Subacute Exposure to Low Pb Doses Promotes Oxidative Stress in the Kidneys and Copper Disturbances in the Liver of Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
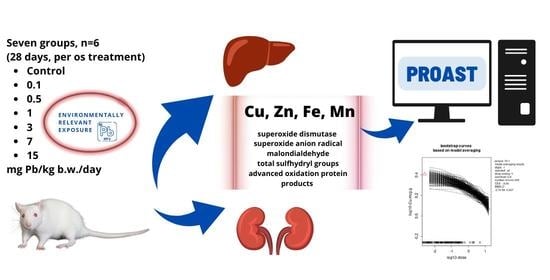
2.2. Animals and Experimental Study Design
2.3. Metal Analyses
2.4. Oxidative Status Analyses
2.5. Benchmark Dose Modeling
2.6. Statistical Analyses
3. Results
3.1. Relative Organ Weight
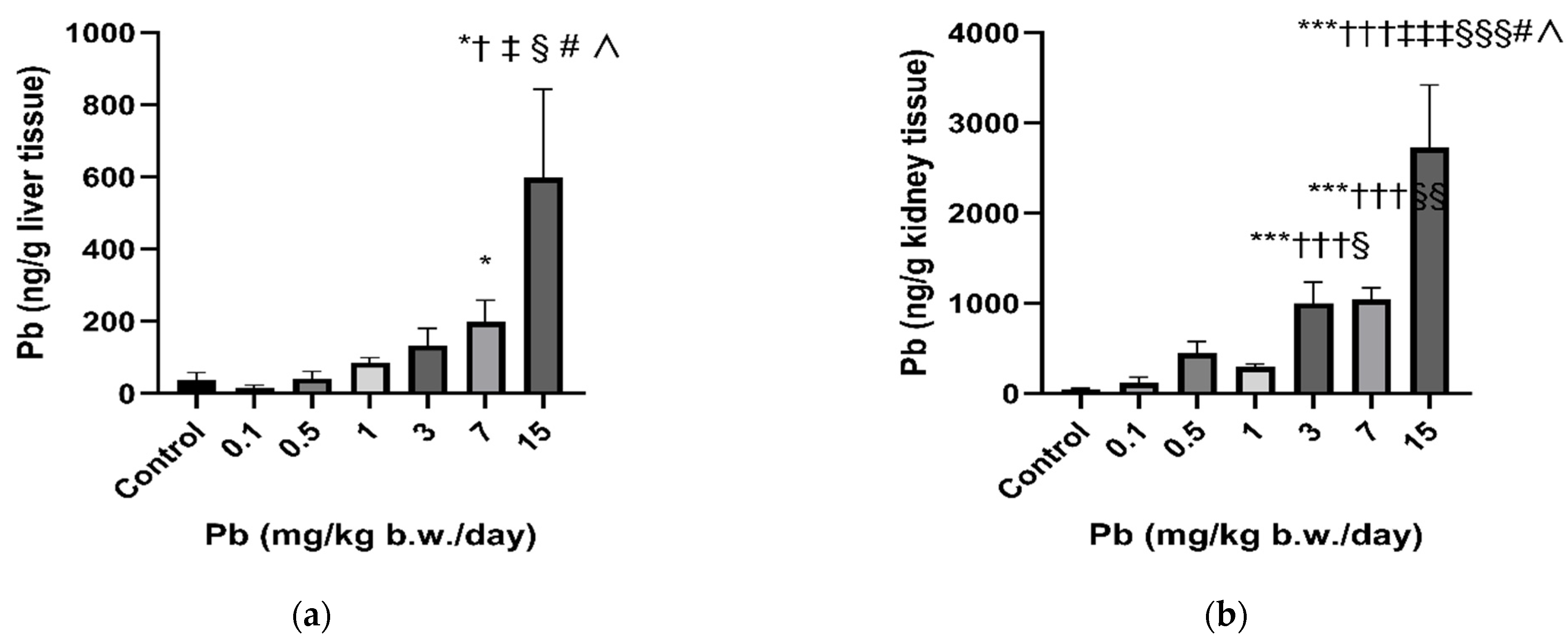
3.2. Lead and Essential Metal Status in Rats’ Liver and Kidneys
3.3. Oxidative Status in Rats’ Liver and Kidney Tissues
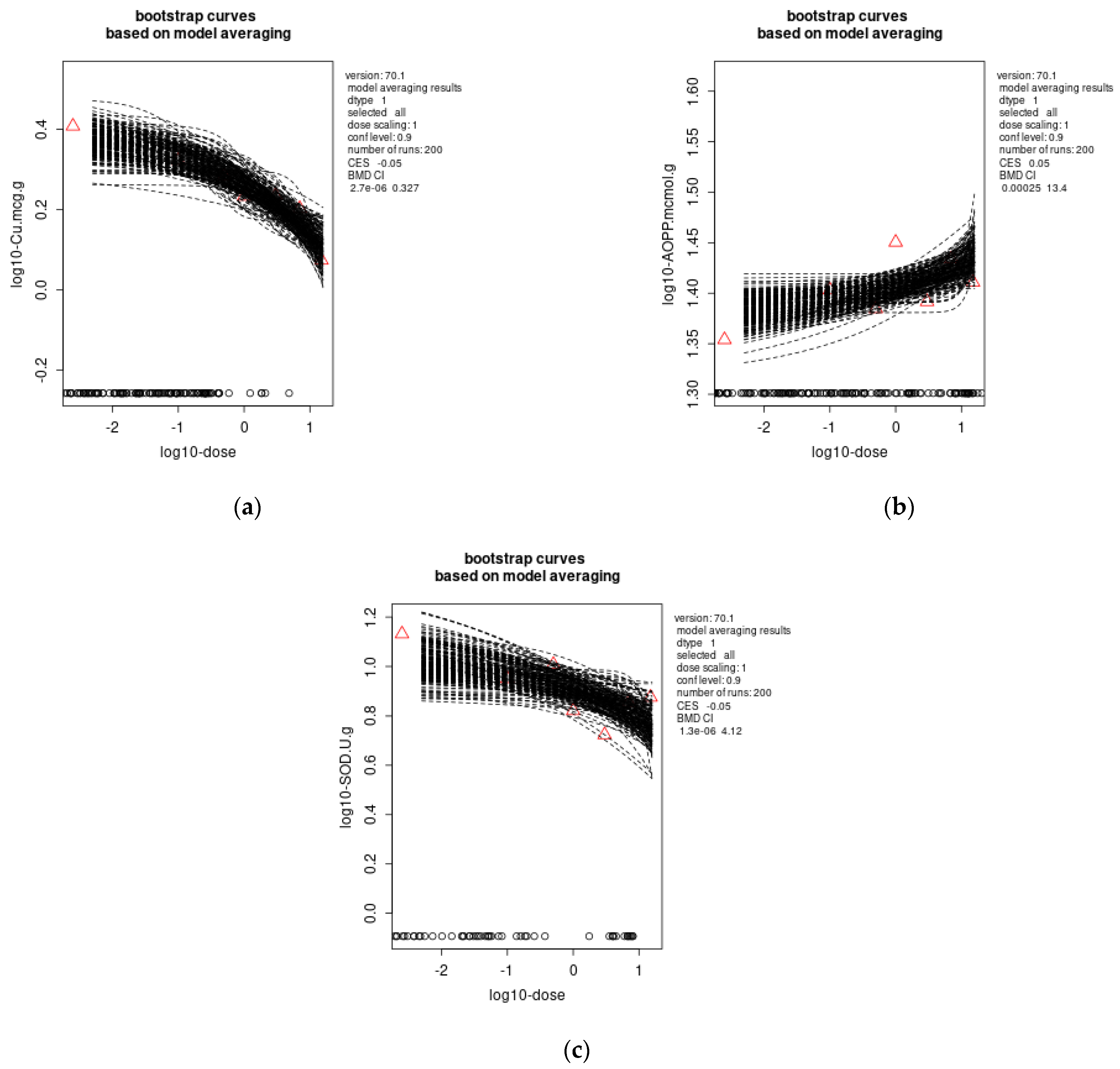
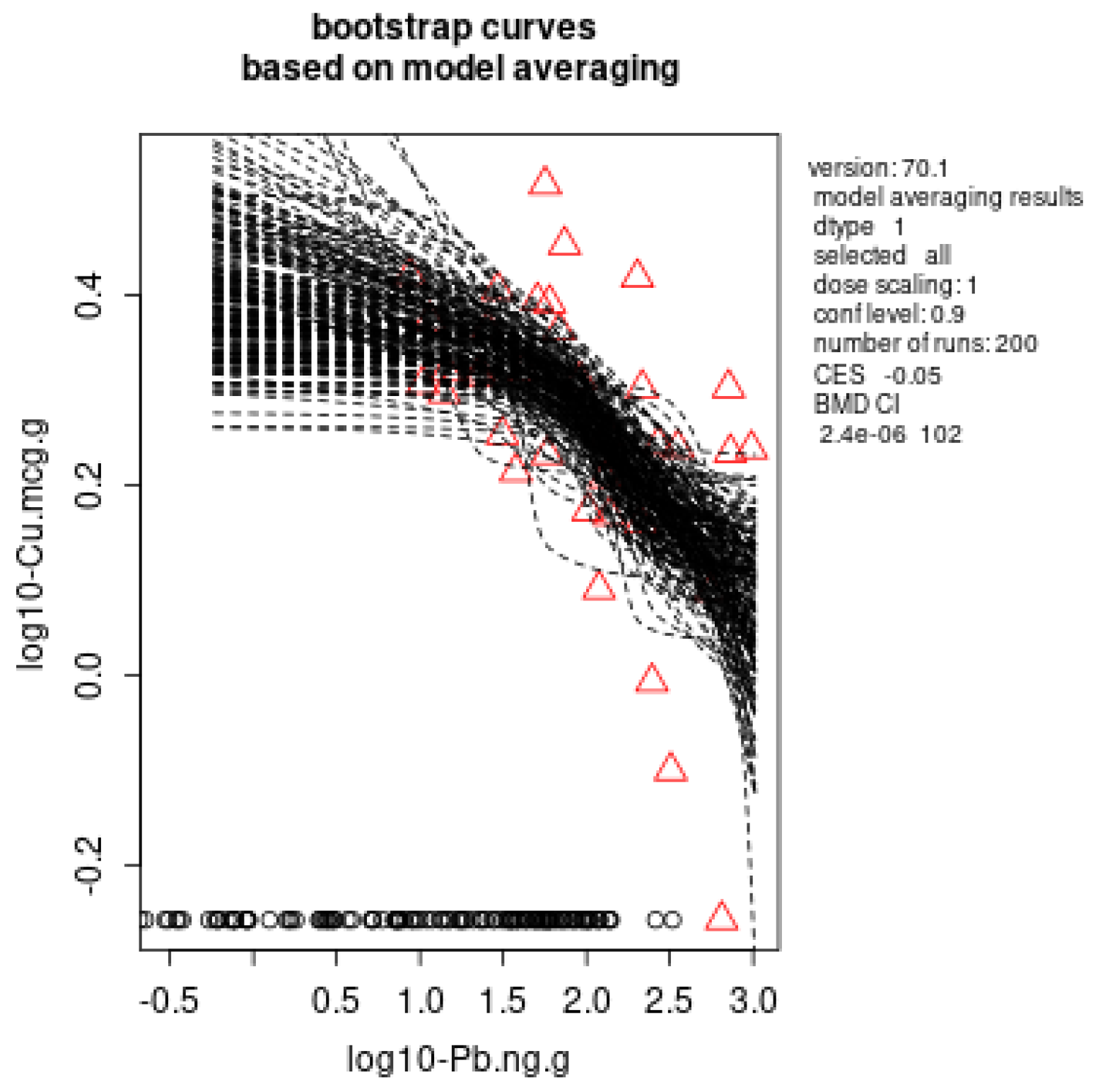
3.4. Benchmark Dose Modelling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; Gopalakrishnan, A.V. Heavy metal and metalloid—Induced reproductive toxicity. Environ. Toxicol. Pharmacol. 2022, 92, 103859. [Google Scholar] [CrossRef] [PubMed]
- Javorac, D.; Anđelković, M.; Repić, A.; Tatović, S.; Djordjevic, A.B.; Miljaković, E.A.; Batinić, B.; Boričić, N.; Đukić-Ćosić, D.; Antonijević, B.; et al. Comprehensive insight into the neurotoxic mechanisms of low dose Pb exposure in Wistar rats: Benchmark dose analysis. Chem.