A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution
Abstract
1. Introduction
2. Cr Removal Methods
2.1. Adsorption
2.1.1. Natural Adsorbents
2.1.2. Bio-Sorbents
Bacteria
Fungi
Algae
Plants
Agricultural Wastes
Composites
Metal–Organic Framework
Mesoporous Silica
Zeolites
2.2. Electrochemical Treatment
2.2.1. Electrocoagulation
Iron Electrodes
Aluminum Electrodes
2.2.2. Electro-Floatation
2.2.3. Electrochemical Reduction
Carbon-Based Electrode
Gold Electrodes
Conducting Polymers
2.2.4. Electrically-Driven Ion Transport
Electrodialysis and Electro-Electrodialysis
Electro-Deionization
2.3. Physico-Chemical Processes
2.3.1. Ion Exchange Method
2.3.2. Reduction Process
Chemical Reduction
- Sulfur Compounds
- 2.
- Iron Salts
Photocatalytic Reduction
- Organic Matter for Cr6+ Reduction
- 2.
- Fe (III) Photocatalytic Reduction of Cr6+ by Organic Acids
- 3.
- TiO2 Photocatalytic Reduction of Cr6+ by Organic Acids
- 4.
- Photocatalytic Reduction of Cr6+ by Ag/Ag3PO4/Reduced Graphene Oxide Microspheres
2.4. Biological Removal
2.4.1. Aerobic Cr6+ Reducing Bacteria
2.4.2. Anaerobic Cr6+ Reducing Bacteria
2.4.3. Cr6+ Reducing Fungi
2.5. Membrane Filtration Process
2.6. Chelation
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef]
- Chrysochoou, M.; Johnston, C.P.; Dahal, G. A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green-tea nanoscale zero-valent iron. J. Hazard. Mater. 2012, 201, 33–42. [Google Scholar] [CrossRef]
- Wittbrodt, P.R.; Palmer, C.D. Effect of temperature, ionic strength, background electrolytes, and Fe (III) on the reduction of hexavalent chromium by soil humic substances. Environ. Sci. Technol. 1996, 30, 2470–2477. [Google Scholar] [CrossRef]
- Ashraf, A.; Bibi, I.; Niazi, N.K.; Ok, Y.S.; Murtaza, G.; Shahid, M.; Kunhikrishnan, A.; Li, D.; Mahmood, T. Chromium (VI) sorption efficiency of acid-activated banana peel over organo-montmorillonite in aqueous solutions. Int. J. Phytoremediation 2017, 19, 605–613. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Bolan, N.; Wang, H. A critical review on bioremediation technologies for Cr (VI)-contaminated soils and wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1027–1078. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr (VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef]
- Antoniadis, V.; Zanni, A.A.; Levizou, E.; Shaheen, S.M.; Dimirkou, A.; Bolan, N.; Rinklebe, J. Modulation of hexavalent chromium toxicity on Origanum vulgare in an acidic soil amended with peat, lime, and zeolite. Chemosphere 2018, 195, 291–300. [Google Scholar] [CrossRef]
- Brown, T.; LeMay, H.; Wilson, R. Chemistry: The Central Science (No. QD 31.2. B76 1988); Prentice Hall: Hoboken, NJ, USA, 1988. [Google Scholar]
- Janus, J.; Krajnc, E. Integrated Criteria Document Chromium: Effects; National Institute of Public Health and Environmental Protection Bilthoven: Bilthoven, The Netherlands, 1990.
- Wallwork, G. The oxidation of alloys. Rep. Prog. Phys. 1976, 39, 401. [Google Scholar] [CrossRef]
- Sharma, S.K.; Petrusevski, B.; Amy, G. Chromium removal from water: A review. J. Water Supply Res. Technol.—AQUA 2008, 57, 541–553. [Google Scholar] [CrossRef]
- Rakhunde, R.; Deshpande, L.; Juneja, H. Chemical speciation of chromium in water: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 776–810. [Google Scholar] [CrossRef]
- Chen, J.; Tian, Y. Hexavalent chromium reducing bacteria: Mechanism of reduction and characteristics. Environ. Sci. Pollut. Res. 2021, 28, 20981–20997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Tian, Y. Characteristics of natural biopolymers and their derivative as sorbents for chromium adsorption: A review. J. Leather Sci. Eng. 2020, 2, 24. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bishop, T.F.; Singh, B. Evaluation of spatial variability of soil arsenic adjacent to a disused cattle-dip site, using model-based geostatistics. Environ. Sci. Technol. 2011, 45, 10463–10470. [Google Scholar] [CrossRef] [PubMed]
- Niazi, N.K.; Singh, B.; Minasny, B. Mid-infrared spectroscopy and partial least-squares regression to estimate soil arsenic at a highly variable arsenic-contaminated site. Int. J. Environ. Sci. Technol. 2015, 12, 1965–1974. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–25. [Google Scholar]
- Vimercati, L.; Gatti, M.F.; Gagliardi, T.; Cuccaro, F.; De Maria, L.; Caputi, A.; Quarato, M.; Baldassarre, A. Environmental exposure to arsenic and chromium in an industrial area. Environ. Sci. Pollut. Res. 2017, 24, 11528–11535. [Google Scholar] [CrossRef]
- Hasan, S.M.; Akber, M.; Bahar, M.; Islam, M.; Akbor, M.; Siddique, M.; Bakar, A. Chromium contamination from tanning industries and Phytoremediation potential of native plants: A study of savar tannery industrial estate in Dhaka, Bangladesh. Bull. Environ. Contam. Toxicol. 2021, 106, 1024–1032. [Google Scholar] [CrossRef]
- Faust, S.; Ali, O. Chemistry of Water Treatment; Ann Arbor Press: Chelsea, MI, USA, 1998. [Google Scholar]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef]
- Barnowski, C.; Jakubowski, N.; Stuewer, D.; Broekaert, J.A. Speciation of chromium by direct coupling of ion exchange chromatography with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1997, 12, 1155–1161. [Google Scholar] [CrossRef]
- Gil, R.; Cerutti, S.; Gásquez, J.; Olsina, R.; Martinez, L. Preconcentration and speciation of chromium in drinking water samples by coupling of on-line sorption on activated carbon to ETAAS determination. Talanta 2006, 68, 1065–1070. [Google Scholar] [CrossRef]
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of hexavalent chromium on the environment and removal techniques: A review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.A.; Salem, H. The Biological and Environmental Chemistry of Chromium; VCH Publishers: Hoboken, NJ, USA, 1994. [Google Scholar]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Nandi, R.; Saha, B. Sources and toxicity of hexavalent chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, B.; Cui, G.; Zhang, L.; Guo, Y.; Yan, S. Synthesis of poly (m-phenylenediamine)/iron oxide/acid oxidized multi-wall carbon nanotubes for removal of hexavalent chromium. RSC Adv. 2015, 5, 2266–2275. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y.; Dundar, M. Adsorption of chromium (VI) on pomace—An olive oil industry waste: Batch and column studies. J. Hazard. Mater. 2006, 138, 142–151. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. A review of hexavalent chromium removal from aqueous solutions by sorption technique using nanomaterials. J. Environ. Chem. Eng. 2020, 8, 104503. [Google Scholar] [CrossRef]
- Mohammadi, H.; Gholami, M.; Rahimi, M. Application and optimization in chromium-contaminated wastewater treatment of the reverse osmosis technology. Desalination Water Treat. 2009, 9, 229–233. [Google Scholar] [CrossRef]
- Narayani, M.; Shetty, K.V. Chromium-resistant bacteria and their environmental condition for hexavalent chromium removal: A review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 955–1009. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J.; Tseng, W.-L. Effective adsorption of chromium (VI)/Cr (III) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent. J. Mater. Chem. A 2015, 3, 7044–7057. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Hashem, A.; Akasha, R.; Ghith, A.; Hussein, D. Adsorbent based on agricultural wastes for heavy metal and dye removal: A review. Energy Educ. Sci. Technol. 2007, 19, e86. [Google Scholar]
- Rahman, M.A.; Lamb, D.; Rahman, M.M.; Bahar, M.M.; Sanderson, P.; Abbasi, S.; Bari, A.F.; Naidu, R. Removal of arsenate from contaminated waters by novel zirconium and zirconium-iron modified biochar. J. Hazard. Mater. 2021, 409, 124488. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Gan, Q.; Matthews, R.; Johnson, P.A. Kinetic modeling of the adsorption of basic dyes by kudzu. J. Colloid Interface Sci. 2005, 286, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Siti, N.; Mohd, H.; Md, L.K.; Shamsul, I. Adsorption process of heavy metals by low-cost adsorbent: A review. World Appl. Sci. J. 2013, 28, 1518–1530. [Google Scholar]
- Dehghani, M.H.; Sanaei, D.; Ali, I.; Bhatnagar, A. Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent: Kinetic modeling and isotherm studies. J. Mol. Liq. 2016, 215, 671–679. [Google Scholar] [CrossRef]
- Bentchikou, L.; Mechelouf, F.-Z.; NEGGAZ, F.; Mellah, A. Removal of hexavalent chromium from water by using natural brown clay. J. Turk. Chem. Soc. Sect. B Chem. Eng. 2017, 1, 43–52. [Google Scholar]
- Zhao, Y.; Qi, W.; Chen, G.; Ji, M.; Zhang, Z. Behavior of Cr (VI) removal from wastewater by adsorption onto HCl activated Akadama clay. J. Taiwan Inst. Chem. Eng. 2015, 50, 190–197. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.V.; Dal Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Adam, M.R.; Salleh, N.M.; Othman, M.H.D.; Matsuura, T.; Ali, M.H.; Puteh, M.H.; Ismail, A.; Rahman, M.A.; Jaafar, J. The adsorptive removal of chromium (VI) in aqueous solution by novel natural zeolite based hollow fibre ceramic membrane. J. Environ. Manag. 2018, 224, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Mondal, A.; Mishra, U.; Bar, N.; Das, S.K. Removal of chromium (VI) from aqueous solutions using rubber leaf powder: Batch and column studies. Desalination Water Treat. 2016, 57, 16927–16942. [Google Scholar] [CrossRef]
- Gottipati, R.; Mishra, S. Preparation of microporous activated carbon from Aegle Marmelos fruit shell and its application in removal of chromium (VI) from aqueous phase. J. Ind. Eng. Chem. 2016, 36, 355–363. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, P.K. Adsorption study of Cr (II) from aqueous solution using animal bone charcoal as low cost adsorbent. Int. J. Eng. Technol. Manag. Appl. Sci. 2015, 3, 151–163. [Google Scholar]
- Zafarani, H.R.; Bahrololoom, M.E.; Noubactep, C.; Tashkhourian, J. Green walnut shell as a new material for removal of Cr (VI) ions from aqueous solutions. Desalination Water Treat. 2015, 55, 431–439. [Google Scholar] [CrossRef]
- Das, N.; Konar, J.; Mohanta, M.; Srivastava, S. Adsorption of Cr (VI) and Se (IV) from their aqueous solutions onto Zr4+-substituted ZnAl/MgAl-layered double hydroxides: Effect of Zr4+ substitution in the layer. J. Colloid Interface Sci. 2004, 270, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.; Bennett, T.; Benjamin, M. Sorption onto and recovery of Cr (VI) using iron-oxide-coated sand. Water Sci. Technol. 1992, 26, 1239–1244. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Nieboer, E. Chromium in the Natural and Human Environments; John Wiley & Sons: Hoboken, NJ, USA, 1988; Volume 20. [Google Scholar]
- Beszedits, S. Chromium removal from industrial wastewaters. Chromium Nat. Hum. Environ. 1988, 20, 231. [Google Scholar]
- USBR. Hexavalent Chromium Treatment Technologies; U.S. Department of the Interior Bureau of Reclamation Research and Development Office: Denver, CO, USA, 2018.
