Exposure to Phthalates in European Children, Adolescents and Adults since 2005: A Harmonized Approach Based on Existing HBM Data in the HBM4EU Initiative
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Harmonization
2.2. Statistical Analysis
3. Results
3.1. BBzP, DEHP, DnBP, DiBP, and DEP (Category A Phthalates)
3.2. DnOP, DnPeP, DCHP, DPHP, DMP, 3cx-MPP, DiNP, DiDP (Category B Phthalates)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Phthalate Diester | Acronym | Classification as Reproductive Toxicant According to Regulation (EC) No 1272/2008 | Identification as SVHC under REACH | REACH | CoRAP |
|---|---|---|---|---|---|
| Di(2-ethylhexyl) phthalate | DEHP | Repr. 1B (2001) | Toxic for reproduction (2008); Endocrine disrupting properties ENV, HH (2014, 2017) | Subject to Authorization since 2015 (Annex XVI); Restriction in toys, childcare (2005) and any plasticized articles since 2020 (Annex XVII) | |
| Di-n-butyl phthalate | DnBP | Repr. 1B (2001) | Toxic for reproduction (2008); Endocrine disrupting properties HH (2017) | Restriction in toys, childcare (2005) and any plasticized articles since 2020 (Annex XVII) | |
| Di-isobutyl phthalate | DiBP | Repr. 1B (2009) | Toxic for reproduction (2008); Endocrine disrupting properties HH (2017) | Subject to Authorization since 2015 (Annex XVI); Restriction in toys, childcare (2005) and any plasticized articles since 2020 | |
| Butyl benzyl phthalate | BBzP | Repr. 1B (2004) | Toxic for reproduction (2008); Endocrine disrupting properties HH (2017) | Subject to Authorization since 2015 (Annex XVI); Restriction in toys, childcare (2005) and any plasticized articles since 2020 (Annex XVII) | |
| Diethyl phthalate | DEP | No ED properties HH | |||
| Di-isodecyl phthalate (and 1 DPHP) | DiDP | Restriction in toys, childcare articles since 2005 (Annex XVII) 2 | |||
| Di-isononyl phthalate | DiNP | Restriction in toys, childcare articles since 2005 (Annex XVII) | |||
| Bis(2-propylheptyl) phthalate | DPHP | Evaluation for ED potential | |||
| Dicyclohexyl phthalate | DCHP | Repr. 1B (2016) | Toxic for reproduction (2018); Endocrine disrupting properties HH (2018) | Evaluation for ED properties ENV | |
| Dimethyl phthalate | DMP | ||||
| Di-n-pentyl phthalate | DnPeP | Repr. 1B (2004) | Toxic for reproduction (2013) | Subject to Authorization since 2020 (Annex XVI) | |
| Di-n-octyl phthalate | DnOP | Restriction in toys, childcare articles since 2005 (Annex XVII) |
References
- Angerer, J.; Bird, M.G.; Burke, T.A.; Doerrer, N.G.; Needham, L.; Robison, S.H.; Sheldon, L.; Zenick, H. Strategic Biomonitoring Initiatives: Moving the Science Forward. Toxicol. Sci. 2006, 93, 3–10. [Google Scholar] [CrossRef]
- Bevan, R.; Angerer, J.; Cocker, J.; Jones, K.; Koch, H.M.; Sepai, O.; Schoeters, G.; Smolders, R.; Levy, L. Framework for the development and application of environmental biological monitoring guidance values. Regul. Toxicol. Pharmacol. 2012, 63, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Louro, H.; Heinälä, M.; Bessems, J.; Buekers, J.; Vermeire, T.; Woutersen, M.; Van Engelen, J.; Borges, T.; Rousselle, C.; Ougier, E.; et al. Human biomonitoring in health risk assessment in Europe: Current practices and recommendations for the future. Int. J. Hyg. Environ. Health 2019, 222, 727–737. [Google Scholar] [CrossRef] [PubMed]
- WHO. Human Biomonitoring: Facts and Figures; WHO Regional Office for Europe: Copenhagen, Denmark, 2015.
- Casteleyn, L.; Dumez, B.; Becker, K.; Kolossa-Gehring, M.; Den Hond, E.; Schoeters, G.; Castaño, A.; Koch, H.M.; Angerer, J.; Esteban, M.; et al. A pilot study on the feasibility of European harmonized human biomonitoring: Strategies towards a common approach, challenges and opportunities. Environ. Res. 2015, 141, 3–14. [Google Scholar] [CrossRef]
- Joas, A.; Knudsen, L.E.; Kolossa-Gehring, M.; Sepai, O.; Casteleyn, L.; Schoeters, G.; Angerer, J.; Castaño, A.; Aerts, D.; Biot, P.; et al. Policy recommendations and cost implications for a more sustainable framework for European human biomonitoring surveys. Environ. Res. 2015, 141, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Göen, T.; Seiwert, M.; Conrad, A.; Pick-Fuss, H.; Müller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef]
- Ganzleben, C.; Antignac, J.P.; Barouki, R.; Castano, A.; Fiddicke, U.; Klanova, J.; Lebret, E.; Olea, N.; Sarigiannis, D.; Schoeters, G.R.; et al. Human biomonitoring as a tool to support chemicals regulation in the European Union. Int. J. Hyg. Environ. Health 2017, 220, 94–97. [Google Scholar] [CrossRef]
- Ougier, E.; Ganzleben, C.; Lecoq, P.; Bessems, J.; David, M.; Schoeters, G.; Lange, R.; Meslin, M.; Uhl, M.; Kolossa-Gehring, M.; et al. Chemical prioritisation strategy in the European Human Biomonitoring Initiative (HBM4EU)—Development and results. Int. J. Hyg. Environ. Health 2021, 236, 113778. [Google Scholar] [CrossRef]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2063–2078. [Google Scholar] [CrossRef]
- Schütze, A.; Kolossa-Gehring, M.; Apel, P.; Brüning, T.; Koch, H.M. Entering markets and bodies: Increasing levels of the novel plasticizer Hexamoll® DINCH® in 24h urine samples from the German Environmental Specimen Bank. Int. J. Hyg. Environ. Health 2014, 217, 421–426. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a Request Related to a 12th List of Substances for Food Contact Materials; Question N°EFSA-Q-2004-073, EFSA-Q-2006-024, EFSA-Q-2005-238a, EFSA-Q-2005-238b, EFSA-Q-2005-238c, EFSA-Q-2006-012, EFSA-Q-2006-027; EFSA: Parma, Italy, 2006; pp. 1–21.