-Biol. Interact. 2022, 360, 109932. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Patrick, L. Lead toxicity, a review of the literature. Part I: Exposure, evaluation, and treatment. Altern. Med. Rev. 2006, 11, 2–22. [Google Scholar] [PubMed]
- Huang, Z.; Yin, X.-C.; Chen, M.; Li, M.-L.; Chen, B.; Hu, Y.-M. Relationships Between Dietary Patterns and Low-Level Lead Exposure Among Children from Hunan Province of China. Expo. Health 2021, 14, 531–541. [Google Scholar] [CrossRef]
- Abdelhamid, F.M.; Mahgoub, H.A.; Ateya, A.I. Ameliorative effect of curcumin against lead acetate–induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ. Sci. Pollut. Res. 2020, 27, 10950–10965. [Google Scholar] [CrossRef]
- Cherkani-Hassani, A.; Slaoui, M.; Ghanname, I.; Mojemmi, B.; Eljaoudi, R.; Belhaj, A.; Kers, B.; Flayou, M.; Mouane, N. Lead concentrations in breast milk of Moroccan nursing mothers and associated factors of exposure: CONTAMILK STUDY. Environ. Toxicol. Pharmacol. 2021, 85, 103629. [Google Scholar] [CrossRef] [PubMed]
- ATSDR Toxicological profile for Lead; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2020.
- Mostafalou, S.; Baeeri, M.; Bahadar, H.; Soltany-Rezaee-Rad, M.; Gholami, M.; Abdollahi, M. Molecular mechanisms involved in lead induced disruption of hepatic and pancreatic glucose metabolism. Environ. Toxicol. Pharmacol. 2014, 39, 16–26. [Google Scholar] [CrossRef]
- Nolan, C.; Shaikh, Z. Lead nephrotoxicity and associated disorders: Biochemical mechanisms. Toxicology 1992, 73, 127–146. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Lundh, T.; Mölne, J. Low-level exposure to lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ. Res. 2022, 211, 113119. [Google Scholar] [CrossRef]
- Rastogi, S.K. Renal effects of environmental and occupational lead exposure. Indian J. Occup. Environ. Med. 2008, 12, 103–106. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Dukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Goyer, R.A. Toxic and Essential Metal Interactions. Annu. Rev. Nutr. 1997, 17, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Bressler, J.P.; Goldstein, G.W. Mechanisms of lead neurotoxicity. Biochem. Pharmacol. 1991, 41, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Kwon, S.-Y.; Bae, O.-N.; Noh, J.-Y.; Kim, K.; Kang, S.; Shin, Y.-J.; Lim, K.-M.; Chung, J.-H. Erythrophagocytosis of Lead-Exposed Erythrocytes by Renal Tubular Cells: Possible Role in Lead-Induced Nephrotoxicity. Environ. Health Perspect. 2015, 123, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Trujillo, K.A. Neurotoxicity of low-level lead exposure: History, mechanisms of action, and behavioral effects in humans and preclinical models. Neurotoxicology 2019, 73, 58–80. [Google Scholar] [CrossRef]
- Hernberg, S. Lead poisoning in a historical perspective. Am. J. Ind. Med. 2000, 38, 244–254. [Google Scholar] [CrossRef]
- CDC. What Do Parents Need to Know to Protect Their Children? 2017. Available online: http://www.cdc.gov/nceh/lead/ACCLPP/blood_lead_levels.htm (accessed on 10 May 2020).