- Sillanpää, M.; Shestakova, M. Electrochemical Water Treatment Methods: Fundamentals, Methods and Full Scale Applications; Butterworth-Heinemann: Oxford, UK, 2017. [Google Scholar]
- Mukhopadhyay, B.; Sundquist, J.; Schmitz, R.J. Removal of Cr (VI) from Cr-contaminated groundwater through electrochemical addition of Fe (II). J. Environ. Manag. 2007, 82, 66–76. [Google Scholar] [CrossRef]
- Sengupta, A.K.; Clifford, D. Important process variables in chromate ion exchange. Environ. Sci. Technol. 1986, 20, 149–155. [Google Scholar] [CrossRef]
- Sengupta, A.K.; Clifford, D.; Subramonian, S. Chromate ion-exchange process at alkaline pH. Water Res. 1986, 20, 1177–1184. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, K.-H.; Moon, S.-H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Galán, B.; Castañeda, D.; Ortiz, I. Removal and recovery of Cr (VI) from polluted ground waters: A comparative study of ion-exchange technologies. Water Res. 2005, 39, 4317–4324. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Pehlivan, E. A comparative study of two chelating ion-exchange resins for the removal of chromium (III) from aqueous solution. J. Hazard. Mater. 2003, 100, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Faust, S.D.; Aly, O.M. Chemistry of Water Treatment, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Yasmeen, S.; Kabiraz, M.K.; Saha, B.; Qadir, M.; Gafur, M.; Masum, S. Chromium (VI) ions removal from tannery effluent using chitosan-microcrystalline cellulose composite as adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Wan, Z.; Li, M.; Zhang, Q.; Fan, Z.; Verpoort, F. Concurrent reduction-adsorption of chromium using m-phenylenediamine-modified magnetic chitosan: Kinetics, isotherm, and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 17830–17841. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, F.; Huang, L.; Chang, Z.; Yang, B.; Liu, S.; Zheng, M.; Lu, Y.; Chen, J. Cyclodextrin functionalized 3D-graphene for the removal of Cr (VI) with the easy and rapid separation strategy. Environ. Pollut. 2019, 254, 112854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Forster, C. A preliminary examination into the adsorption of hexavalent chromium using low-cost adsorbents. Bioresour. Technol. 1994, 47, 257–264. [Google Scholar] [CrossRef]
- Singh, K.; Rastogi, R.; Hasan, S. Removal of Cr (VI) from wastewater using rice bran. J. Colloid Interface Sci. 2005, 290, 61–68. [Google Scholar] [CrossRef]
- Dupont, L.; Guillon, E. Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environ. Sci. Technol. 2003, 37, 4235–4241. [Google Scholar] [CrossRef]
- Dupont, L.; Bouanda, J.; Ghanbaja, J.; Dumonceau, J.; Aplincourt, M. Use of analytical microscopy to analyze the speciation of copper and chromium ions onto a low-cost biomaterial. J. Colloid Interface Sci. 2004, 279, 418–424. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’Eb, M. Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- Meena, A.K.; Kadirvelu, K.; Mishra, G.; Rajagopal, C.; Nagar, P. Adsorptive removal of heavy metals from aqueous solution by treated sawdust (Acacia arabica). J. Hazard. Mater. 2008, 150, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Bhuptawat, H.K.; Chaudhari, S. Biosorption of aqueous chromium (VI) by Tamarindus indica seeds. Bioresour. Technol. 2006, 97, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Dost, K.; Sezer, H. An investigation of chromium(VI) ion removal from wastewaters by adsorption on residual lignin. Fresenius Environ. Bull. 2004, 13, 124–127. [Google Scholar]
- Daneshvar, N.; Salari, D.; Aber, S. Chromium adsorption and Cr (VI) reduction to trivalent chromium in aqueous solutions by soya cake. J. Hazard. Mater. 2002, 94, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.H.; Araújo, G.C.; Pelizaro, C.B.; Menezes, E.A.; Lemos, S.G.; De Sousa, G.B.; Nogueira, A.R.A. Coconut coir as biosorbent for Cr (VI) removal from laboratory wastewater. J. Hazard. Mater. 2008, 159, 252–256. [Google Scholar] [CrossRef]
- Ahalya, N.; Kanamadi, R.; Ramachandra, T. Biosorption of chromium (VI) from aqueous solutions by the husk of Bengal gram (Cicer arientinum). Electron. J. Biotechnol. 2005, 8, 258–264. [Google Scholar] [CrossRef]
- Ali, A.; Saeed, K.; Mabood, F. Removal of chromium (VI) from aqueous medium using chemically modified banana peels as efficient low-cost adsorbent. Alex. Eng. J. 2016, 55, 2933–2942. [Google Scholar] [CrossRef]
- Yari, A.R.; Kord Mostafapour, F.; Mahdavi, Y.; Joghataei, A. Agricultural Waste as Adsorbent for Removal of Chromium (VI) from aqueous solution. Arch. Hyg. Sci. 2016, 5, 310–318. [Google Scholar]
- Altun, T.; Parlayıcı, Ş.; Pehlivan, E. Hexavalent chromium removal using agricultural waste “rye husk”. Desalination Water Treat. 2016, 57, 17748–17756. [Google Scholar] [CrossRef]
- Begum, H.A.; Haque, A.M.; Islam, M.D.; Hasan, M.M.; Ahmed, S.; Razzak, M.; Khan, R.A. Analysis of the adsorption of toxic chromium (VI) by untreated and chitosan treated banana and areca fiber. J. Text. Sci. Technol. 2020, 6, 81. [Google Scholar] [CrossRef]
- Pourfadakari, S.; Jorfi, S.; Ahmadi, M.; Takdastan, A. Experimental data on adsorption of Cr (VI) from aqueous solution using nanosized cellulose fibers obtained from rice husk. Data Brief 2017, 15, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Verma, R.; Asthana, A.; Vidya, S.S.; Singh, A.K. Adsorption of hazardous chromium (VI) ions from aqueous solutions using modified sawdust: Kinetics, isotherm and thermodynamic modelling. Int. J. Environ. Anal. Chem. 2021, 101, 911–928. [Google Scholar] [CrossRef]
- Ahmed, N.; Islam, M.; Hossain, M.; Rahman, A.; Sultana, A. Modified coconut coir to remove hexavalent chromium from aqueous solution. Bangladesh J. Sci. Ind. Res. 2019, 54, 89–98. [Google Scholar] [CrossRef]
- Krishnani, K.; Meng, X.; Dupont, L. Metal ions binding onto lignocellulosic biosorbent. J. Environ. Sci. Health Part A 2009, 44, 688–699. [Google Scholar] [CrossRef]
- Jain, M.; Garg, V.; Kadirvelu, K. Chromium (VI) removal from aqueous system using Helianthus annuus (sunflower) stem waste. J. Hazard. Mater. 2009, 162, 365–372. [Google Scholar] [CrossRef]
- Aoyama, M. Removal of Cr (VI) from aqueous solution by London plane leaves. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2003, 78, 601–604. [Google Scholar] [CrossRef]
- Uysal, M.; Ar, I. Removal of Cr (VI) from industrial wastewaters by adsorption: Part I: Determination of optimum conditions. J. Hazard. Mater. 2007, 149, 482–491. [Google Scholar] [CrossRef]
- Pehlivan, E.; Altun, T. Biosorption of chromium (VI) ion from aqueous solutions using walnut, hazelnut and almond shell. J. Hazard. Mater. 2008, 155, 378–384. [Google Scholar] [CrossRef]
- Raji, C.; Anirudhan, T. Batch Cr (VI) removal by polyacrylamide-grafted sawdust: Kinetics and thermodynamics. Water Res. 1998, 32, 3772–3780. [Google Scholar] [CrossRef]
- Sinha, V.; Pakshirajan, K.; Chaturvedi, R. Evaluation of Cr (VI) exposed and unexposed plant parts of Tradescantia pallida (Rose) DR Hunt. for Cr removal from wastewater by biosorption. Int. J. Phytoremediation 2015, 17, 1204–1211. [Google Scholar] [CrossRef]
- Suganya, E.; Rangabhashiyam, S.; Lity, A.; Selvaraju, N. Removal of hexavalent chromium from aqueous solution by a novel biosorbent Caryota urens seeds: Equilibrium and kinetic studies. Desalination Water Treat. 2016, 57, 23940–23950. [Google Scholar] [CrossRef]
- Lin, C.; Luo, W.; Luo, T.; Zhou, Q.; Li, H.; Jing, L. A study on adsorption of Cr (VI) by modified rice straw: Characteristics, performances and mechanism. J. Clean. Prod. 2018, 196, 626–634. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Meng, X.; Christodoulatos, C.; Boddu, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Losada, V.A.R.; Bonilla, E.P.; Pinilla, L.A.C.; Serrezuela, R.R. Removal of chromium in wastewater from tanneries applying bioremediation with algae, orange peels and citrus pectin. Contemp. Eng. Sci. 2018, 11, 433–449. [Google Scholar] [CrossRef]
- Shokri Khoubestani, R.; Mirghaffari, N.; Farhadian, O. Removal of three and hexavalent chromium from aqueous solutions using a microalgae biomass-derived biosorbent. Environ. Prog. Sustain. Energy 2015, 34, 949–956. [Google Scholar] [CrossRef]
- Jayakumar, R.; Rajasimman, M.; Karthikeyan, C. Sorption and desorption of hexavalent chromium using a novel brown marine algae Sargassum myriocystum. Korean J. Chem. Eng. 2015, 32, 2031–2046. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Al-Qahtani, H.S.; Al-Ghanayem, A.A.; Ameen, F.; Ibraheem, I.B. Potential use of green algae as a biosorbent for hexavalent chromium removal from aqueous solutions. Saudi J. Biol. Sci. 2018, 25, 1733–1738. [Google Scholar] [CrossRef]
- Uluozlu, O.D.; Sari, A.; Tuzen, M.; Soylak, M. Biosorption of Pb (II) and Cr (III) from aqueous solution by lichen (Parmelina tiliaceae) biomass. Bioresour. Technol. 2008, 99, 2972–2980. [Google Scholar] [CrossRef]
- Bernardo, G.-R.R.; Rene, R.-M.J. Chromium (III) uptake by agro-waste biosorbents: Chemical characterization, sorption–desorption studies, and mechanism. J. Hazard. Mater. 2009, 170, 845–854. [Google Scholar] [CrossRef]
- Onyancha, D.; Mavura, W.; Ngila, J.C.; Ongoma, P.; Chacha, J. Studies of chromium removal from tannery wastewaters by algae biosorbents, Spirogyra condensata and Rhizoclonium hieroglyphicum. J. Hazard. Mater. 2008, 158, 605–614. [Google Scholar] [CrossRef]
- Barkhordar, B.; Ghias, A.M. Comparison of Langmuir and Freundlich equilibriums in Cr, Cu and Ni adsorption by Sargassum. 2004, 1, 58–64. Iran. J. Environ. Health Sci. 2004, 1, 58–64. [Google Scholar]
- Pena-Castro, J.; Martınez-Jerónimo, F.; Esparza-Garcıa, F.; Canizares-Villanueva, R. Heavy metals removal by the microalga Scenedesmus incrassatulus in continuous cultures. Bioresour. Technol. 2004, 94, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Shrivastava, A.; Jain, N. Biosorption of chromium (VI) from aqueous solutions by green algae Spirogyra species. Water Res. 2001, 35, 4079–4085. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.; Yin, P.; Li, X.; Shan, Y. Exploring the mechanisms of biosorption of Cr (Vi) by marine-derived Penicillium janthinellum P1. Int. J. Agric. Biol. 2019, 22, 913–920. [Google Scholar]
- Mondal, N.K.; Samanta, A.; Dutta, S.; Chattoraj, S. Optimization of Cr (VI) biosorption onto Aspergillus niger using 3-level Box-Behnken design: Equilibrium, kinetic, thermodynamic and regeneration studies. J. Genet. Eng. Biotechnol. 2017, 15, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Senthil Kumar, P.; Preetha, B. Optimization of process parameters for the removal of chromium (VI) and nickel (II) from aqueous solutions by mixed biosorbents (custard apple seeds and Aspergillus niger) using response surface methodology. Desalination Water Treat. 2016, 57, 14530–14543. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Yakout, A.A.; Abdel-Aal, H.; Osman, M.M. Speciation and selective biosorption of Cr (III) and Cr (VI) using nanosilica immobilized-fungi biosorbents. J. Environ. Eng. 2015, 141, 04014079. [Google Scholar] [CrossRef]