- Lange, R.; Vogel, N.; Schmidt, P.; Gerofke, A.; Luijten, M.; Bil, W.; Santonen, T.; Schoeters, G.; Gilles, L.; Sakhi, A.K.; et al. Cumulative risk assessment of five phthalates in European children and adolescents. Int. J. Hyg. Environ. Health 2022, 246, 114052. [Google Scholar] [CrossRef] [PubMed]
- Gerofke, A.; David, M.; Schmidt, P.; Vicente, J.L.; Buekers, J.; Gilles, L.; Colles, A.; Bessems, J.; Bastiaensen, M.; Covaci, A.; et al. From science to policy: How European HBM indicators help to answer policy questions related to phthalates and DINCH exposure. Int. J. Hyg. Environ. Health 2022, 247, 114073. [Google Scholar] [CrossRef]
- Vogel, N.; Frederiksen, H.; Lange, R.; Jorgensen, N.; Koch, H.M.; Weber, T.; Andersson, A.; Kolossa-Gehring, M. Urinary excretion of phthalates and the substitutes DINCH and DEHTP in Danish and German young adults between 2000 and 2017—A time trend analysis. Int. J. Hyg. Environ. Health 2023, 248, 114080. [Google Scholar] [CrossRef] [PubMed]
- Vogel, N.; Schmidt, P.; Lange, R.; Gerofke, A.; Sakhi, A.K.; Haug, L.S.; Jensen, T.K.; Frederiksen, H.; Szigeti, T.; Csákó, Z.; et al. Current Exposure to Phthalates and DINCH in European Children and Adolescents—Results from the HBM4EU Aligned Studies 2014 to 2021. Int. J. Hyg. Environ. Health 2023, 249, 114101. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Govarts, E.; Willems, H.; Smolders, R.; Casteleyn, L.; Kolossa-Gehring, M.; Schwedler, G.; Seiwert, M.; Fiddicke, U.; Castaño, A.; et al. First Steps toward Harmonized Human Biomonitoring in Europe: Demonstration Project to Perform Human Biomonitoring on a European Scale. Environ. Health Perspect. 2015, 123, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Vorkamp, K.; Castaño, A.; Antignac, J.-P.; Boada, L.D.; Cequier, E.; Covaci, A.; Esteban López, M.; Haug, L.S.; Kasper-Sonnenberg, M.; Koch, H.M.; et al. Biomarkers, matrices and analytical methods targeting human exposure to chemicals selected for a European human biomonitoring initiative. Environ. Int. 2021, 146, 106082. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Public Health. Environmental Health Monitoring System in the Czech Republic—Summary Report 2016; National Institute of Public Health: Prague, Czech Republic, 2017; ISBN 978-80-7071-365-5. [Google Scholar]
- Puklová, V.; Janoš, T.; Sochorová, L.; Vavrouš, A.; Vrbík, K.; Fialová, A.; Hanzlíková, L.; Černá, M. Exposure to Mixed Phthalates in Czech Preschool and School Children. Arch. Environ. Contam. Toxicol. 2019, 77, 471–479. [Google Scholar] [CrossRef]
- Černá, M.; Malý, M.; Rudnai, P.; Középesy, S.; Náray, M.; Halzlová, K.; Jajcaj, M.; Grafnetterová, A.; Krsková, A.; Antošová, D.; et al. Case study: Possible differences in phthalates exposure among the Czech, Hungarian, and Slovak populations identified based on the DEMOCOPHES pilot study results. Environ. Res. 2015, 141, 118–124. [Google Scholar] [CrossRef]
- National Institute of Public Health. Environmental Health Monitoring System in the Czech Republic—Summary Report 2018; National Institute of Public Health: Prague, Czech Republic, 2019; ISBN 978-80-7071-384-6. [Google Scholar]
- Hertz-Picciotto, I.; Trnovec, T.; Koèan, A.; Charles, M.J.; Èižnar, P.; Langer, P.; Sovèikova, E.; James, R.A. PCBs and Early Childhood Development in Slovakia: Study Design and Background. Fresenius Environ. Bull. 2003, 12, 208–214. [Google Scholar]
- Cutanda, F.; Koch, H.M.; Esteban, M.; Sánchez, J.; Angerer, J.; Castaño, A. Urinary levels of eight phthalate metabolites and bisphenol A in mother–child pairs from two Spanish locations. Int. J. Hyg. Environ. Health 2015, 218, 47–57. [Google Scholar] [CrossRef]
- Runkel, A.A.; Snoj-Tratnik, J.; Mazej, D.; Horvat, M. Urinary phthalate concentrations in the slovenian population: An attempt to exposure assessment of family units. Environ. Res. 2020, 186, 109548. [Google Scholar] [CrossRef]
- Frederiksen, H.; Nielsen, J.K.S.; Mørck, T.A.; Hansen, P.W.; Jensen, J.F.; Nielsen, O.; Andersson, A.-M.; Knudsen, L.E. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother–child pairs. Int. J. Hyg. Environ. Health 2013, 216, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Boas, M.; Frederiksen, H.; Feldt-Rasmussen, U.; Skakkebæk, N.E.; Hegedüs, L.; Hilsted, L.; Juul, A.; Main, K.M. Childhood Exposure to Phthalates: Associations with Thyroid Function, Insulin-like Growth Factor I, and Growth. Environ. Health Perspect. 2010, 118, 1458–1464. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jensen, T.K.; Jørgensen, N.; Kyhl, H.B.; Husby, S.; Skakkebæk, N.E.; Main, K.M.; Juul, A.; Andersson, A.M. Human urinary excretion of non-persistent environmental chemicals: An overview of Danish data collected between 2006 and 2012. Reproduction 2014, 147, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Nielsen, O.; Koch, H.M.; Skakkebaek, N.E.; Juul, A.; Jørgensen, N.; Andersson, A.-M. Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009–2017. Int. J. Hyg. Environ. Health 2020, 223, 93–105. [Google Scholar] [CrossRef]