- Becker, F.; Marcantonio, F.; Datta, S.; Wichterich, C.; Cizmas, L.; Surber, J.; Kennedy, K.; Bowles, E. Tracking the source of contaminant lead in children’s blood. Environ. Res. 2022, 212, 113307. [Google Scholar] [CrossRef]
- Korchevskiy, A. Using benchmark dose modeling for the quantitative risk assessment: Carbon nanotubes, asbestos, glyphosate. J. Appl. Toxicol. 2020, 41, 148–160. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, K.H.; More, S.; Mortensen, A.; Naegeli, H.; Noteborn, H.; et al. Update: Use of the benchmark dose approach in risk assessment. EFSA J. 2017, 15, e04658. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, N.-H.; Chung, S.-H.; Chen, S.-C.; Chen, W.-Y.; Cheng, Y.-H.; Lin, Y.-J.; You, S.-H.; Liao, C.-M. Anemia risk in relation to lead exposure in lead-related manufacturing. BMC Public Health 2017, 17, 389. [Google Scholar] [CrossRef]
- Zubero, M.B.; Aurrekoetxea, J.J.; Ibarluzea, J.M.; Arenaza, M.J.; Rodríguez, C.; Sáenz, J.R. Heavy metal levels (Pb, Cd, Cr and Hg) in the adult general population near an urban solid waste incinerator. Sci. Total Environ. 2010, 408, 4468–4474. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.O.; Schwartz, J.; Wright, R.J.; Bollati, V.; Tarantini, L.; Park, S.K.; Hu, H.; Sparrow, D.; Vokonas, P.; Baccarelli, A. Biomarkers of Lead Exposure and DNA Methylation within Retrotransposons. Environ. Health Perspect. 2010, 118, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.A. Handbook Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Girotti, M.J.; Khan, N.; Mclellan, B.A. Early Measurement of Systemic Lipid Peroxidation Products in the Plasma of Major Blunt Trauma Patients. J. Trauma: Inj. Infect. Crit. Care 1991, 31, 32–35. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Haber, L.T.; Dourson, M.L.; Allen, B.C.; Hertzberg, R.C.; Parker, A.; Vincent, M.J.; Maier, A.; Boobis, A.R. Benchmark dose (BMD) modeling: Current practice, issues, and challenges. Crit. Rev. Toxicol. 2018, 48, 387–415. [Google Scholar] [CrossRef]
- Javorac, D.; Antonijević, B.; Anđelković, M.; Repić, A.; Bulat, P.; Djordjevic, A.B.; Baralić, K.; Đukić-Ćosić, D.; Antonić, T.; Bulat, Z. Oxidative stress, metallomics and blood toxicity after subacute low-level lead exposure in Wistar rats: Benchmark dose analyses. Environ. Pollut. 2021, 291, 118103. [Google Scholar] [CrossRef] [PubMed]
- Sallsten, G.; Ellingsen, D.G.; Berlinger, B.; Weinbruch, S.; Barregard, L. Variability of lead in urine and blood in healthy individuals. Environ. Res. 2022, 212, 113412. [Google Scholar] [CrossRef] [PubMed]