- Sivakumar, D. Biosorption of hexavalent chromium in a tannery industry wastewater using fungi species. Glob. J. Environ. Sci. Manag. 2016, 2, 105–124. [Google Scholar]
- Ertugay, N.; Bayhan, Y. Biosorption of Cr (VI) from aqueous solutions by biomass of Agaricus bisporus. J. Hazard. Mater. 2008, 154, 432–439. [Google Scholar] [CrossRef]
- Preetha, B.; Viruthagiri, T. Batch and continuous biosorption of chromium (VI) by Rhizopus arrhizus. Sep. Purif. Technol. 2007, 57, 126–133. [Google Scholar] [CrossRef]
- Elangovan, R.; Philip, L.; Chandraraj, K. Biosorption of chromium species by aquatic weeds: Kinetics and mechanism studies. J. Hazard. Mater. 2008, 152, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Rastogi, A. Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J. Hazard. Mater. 2009, 163, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Dastidar, M.G. Adsorption-desorption studies on Cr (VI) using non-living fungal biomass. Asian J. Chem. 2010, 22, 2331. [Google Scholar]
- Sen, M.; Dastidar, M.G.; Roychoudhury, P.K. Biological removal of Cr (VI) using Fusarium solani in batch and continuous modes of operation. Enzym. Microb. Technol. 2007, 41, 51–56. [Google Scholar] [CrossRef]
- Rezaei, H. Biosorption of chromium by using Spirulina sp. Arab. J. Chem. 2016, 9, 846–853. [Google Scholar] [CrossRef]
- Quiton, K.G.; Doma, B., Jr.; Futalan, C.M.; Wan, M.-W. Removal of chromium (VI) and zinc (II) from aqueous solution using kaolin-supported bacterial biofilms of Gram-negative E. coli and Gram-positive Staphylococcus epidermidis. Sustain. Environ. Res. 2018, 28, 206–213. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Szcześ, A.; Czemierska, M.; Jarosz-Wikołazka, A. Studies of cadmium (II), lead (II), nickel (II), cobalt (II) and chromium (VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresour. Technol. 2017, 225, 113–120. [Google Scholar] [CrossRef]
- Emran, F.K.; Walli, H.A.; Shinjar, F.J.; Abdulameer, M.A. Chromium Bioremoval by Staphylococcus sp. and Pseudomonas sp. Isolated from Industrial Waste Water in Baghdad, Iraq. J. Phys. Conf. Ser. 2019, 1294, 072014. [Google Scholar] [CrossRef]
- Chug, R.; Gour, V.S.; Mathur, S.; Kothari, S. Optimization of extracellular polymeric substances production using Azotobacter beijreinckii and Bacillus subtilis and its application in chromium (VI) removal. Bioresour. Technol. 2016, 214, 604–608. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Y.; Zeng, G.; Li, X.; Xu, W.; Fan, T. Kinetic and equilibrium studies of Cr (VI) biosorption by dead Bacillus licheniformis biomass. World J. Microbiol. Biotechnol. 2007, 23, 43–48. [Google Scholar] [CrossRef]
- Sivaprakash, A.; Aravindhan, R.; Rao, J.R.; Nair, B.U. Kinetics and equilibrium studies on the biosorption of hexavalent chromium from aqueous solutions using Bacillus subtilis biomass. Appl. Ecol. Environ. Res. 2009, 7, 45–57. [Google Scholar] [CrossRef]
- Ziagova, M.; Dimitriadis, G.; Aslanidou, D.; Papaioannou, X.; Tzannetaki, E.L.; Liakopoulou-Kyriakides, M. Comparative study of Cd (II) and Cr (VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour. Technol. 2007, 98, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Bueno, B.; Torem, M.; Molina, F.; De Mesquita, L. Biosorption of lead (II), chromium (III) and copper (II) by R. opacus: Equilibrium and kinetic studies. Miner. Eng. 2008, 21, 65–75. [Google Scholar] [CrossRef]
- Sahmoune, M.; Louhab, K.; Boukhiar, A. Biosorption of Cr (III) from aqueous solutions using bacterium biomass Streptomyces rimosus. Int. J. Environ. Res. 2009, 3, 229–238. [Google Scholar]
- Khanafari, A.; Eshgh, D.S.; Mashinchian, A. Removal of lead and chromium from aqueous solution by Bacillus circulans biofilm. Iran. J. Environ. Health. Sci. Eng. 2008, 5, 195–200. [Google Scholar]
- Srinath, T.; Verma, T.; Ramteke, P.; Garg, S. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere 2002, 48, 427–435. [Google Scholar] [CrossRef]
- Srinath, T.; Garg, S.; Ramteke, P. Chromium (VI) accumulation by Bacillus circulans: Effect of growth conditions. Indian J. Microbiol. 2002, 42, 141–146. [Google Scholar]
- Pattanapipitpaisal, P.; Brown, N.; Macaskie, L. Chromate reduction by Microbacterium liquefaciens immobilised in polyvinyl alcohol. Biotechnol. Lett. 2001, 23, 61–65. [Google Scholar] [CrossRef]
- Islam, M.M.; Khan, M.N.; Biswas, S.; Choudhury, T.R.; Haque, P.; Rashid, T.U.; Rahman, M.M. Preparation and characterization of bijoypur clay-crystalline cellulose composite for application as an adsorbent. Adv. Mater. Sci 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr (VI) from aqueous solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
- Islam, M.M.; Islam, M.S.; Maniruzzaman, M.; Haque, M.M.-U.; Mohana, A.A. Banana rachis CNC/Clay composite filter for dye and heavy metals adsorption from industrial wastewater. Eng. Sci. Technol. 2021, 2, 140–152. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Islam, M.A.; Islam, M.M.; Rahman, M.A.; Alam, S.N. Biodegradable composite adsorbent of modified cellulose and chitosan to remove heavy metal ions from aqueous solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Choudhury, P.R.; Majumdar, S.; Sahoo, G.C.; Saha, S.; Mondal, P. High pressure ultrafiltration CuO/hydroxyethyl cellulose composite ceramic membrane for separation of Cr (VI) and Pb (II) from contaminated water. Chem. Eng. J. 2018, 336, 570–578. [Google Scholar] [CrossRef]
- Dokmaji, T.; Ibrahim, T.; Khamis, M.; Abouleish, M.; Alam, I. Chemically modified nanoparticles usage for removal of chromium from sewer water. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100319. [Google Scholar] [CrossRef]
- Subedi, N.; Lähde, A.; Abu-Danso, E.; Iqbal, J.; Bhatnagar, A. A comparative study of magnetic chitosan (Chi@ Fe3O4) and graphene oxide modified magnetic chitosan (Chi@ Fe3O4GO) nanocomposites for efficient removal of Cr (VI) from water. Int. J. Biol. Macromol. 2019, 137, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhuang, M.; Shan, L.; Wu, J.; Quan, G.; Cui, L.; Zhang, Y.; Yan, J. Bimetallic FeNi nanoparticles immobilized by biomass-derived hierarchically porous carbon for efficient removal of Cr (VI) from aqueous solution. J. Hazard. Mater. 2022, 423, 127098. [Google Scholar] [CrossRef]
- Jabłońska, B. Removing of Cr (III) and Cr (VI) compounds from aqueous solutions by shale waste rocks. Desalination Water Treat. 2020, 186, 234–246. [Google Scholar] [CrossRef]
- Qiao, K.; Tian, W.; Bai, J.; Zhao, J.; Du, Z.; Song, T.; Chu, M.; Wang, L.; Xie, W. Synthesis of floatable magnetic iron/biochar beads for the removal of chromium from aqueous solutions. Environ. Technol. Innov. 2020, 19, 100907. [Google Scholar] [CrossRef]
- Li, F.; Zimmerman, A.R.; Hu, X.; Gao, B. Removal of aqueous Cr (VI) by Zn-and Al-modified hydrochar. Chemosphere 2020, 260, 127610. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption and me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. CLEAN–Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Lesmana, S.O.; Febriana, N.; Soetaredjo, F.E.; Sunarso, J.; Ismadji, S. Studies on potential applications of biomass for the separation of heavy metals from water and wastewater. Biochem. Eng. J. 2009, 44, 19–41. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions—A review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Gupta, V.; Rastogi, A. Sorption and desorption studies of chromium (VI) from nonviable cyanobacterium Nostoc muscorum biomass. J. Hazard. Mater. 2008, 154, 347–354. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr (VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Karishma, S.; Jeevanantham, S.; Yaashikaa, P. Effective removal of Cr (VI) ions from synthetic solution using mixed biomasses: Kinetic, equilibrium and thermodynamic study. J. Water Process Eng. 2021, 40, 101905. [Google Scholar] [CrossRef]
- Tytłak, A.; Oleszczuk, P.; Dobrowolski, R. Sorption and desorption of Cr (VI) ions from water by biochars in different environmental conditions. Environ. Sci. Pollut. Res. 2015, 22, 5985–5994. [Google Scholar] [CrossRef]
- Imran, M.; Khan, Z.U.H.; Iqbal, M.M.; Iqbal, J.; Shah, N.S.; Munawar, S.; Ali, S.; Murtaza, B.; Naeem, M.A.; Rizwan, M. Effect of biochar modified with magnetite nanoparticles and HNO3 for efficient removal of Cr (VI) from contaminated water: A batch and column scale study. Environ. Pollut. 2020, 261, 114231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yan, C.; Luo, W. Polypyrrole coated secondary fly ash–iron composites: Novel floatable magnetic adsorbents for the removal of chromium (VI) from wastewater. Mater. Des. 2016, 92, 701–709. [Google Scholar] [CrossRef]
- Mohamed, A.; Nasser, W.; Osman, T.; Toprak, M.; Muhammed, M.; Uheida, A. Removal of chromium (VI) from aqueous solutions using surface modified composite nanofibers. J. Colloid Interface Sci. 2017, 505, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lee, X.; Macazo, F.C.; Grattieri, M.; Cai, R.; Minteer, S.D. Fast and efficient removal of chromium (VI) anionic species by a reusable chitosan-modified multi-walled carbon nanotube composite. Chem. Eng. J. 2018, 339, 259–267. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Wang, H.; Dong, J.; Chen, W.; Wang, X.; Wang, S.; Hayat, T.; Alsaedi, A.; Wang, X. Preparation of molybdenum disulfide coated Mg/Al layered double hydroxide composites for efficient removal of chromium (VI). ACS Sustain. Chem. Eng. 2017, 5, 7165–7174. [Google Scholar] [CrossRef]
- Kumar, N.; Kardam, A.; Jain, V.; Nagpal, S. A rapid, reusable polyaniline-impregnated nanocellulose composite-based system for enhanced removal of chromium and cleaning of waste water. Sep. Sci. Technol. 2020, 55, 1436–1448. [Google Scholar] [CrossRef]
- Wang, H.; Cui, T.; Chen, D.; Luo, Q.; Xu, J.; Sun, R.; Zi, W.; Xu, R.; Liu, Y.; Zhang, Y. Hexavalent chromium elimination from wastewater by integrated micro-electrolysis composites synthesized from red mud and rice straw via a facile one-pot method. Sci. Rep. 2022, 12, 14242. [Google Scholar] [CrossRef]