- Joensen, U.N.; Frederiksen, H.; Blomberg Jensen, M.; Lauritsen, M.P.; Olesen, I.A.; Lassen, T.H.; Andersson, A.M.; Jørgensen, N. Phthalate excretion pattern and testicular function: A study of 881 healthy Danish men. Environ. Health Perspect. 2012, 120, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; Sabaredzovic, A.; Cequier, E.; Thomsen, C. Phthalate metabolites in Norwegian mothers and children: Levels, diurnal variation and use of personal care products. Sci. Total Environ. 2017, 599–600, 1984–1992. [Google Scholar] [CrossRef]
- Larsson, K.; Ljung Björklund, K.; Palm, B.; Wennberg, M.; Kaj, L.; Lindh, C.H.; Jönsson, B.A.G.; Berglund, M. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ. Int. 2014, 73, 323–333. [Google Scholar] [CrossRef]
- Hartmann, C.; Uhl, M.; Weiss, S.; Koch, H.M.; Scharf, S.; König, J. Human biomonitoring of phthalate exposure in Austrian children and adults and cumulative risk assessment. Int. J. Hyg. Environ. Health 2015, 218, 489–499. [Google Scholar] [CrossRef]
- Hohenblum, P.; Steinbichl, P.; Raffesberg, W.; Weiss, S.; Moche, W.; Vallant, B.; Scharf, S.; Haluza, D.; Moshammer, H.; Kundi, M.; et al. Pollution gets personal! A first population-based human biomonitoring study in Austria. Int. J. Hyg. Environ. Health 2012, 215, 176–179. [Google Scholar] [CrossRef]
- The Belgian Steering Committee on HBM. DEMOCOPHES NATIONAL REPORT ON HUMAN BIOMONITORING IN MOTHERS AND CHILDREN BELGIUM; FPS n°1 – D17 – A52. January. 2013. Available online: https://www.leefmilieu-gezondheid.be/sites/default/files/public/content/democophes_samenvatting_1.pdf (accessed on 26 September 2022).
- Koppen, G.; Govarts, E.; Vanermen, G.; Voorspoels, S.; Govindan, M.; Dewolf, M.C.; Den Hond, E.; Biot, P.; Casteleyn, L.; Kolossa-Gehring, M.; et al. Mothers and children are related, even in exposure to chemicals present in common consumer products. Environ. Res. 2019, 175, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Bruckers, L.; Covaci, A.; Schoeters, G.; Fierens, T.; Sioen, I.; Vanermen, G.; Baeyens, W.; Morrens, B.; Loots, I.; et al. Determinants of bisphenol A and phthalate metabolites in urine of Flemish adolescents. Environ. Res. 2014, 134, 110–117. [Google Scholar] [CrossRef]
- Den Hond, E.; Paulussen, M.; Geens, T.; Bruckers, L.; Baeyens, W.; David, F.; Dumont, E.; Loots, I.; Morrens, B.; de Bellevaux, B.N.; et al. Biomarkers of human exposure to personal care products: Results from the Flemish Environment and Health Study (FLEHS 2007–2011). Sci. Total Environ. 2013, 463–464, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Schoeters, G.; Govarts, E.; Bruckers, L.; Den Hond, E.; Nelen, V.; De Henauw, S.; Sioen, I.; Nawrot, T.S.; Plusquin, M.; Vriens, A.; et al. Three cycles of human biomonitoring in Flanders—Time trends observed in the Flemish Environment and Health Study. Int. J. Hyg. Environ. Health 2017, 220, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Schwedler, G.; Rucic, E.; Lange, R.; Conrad, A.; Koch, H.M.; Pälmke, C.; Brüning, T.; Schulz, C.; Schmied-Tobies, M.I.H.; Daniels, A.; et al. Phthalate metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014–2017. Int. J. Hyg. Environ. Health 2020, 225, 113444. [Google Scholar] [CrossRef]
- Schwedler, G.; Seiwert, M.; Fiddicke, U.; Ißleb, S.; Hölzer, J.; Nendza, J.; Wilhelm, M.; Wittsiepe, J.; Koch, H.M.; Schindler, B.K.; et al. Human biomonitoring pilot study DEMOCOPHES in Germany: Contribution to a harmonized European approach. Int. J. Hyg. Environ. Health 2017, 220, 686–696. [Google Scholar] [CrossRef]
- Kasper-Sonnenberg, M.; Koch, H.M.; Apel, P.; Rüther, M.; Pälmke, C.; Brüning, T.; Kolossa-Gehring, M. Time trend of exposure to the phthalate plasticizer substitute DINCH in Germany from 1999 to 2017: Biomonitoring data on young adults from the Environmental Specimen Bank (ESB). Int. J. Hyg. Environ. Health 2019, 222, 1084–1092. [Google Scholar] [CrossRef]
- Koch, H.M.; Rüther, M.; Schütze, A.; Conrad, A.; Pälmke, C.; Apel, P.; Brüning, T.; Kolossa-Gehring, M. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 2017, 220, 130–141. [Google Scholar] [CrossRef]
