R.; D′Ambrosio, F.; Pisano, M.; Di Palo, M.P.; Contaldo, M. Smoking Cessation on Periodontal and Peri-Implant Health Status: A Systematic Review. Dent. J. 2022, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Mackenbach, J.P.; Damhuis, R.A.; Been, J.V. The effects of smoking on health: Growth of knowledge reveals even grimmer picture. Ned. Tijdschr. Geneeskd. 2017, 160, D869. [Google Scholar]
- Lavacchi, D.; Roviello, G.; Rodriquenz, M.G. Electronic nicotine delivery systems (ENDS): Not still ready to put on END. J. Thorac. Dis. 2020, 12, 3857–3865. [Google Scholar] [CrossRef]
- Rüther, T.; Hagedorn, D.; Schiela, K.; Schettgen, T.; Osiander-Fuchs, H.; Schober, W. Nicotine delivery efficiency of first- and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int. J. Hyg. Environ. Health 2018, 221, 191–198. [Google Scholar] [CrossRef]
- de Wit, H.; Bodker, B.; Ambre, J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology 1992, 107, 352–358. [Google Scholar] [CrossRef]
- Henningfield, J.E.; Keenan, R.M. Nicotine delivery kinetics and abuse liability. J. Consult. Clin. Psychol. 1993, 61, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, L.; Cox, S.; Goniewicz, M.; McRobbie, H.; Kimber, C.; Doig, M.; Kośmider, L. ‘Real-world’ compensatory behaviour with low nicotine concentration e-liquid: Subjective effects and nicotine, acrolein and formaldehyde exposure. Addiction 2018, 113, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Kosmider, L.; Kimber, C.F.; Kurek, J.; Corcoran, O.; Dawkins, L.E. Compensatory Puffing With Lower Nicotine Concentration E-liquids Increases Carbonyl Exposure in E-cigarette Aerosols. Nicotine Tob. Res. 2018, 20, 998–1003. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public Health Consequences of E-Cigarettes; Eaton, D.L., Kwan, L.Y., Stratton, K., Eds.; National Academies Press (US): Washington, DC, USA, 2018.
- Wang, T.W.; Gentzke, A.S.; Creamer, M.R.; Cullen, K.A.; Holder-Hayes, E.; Sawdey, M.D.; Anic, G.M.; Portnoy, D.B.; Hu, S.; Homa, D.M.; et al. Tobacco Product Use and Associated Factors Among Middle and High School Students—United States, 2019. MMWR Surveill. Summ. 2019, 68, 1–22. [Google Scholar] [CrossRef]
- Czaplicki, L.; Kostygina, G.; Kim, Y.; Perks, S.N.; Szczypka, G.; Emery, S.L.; Vallone, D.; Hair, E.C. Characterising JUUL-related posts on Instagram. Tobacco Control 2020, 29, 612. [Google Scholar] [CrossRef] [PubMed]
- Ickes, M.; Hester, J.W.; Wiggins, A.T.; Rayens, M.K.; Hahn, E.J.; Kavuluru, R. Prevalence and reasons for Juul use among college students. J. Am. Coll. Health 2020, 68, 455–459. [Google Scholar] [CrossRef]
- Kavuluru, R.; Han, S.; Hahn, E.J. On the popularity of the USB flash drive-shaped electronic cigarette Juul. Tobacco Control 2019, 28, 110. [Google Scholar] [CrossRef]
- Ramamurthi, D.; Chau, C.; Jackler, R.K. JUUL and other stealth vaporisers: Hiding the habit from parents and teachers. Tobacco Control 2019, 28, 610. [Google Scholar] [CrossRef]
- Hajek, P.; Pittaccio, K.; Pesola, F.; Myers Smith, K.; Phillips-Waller, A.; Przulj, D. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e-cigarette products. Addiction 2020, 115, 1141–1148. [Google Scholar] [CrossRef]
- Mallock, N.; Rabenstein, A.; Gernun, S.; Laux, P.; Hutzler, C.; Karch, S.; Koller, G.; Henkler-Stephani, F.; Parr, M.K.; Pogarell, O.; et al. Nicotine delivery and relief of craving after consumption of European JUUL e-cigarettes prior and after pod modification. Sci. Rep. 2021, 11, 12078. [Google Scholar] [CrossRef]
- St Helen, G.; Ross, K.C.; Dempsey, D.A.; Havel, C.M.; Jacob, P., 3rd; Benowitz, N.L. Nicotine Delivery and Vaping Behavior During ad Libitum E-cigarette Access. Tob. Regul. Sci. 2016, 2, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Mallock, N.; Pieper, E.; Hutzler, C.; Henkler-Stephani, F.; Luch, A. Heated Tobacco Products: A Review of Current Knowledge and Initial Assessments. Front. Public. Health 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Vukas, J.; Mallock-Ohnesorg, N.; Rüther, T.; Romano-Brandt, L.; Stoll, Y.; Hoehne, L.; Laux, P.; Luch, A.; Rabenstein, A. Two different Heated Tobacco Products vs. cigarette: Comparison of nicotin delivery and subjective effects in experiences users. 2023; submitted. [Google Scholar]