- Wu, S.; Li, M.; Xin, L.; Long, H.; Gao, X. Efficient removal of Cr (VI) by triethylenetetramine modified sodium alginate/carbonized chitosan composite via adsorption and photocatalytic reduction. J. Mol. Liq. 2022, 366, 120160. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken experimental design for chromium (VI) ions removal by bacterial cellulose-magnetite composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244, 444–456. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, H.-C. Recent progress in the synthesis of metal–organic frameworks. Sci. Technol. Adv. Mater. 2015, 16, 054202. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Seleim, S.M.; Mohamed, A.K. Amino-decorated magnetic metal-organic framework as a potential novel platform for selective removal of chromium (Vl), cadmium (II) and lead (II). J. Hazard. Mater. 2020, 381, 120979. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, J.-J.; Wang, C.-C. Adsorptive removal of Cr (VI) from simulated wastewater in MOF BUC-17 ultrafine powder. J. Environ. Chem. Eng. 2019, 7, 102909. [Google Scholar] [CrossRef]
- Han, C.; Xie, J.; Min, X. Efficient adsorption H3AsO4 and Cr (VI) from strongly acidic solutions by La-Zr bimetallic MOFs: Crystallinity role and mechanism. J. Environ. Chem. Eng. 2022, 10, 108982. [Google Scholar] [CrossRef]
- Meier, M.; Ungerer, J.; Klinge, M.; Nirschl, H. Formation of porous silica nanoparticles at higher reaction kinetics. Powder Technol. 2018, 339, 801–808. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Peng, J.; Zhao, Y.; Yang, Q. Highly efficient solid catalysts for asymmetric hydrogenation fabricated via facile adsorption of Rh–MonoPhos on porous silicas. Catal. Sci. Technol. 2014, 4, 1012–1016. [Google Scholar] [CrossRef]
- Prieto, G.; Shakeri, M.; De Jong, K.P.; De Jongh, P.E. Quantitative relationship between support porosity and the stability of pore-confined metal nanoparticles studied on CuZnO/SiO2 methanol synthesis catalysts. ACS Nano 2014, 8, 2522–2531. [Google Scholar] [CrossRef]
- Buntara, T.; Melián-Cabrera, I.; Tan, Q.; Fierro, J.L.; Neurock, M.; De Vries, J.G.; Heeres, H.J. Catalyst studies on the ring opening of tetrahydrofuran–dimethanol to 1,2,6-hexanetriol. Catal. Today 2013, 210, 106–116. [Google Scholar] [CrossRef]
- Fechete, I.; Donnio, B.; Ersen, O.; Dintzer, T.; Djeddi, A.; Garin, F. Single crystals of mesoporous tungstenosilicate W-MCM-48 molecular sieves for the conversion of methylcyclopentane (MCP). Appl. Surf. Sci. 2011, 257, 2791–2800. [Google Scholar] [CrossRef]
- Staub, H.L.N.; Del Rosal, I.; Maron, L.; Kleitz, F.; Fontaine, F.D.R.-G. On the interaction of phosphines with high surface area mesoporous silica. J. Phys. Chem. C 2012, 116, 25919–25927. [Google Scholar] [CrossRef]
- Shi, Y.-T.; Cheng, H.-Y.; Geng, Y.; Nan, H.-M.; Chen, W.; Cai, Q.; Chen, B.-H.; Sun, X.-D.; Yao, Y.-W. The size-controllable synthesis of nanometer-sized mesoporous silica in extremely dilute surfactant solution. Mater. Chem. Phys. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Li, C.M.; Wang, X.P.; Jiao, Z.H.; Zhang, Y.S.; Yin, X.B.; Cui, X.M.; Wei, Y.Z. Functionalized porous silica-based nano/micro particles for environmental remediation of hazard ions. Nanomaterials 2019, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, J.-K.; Lee, S.-C.; Kim, S.-B. Synthesis of powdered and granular N-(3-trimethoxysilylpropyl) diethylenetriamine-grafted mesoporous silica SBA-15 for Cr (VI) removal from industrial wastewater. J. Taiwan Inst. Chem. Eng. 2018, 87, 140–149. [Google Scholar] [CrossRef]
- Shariati, S.; Khabazipour, M.; Safa, F. Synthesis and application of amine functionalized silica mesoporous magnetite nanoparticles for removal of chromium (VI) from aqueous solutions. J. Porous Mater. 2017, 24, 129–139. [Google Scholar] [CrossRef]
- Idris, S.A.; Alotaibi, K.; Peshkur, T.A.; Anderson, P.; Gibson, L.T. Preconcentration and selective extraction of chromium species in water samples using amino modified mesoporous silica. J. Colloid Interface Sci. 2012, 386, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Marjani, A.; Hosseini, M.; Shirazian, S. Synthesis and characterization of novel N-methylimidazolium-functionalized KCC-1: A highly efficient anion exchanger of hexavalent chromium. Chemosphere 2020, 239, 124735. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Marjani, A.; Soltani, R.; Shirazian, S. Hierarchical multi-shell hollow micro–meso–macroporous silica for Cr (VI) adsorption. Sci. Rep. 2020, 10, 9788. [Google Scholar] [CrossRef] [PubMed]
- Atkovska, K.; Lisichkov, K.; Ruseska, G.; Dimitrov, A.T.; Grozdanov, A. Removal of heavy metal ions from wastewater using conventional and nanosorbents: A review. J. Chem. Technol. Metall. 2018, 53, 202–219. [Google Scholar]
- Marantos, I.; Christidis, G.; Ulmanu, M. Zeolite formation and deposits. In Handbook of Natural Zeolites; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012. [Google Scholar]
- Alvarez-Ayuso, E.; Garcıa-Sánchez, A.; Querol, X. Purification of metal electroplating waste waters using zeolites. Water Res. 2003, 37, 4855–4862. [Google Scholar] [CrossRef]
- Dal Bosco, S.M.; Jimenez, R.S.; Carvalho, W.A. Removal of toxic metals from wastewater by Brazilian natural scolecite. J. Colloid Interface Sci. 2005, 281, 424–431. [Google Scholar] [CrossRef]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C. Zeolite application in wastewater treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Zeng, Y.; Woo, H.; Lee, G.; Park, J. Adsorption of Cr (VI) on hexadecylpyridinium bromide (HDPB) modified natural zeolites. Microporous Mesoporous Mater. 2010, 130, 83–91. [Google Scholar] [CrossRef]
- Neolaka, Y.A.; Lawa, Y.; Naat, J.; Riwu, A.A.; Mango, A.W.; Darmokoesoemo, H.; Widyaningrum, B.A.; Iqbal, M.; Kusuma, H.S. Efficiency of activated natural zeolite-based magnetic composite (ANZ-Fe3O4) as a novel adsorbent for removal of Cr (VI) from wastewater. J. Mater. Res. Technol. 2022, 18, 2896–2909. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, Y.; Wang, H.; Tang, J.; Li, Y.; Wang, S. Removal of Cr (VI) from wastewater by artificial zeolite spheres loaded with nano Fe–Al bimetallic oxide in constructed wetland. Chemosphere 2020, 257, 127224. [Google Scholar] [CrossRef]
- Pacheco, S.; Tapia, J.; Medina, M.; Rodriguez, R. Cadmium ions adsorption in simulated wastewater using structured alumina–silica nanoparticles. J. Non-Cryst. Solids 2006, 352, 5475–5481. [Google Scholar] [CrossRef]
- Di, Z.-C.; Ding, J.; Peng, X.-J.; Li, Y.-H.; Luan, Z.-K.; Liang, J. Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere 2006, 62, 861–865. [Google Scholar] [CrossRef]
- Skubal, L.; Meshkov, N.; Rajh, T.; Thurnauer, M. Cadmium removal from water using thiolactic acid-modified titanium dioxide nanoparticles. J. Photochem. Photobiol. A Chem. 2002, 148, 393–397. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.; Chen, G. Fast removal and recovery of Cr (VI) using surface-modified jacobsite (MnFe2O4) nanoparticles. Langmuir 2005, 21, 11173–11179. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.; Chen, G. Performance and mechanism of chromate (VI) adsorption by δ-FeOOH-coated maghemite (γ-Fe2O3) nanoparticles. Sep. Purif. Technol. 2007, 58, 76–82. [Google Scholar] [CrossRef]
- Jayson, G.; Sangster, J.; Thompson, G.; Wilkinson, M. Adsorption of chromium from aqueous solution onto activated charcoal cloth. Carbon 1993, 31, 487–492. [Google Scholar] [CrossRef]
- Selomulya, C.; Meeyoo, V.; Amal, R. Mechanisms of Cr (VI) removal from water by various types of activated carbons. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1999, 74, 111–122. [Google Scholar] [CrossRef]
- Hamadi, N.K.; Chen, X.D.; Farid, M.M.; Lu, M.G. Adsorption kinetics for the removal of chromium (VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chem. Eng. J. 2001, 84, 95–105. [Google Scholar] [CrossRef]
- Cimino, G.; Passerini, A.; Toscano, G. Removal of toxic cations and Cr (VI) from aqueous solution by hazelnut shell. Water Res. 2000, 34, 2955–2962. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Taher, M.M.; Bajpai, A.K.; Heibati, B.; Tyagi, I.; Asif, M.; Agarwal, S.; Gupta, V.K. Removal of noxious Cr (VI) ions using single-walled carbon nanotubes and multi-walled carbon nanotubes. Chem. Eng. J. 2015, 279, 344–352. [Google Scholar] [CrossRef]
- Pillay, K.; Cukrowska, E.; Coville, N. Multi-walled carbon nanotubes as adsorbents for the removal of parts per billion levels of hexavalent chromium from aqueous solution. J. Hazard. Mater. 2009, 166, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, C.; Zhu, X.; Wang, X. Removal of chromium from aqueous solution by using oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2009, 162, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Agarwal, S.; Saleh, T.A. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011, 45, 2207–2212. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Tawabini, B.S.; Bukhari, A.A.; Khaled, M.; Alharthi, M.; Fettouhi, M.; Abuilaiwi, F.A. Removal of chromium (III) from water by using modified and nonmodified carbon nanotubes. J. Nanomater. 2010, 2010, 232378. [Google Scholar] [CrossRef]
- Atieh, M.A. Removal of chromium (VI) from polluted water using carbon nanotubes supported with activated carbon. Procedia Environ. Sci. 2011, 4, 281–293. [Google Scholar] [CrossRef]
- Park, H.J.; Jeong, S.W.; Yang, J.K.; Kim, B.G.; Lee, S.M. Removal of heavy metals using waste eggshell. J. Environ. Sci. 2007, 19, 1436–1441. [Google Scholar] [CrossRef]
- Amuda, O.; Adelowo, F.; Ologunde, M. Kinetics and equilibrium studies of adsorption of chromium (VI) ion from industrial wastewater using Chrysophyllum albidum (Sapotaceae) seed shells. Colloids Surf. B Biointerfaces 2009, 68, 184–192. [Google Scholar] [CrossRef] [PubMed]
- El-Sikaily, A.; El Nemr, A.; Khaled, A.; Abdelwehab, O. Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J. Hazard. Mater. 2007, 148, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Quintelas, C.; Rocha, Z.; Silva, B.; Fonseca, B.; Figueiredo, H.; Tavares, T. Biosorptive performance of an Escherichia coli biofilm supported on zeolite NaY for the removal of Cr (VI), Cd (II), Fe (III) and Ni (II). Chem. Eng. J. 2009, 152, 110–115. [Google Scholar] [CrossRef]
- Tuzen, M.; Saygi, K.O.; Usta, C.; Soylak, M. Pseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ions. Bioresour. Technol. 2008, 99, 1563–1570. [Google Scholar] [CrossRef]
- Baytak, S.; Türker, A.R. Determination of iron (III), cobalt (II) and chromium (III) in various water samples by flame atomic absorption spectrometry after preconcentration by means of Saccharomyces carlsbergensis immobilized on amberlite XAD-4. Microchim. Acta 2005, 149, 109–116. [Google Scholar] [CrossRef]
- Gupta, V.; Pathania, D.; Agarwal, S.; Sharma, S. Removal of Cr (VI) onto Ficus carica biosorbent from water. Environ. Sci. Pollut. Res. 2013, 20, 2632–2644. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption of Cr (III) Ions by Wheat Straw and Grass: A Systematic Characterization of New Biosorbents. Pol. J. Environ. Stud. 2006, 15, 845–852. [Google Scholar]