- Berman, T.; Goldsmith, R.; Göen, T.; Spungen, J.; Novack, L.; Levine, H.; Amitai, Y.; Shohat, T.; Grotto, I. Urinary concentrations of environmental contaminants and phytoestrogens in adults in Israel. Environ. Int. 2013, 59, 478–484. [Google Scholar] [CrossRef]
- Berman, T.; Goldsmith, R.; Levine, H.; Grotto, I. Human biomonitoring in Israel: Recent results and lessons learned. Int. J. Hyg. Environ. Health 2017, 220, 6–12. [Google Scholar] [CrossRef]
- Esteban López, M.; Göen, T.; Mol, H.; Nübler, S.; Haji-Abbas-Zarrabi, K.; Koch, H.M.; Kasper-Sonnenberg, M.; Dvorakova, D.; Hajslova, J.; Antignac, J.-P.; et al. The European human biomonitoring platform—Design and implementation of a laboratory quality assurance/quality control (QA/QC) programme for selected priority chemicals. Int. J. Hyg. Environ. Health 2021, 234, 113740. [Google Scholar] [CrossRef]
- Mol, H.G.J.; Elbers, I.; Pälmke, C.; Bury, D.; Göen, T.; López, M.E.; Nübler, S.; Vaccher, V.; Antignac, J.-P.; Dvořáková, D.; et al. Proficiency and Interlaboratory Variability in the Determination of Phthalate and DINCH Biomarkers in Human Urine: Results from the HBM4EU Project. Toxics 2022, 10, 57. [Google Scholar] [CrossRef]
- Schindler, B.K.; Esteban, M.; Koch, H.M.; Castano, A.; Koslitz, S.; Cañas, A.; Casteleyn, L.; Kolossa-Gehring, M.; Schwedler, G.; Schoeters, G.; et al. The European COPHES/DEMOCOPHES project: Towards transnational comparability and reliability of human biomonitoring results. Int. J. Hyg. Environ. Health 2014, 217, 653–661. [Google Scholar] [CrossRef]
- Göen, T.; Schaller, K.H.; Drexler, H. External quality assessment of human biomonitoring in the range of environmental exposure levels. Int. J. Hyg. Environ. Health 2012, 215, 229–232. [Google Scholar] [CrossRef]
- Flemish Institute for Technological Research (VITO). Dashboard European Human Biomonitoring Data for Visualization of Aggregated Data; Flemish Institute for Technological Research (VITO): Mol, Belgium, 2021. [Google Scholar]
- Schmidtkunz, C.; Gries, W.; Weber, T.; Leng, G.; Kolossa-Gehring, M. Internal exposure of young German adults to di(2-propylheptyl) phthalate (DPHP): Trends in 24-h urine samples from the German Environmental Specimen Bank 1999–2017. Int. J. Hyg. Environ. Health 2019, 222, 419–424. [Google Scholar] [CrossRef]
- Schwedler, G.; Conrad, A.; Rucic, E.; Koch, H.M.; Leng, G.; Schulz, C.; Schmied-Tobies, M.I.H.; Kolossa-Gehring, M. Hexamoll® DINCH and DPHP metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014–2017. Int. J. Hyg. Environ. Health 2020, 229, 113397. [Google Scholar] [CrossRef]
- Gilles, L.; Govarts, E.; Martin, L.R.; Andersson, A.-M.; Appenzeller, B.M.R.; Nina, V.; Wąsowicz, W.; Weber, T.; Zock, J.-P.; Sepai, O.; et al. Harmonisation of Human Biomonitoring Studies in Europe: Characteristics of the HBM4EU Aligned Studies Participants. Int. J. Environ. Res. Public Health 2022, 19, 6787. [Google Scholar] [CrossRef]
- Gilles, L.; Govarts, E.; Rambaud, L.; Vogel, N.; Castaño, A.; Esteban López, M.; Rodriguez Martin, L.; Koppen, G.; Remy, S.; Vrijheid, M.; et al. HBM4EU combines and harmonises human biomonitoring data across the EU, building on existing capacity—The HBM4EU survey. Int. J. Hyg. Environ. Health 2021, 237, 113809. [Google Scholar] [CrossRef]
- Domínguez-Romero, E.; Komprdová, K.; Kalina, J.; Bessems, J.; Karakitsios, S.; Sarigiannis, D.A.; Scheringer, M. Time-trends in human urinary concentrations of phthalates and substitutes DEHT and DINCH in Asian and North American countries (2009–2019). J. Expo. Sci. Environ. Epidemiol. 2022. [Google Scholar] [CrossRef]
- Govarts, E.; Gilles, L.; Rodriguez-Martin, L.; Santonen, T.; Apel, P.; Alvito, P.; Anastasi, E.; Andersson, A.M.; Andryskova, L.; Antignac, J.P. Human Biomonitoring Data in European children, teenagers and adults: Results from the HBM4EU aligned studies (2014–2021). Int. J. Hyg. Environ. Health 2022. in review. [Google Scholar]
- ECHA. Evaluation of New Scientific Evidence Concerning DINP and DIDP in Relation to Entry 52 of Annex XVII to REACH Regulation (EC) No 1907/2006; ECHA: Helsinki, Finland, 2013.
- Bureau for Chemical Substances Poland. Annex XV dossier—Proposal for a Identificaton of a Substance as a CMR 1A or 1B, PBT, vPBT or a Substance of Equivalent Concern: Dipentyl Phthalate (DPP); Bureau for Chemical Substances Poland: Łódź, Poland, 2012. [Google Scholar]
- ECHA. Brief Profile for Dicyclohexyl Phthalate. Available online: https://echa.europa.eu/de/brief-profile/-/briefprofile/100.001.405 (accessed on 26 September 2022).