- Ademuyiwa, O.; Agarwal, R.; Chandra, R.; Behari, J.R. Effects of sub-chronic low-level lead exposure on the homeostasis of copper and zinc in rat tissues. J. Trace Elements Med. Biol. 2010, 24, 207–211. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Djordjevic, A.B.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Javorac, D.; Tatović, S.; Anđelković, M.; Repić, A.; Baralić, K.; Djordjevic, A.B.; Mihajlović, M.; Stevuljević, J.K.; Đukić-Ćosić, D.; Ćurčić, M.; et al. Low-lead doses induce oxidative damage in cardiac tissue: Subacute toxicity study in Wistar rats and Benchmark dose modelling. Food Chem. Toxicol. 2022, 161, 112825. [Google Scholar] [CrossRef]
- Mudipalli, A. Lead hepatotoxicity & potential health effects. Indian J. Med. Res. 2007, 126, 518–527. [Google Scholar] [PubMed]
- Morrell, A.; Tallino, S.; Yu, L.; Burkhead, J.L. The role of insufficient copper in lipid synthesis and fatty-liver disease. IUBMB Life 2017, 69, 263–270. [Google Scholar] [CrossRef]
- Sternlieb, I. Copper and the Liver. Gastroenterology 1980, 78, 1615–1628. [Google Scholar] [CrossRef]
- Klauder, D.S.; Petering, H.G. Anemia of Lead Intoxication: A Role for Copper. J. Nutr. 1977, 107, 1779–1785. [Google Scholar] [CrossRef]
- Klevay, L.M. Copper Deficiency, Lead, and Paraoxonase. Environ. Health Perspect. 2007, 115, A341–A342. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Coulombe, R.A.; Sharma, R.P.; Tandon, S.K. Influence of dietary protein deficiency on lead-copper interaction in rats. Ecotoxicol. Environ. Saf. 1989, 18, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Ouyang, X.; Jiang, Z.; Xie, Z.; Fan, L.; Zhu, D.; Li, L. Advanced oxidation protein products play critical roles in liver diseases. Eur. J. Clin. Investig. 2019, 49, e13098. [Google Scholar] [CrossRef]
- Jin, Y.; Liao, Y.; Lu, C.; Li, G.; Yu, F.; Zhi, X.; Xu, J.; Liu, S.; Liu, M.; Yang, J. Health effects in children aged 3–6 years induced by environmental lead exposure. Ecotoxicol. Environ. Saf. 2006, 63, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E. Indigofera oblongifolia Prevents Lead Acetate-Induced Hepatotoxicity, Oxidative Stress, Fibrosis and Apoptosis in Rats. PLoS ONE 2016, 11, e0158965. [Google Scholar] [CrossRef] [PubMed]
- Elrasoul, A.; Mousa, A.; Orabi, S.; Mohamed, M.; Gad-Allah, S.; Almeer, R.; Abdel-Daim, M.; Khalifa, S.; El-Seedi, H.; Eldaim, M. Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects of Azolla pinnata Ethanolic Extract against Lead-Induced Hepatotoxicity in Rats. Antioxidants 2020, 9, 1014. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, X.; Yu, J.; Xie, J.; Li, C.; Liu, D.; Tang, C.; Wang, C. Lead-induced oxidative damage in rats/mice: A meta-analysis. J. Trace Elements Med. Biol. 2019, 58, 126443. [Google Scholar] [CrossRef]
- Albarakati, A.J.A.; Baty, R.S.; Aljoudi, A.M.; Habotta, O.A.; Elmahallawy, E.K.; Kassab, R.B.; Moneim, A.E.A. Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol. Biol. Rep. 2020, 47, 2591–2603. [Google Scholar] [CrossRef]
- Baş, H.; Kalender, Y. Nephrotoxic effects of lead nitrate exposure in diabetic and nondiabetic rats: Involvement of oxidative stress and the protective role of sodium selenite. Environ. Toxicol. 2015, 31, 1229–1240. [Google Scholar] [CrossRef]