- Wang, X.S.; Li, Z.Z.; Sun, C. Removal of Cr (VI) from aqueous solutions by low-cost biosorbents: Marine macroalgae and agricultural by-products. J. Hazard. Mater. 2008, 153, 1176–1184. [Google Scholar] [CrossRef]
- Singh, K.; Hasan, S.; Talat, M.; Singh, V.; Gangwar, S. Removal of Cr (VI) from aqueous solutions using wheat bran. Chem. Eng. J. 2009, 151, 113–121. [Google Scholar] [CrossRef]
- Nameni, M.; Alavi Moghadam, M.; Arami, M. Adsorption of hexavalent chromium from aqueous solutions by wheat bran. Int. J. Environ. Sci. Technol. 2008, 5, 161–168. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Monji, A.B. Adsorption characteristics of wheat bran towards heavy metal cations. Sep. Purif. Technol. 2004, 38, 197–207. [Google Scholar] [CrossRef]
- Sumathi, K.; Mahimairaja, S.; Naidu, R. Use of low-cost biological wastes and vermiculite for removal of chromium from tannery effluent. Bioresour. Technol. 2005, 96, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, M.; Sag, Y.; Özer, D.; Aksu, Z.; Kutsal, T.; Caglar, A. A comparative study of various biosorbents for removal of chromium (VI) ions from industrial waste waters. Process Biochem. 1994, 29, 1–5. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003, 37, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, C.; Sureshkumar, M. Removal of chromium (VI) from water and wastewater using surfactant modified coconut coir pith as a biosorbent. Bioresour. Technol. 2008, 99, 2218–2225. [Google Scholar] [CrossRef]
- Sankararamakrishnan, N.; Dixit, A.; Iyengar, L.; Sanghi, R. Removal of hexavalent chromium using a novel cross linked xanthated chitosan. Bioresour. Technol. 2006, 97, 2377–2382. [Google Scholar] [CrossRef]
- Daraei, H.; Mittal, A.; Mittal, J.; Kamali, H. Optimization of Cr (VI) removal onto biosorbent eggshell membrane: Experimental & theoretical approaches. Desalination Water Treat. 2014, 52, 1307–1315. [Google Scholar]
- Nasernejad, B.; Zadeh, T.E.; Pour, B.B.; Bygi, M.E.; Zamani, A. Camparison for biosorption modeling of heavy metals (Cr (III), Cu (II), Zn (II)) adsorption from wastewater by carrot residues. Process Biochem. 2005, 40, 1319–1322. [Google Scholar] [CrossRef]
- Bai, R.S.; Abraham, T.E. Studies on chromium (VI) adsorption–desorption using immobilized fungal biomass. Bioresour. Technol. 2003, 87, 17–26. [Google Scholar]
- Wen, Y.; Tang, Z.; Chen, Y.; Gu, Y. Adsorption of Cr (VI) from aqueous solutions using chitosan-coated fly ash composite as biosorbent. Chem. Eng. J. 2011, 175, 110–116. [Google Scholar] [CrossRef]
- Chand, R.; Narimura, K.; Kawakita, H.; Ohto, K.; Watari, T.; Inoue, K. Grape waste as a biosorbent for removing Cr (VI) from aqueous solution. J. Hazard. Mater. 2009, 163, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Dönmez, G.; Aksu, Z. Removal of chromium (VI) from saline wastewaters by Dunaliella species. Process Biochem. 2002, 38, 751–762. [Google Scholar] [CrossRef]
- Hadjmohammadi, M.R.; Salary, M.; Biparva, P. Removal of Cr (VI) from aqueous solution using pine needles powder as a biosorbent. J. Appl. Sci. Environ. Sanit. 2011, 6, 1–13. [Google Scholar]
- Zhou, L.; Yi, Y.; Fang, Z. Nanoscale zero-valent iron immobilized by ZIF-8 metal-organic frameworks for enhanced removal of hexavalent chromium. Chemosphere 2022, 306, 135456. [Google Scholar] [CrossRef]
- Maksoud, M.A.; Elgarahy, A.M.; Farrell, C.; Ala’a, H.; Rooney, D.W.; Osman, A.I. Insight on water remediation application using magnetic nanomaterials and biosorbents. Coord. Chem. Rev. 2020, 403, 213096. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Siddiqui, M.; Agarwal, S. Chromium removal from water by activated carbon developed from waste rubber tires. Environ. Sci. Pollut. Res. 2013, 20, 1261–1268. [Google Scholar] [CrossRef]
- Holt, P.K.; Barton, G.W.; Mitchell, C.A. The future for electrocoagulation as a localised water treatment technology. Chemosphere 2005, 59, 355–367. [Google Scholar] [CrossRef]
- Wang, L.K.; Hung, Y.-T.; Shammas, N.K. Advanced Physicochemical Treatment Technologies; Springer: Berlin/Heidelberg, Germany, 2007; Volume 5. [Google Scholar]
- Jin, W.; Du, H.; Zheng, S.; Zhang, Y. Electrochemical processes for the environmental remediation of toxic Cr (VI): A review. Electrochim. Acta 2016, 191, 1044–1055. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr (VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009, 90, 1663–1679. [Google Scholar] [CrossRef]
- Arroyo, M.; Pérez-Herranz, V.; Montanes, M.; García-Antón, J.; Guinon, J. Effect of pH and chloride concentration on the removal of hexavalent chromium in a batch electrocoagulation reactor. J. Hazard. Mater. 2009, 169, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, T.-K.; Zoh, K.-D. Removal mechanism of heavy metal (Cu, Ni, Zn, and Cr) in the presence of cyanide during electrocoagulation using Fe and Al electrodes. J. Water Process Eng. 2020, 33, 101109. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.-R.; Liu, Y.-L.; Tang, Q. Removal of Cr ions from aqueous solution using batch electrocoagulation: Cr removal mechanism and utilization rate of in situ generated metal ions. Process Saf. Environ. Prot. 2016, 104, 436–443. [Google Scholar] [CrossRef]
- Verma, S.K.; Khandegar, V.; Saroha, A.K. Removal of chromium from electroplating industry effluent using electrocoagulation. J. Hazard. Toxic Radioact. Waste 2013, 17, 146–152. [Google Scholar] [CrossRef]
- Aber, S.; Amani-Ghadim, A.; Mirzajani, V. Removal of Cr (VI) from polluted solutions by electrocoagulation: Modeling of experimental results using artificial neural network. J. Hazard. Mater. 2009, 171, 484–490. [Google Scholar] [CrossRef]
- Akbal, F.; Camcı, S. Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 2011, 269, 214–222. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Golder, A.K.; Samanta, A.N.; Ray, S. Trivalent chromium removal by electrocoagulation and characterization of the process sludge. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 496–503. [Google Scholar] [CrossRef]
- Heidmann, I.; Calmano, W. Removal of Ni, Cu and Cr from a galvanic wastewater in an electrocoagulation system with Fe-and Al-electrodes. Sep. Purif. Technol. 2010, 71, 308–314. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Studies on the Al–Zn–In-alloy as anode material for the removal of chromium from drinking water in electrocoagulation process. Desalination 2011, 275, 260–268. [Google Scholar] [CrossRef]
- Mouedhen, G.; Feki, M.; De Petris-Wery, M.; Ayedi, H. Electrochemical removal of Cr (VI) from aqueous media using iron and aluminum as electrode materials: Towards a better understanding of the involved phenomena. J. Hazard. Mater. 2009, 168, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J. Removal of lead and zinc from battery industry wastewater using electrocoagulation process: Influence of direct and alternating current by using iron and stainless steel rod electrodes. Sep. Purif. Technol. 2014, 135, 165–175. [Google Scholar] [CrossRef]
- Elabbas, S.; Ouazzani, N.; Mandi, L.; Berrekhis, F.; Perdicakis, M.; Pontvianne, S.; Pons, M.-N.; Lapicque, F.; Leclerc, J.-P. Treatment of highly concentrated tannery wastewater using electrocoagulation: Influence of the quality of aluminium used for the electrode. J. Hazard. Mater. 2016, 319, 69–77. [Google Scholar] [CrossRef]
- Lu, J.; Fan, R.; Wu, H.; Zhang, W.; Li, J.; Zhang, X.; Sun, H.; Liu, D. Simultaneous removal of Cr (VI) and Cu (II) from acid wastewater by electrocoagulation using sacrificial metal anodes. J. Mol. Liq. 2022, 359, 119276. [Google Scholar] [CrossRef]
- Hanay, Ö.; Hasar, H. Effect of anions on removing Cu2+, Mn2+ and Zn2+ in electrocoagulation process using aluminum electrodes. J. Hazard. Mater. 2011, 189, 572–576. [Google Scholar] [CrossRef]
- Mahmad, M.K.N.; Rozainy, M.M.R.; Abustan, I.; Baharun, N. Electrocoagulation process by using aluminium and stainless steel electrodes to treat total chromium, colour and turbidity. Procedia Chem. 2016, 19, 681–686. [Google Scholar] [CrossRef]
- Merzouk, B.; Gourich, B.; Sekki, A.; Madani, K.; Chibane, M. Removal turbidity and separation of heavy metals using electrocoagulation–electroflotation technique: A case study. J. Hazard. Mater. 2009, 164, 215–222. [Google Scholar] [CrossRef]
- Oussedik, S.M.; Khelifa, A. Reduction of copper ions concentration in wastewaters of galvanoplastic industry by electroflotation. Desalination 2001, 139, 383. [Google Scholar] [CrossRef]
- Gao, P.; Chen, X.; Shen, F.; Chen, G. Removal of chromium (VI) from wastewater by combined electrocoagulation–electroflotation without a filter. Sep. Purif. Technol. 2005, 43, 117–123. [Google Scholar] [CrossRef]
- da Mota, I.d.O.; de Castro, J.A.; de Góes Casqueira, R.; de Oliveira Junior, A.G. Study of electroflotation method for treatment of wastewater from washing soil contaminated by heavy metals. J. Mater. Res. Technol. 2015, 4, 109–113. [Google Scholar] [CrossRef]
- Zodi, S.; Potier, O.; Lapicque, F.; Leclerc, J.-P. Treatment of the textile wastewaters by electrocoagulation: Effect of operating parameters on the sludge settling characteristics. Sep. Purif. Technol. 2009, 69, 29–36. [Google Scholar] [CrossRef]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent—A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, A.; Matis, K.; Lazaridis, N.; Golyshin, P. The use of biosurfactants in flotation: Application for the removal of metal ions. Miner. Eng. 2003, 16, 1231–1236. [Google Scholar] [CrossRef]
- Frenzel, I.; Holdik, H.; Barmashenko, V.; Stamatialis, D.F.; Wessling, M. Electrochemical reduction of dilute chromate solutions on carbon felt electrodes. J. Appl. Electrochem. 2006, 36, 323–332. [Google Scholar] [CrossRef]
- Rodriguez-Valadez, F.; Ortiz-Éxiga, C.; Ibanez, J.G.; Alatorre-Ordaz, A.; Gutierrez-Granados, S. Electroreduction of Cr (VI) to Cr (III) on reticulated vitreous carbon electrodes in a parallel-plate reactor with recirculation. Environ. Sci. Technol. 2005, 39, 1875–1879. [Google Scholar] [CrossRef]
- Almaguer-Busso, G.; Velasco-Martínez, G.; Carreño-Aguilera, G.; Gutiérrez-Granados, S.; Torres-Reyes, E.; Alatorre-Ordaz, A. A comparative study of global hexavalent chromium removal by chemical and electrochemical processes. Electrochem. Commun. 2009, 11, 1097–1100. [Google Scholar] [CrossRef]
- Wang, H.; Na, C. Binder-free carbon nanotube electrode for electrochemical removal of chromium. ACS Appl. Mater. Interfaces 2014, 6, 20309–20316. [Google Scholar] [CrossRef]
- Walsh, F.C. A First Course in Electrochemical Engineering; Electrochemical Consultancy: Romsey, UK, 1993. [Google Scholar]