- Haug, L.S.; Sakhi, A.K.; Cequier, E.; Casas, M.; Maitre, L.; Basagana, X.; Andrusaityte, S.; Chalkiadaki, G.; Chatzi, L.; Coen, M.; et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ. Int. 2018, 121, 751–763. [Google Scholar] [CrossRef]
- ECHA. C&L Inventory. 2023. Available online: https://echa.europa.eu/information-on-chemicals/cl-inventory-database (accessed on 26 September 2022).
- Frederiksen, H.; Kranich, S.K.; Jørgensen, N.; Taboureau, O.; Petersen, J.H.; Andersson, A.M. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: Considerations for epidemiological studies. Environ. Sci. Technol. 2013, 47, 958–967. [Google Scholar] [CrossRef] [PubMed]

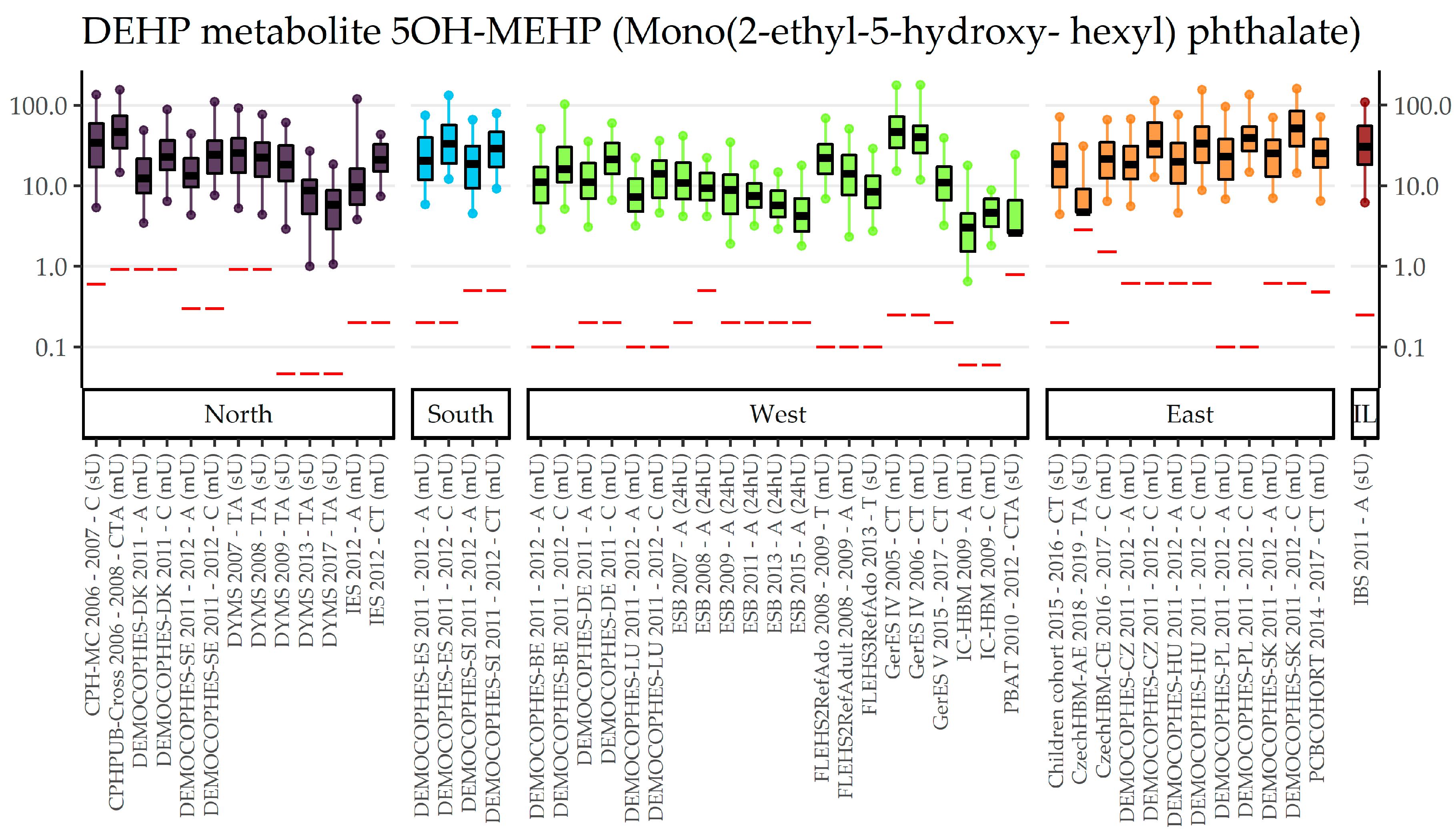


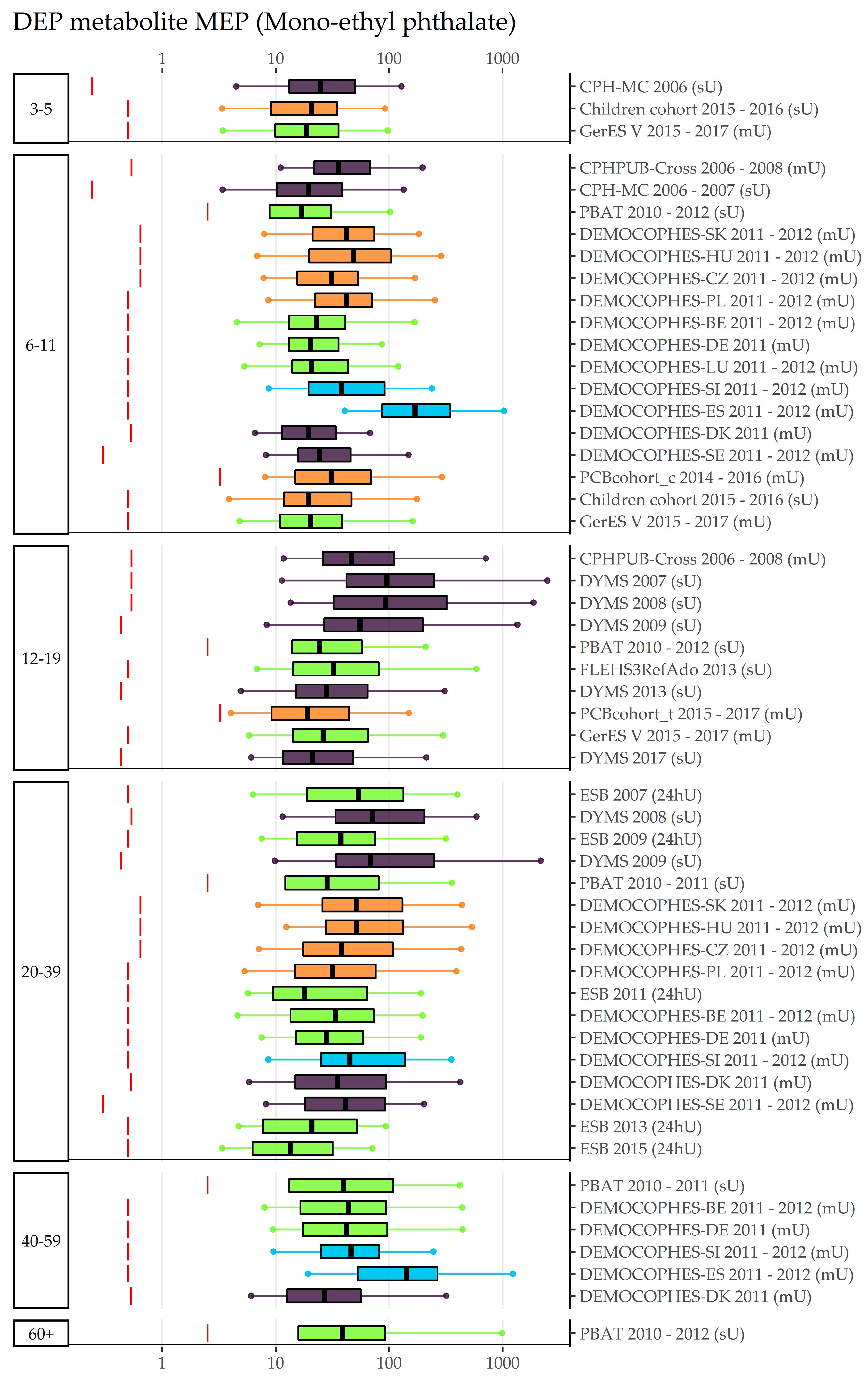
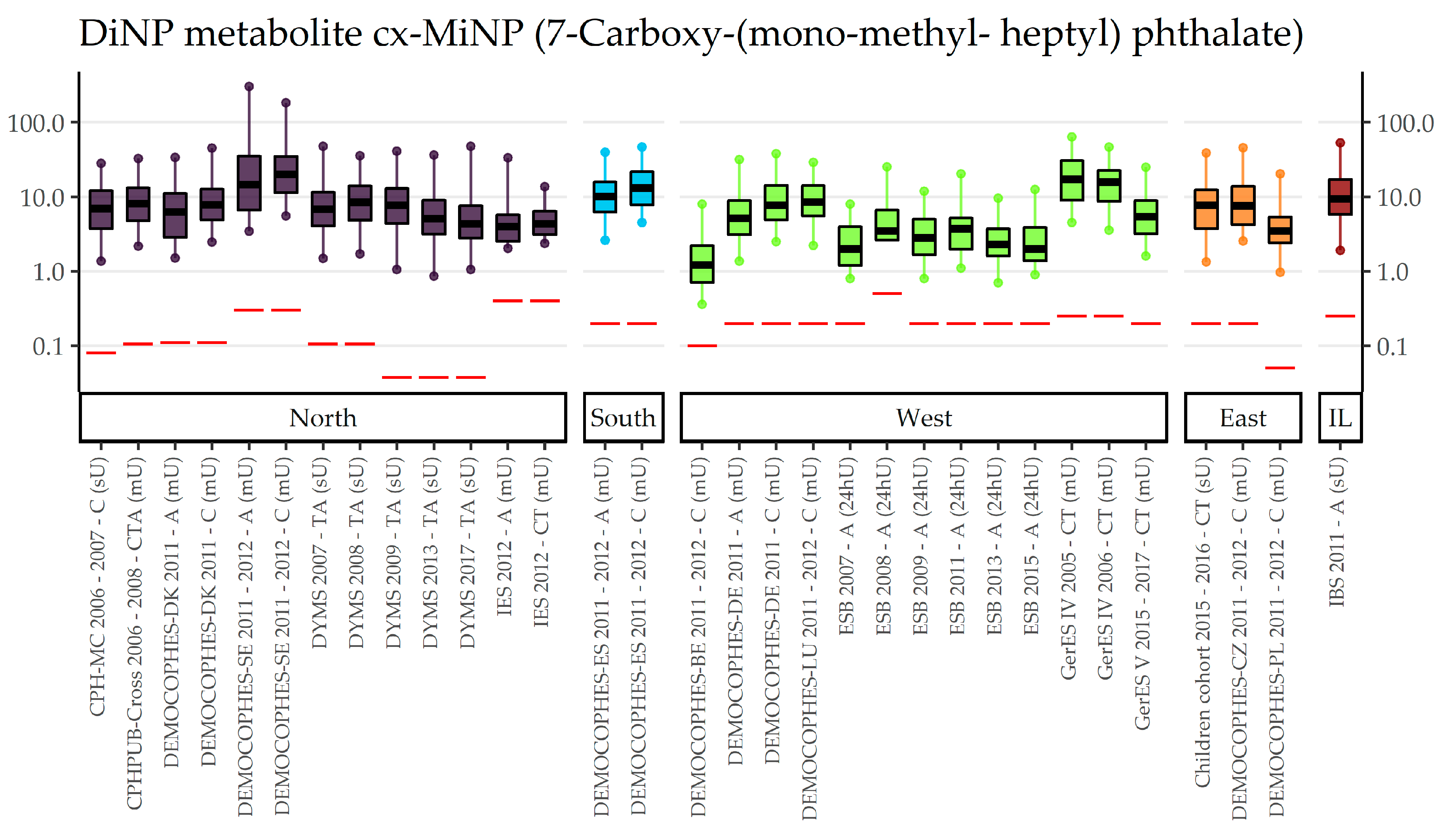
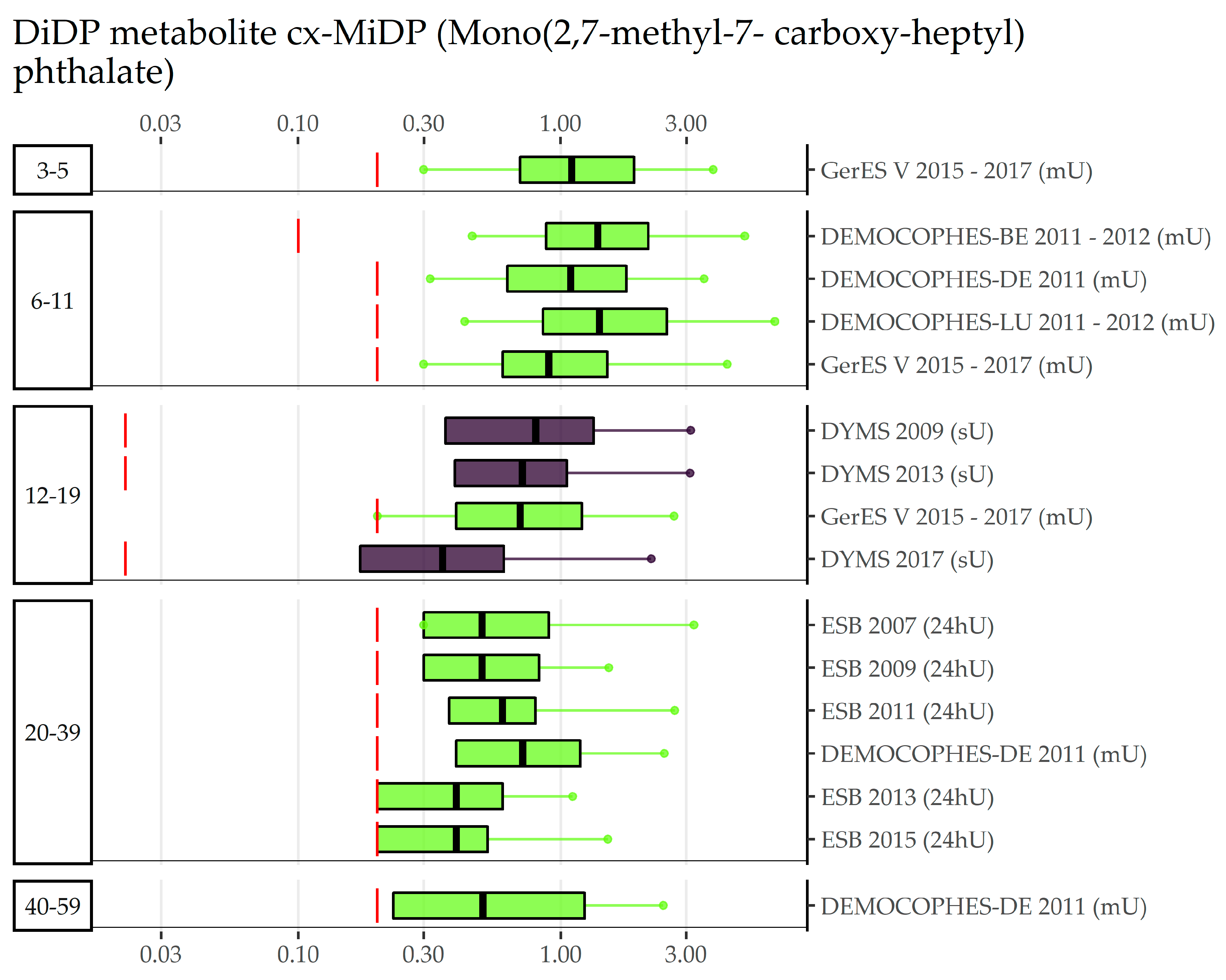
| EU Region | Country | Data Collection | Age Groups with Phthalates Data | (Range of) Sampling Years | Publications |
|---|---|---|---|---|---|
| East | Czech Republic | CzechHBM-CE | Children | 2016–2017 | [19,20] |
| Czech Republic | DEMOCOPHES-CZ | Children, adults | 2011–2012 | [21] | |
| Czech Republic | CzechHBM-AE | Adults | 2018–2019 | [22] | |
| Hungary | DEMOCOPHES-HU | Children, adults | 2011–2012 | [21] | |
| Poland | DEMOCOPHES-PL | Children, adults | 2011–2012 | [17] | |
| Slovakia | PCBcohort | Children, teenagers | 2014–2017 | [23] | |
| Slovakia | Children cohort | Children | 2015–2016 | - | |