- Wang, T.; Tu, Y.; Zhang, G.; Gong, S.; Wang, K.; Zhang, Y.; Meng, Y.; Wang, T.; Li, A.; Christiani, D.C.; et al. Development of a benchmark dose for lead-exposure based on its induction of micronuclei, telomere length changes and hematological toxicity. Environ. Int. 2020, 145, 106129. [Google Scholar] [CrossRef]
- Javorac, D.; Baralić, K.; Marić, Đ.; Mandić-Rajčević, S.; Đukić-Ćosić, D.; Bulat, Z.; Djordjevic, A.B. Exploring the endocrine disrupting potential of lead through benchmark modelling—Study in humans. Environ. Pollut. 2023, 316, 120428. [Google Scholar] [CrossRef] [PubMed]

| Dose (mg Pb/kg b.w./Day) | Liver (g/100 g Body Weight) | Kidneys (g/100 g Body Weight) |
|---|---|---|
| Control | 4.13 ± 0.50 | 0.72 ± 0.06 |
| 0.1 | 4.29 ± 0.32 | 0.69 ± 0.08 |
| 0.5 | 4.26 ± 0.29 | 0.66 ± 0.05 |
| 1 | 4.17 ± 0.24 | 0.66 ± 0.04 |
| 3 | 4.04 ± 0.11 | 0.64 ± 0.04 |
| 7 | 4.01 ± 0.28 | 0.69 ± 0.06 |
| 15 | 4.50 ± 0.24 | 0.72 ± 0.03 |
| Liver | Dose (mg Pb/kg b.w./Day) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.1 | 0.5 | 1 | 3 | 7 | 15 | ||
| Cu | Mean | 2.58 | 2.11 | 1.94 * | 1.78 ** | 1.73 ** | 1.68 *** | 1.29 ***††‡ |
| µg/g | SD | 0.39 | 0.19 | 0.26 | 0.59 | 0.26 | 0.59 | 0.52 |
| Zn | Mean | 25.22 | 27.58 | 24.60 | 26.71 | 23.28 | 26.67 | 22.24 |
| µg/g | SD | 2.24 | 1.63 | 2.13 | 5.55 | 2.56 | 5.87 | 4.48 |
| Fe | Mean | 88.19 | 103.5 | 92.85 | 92.79 | 76.57 †† | 103.1 # | 86.89 |
| µg/g | SD | 8.12 | 13.01 | 17.74 | 23.04 | 8.89 | 13.83 | 19.49 |
| Mn | Mean | 4.21 | 4.77 | 2.07 † | 2.49 | 2.15 | 5.60 ‡§# | 2.70 ∧ |
| µg/g | SD | 1.38 | 1.22 | 1.19 | 1.52 | 1.37 | 2.42 | 1.19 |
| Kidney | Control | 0.1 | 0.5 | 1 | 3 | 7 | 15 | |
| Cu | Mean | 1.79 | 1.65 | 1.88 | 1.36 | 2.04 | 1.38 | 1.43 |
| µg/g | SD | 0.82 | 0.58 | 0.52 | 0.62 | 0.78 | 0.49 | 0.35 |
| Zn | Mean | 9.48 | 8.52 | 10.27 | 8.91 | 11.87 | 8.02 | 7.97 |
| µg/g | SD | 4.22 | 2.84 | 2.92 | 4.34 | 3.29 | 2.51 | 0.67 |
| Fe | Mean | 5.15 | 6.07 | 8.93 | 5.28 | 8.38 | 6.14 | 5.52 |
| µg/g | SD | 1.59 | 2.13 | 3.99 | 2.35 | 2.80 | 2.25 | 1.88 |
| Mn | Mean | 3.47 | 1.95 | 2.41 | 3.13 | 2.86 | 2.40 | 2.62 |
| µg/g | SD | 1.52 | 0.98 | 0.33 | 1.24 | 1.48 | 0.58 | 0.98 |
| Liver | Dose (mg Pb/kg b.w./Day) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | 0.1 | 0.5 | 1 | 3 | 7 | 15 | ||
| AOPP | Mean | 21.80 | 24.25 | 23.10 | 28.00 ***‡ | 24.60 # | 27.05 **‡ | 23.60 #∧ |
| (μmol/min/g) | SD | 2.41 | 2.81 | 2.85 | 3.14 | 0.98 | 1.94 | 1.34 |
| O2− | Mean | 32.89 | 36.47 | 36.51 | 34.90 | 38.07 | 33.88 | 39.05 |
| (μmol/min/g) | SD | 2.73 | 3.98 | 5.91 | 3.04 | 1.89 | 3.75 | 7.73 |
| SOD | Mean | 5.753 | 10.44 *** | 7.29 † | 7.30 † | 10.31 **‡§ | 7.84 † | 13.28 ***†‡§#∧ |
| (U/g) | SD | 2.63 | 3.26 | 2.92 | 0.94 | 2.37 | 0.55 | 0.44 |
| TOS | Mean | 0.21 | 0.15 * | 0.24 ††† | 0.17 ‡ | 0.22 ††§ | 0.16 *‡‡‡# | 0.23 ††§∧ |