- Jin, W.; Yan, K. Recent advances in electrochemical detection of toxic Cr (VI). RSC Adv. 2015, 5, 37440–37450. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Z.; Wu, G.; Tolba, R.; Chen, A. Integrated lignin-mediated adsorption-release process and electrochemical reduction for the removal of trace Cr (VI). RSC Adv. 2014, 4, 27843–27849. [Google Scholar] [CrossRef]
- Wei, C.; German, S.; Basak, S.; Rajeshwar, K. Reduction of hexavalent chromium in aqueous solutions by polypyrrole. J. Electrochem. Soc. 1993, 140, L60–L62. [Google Scholar] [CrossRef]
- Conroy, K.G.; Breslin, C.B. Reduction of hexavalent chromium at a polypyrrole-coated aluminium electrode: Synergistic interactions. J. Appl. Electrochem. 2004, 34, 191–195. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, L.; Zhou, X.; Wu, C. Electroreduction of hexavalent chromium using a polypyrrole-modified electrode under potentiostatic and potentiodynamic conditions. J. Hazard. Mater. 2012, 225, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, L.; Gubulin, J. Reduction of hexavalent chromium using polyaniline films. Effect of film thickness, potential and flow velocity on the reaction rate and polymer stability. J. Appl. Electrochem. 2003, 33, 1217–1222. [Google Scholar] [CrossRef]
- Ruotolo, L.; Liao, A. Reaction rate and electrochemical stability of conducting polymer films used for the reduction of hexavalent chromium. J. Appl. Electrochem. 2004, 34, 1259–1263. [Google Scholar] [CrossRef]
- Ruotolo, L.; Santos-Júnior, D.; Gubulin, J. Electrochemical treatment of effluents containing Cr (VI). Influence of pH and current on the kinetic. Water Res. 2006, 40, 1555–1560. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, G.; Zhang, G.; Wang, X.; Wang, S.; Yang, Y. The resistance to over-oxidation for polyaniline initiated by the resulting quinone-like molecules. Polym. Degrad. Stab. 2011, 96, 1799–1804. [Google Scholar] [CrossRef]
- Kulikov, S.; Kulikova, O.; Scharkova, O.; Maximovskaya, R.; Kozhevnikov, I. Use of electromembrane technology for waste water treatment and modern acidic catalyst synthesis. Desalination 1996, 104, 107–111. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; García-Gabaldón, M.; Pérez-Herranz, V. Study of the effects of the applied current regime and the concentration of chromic acid on the transport of Ni2+ ions through Nafion 117 membranes. J. Membr. Sci. 2012, 392, 137–149. [Google Scholar] [CrossRef]
- Nataraj, S.; Hosamani, K.; Aminabhavi, T. Potential application of an electrodialysis pilot plant containing ion-exchange membranes in chromium removal. Desalination 2007, 217, 181–190. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, L.; Ramírez, A.; Rodríguez-Torres, I. Cr (VI) removal by continuous electrodeionization: Study of its basic technologies. Desalination 2009, 249, 423–428. [Google Scholar] [CrossRef]
- Xu, T.; Huang, C. Electrodialysis-based separation technologies: A critical review. AIChE J. 2008, 54, 3147–3159. [Google Scholar] [CrossRef]
- Schlichter, B.; Mavrov, V.; Erwe, T.; Chmiel, H. Regeneration of bonding agents loaded with heavy metals by electrodialysis with bipolar membranes. J. Membr. Sci. 2004, 232, 99–105. [Google Scholar] [CrossRef]
- Liu, L.; Chen, H.; Yang, X.; Tan, W.; Liu, C.; Dang, Z.; Qiu, G. High-efficiency As (III) oxidation and electrocoagulation removal using hematite with a charge-discharge technique. Sci. Total Environ. 2020, 703, 135678. [Google Scholar] [CrossRef]
- Zaied, B.; Rashid, M.; Nasrullah, M.; Zularisam, A.; Pant, D.; Singh, L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Ingelsson, M.; Yasri, N.; Roberts, E.P. Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation—A review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef]
- Korngold, E.; Belayev, N.; Aronov, L. Removal of chromates from drinking water by anion exchangers. Sep. Purif. Technol. 2003, 33, 179–187. [Google Scholar] [CrossRef]
- Tiravanti, G.; Petruzzelli, D.; Passino, R. Pretreatment of tannery wastewaters by an ion exchange process for Cr (III) removal and recovery. Water Sci. Technol. 1997, 36, 197–207. [Google Scholar] [CrossRef]
- El-Moselhy, M.M.; Hakami, O.M. Selective removal of chromate using hybrid anion exchanger. Desalination Water Treat. 2015, 56, 2917–2924. [Google Scholar] [CrossRef]
- Zang, Y.; Yue, Q.; Kan, Y.; Zhang, L.; Gao, B. Research on adsorption of Cr (Ⅵ) by Poly-epichlorohydrin-dimethylamine (EPIDMA) modified weakly basic anion exchange resin D301. Ecotoxicol. Environ. Saf. 2018, 161, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Canning, W.; Co., L. The Canning Handbook: Surface Finishing Technology, Integrated Design; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Jardine, P.; Fendorf, S.; Mayes, M.; Larsen, I.; Brooks, S.; Bailey, W. Fate and transport of hexavalent chromium in undisturbed heterogeneous soil. Environ. Sci. Technol. 1999, 33, 2939–2944. [Google Scholar] [CrossRef]
- Agrawal, S.G.; Fimmen, R.L.; Chin, Y.-P. Reduction of Cr (VI) to Cr (III) by Fe (II) in the presence of fulvic acids and in lacustrine pore water. Chem. Geol. 2009, 262, 328–335. [Google Scholar] [CrossRef]
- Wang, Q.; Cissoko, N.; Zhou, M.; Xu, X. Effects and mechanism of humic acid on chromium (VI) removal by zero-valent iron (Fe0) nanoparticles. Phys. Chem. Earth Parts A/B/C 2011, 36, 442–446. [Google Scholar] [CrossRef]
- Rivero-Huguet, M.; Marshall, W.D. Influence of various organic molecules on the reduction of hexavalent chromium mediated by zero-valent iron. Chemosphere 2009, 76, 1240–1248. [Google Scholar] [CrossRef]
- Sun, J.; Mao, J.-D.; Gong, H.; Lan, Y. Fe (III) photocatalytic reduction of Cr (VI) by low-molecular-weight organic acids with α-OH. J. Hazard. Mater. 2009, 168, 1569–1574. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Liu, L.; Zong, R.; Yao, W.; Liang, Y.; Zhu, Y. Removal of Cr (VI) by 3D TiO2-graphene hydrogel via adsorption enriched with photocatalytic reduction. Appl. Catal. B Environ. 2016, 199, 412–423. [Google Scholar] [CrossRef]
- Naimi-Joubani, M.; Shirzad-Siboni, M.; Yang, J.-K.; Gholami, M.; Farzadkia, M. Photocatalytic reduction of hexavalent chromium with illuminated ZnO/TiO2 composite. J. Ind. Eng. Chem. 2015, 22, 317–323. [Google Scholar] [CrossRef]
- de Bittencourt, M.A.; Novack, A.M.; Scherer Filho, J.A.; Mazur, L.P.; Marinho, B.A.; da Silva, A.; de Souza, A.A.U.; de Souza, S.M.G.U. Application of FeCl3 and TiO2-coated algae as innovative biophotocatalysts for Cr (VI) removal from aqueous solution: A process intensification strategy. J. Clean. Prod. 2020, 268, 122164. [Google Scholar] [CrossRef]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, D.; Xu, T.; Shi, Y.; Song, L.; Yu, Z.-Z. Continuous photocatalytic removal of chromium (VI) with structurally stable and porous Ag/Ag3PO4/reduced graphene oxide microspheres. Chem. Eng. J. 2020, 379, 122200. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zhang, J.; Zuo, W.; Ding, Y. Efficient removal of chromium from water by Mn3O4@ ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Appl. Catal. B Environ. 2017, 214, 126–136. [Google Scholar] [CrossRef]
- Du, X.-D.; Yi, X.-H.; Wang, P.; Zheng, W.; Deng, J.; Wang, C.-C. Robust photocatalytic reduction of Cr (VI) on UiO-66-NH2 (Zr/Hf) metal-organic framework membrane under sunlight irradiation. Chem. Eng. J. 2019, 356, 393–399. [Google Scholar] [CrossRef]
- Qi, Y.; Fan, Y.; Liu, T.; Zheng, X. Flower-like hierarchical ZnS-Ga2S3 heterojunction for the adsorption-photo-reduction of Cr (VI). Chemosphere 2020, 261, 127824. [Google Scholar] [CrossRef]
- Tian, X.; Gao, X.; Yang, F.; Lan, Y.; Mao, J.-D.; Zhou, L. uCatalytic role of soils in the transformation of Cr (VI) to Cr (III) in the presence of organic acids containing α-OH groups. Geoderma 2010, 159, 270–275. [Google Scholar] [CrossRef]
- Idris, A.; Hassan, N.; Ismail, N.S.M.; Misran, E.; Yusof, N.M.; Ngomsik, A.-F.; Bee, A. Photocatalytic magnetic separable beads for chromium (VI) reduction. Water Res. 2010, 44, 1683–1688. [Google Scholar] [CrossRef]
- Nasrallah, N.; Kebir, M.; Koudri, Z.; Trari, M. Photocatalytic reduction of Cr (VI) on the novel hetero-system CuFe2O4/CdS. J. Hazard. Mater. 2011, 185, 1398–1404. [Google Scholar] [CrossRef]
- Wang, J.; Lv, Y.; Zhang, Z.; Deng, Y.; Zhang, L.; Liu, B.; Xu, R.; Zhang, X. Sonocatalytic degradation of azo fuchsine in the presence of the Co-doped and Cr-doped mixed crystal TiO2 powders and comparison of their sonocatalytic activities. J. Hazard. Mater. 2009, 170, 398–404. [Google Scholar] [CrossRef]
- Mu, R.; Xu, Z.; Li, L.; Shao, Y.; Wan, H.; Zheng, S. On the photocatalytic properties of elongated TiO2 nanoparticles for phenol degradation and Cr (VI) reduction. J. Hazard. Mater. 2010, 176, 495–502. [Google Scholar] [CrossRef]
- Yang, Q.-L.; Kang, S.-Z.; Chen, H.; Bu, W.; Mu, J. La2Ti2O7: An efficient and stable photocatalyst for the photoreduction of Cr (VI) ions in water. Desalination 2011, 266, 149–153. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Deng, K.; She, Y.; Yu, Y.; Tang, H. Visible light photocatalytic reduction of Cr (VI) on TiO2 in situ modified with small molecular weight organic acids. Appl. Catal. B Environ. 2010, 95, 400–407. [Google Scholar] [CrossRef]
- Kim, G.; Choi, W. Charge-transfer surface complex of EDTA-TiO2 and its effect on photocatalysis under visible light. Appl. Catal. B Environ. 2010, 100, 77–83. [Google Scholar] [CrossRef]
- Gherbi, R.; Nasrallah, N.; Amrane, A.; Maachi, R.; Trari, M. Photocatalytic reduction of Cr (VI) on the new hetero-system CuAl2O4/TiO2. J. Hazard. Mater. 2011, 186, 1124–1130. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, Y.; Liu, S.; Li, Y.; Cai, Q.; Luo, S.; Zeng, G. Photocatalytic reduction of Cr (VI) on WO3 doped long TiO2 nanotube arrays in the presence of citric acid. Appl. Catal. B Environ. 2010, 94, 142–149. [Google Scholar] [CrossRef]
- Qamar, M.; Gondal, M.; Yamani, Z. Synthesis of nanostructured NiO and its application in laser-induced photocatalytic reduction of Cr (VI) from water. J. Mol. Catal. A Chem. 2011, 341, 83–88. [Google Scholar] [CrossRef]
- Qamar, M.; Gondal, M.; Yamani, Z. Laser-induced efficient reduction of Cr (VI) catalyzed by ZnO nanoparticles. J. Hazard. Mater. 2011, 187, 258–263. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, A.; Kirrolia, A.; Kumar, R.; Yadav, N.; Bishnoi, N.R.; Lohchab, R.K. Removal of sulphate, COD and Cr (VI) in simulated and real wastewater by sulphate reducing bacteria enrichment in small bioreactor and FTIR study. Bioresour. Technol. 2011, 102, 677–682. [Google Scholar] [CrossRef]
- Cheung, K.; Gu, J.-D. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. Int. Biodeterior. Biodegrad. 2007, 59, 8–15. [Google Scholar] [CrossRef]
- Zahoor, A.; Rehman, A. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci. 2009, 21, 814–820. [Google Scholar] [CrossRef]