- Mallock, N.; Rabenstein, A.; Laux, P.; Rüther, T.; Hutzler, C.; Parr, M.K.; Luch, A. Rapid, sensitive, and reliable quantitation of nicotine and its main metabolites cotinine and trans-3′-hydroxycotinine by LC-MS/MS: Method development and validation for human plasma. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2021, 1179, 122736. [Google Scholar] [CrossRef] [PubMed]
- Mallock, N.; Trieu, H.L.; Macziol, M.; Malke, S.; Katz, A.; Laux, P.; Henkler-Stephani, F.; Hahn, J.; Hutzler, C.; Luch, A. Trendy e-cigarettes enter Europe: Chemical characterization of JUUL pods and its aerosols. Arch. Toxicol. 2020, 94, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Bekki, K.; Inaba, Y.; Uchiyama, S.; Kunugita, N. Comparison of Chemicals in Mainstream Smoke in Heat-not-burn Tobacco and Combustion Cigarettes. J. UOEH 2017, 39, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.T.; Mehta, N.Y.; Sweeney, C.T.; Schwartz, S.S.; Vogler, G.P.; Jarvis, M.J.; West, R.J. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob. Control 1998, 7, 369–375. [Google Scholar] [CrossRef]
- Fagerstrom, K.O.; Schneider, N.G. Measuring nicotine dependence: A review of the Fagerstrom Tolerance Questionnaire. J. Behav. Med. 1989, 12, 159–182. [Google Scholar] [CrossRef]
- Müller, V.; Mucha, R.; Ackermann, K.; Pauli, P. Die Erfassung des Cravings bei Rauchern mit einer deutschen Version des “Questionaire on Smoking Urges” (QSU-G). Z. Für Klin. Psychol. Psychother. 2001, 30, 164–171. [Google Scholar] [CrossRef]
- Tiffany, S.T.; Drobes, D.J. The development and initial validation of a questionnaire on smoking urges. Br. J. Addict. 1991, 86, 1467–1476. [Google Scholar] [CrossRef]
- Allenby, C.E.; Boylan, K.A.; Lerman, C.; Falcone, M. Precision Medicine for Tobacco Dependence: Development and Validation of the Nicotine Metabolite Ratio. J. Neuroimmune Pharmacol. 2016, 11, 471–483. [Google Scholar] [CrossRef]
- Dempsey, D.; Tutka, P.; Jacob, P., 3rd; Allen, F.; Schoedel, K.; Tyndale, R.F.; Benowitz, N.L. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin. Pharmacol. Ther. 2004, 76, 64–72. [Google Scholar] [CrossRef]
- Spindle, T.R.; Breland, A.B.; Karaoghlanian, N.V.; Shihadeh, A.L.; Eissenberg, T. Preliminary results of an examination of electronic cigarette user puff topography: The effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob. Res. 2015, 17, 142–149. [Google Scholar] [CrossRef]
- Maloney, S.; Eversole, A.; Crabtree, M.; Soule, E.; Eissenberg, T.; Breland, A. Acute effects of JUUL and IQOS in cigarette smokers. Tobacco Control 2021, 30, 449. [Google Scholar] [CrossRef]
- Behar, R.Z.; Hua, M.; Talbot, P. Puffing topography and nicotine intake of electronic cigarette users. PLoS ONE 2015, 10, e0117222. [Google Scholar] [CrossRef]
- Hua, M.; Yip, H.; Talbot, P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob. Control 2013, 22, 103–106. [Google Scholar] [CrossRef]
- DeVito, E.E.; Krishnan-Sarin, S. E-cigarettes: Impact of E-Liquid Components and Device Characteristics on Nicotine Exposure. Curr. Neuropharmacol. 2018, 16, 438–459. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Romagna, G.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities’ regulation. Int. J. Environ. Res. Public. Health 2013, 10, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, A.M.; Madden, D.R.; Peraza, N.; Schiff, S.J.; Lebovitz, L.; Whitted, L.; Barrington-Trimis, J.; Mason, T.B.; Anderson, M.K.; Tackett, A.P. Effect of Exposure to e-Cigarettes With Salt vs Free-Base Nicotine on the Appeal and Sensory Experience of Vaping: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2032757. [Google Scholar] [CrossRef] [PubMed]
- Duell, A.K.; Pankow, J.F.; Peyton, D.H. Free-Base Nicotine Determination in Electronic Cigarette Liquids by (1)H NMR Spectroscopy. Chem. Res. Toxicol. 2018, 31, 431–434. [Google Scholar] [CrossRef]