| (μmol H2O2 Equiv./g) | SD | 0.04 | 0.04 | 0.03 | 0.03 | 0.03 | 0.04 | 0.06 |
| MDA | Mean | 0.090 | 0.100 | 0.095 | 0.110 | 0.110 | 0.095 | 0.115 |
| (μmol/g) | SD | 0.040 | 0.140 | 0.080 | 0.030 | 0.020 | 0.050 | 0.070 |
| SHG | Median | 21.80 | 24.25 | 23.10 | 28.00 ***‡ | 24.60 # | 27.05 **‡ | 23.60 #∧ |
| (mmol/g) | Range | 6.20–32.89 | 7.50–36.47 | 7.30–36.51 | 9.40–34.90 | 2.70–38.07 | 4.70–33.88 | 3.80–39.05 |
| KIDNEY | Control | 0.1 | 0.5 | 1 | 3 | 7 | 15 | |
| AOPP | Mean | 6.75 | 6.04 | 4.66 | 6.86 | 5.56 | 7.40 | 5.71 |
| (μmol/min/g) | SD | 1.21 | 1.26 | 0.817 | 0.74 | 1.06 | 2.20 | 1.57 |
| O2− | Mean | 34.91 | 35.24 | 33.61 | 27.13 **††‡ | 33.64 | 29.13 **†† | 33.87 §∧ |
| (μmol/min/g) | SD | 3.60 | 6.01 | 4.70 | 1.56 | 9.782 | 4.69 | 5.75 |
| SOD | Mean | 13.56 | 9.20 * | 10.64 | 7.20 ** | 7.18 ** | 7.45 ** | 8.10 ** |
| (U/g) | SD | 0.95 | 2.36 | 3.44 | 2.80 | 4.24 | 2.53 | 3.23 |
| TOS | Mean | 3.30 | 3.30 | 3.60 | 3.11 | 3.84 | 3.35 | 3.78 |
| (μmol H2O2 Equiv./g) | SD | 0.38 | 0.37 | 0.34 | 0.20 | 0.41 | 0.71 | 0.66 |
| MDA | Mean | 1.66 | 1.42 | 1.02 ** | 0.73 *** | 1.10 * | 0.69 *** | 1.14 * |
| (μmol/g) | SD | 0.32 | 0.37 | 0.23 | 0.55 | 0.31 | 0.14 | 0.22 |
| SHG | Median | 0.08 | 0.09 | 0.07 | 0.09 | 0.09 | 0.11 | 0.09 |
| (mmol/g) | Range | 0.07–0.10 | 0.06–0.11 | 0.05–0.08 | 0.07–0.13 | 0.06–0.09 | 0.10–0.19 | 0.05–0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vukelić, D.; Djordjevic, A.B.; Anđelković, M.; Antonijević Miljaković, E.; Baralić, K.; Živančević, K.; Bulat, P.; Radovanović, J.; Đukić-Ćosić, D.; Antonijević, B.; et al. Subacute Exposure to Low Pb Doses Promotes Oxidative Stress in the Kidneys and Copper Disturbances in the Liver of Male Rats. Toxics 2023, 11, 256. https://doi.org/10.3390/toxics11030256
Vukelić D, Djordjevic AB, Anđelković M, Antonijević Miljaković E, Baralić K, Živančević K, Bulat P, Radovanović J, Đukić-Ćosić D, Antonijević B, et al. Subacute Exposure to Low Pb Doses Promotes Oxidative Stress in the Kidneys and Copper Disturbances in the Liver of Male Rats. Toxics. 2023; 11(3):256. https://doi.org/10.3390/toxics11030256
Chicago/Turabian StyleVukelić, Dragana, Aleksandra Buha Djordjevic, Milena Anđelković, Evica Antonijević Miljaković, Katarina Baralić, Katarina Živančević, Petar Bulat, Jelena Radovanović, Danijela Đukić-Ćosić, Biljana Antonijević, and et al. 2023. "Subacute Exposure to Low Pb Doses Promotes Oxidative Stress in the Kidneys and Copper Disturbances in the Liver of Male Rats" Toxics 11, no. 3: 256. https://doi.org/10.3390/toxics11030256
APA StyleVukelić, D., Djordjevic, A. B., Anđelković, M., Antonijević Miljaković, E., Baralić, K., Živančević, K., Bulat, P., Radovanović, J., Đukić-Ćosić, D., Antonijević, B., & Bulat, Z. (2023). Subacute Exposure to Low Pb Doses Promotes Oxidative Stress in the Kidneys and Copper Disturbances in the Liver of Male Rats. Toxics, 11(3), 256. https://doi.org/10.3390/toxics11030256