- Kathiravan, M.N.; Karthick, R.; Muthukumar, K. Ex situ bioremediation of Cr (VI) contaminated soil by Bacillus sp.: Batch and continuous studies. Chem. Eng. J. 2011, 169, 107–115. [Google Scholar] [CrossRef]
- Ng, T.W.; Cai, Q.; Wong, C.-K.; Chow, A.T.; Wong, P.-K. Simultaneous chromate reduction and azo dye decolourization by Brevibacterium casei: Azo dye as electron donor for chromate reduction. J. Hazard. Mater. 2010, 182, 792–800. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, Y.; Huang, H.; Shi, J.; Wu, K.; Zhang, P.; Lv, J.; Li, H.; He, H.; Liu, P. Simultaneous aerobic denitrification and Cr (VI) reduction by Pseudomonas brassicacearum LZ-4 in wastewater. Bioresour. Technol. 2016, 221, 121–129. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Deng, S.; Xu, J.; Nan, H.; Li, Z.; Song, J.-L. Simultaneous reduction of nitrate and Cr (VI) by Pseudomonas aeruginosa strain G12 in wastewater. Ecotoxicol. Environ. Saf. 2020, 191, 110001. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Luo, M.; Li, W.; Wei, X.; Xie, K.; Liu, L.; Jiang, C.; Liu, H. Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 stimulated with external electron donors under alkaline conditions. J. Hazard. Mater. 2011, 185, 1169–1176. [Google Scholar] [CrossRef]
- Xu, L.; Yang, L.; Luo, M.; Liang, X.; Wei, X.; Zhao, J.; Liu, H. Reduction of hexavalent chromium by Pannonibacter phragmitetus LSSE-09 coated with polyethylenimine-functionalized magnetic nanoparticles under alkaline conditions. J. Hazard. Mater. 2011, 189, 787–793. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, Y.; Li, F.-B.; Feng, C.-H. In-situ Cr (VI) reduction with electrogenerated hydrogen peroxide driven by iron-reducing bacteria. Bioresour. Technol. 2011, 102, 2468–2473. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wu, C.-L.; Li, H.-L.; Hsu, C.-H. Verification of model for adsorption and reduction of chromium (VI) by Escherichia coli 33456 using chitosan bead as a supporting medium. Appl. Math. Model. 2011, 35, 2736–2751. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.; Wang, J.; Jin, R.; Zhou, J.; Lv, H. Enhanced chromate reduction by resting Escherichia coli cells in the presence of quinone redox mediators. Bioresour. Technol. 2010, 101, 8127–8131. [Google Scholar] [CrossRef]
- Somasundaram, V.; Philip, L.; Bhallamudi, S.M. Laboratory scale column studies on transport and biotransformation of Cr (VI) through porous media in presence of CRB, SRB and IRB. Chem. Eng. J. 2011, 171, 572–581. [Google Scholar] [CrossRef]
- Mangaiyarkarasi, M.M.; Vincent, S.; Janarthanan, S.; Rao, T.S.; Tata, B. Bioreduction of Cr (VI) by alkaliphilic Bacillus subtilis and interaction of the membrane groups. Saudi J. Biol. Sci. 2011, 18, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ishino, Y.; Ogawa, A.; Tsutsumi, K.; Morita, H. Cr (VI) reduction from contaminated soils by Aspergillus sp. N2 and Penicillium sp. N3 isolated from chromium deposits. J. Gen. Appl. Microbiol. 2008, 54, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Gola, D.; Dey, P.; Bhattacharya, A.; Mishra, A.; Malik, A.; Namburath, M.; Ahammad, S.Z. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresour. Technol. 2016, 218, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, V.; Sengupta, S.; Chaudhuri, P.; Das, P. Assessment on removal efficiency of chromium by the isolated manglicolous fungi from Indian Sundarban mangrove forest: Removal and optimization using response surface methodology. Environ. Technol. Innov. 2018, 10, 335–344. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mahanty, S.; Das, P.; Chaudhuri, P.; Das, S. Biofabrication of iron oxide nanoparticles using manglicolous fungus Aspergillus niger BSC-1 and removal of Cr (VI) from aqueous solution. Chem. Eng. J. 2020, 385, 123790. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Hafiane, A.; Lemordant, D.; Dhahbi, M. Removal of hexavalent chromium by nanofiltration. Desalination 2000, 130, 305–312. [Google Scholar] [CrossRef]
- Gasemloo, S.; Khosravi, M.; Sohrabi, M.R.; Dastmalchi, S.; Gharbani, P. Response surface methodology (RSM) modeling to improve removal of Cr (VI) ions from tannery wastewater using sulfated carboxymethyl cellulose nanofilter. J. Clean. Prod. 2019, 208, 736–742. [Google Scholar] [CrossRef]
- Wu, S.; Ge, Y.; Wang, Y.; Chen, X.; Li, F.; Xuan, H.; Li, X. Adsorption of Cr (VI) on nano Uio-66-NH2 MOFs in water. Environ. Technol. 2018, 39, 1937–1948. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Yu, T.; Zhang, J.; Zhang, X.; Zhang, H.; Zhao, K.; Wei, J. Plant-mediated biosynthesis of iron nanoparticles-calcium alginate hydrogel membrane and its eminent performance in removal of Cr (VI). Chem. Eng. J. 2019, 378, 122120. [Google Scholar] [CrossRef]
- Koushkbaghi, S.; Zakialamdari, A.; Pishnamazi, M.; Ramandi, H.F.; Aliabadi, M.; Irani, M. Aminated-Fe3O4 nanoparticles filled chitosan/PVA/PES dual layers nanofibrous membrane for the removal of Cr (VI) and Pb (II) ions from aqueous solutions in adsorption and membrane processes. Chem. Eng. J. 2018, 337, 169–182. [Google Scholar] [CrossRef]
- Zhijiang, C.; Xianyou, S.; Qing, Z.; Yuanpei, L. Amidoxime surface modification of polyindole nanofiber membrane for effective removal of Cr (VI) from aqueous solution. J. Mater. Sci. 2017, 52, 5417–5434. [Google Scholar] [CrossRef]
- Jo, J.H.; Shin, S.S.; Jeon, S.; Park, S.-J.; Park, H.; Park, Y.-I.; Lee, J.-H. Star polymer-assembled adsorptive membranes for effective Cr (VI) removal. Chem. Eng. J. 2022, 449, 137883. [Google Scholar] [CrossRef]
- Okhovat, A.; Mousavi, S.M. Modeling of arsenic, chromium and cadmium removal by nanofiltration process using genetic programming. Appl. Soft Comput. 2012, 12, 793–799. [Google Scholar] [CrossRef]
- Ozaki, H.; Sharma, K.; Saktaywin, W. Performance of an ultra-low-pressure reverse osmosis membrane (ULPROM) for separating heavy metal: Effects of interference parameters. Desalination 2002, 144, 287–294. [Google Scholar] [CrossRef]
- Li, Y.; Lan, L.; Zhou, F.; Peng, J.; Guo, L.; Wang, F.; Zhang, Z.; Wang, L.; Mao, J. Flexible and easy-handling pristine polypyrrole membranes with bayberry-like vesicle structure for enhanced Cr (VI) removal from aqueous solution. J. Hazard. Mater. 2022, 439, 129598. [Google Scholar] [CrossRef]
- Malek, A.; Hachemi, M.; Didier, V. New approach of depollution of solid chromium leather waste by the use of organic chelates: Economical and environmental impacts. J. Hazard. Mater. 2009, 170, 156–162. [Google Scholar] [CrossRef]
- Shukla, M.; Baksi, B.; Mohanty, S.P.; Mahanty, B.; Mansi, A.; Rene, E.R.; Behera, S.K. Remediation of chromium contaminated soil by soil washing using EDTA and N-acetyl-L-cysteine as the chelating agents. Prog. Org. Coat. 2022, 165, 106704. [Google Scholar] [CrossRef]
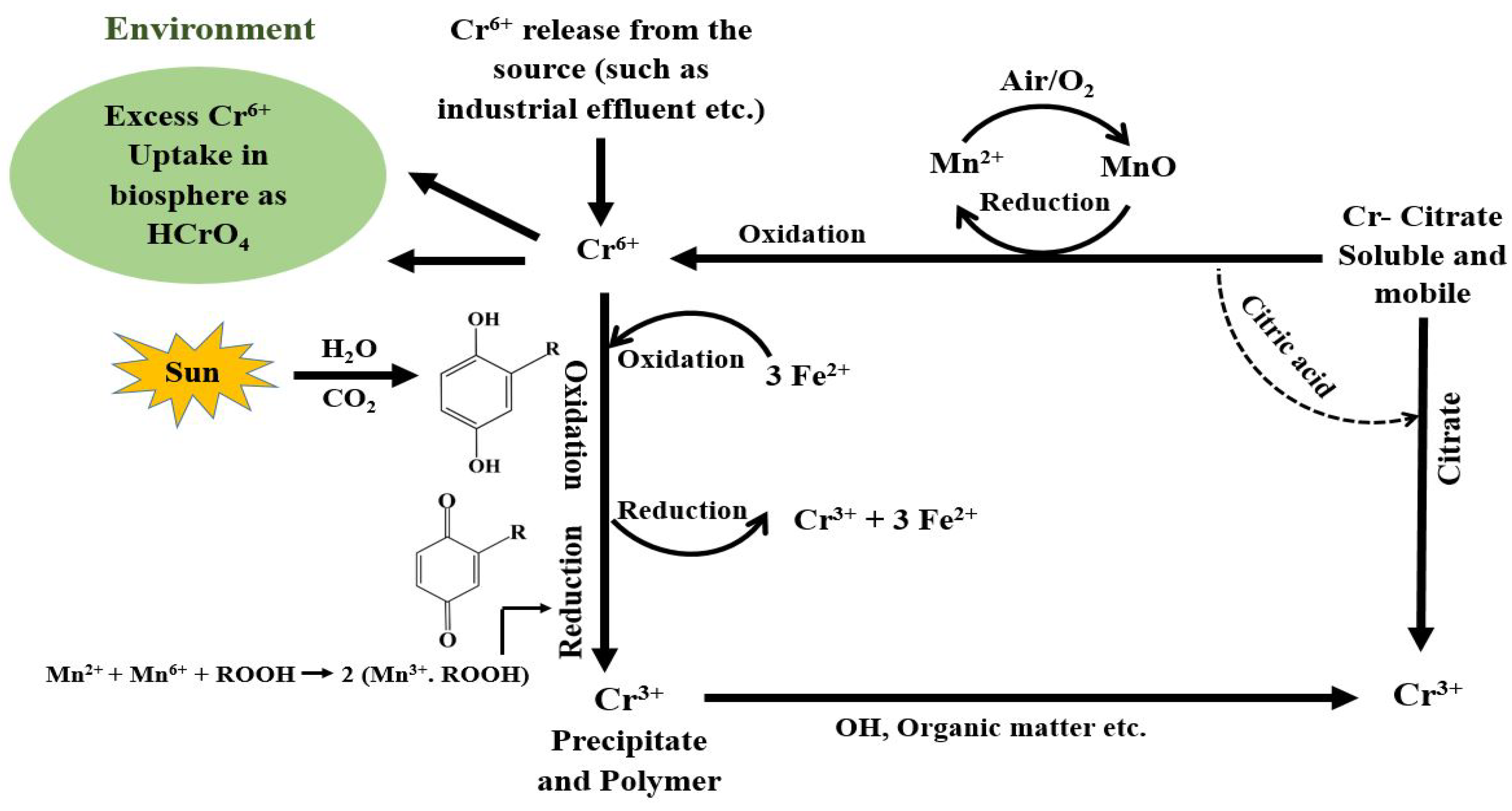
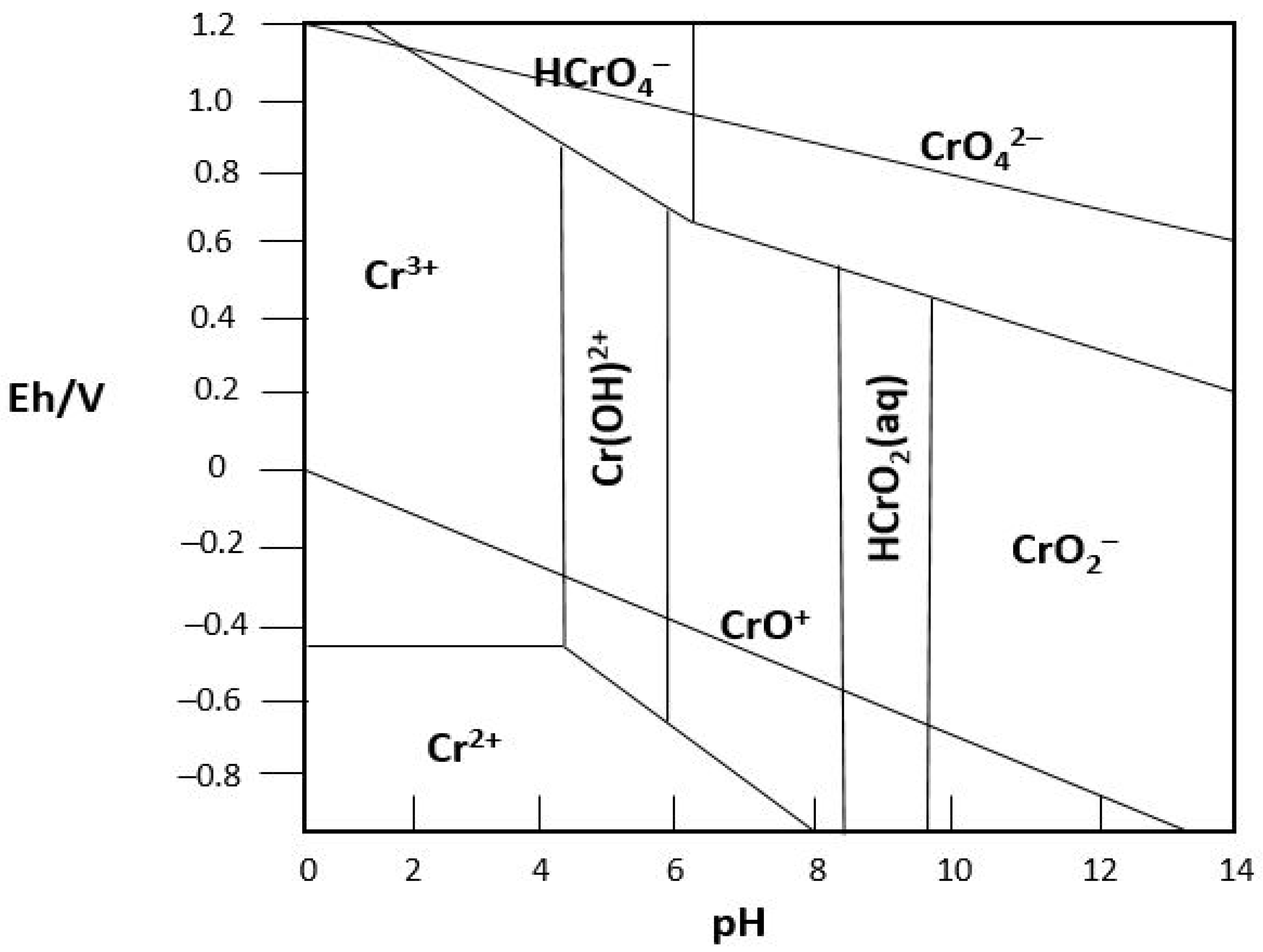
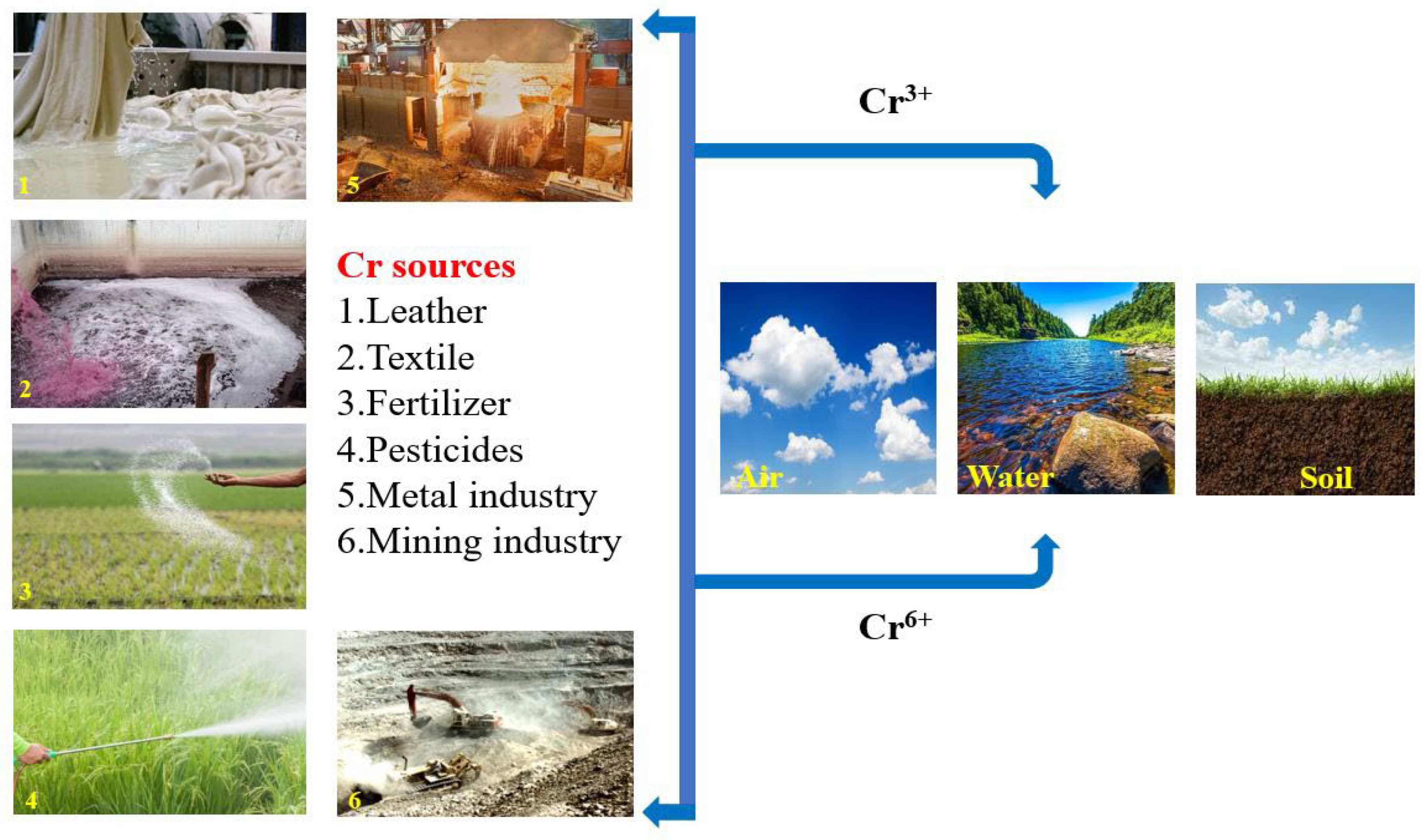
| Name of the Technique | Name of Methods | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Adsorption/Sorption | Adsorption |
|
| [34,35] |
| Electro-chemical treatment | Electrocoagulation |
|
| [36,37,38] |
| Electro-floatation |
|
| [39] | |
| Electro chemical reduction |
|
| [36,40] | |
| Physico-chemical treatment | Ion exchange method |
|
| [41,42,43,44,45] |
| Reduction process |
|
| ||
| Biological removal |
|
| [6] | |
| Membrane filtration | Reverse osmosis |
|
| [38,46] |
| Nano filter |
|
|
| Type of Adsorbent | Adsorbent | pH | Removal Quantity (mg/g) | Isotherm | References |
|---|---|---|---|---|---|
| Agricultural Wastes | Bagasse sugarcane | 2.0 | 13.4 | L, F | [62] |
| Bran rice | 2.0 | 285.7 | L, F | [63] | |
| Wheat bran | 2.1 | 35 | L | [64,65] | |
| Olive cake | 2.0 | 35.44 | L, F | [66] | |
| Sawdust Acacia arabica (pretreated) | 6.0 | 111.6 | L, F | [67] | |
| Shell Almond | 4.0 | 22.20 | L, F | [68] | |
| Lignin from paper Industry | 3.0 | 13.48 | L | [69] | |
| Soya cake | <1.0 | 0.28 | L, F | [70] | |
| Coir Coconut | 2.0 | 6.3 | L, F, R–P, T, etc. | [71] | |
| Husk and hull Bengal gram husk | 2.0 | 91.64 | L, F | [72] | |
| Acrylonitrile grafted banana peels | 3.0 | 96 a | L, F | [73] | |
| Rice husk | 5.0 | 38.4 | - | [74] | |
| Rye husk | 3.0 | 80 a | L | [75] | |
| Chitosan-coated banana and areca fiber | 4.5 | 75 a | - | [76] | |
| Rice husk | 6 | 92.99 a | L | [77] | |
| Saw dust | 2 | 100 a | F | [78] | |
| SCM-CC | 2 | 99.92 a | F | [79] | |
| Green walnut shell | 3.6 | 95 a | - | [61] | |
| Plants | Sugarcane bagasse | 1.9 | 23 | L, D, S | [80] |
| Sunflower stem waste (pretreated) | 2.0 | 5.37–4.81 | L, F, D-R | [81] | |
| London plane leaves | 3.0 | 68.03 | L | [82] | |
| Modified Pine sawdust | 2.0 | 30.49 | L, F | [83] | |
| Hazelnut shell | 3.5 | 8.28 | L, F | [84] | |
| Almond shell | 2.0 | 10.61 | L, F | [66] | |
| Rubber wood sawdust (Polyacrylamide grafted) | 3.0 | 22.6 | F | [85] | |
| Tradescantia pallida leaf | 2.0 | 94 a | L | [86] | |
| Caryota urens seeds | 2.0 | 52.63 | L | [87] | |
| Aminated rice straw-grafted-poly (vinyl alcohol) (A-RS/PVA) | 2.0 | 140.39 | E, F | [88] | |
| Paddy straw | 2.0 | 21.50 | L, D, S | [89] | |
| Rubber leaf | 1.5 | 96 a | - | [58] | |
| Algae | Scenedesmus sp. | 6.0 | 98.63 a | - | [90] |
| Scenedesmus quadri-cauda (Chlorophyta) | 6.0 | 98.3 a | L, F | [91] | |
| Sargassum myriocystum | 5.2 | 66.66 | L, T | [92] | |
| Cladophora glomerata | 2.0 | 66.6 | L, F | [93] | |
| Oedogonium hatei | 2.0 | 35.2 | L | [94] | |
| Sargassum sp | 2.0 | 39.61 | L | [95] | |
| Nostoc calcicola HH-12 | 3.0–4.0 | 12.23 | L | [96] | |
| Chroococcus sp. HH-11 | 3.0–4.0 | 21.36 | L | [96] | |
| Sargassum seaweed (marine algae) | 3.5 | 60 b | L, F | [97] | |
| Scenedesmus incrassalulus (green micro algae) | - | 52.7 a | - | [98] | |
| Spyrogyra species (green filamentous algae) | 2.0 | 90 a | L | [99] | |
| Fungi | Penicillium janthinellum | 1.0 | 58.6 a | F, D-R | [100] |
| Aspergillus niger | 2.0 | 11.79 | Tm, F | [101] | |
| Aspergillus niger | 3.0 | 95.7 a | - | [102] | |
| Aspergillus ustus | 2.0 | 6466.7 c | L, F | [103] | |
| Fusarium verticillioides | 2.0 | 6400.0 c | L, F | [103] | |
| Pencillium funiculosum | 2.0 | 3800.0 c | L, F | [103] | |
| Aspergillus niger | 3.0 | 96.3 a | - | [104] | |
| Agaricus bisporus | 1.0 | 8.0 | F | [105] | |
| Aspergillus niger | 2.0 | 17.92 | F | [106] | |
| Aspergillus sydoni | 2.0 | 9.07 | L | [106] | |
| Marine Aspergillus niger | 1.0 | 117.33 | L | [107] | |
| Basidiomycete, BDT-14 | 6.5 | 83.33 | L | [108] | |
| Aspergillus sp. (filamentous) | 2.0 | 10.0–27.5 | L, F | [109] | |
| Fusarium sp. (filamentous) | 5.0 | 18.2–71.0 | - | [110] | |
| Bacteria | Spirulina sp. | 5.0 | 90.91 | F | [111] |
| Eshcherichia coli and Staphylococcus epidermidis | 3.0–6.0 | 16.9 | L, F | [112] | |
| Rhodococcus opacus | 5 | 82 | F | [113] | |