| Slovakia | DEMOCOPHES-SK | Children, adults | 2011–2012 | [21] | |
| South | Spain | DEMOCOPHES-ES | Children, adults | 2011–2012 | [24] |
| Slovenia | DEMOCOPHES-SI | Children, adults | 2011–2012 | [25] | |
| North | Denmark | DEMOCOPHES-DK | Children, adults | 2011 | [17,26] |
| Denmark | CPH-MC | Children | 2006 | [27] | |
| Denmark | CPHPUB-Cross | Children, teenagers | 2006–2008 | [28] | |
| Denmark | DYMS | Teenagers, adults | 2007–2017 | [29,30] | |
| Norway | IES | Children, adults | 2012 | [31] | |
| Sweden | DEMOCOPHES-SE | Children, adults | 2011–2012 | [32] | |
| West | Austria | PBAT | Children, teenagers, adults, elderly | 2010–2012 | [33] |
| Austria | IC-HBM | Children, adults | 2009 | [34] | |
| Belgium | DEMOCOPHES-BE | Children, adults | 2011–2012 | [35,36] | |
| Belgium | FLEHS2RefAdo | Teenagers | 2008–2009 | [37] | |
| Belgium | FLEHS2RefAdult | Adults | 2008–2009 | [38] | |
| Belgium | FLEHS3RefAdo | Teenagers | 2013 | [39] | |
| Germany | GerES IV (unweighted) | Children, teenagers | 2003–2006 | [7] | |
| Germany | GerES V (unweighted) | Children, teenagers | 2015–2017 | [40] | |
| Germany | DEMOCOPHES-DE | Children, adults | 2011 | [41] | |
| Germany | ESB | Adults | 2007–2019 | [42,43] | |
| Luxemburg | DEMOCOPHES-LU | Children, adults | 2011–2012 | [35] | |
| Other | Israel | IBS | Adults, elderly | 2011 | [44] |
| Phthalate Diester | Acronym | CAS-No. | Metabolite of Diester | Acronym of Metabolite |