- Gholap, V.V.; Kosmider, L.; Golshahi, L.; Halquist, M.S. Nicotine forms: Why and how do they matter in nicotine delivery from electronic cigarettes? Expert. Opin. Drug. Deliv. 2020, 17, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Picavet, P.; Haziza, C.; Lama, N.; Weitkunat, R.; Lüdicke, F. Comparison of the Pharmacokinetics of Nicotine Following Single and Ad Libitum Use of a Tobacco Heating System or Combustible Cigarettes. Nicotine Tob. Res. 2016, 18, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Scherer, G.; Lee, P.N. Smoking behaviour and compensation: A review of the literature with meta-analysis. Regul. Toxicol. Pharmacol. 2014, 70, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Lüdicke, F.; Baker, G.; Magnette, J.; Picavet, P.; Weitkunat, R. Reduced Exposure to Harmful and Potentially Harmful Smoke Constituents With the Tobacco Heating System 2.1. Nicotine Tob. Res. 2017, 19, 168–175. [Google Scholar] [CrossRef]
- Haziza, C.; de La Bourdonnaye, G.; Skiada, D.; Ancerewicz, J.; Baker, G.; Picavet, P.; Lüdicke, F. Evaluation of the Tobacco Heating System 2.2. Part 8: 5-Day randomized reduced exposure clinical study in Poland. Regul. Toxicol. Pharmacol. 2016, 81 (Suppl. S2), S139–S150. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Hall, S.M.; Herning, R.I.; Jacob, P., 3rd; Jones, R.T.; Osman, A.L. Smokers of low-yield cigarettes do not consume less nicotine. N. Engl. J. Med. 1983, 309, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Darby, T.D.; McNamee, J.E.; van Rossum, J.M. Cigarette smoking pharmacokinetics and its relationship to smoking behaviour. Clin. Pharmacokinet. 1984, 9, 435–449. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Kuyt, F.; Jacob, P., 3rd. Circadian blood nicotine concentrations during cigarette smoking. Clin. Pharmacol. Ther. 1982, 32, 758–764. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Jacob, P., 3rd. Effects of cigarette smoking and carbon monoxide on nicotine and cotinine metabolism. Clin. Pharmacol. Ther. 2000, 67, 653–659. [Google Scholar] [CrossRef]

| Pod E-Cig Group | HTP Group | CC Group | |
|---|---|---|---|
| Recruited | 19 | 24 | 30 |
| Included | 16 | 18 | 18 |
| Drop-out | 1 | 3 | 3 |
| Tested | 15 | 15 | 15 |
| Age | 25.7 (9.5) | 27.1 (8.5) | 26.7 (7.5) |
| Sex, female, n (%) | 6 (40) | 7 (47) | 7 (47) |
| Sex, male, n (%) | 9 (60) | 8 (53) | 8 (53) |
| FTND | 5.2 * (3.5) | 4.4 * (1) | 4.3 (2.5) |
| NMR | 0.38 (0.19) | 0.38 (0.53) | 0.36 (0.54) |
| Days of product use in past 30 days | 29.7 (0) | 29.9 (0) | 29.1 (0) |
| Pod E-Cig | HTP | CC | Pod E-Cig vs. HTP | Pod E-Cig vs. CC | HTP vs. CC | |
|---|---|---|---|---|---|---|
| Cmax (ng/mL) | 8.0 (136%) | 17.7 (66%) | 24.0 (45%) | p = 0.018 ** | p < 0.001 *** | p = 0.130 |
| AUC0–90min (ng/mL * h) | 8.3 (126%) | 17.3 (68%) | 24.8 (51%) | p = 0.014 ** | p < 0.001 *** | p = 0.226 |
| tmax (min) | 90 (5–90) | 75 (5–90) | 75 (5–90) | p = 2.2 | p = 1.1 | p = 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabenstein, A.; Rahofer, A.; Vukas, J.; Rieder, B.; Störzenhofecker, K.; Stoll, Y.; Burgmann, N.; Pieper, E.; Laux, P.; Luch, A.; et al. Usage Pattern and Nicotine Delivery during Ad Libitum Consumption of Pod E-Cigarettes and Heated Tobacco Products. Toxics 2023, 11, 434. https://doi.org/10.3390/toxics11050434
Rabenstein A, Rahofer A, Vukas J, Rieder B, Störzenhofecker K, Stoll Y, Burgmann N, Pieper E, Laux P, Luch A, et al. Usage Pattern and Nicotine Delivery during Ad Libitum Consumption of Pod E-Cigarettes and Heated Tobacco Products. Toxics. 2023; 11(5):434. https://doi.org/10.3390/toxics11050434
Chicago/Turabian StyleRabenstein, Andrea, Anna Rahofer, Jochen Vukas, Benedikt Rieder, Kristin Störzenhofecker, Yvonne Stoll, Nestor Burgmann, Elke Pieper, Peter Laux, Andreas Luch, and et al. 2023. "Usage Pattern and Nicotine Delivery during Ad Libitum Consumption of Pod E-Cigarettes and Heated Tobacco Products" Toxics 11, no. 5: 434. https://doi.org/10.3390/toxics11050434
APA StyleRabenstein, A., Rahofer, A., Vukas, J., Rieder, B., Störzenhofecker, K., Stoll, Y., Burgmann, N., Pieper, E., Laux, P., Luch, A., Rüther, T., & Mallock-Ohnesorg, N. (2023). Usage Pattern and Nicotine Delivery during Ad Libitum Consumption of Pod E-Cigarettes and Heated Tobacco Products. Toxics, 11(5), 434. https://doi.org/10.3390/toxics11050434