| Rhodococcus rhodochrous | 5 | 62 | F | [113] | |
| Staphylococcus sp. and Pseudomonas sp. | 5 | L, F | [114] | ||
| Azotobacter beijreinckii | - | 26 a | - | [115] | |
| Bacillus subtilis | - | 48 a | - | [115] | |
| Bacillus licheniformis | 2.5 | 60.5 | L | [116] | |
| Bacillus subtilis | 2.0 | 14.54 | L | [117] | |
| Staphylococcus xylosus | 1.0 | 143 | L, F | [118] | |
| Pseudomonas sp. | 4.0 | 95 | L, F | [118] | |
| Rhodococcus opacus | 6.0 | 72.9 | L | [119] | |
| Streptomyces rimosus | 4.8 | 83 | L | [120] | |
| Bacillus circulans biofilm | 7.0 | 48 a | - | [121] | |
| Bacillus circulan | 2.5 | 34.5 | - | [122] | |
| Bacillus megaterium | 2.5 | 32 | - | [122] | |
| Bacillus coaglans | 2.5 | 23.8 | - | [123] | |
| Microbacterium liquuefaciens MP30 | - | 90 a | - | [124] | |
| Activated Carbon | Aegle marmelos fruit shell | 2.0 | 82.3 a | - | [59] |
| Animal bone charcoal | 2 | 92 a | - | [60] | |
| Cellulose-clay composite | 4 | 2.37 | - | [125] | |
| CHA/MFC | - | 2.208 d | - | [126] | |
| CNC/clay composite | 4 | 100 a | - | [127] | |
| Cellulose/chitosan composite | 4 | 56 a | - | [128] | |
| Clay-alumina ceramic membrane | - | 91.44 a | - | [129] | |
| MWCNTs-CTAB | 5 | 98 a | L | [130] | |
| MWCNTs-M-SLS | 5 | 99 a | L | [130] | |
| Chi@Fe3O4 | - | 142.32 | - | [131] | |
| Chi@Fe3O4GO | - | 100.51 | - | [131] | |
| FeNi@HPC | 4 | 30 b | - | [132] | |
| Miscellaneous | Shale waste rock | 3–6 | 90–91 a | L, F | [133] |
| Iron/biochar beads (FMIB) | 4 | 87.7 a | L | [134] | |
| Zn and Al modified pristine hydrochar | 2–4 | 65 a (Zn-HC) 50 a (Al-HC) | L | [135] | |
| CDGF | 3 | 99.8 a | - | [136] | |
| m-phenylenediamine-modified magnetic chitosan | <4 | 227.27 | - | [137] | |
| TWNP | 3.0 | 59.88 (64 a) | L | [53] | |
| HFCM | 4.0 | 40 a | - | [57] | |
| Brown clay | 4.0 | 90 a | - | [54] | |
| Clinoptilolite | 4.0 | 85 a | - | [56] |
| Adsorbents | pH | Removing Capacities (mg/g) | References |
|---|---|---|---|
| Iron (nanoparticles) | 7 | - | [152] |
| Maghemite (10 nm) | 2.5 | - | [153] |
| Akaganeite (3−6 nm) | 5.5 | 80 | [154] |
| MnFe2O4 (10 nm) | 2 | 31.5 | [155] |
| Carbon nanotube-supported ceria (6 nm) | 3.0–7.4 | 30.2 | [153] |
| δ-FeOOH coated maghemite γ-Fe2O3 (15 nm) | 2.5 | 25.8 | [156] |
| Activated charcoal cloth | 1 | 5 mmol/g | [157] |
| Coconut shell | 2–6 | 75.0–107.1 | [158] |
| Wood | 2–6 | 71.6–87.6 | [158] |
| Dust coal | 2–6 | 60.5–101.9 | [158] |
| Sawdust and waste tires (0.38 mm) | 2 | 30–43 | [159] |
| Cactus | 2 | 8.5–34.5 | [66] |
| Wool | 2 | 8.5–34.5 | [66] |
| Charcoal | 2 | 8.5–34.5 | [66] |
| Pine needles | 2 | 8.5–34.5 | [66] |
| Hazelnut Shell | 2.5–3.5 | 17.7 | [160] |
| Single-walled Carbon Nanotubes (SWCNT) | 2.5 | 2.35 | [161] |
| Multi-walled Carbon Nanotubes (MWCNT) | 2.5 | 1.26 | [161] |
| Unfunctionalized MWCNT | 6 | 98 a | [162] |
| Oxidized MWCNT | 2.05 | 4.26 | [163] |
| MWCNTs/nano iron oxide | 5–6 | - | [164] |
| Modified MWCNT | 7 | [165] | |
| Activated carbon-coated CNT | 4 | 9.0 | [166] |
| Waste eggshell | 6–12 | 90 a | [167] |
| Chitosan-coated acid-treated seed shells | 4.5–5.0 | 60–85 | [168] |
| Green algae (Ulva lactuca) | 1 | 10.61 | [169] |
| Activated carbon from green algae (Ulva lactuca) | 1 | 112.36 | [169] |
| Biofilm of E. coli supported on NaY zeolite | 4.6–5.1 | >85.5 a | [170] |
| Pseudomonas aeruuginosa immobilized MWCNT | 8.5–9.5 | 6.23 | [171] |
| Saccharomyces carlsbergensis immobilized on amberlite | 8 | 95 a | [172] |
| Wallnut shell | 3.5 | 8.01 | [84] |
| Hazelnut shell | 3.5 | 8.28 | [84] |
| Almond shell | 3.2 | 3.40 | [84] |
| Ficus carica biosorbent | 1.0–3.0 | 19.68 | [173] |
| Straw from Triticum aestivum | 5 | 21 | [174] |
| Wheat Bran from Triticum aestivum | >4 | 35 | [64] |
| Wheat Bran from Triticum aestivum | 1 | 40.8 | [175] |
| Wheat Bran from Triticum aestivum | 2 | 310.58 | [176] |
| Wheat Bran from Triticum aestivum | 2 | 0.942 | [177] |
| Wheat Bran from Triticum aestivum | 5 | 93 | [178] |
| Coconut coir | 1–5 | 26.6–27 | [71] |
| Sawdust | 3 | 1.48 | [179] |
| Rice husks | 3 | 0.63 | [179] |
| Coirpith | 3 | 0.16 | [179] |
| Vermiculite | 3 | 0.26 | [179] |
| Spirogyra condensata | 5 | 14 | [96] |
| Rhizoclonium hieroglyphicum | 4 | 11.81 | [96] |
| Chlorella vulgaris | 1–5 | 2.98 | [180] |
| Clodophara crispata | 1–2 | 6.20 | [180] |
| Zoogloea ramigera | 1–2 | 3.40 | [180] |
| Rhizopus arrhizus | 1–2 | 8.40 | [180] |
| Saccharomyces cerevisiae | 1–2 | 4.30 | [180] |
| Chitosan-ceramic alumina composite | 4 | 153.85 | [181] |
| Surfactant-modified coconut coir pith | 2 | 76.3 | [182] |
| Cross-linked xanthated chitosan beds (CMBC) | 3 | 256.4 | [183] |
| Cross-linked xanthated chitosan flakes (CMCF) | 3 | 625 | [183] |
| Eggshells | 3.54 | 81.47 a | [184] |
| Carrot residue | 4 | 45.09 | [185] |
| Fungal biomass | 2 | 119.2 | [186] |
| Chitosan-coated fly ash | 5 | 33.27 | [187] |
| Grape waste | 4 | 1.91 mol/kg | [188] |
| Dundiella algae | 2 | 45.5 | [189] |
| Pine Needles powder | 3 | 40 | [190] |
| Laminaria japonica | 1 | 96.31 | [175] |
| P. yezoensis Ueda | 1 | 95.81 | [175] |
| Rice bran | 1 | 95.35 | [175] |
| PAN-CNTs-TiO2-NH2 composite | 2 | 80 a | [191] |
| Hydrophobic magnetic adsorbent based on polypyrrole coating on acid-dissolved fly ash (MSFA/PPy) | - | 66.93−119.33 | [151] |
| MWCNTs-COOH | 2 | 143–164 | [192] |
| LDHs@MoS2 | 5 | 76.3 | [193] |
| Shrimp shell and waste cotton rags | 5 | 93 a | [138] |
| PANI-NC | 6 | 92.59 | [194] |
| FeNi@HPC | 4 | 30 b | [132] |
| Cellulose and chitosan composite | 4 | 60 b | [128] |
| Red mud and rice straw | 6 | 97.74 a | [195] |
| Carbonized chitosan into triethylenetetramine-modified sodium alginate (CTS/CS-50) | 1 | 144.49 | [196] |
| nZVI/ZIF-8 | 5 | >99 a | [197] |
| Types of Electrode | pH | Removal (%) | Current Density | References |
|---|---|---|---|---|
| Fe-S 304 | 6.9 | 97 | 50 A m−2 | [233] |
| Al-S 304 | 5 | 15 | 50 A m−2 | [233] |
| Fe-Al | 3 | 100 | 10 mA cm−2 | [234] |
| Fe-Fe | 9.56 | 100 | 4 mA cm−2 | [235] |
| Mild S | 5.91 | 99 | 1000 mA | [236] |
| Al-Fe | 4 | 99 | - | [237] |
| Al alloy-galvanized Fe | 7 | 98.2 | 0.2 A dm−2 | [238] |
| Fe-Fe, Al-Al, Al, Pt, Ti, Pt/Ti/Fe, | <0.5 mg/L | 1 Am−2 | [239] | |
| Fe-Al | 7–9 | >90 | 2 mA cm−2 | [240] |
| Fe-Fe | 4 | 100 | 50 mA cm−2 | [241] |
| Fe-Al | 2 | 100 | 0.73 mA cm−2 | [242] |
| Al-Al/Cu/Mg alloy | 5.3 | 99 | 400 Am−2 | [243] |
| Al-Al | 3.5–4.0 | 90–99.8 | 11.57 Am−2 | [244] |
| Electron Donor | Type/Source of Catalyst | Reduction (%) | Contact Time | Radiation | References |
|---|---|---|---|---|---|
| Tartaric, citric, malic, and n-butyric acids | Diluted and Fe(III) adsorbed onto clay | 100 | 7–80 min | Visible Light | [289] |
| Tartaric and citric acids | Soils | 100 | 4 h | Mimic Solar | [299] |
| Alginate | γ-Fe2O3 nanoparticles | ≈100 | 50 min | Sunlight | [300] |
| Salicylic acid | CuFe2O4 nanoparticles | 60 | 2.8 h | - | [301] |
| TiO2 powder | 95 | 2 h | visible light | [302] | |
| - | TiO2 nanoparticles modified with C60(CHCOOH)2 | 97 | 1.5 h | UV radiation | [303] |
| - | La2Ti2O7 and salts (NaCl, KCl, CaCl2, MgCl2, Na2SO4) | 98 | 3 h | UV light | [304] |
| Salicylic acid | TiO2 powder | - | 300–900 min | UV 253.7 nm | [305] |
| Methanol, formic acid, acetic acid, triethanolamine, EDTA | TiO2 | 100 | - | 550 nm, visible light | [306] |
| Salicylic acid | CuAl2O4/TiO2 | 95 | 3 h | visible light, 1.7–2.5 eV | [307] |
| Citric acid | WO dopped TiO2 nanotube | - | - | UV light | [308] |
| Other photocatalysts Added electron donor | NiO nanoparticles | 90 | 75 min | Laser Radiation | [309] |
| Added electron donor | ZnO nanoparticles | 95 | 60 min | Laser Radiation | [310] |
| Ag/Ag3PO4/reduced graphene oxide microspheres | 90 | 30 h | Visible light | [295] | |
| Mn3O4@ZnO | 95.3 | 110 min | Sunlight | [296] | |
| UiO-66-NH2(Zr/Hf) | 94 | 120 min | Visible light | [297] | |
| ZnS-Ga2S3-3 | 99.1 | 160 min | Solar light | [298] |
| Species | Initial Conc. of Cr6+ | pH | Temperature (°C) | Period (Hours) | Reduction (%) | References |
|---|---|---|---|---|---|---|
| Pseudomonas brassicacearum LZ-4 | 10 a | 7.5 | 37 | - | 80 | [316] |
| Brevibacterium casei | 5 b | 7 | 35 | - | 83.4 ± 0.6 | [315] |
| Bacillus sp. JDM-2-1 | 100 a | 7 | 37 | 96 | 85 | [313] |
| Staphylococcus capitis | 100 a | 7 | 37 | 96 | 81 | [313] |
| Pseudomonas aeruginosa strain G12 | 10 a | 7 | 30 | 72 | 93 | [317] |
| Pannonibacter phragmitetus | 1000 a | 9 | 37 | 24 | 100 | [318] |
| Pannonibacter phragmitetus LSSE-09 coated with polyethylenimine-functionalized magnetic nanoparticles | 350 a | 9 | 37 | 0.33 | 100 | [319] |
| Resting Escherichia coli cells | 150 a | 7 | - | 4 | 97.5 | [322] |
| Bacillus subtilis | 50 a | 9 | 30 | 65 | 100 | [324] |
| Beauveria bassiana | 30 a | 7 | 30 | 12 | 61.1 | [326] |
| Aspergillus sp. | 50 a | 4 | 27 | - | 98.96 | [327] |
| Iron oxide nanoparticles fabricated Aspergillus niger BSC-1 | 10 a | 3 | 40 | 2 | 99.75 | [328] |
| Initial Concentration (mg/L) | Application/Membrane Type | Pressure (bar) | pH | Removal % | References |
|---|---|---|---|---|---|
| 0.4 | Nanofilter | 14 | 7.1 | 95 | [331] |
| 0.4 | Nanofilter | 5 | 7.1 | 85 | [331] |
| 0.1 | Nanofilter | 5 | 7.1 | 52.7 | [331] |
| 0.05 | Reverse osmosis | 100 | N. A. | 98.3 | [332] |
| 0.05 | Reverse osmosis | 500 | N. A. | 100 | [332] |
| 0.05 | Reverse osmosis | 3.5 | 3 | 99.01 | [332] |
| 0.05 | Reverse osmosis | 3.5 | 9 | >99.9 | [332] |
| 10,000 | Reverse osmosis | 200 psi | 6–7 | 100 | [32] |
| SCMC-GA-NF | Nanofilter | 3 | 4 | 79.85 | [333] |
| Nano Uio-66-NH2 | Nanofilter | - | 6.5 | 32.36 a | [334] |
| FeNPs-CaAlg | Hydrogel | 5.41 | 99.5 | [335] | |
| Aminated-Fe3O4 chitosan/polyvinyl alcohol-PES | Nanofilter | - | 3 | 509.7 a | [336] |
| SAMPI | - | - | 3 | [337] | |
| PAH-TFC | - | - | 3 | 99.5 | [338] |
| PPy-B | - | - | 2 | 586.9 a | [339] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.M.; Mohana, A.A.; Rahman, M.A.; Rahman, M.; Naidu, R.; Rahman, M.M. A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution. Toxics 2023, 11, 252. https://doi.org/10.3390/toxics11030252
Islam MM, Mohana AA, Rahman MA, Rahman M, Naidu R, Rahman MM. A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution. Toxics. 2023; 11(3):252. https://doi.org/10.3390/toxics11030252
Chicago/Turabian StyleIslam, Md. Monjurul, Anika Amir Mohana, Md. Aminur Rahman, Mahbubur Rahman, Ravi Naidu, and Mohammad Mahmudur Rahman. 2023. "A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution" Toxics 11, no. 3: 252. https://doi.org/10.3390/toxics11030252
APA StyleIslam, M. M., Mohana, A. A., Rahman, M. A., Rahman, M., Naidu, R., & Rahman, M. M. (2023). A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution. Toxics, 11(3), 252. https://doi.org/10.3390/toxics11030252