|---|---|---|---|---|
| HBM4EU Category A 1 | ||||
| Butyl benzyl phthalate | BBzP | 85-68-7 | Mono-benzyl phthalate | MBzP |
| Di(2-ethylhexyl) phthalate | DEHP | 117-81-7 | Mono(2-ethylhexyl) phthalate | MEHP |
| Mono(2-ethyl-5-carboxy-pentyl) phthalate | 5cx-MEPP | |||
| Mono(2-ethyl-5-hydroxy-hexyl) phthalate | 5OH-MEHP | |||
| Mono(2-ethyl-5-oxohexyl) phthalate | 5oxo-MEHP | |||
| Di-n-butyl phthalate | DnBP | 84-74-2 | Mono-n-butyl phthalate | MnBP |
| Mono-hydroxy-n-butyl phthalate | OH-MnBP | |||
| Di-isobutyl phthalate | DiBP | 84-69-5 | Mono-isobutyl phthalate | MiBP |
| Mono-hydroxy-iso-butylphthalate | OH-MiBP | |||
| Diethyl phthalate | DEP | 84-66-2 | Mono-ethyl phthalate | MEP |
| HBM4EU Category B 2 | ||||
| Di-isodecyl phthalate (and 3 DPHP) | DiDP | 26761-40-0 | Mono-isodecyl phthalate | MiDP |
| Mono(2,7-methyl-7-carboxy-heptyl) phthalate | cx-MiDP | |||
| 6-OH-Mono-propyl-heptyl phthalate | OH-MiDP | |||
| 6-Oxo-Mono-propyl-heptyl phthalate | oxo-MiDP | |||
| Di-isononyl phthalate | DiNP | 28553-12-0 | Mono-methyl-octyl phthalate | MiNP |
| 7-Carboxy-(mono-methyl-heptyl) phthalate | cx-MiNP | |||
| 7-OH-(Mono-methyl-octyl) phthalate | OH-MiNP | |||
| 7-Oxo-(Mono-methyl-octyl) phthalate | oxo-MiNP | |||
| Bis(2-propylheptyl) phthalate | DPHP | 53306-54-0 | mono-(propyl-6-carboxyhexyl) phthalate | cx-MPHP |
| mono-(propyl-6-hydroxyheptyl) phthalate | OH-MPHP | |||
| mono-(propyl-6-oxo-heptyl)-phthalate | oxo-MPHP | |||
| Dicyclohexyl phthalate | DCHP | 84-61-7 | Mono-cyclo-hexyl phthalate | MCHP |
| Dimethyl phthalate | DMP | 131-11-3 | Mono-methyl phthalate | MMP |
| Di-n-pentyl phthalate | DnPeP | 131-18-0 | Mono-n-pentyl phthalate | MnPeP |
| Di-n-octyl phthalate | DnOP | 117-84-0 | Mono-n-octyl phthalate | MnOP |
| Various phthalates | 3-Carboxyl-mono-propyl phthalate | 3cx-MPP | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogel, N.; Lange, R.; Schmidt, P.; Rodriguez Martin, L.; Remy, S.; Springer, A.; Puklová, V.; Černá, M.; Rudnai, P.; Középesy, S.; et al. Exposure to Phthalates in European Children, Adolescents and Adults since 2005: A Harmonized Approach Based on Existing HBM Data in the HBM4EU Initiative. Toxics 2023, 11, 241. https://doi.org/10.3390/toxics11030241
Vogel N, Lange R, Schmidt P, Rodriguez Martin L, Remy S, Springer A, Puklová V, Černá M, Rudnai P, Középesy S, et al. Exposure to Phthalates in European Children, Adolescents and Adults since 2005: A Harmonized Approach Based on Existing HBM Data in the HBM4EU Initiative. Toxics. 2023; 11(3):241. https://doi.org/10.3390/toxics11030241
Chicago/Turabian StyleVogel, Nina, Rosa Lange, Phillipp Schmidt, Laura Rodriguez Martin, Sylvie Remy, Andrea Springer, Vladimíra Puklová, Milena Černá, Péter Rudnai, Szilvia Középesy, and et al. 2023. "Exposure to Phthalates in European Children, Adolescents and Adults since 2005: A Harmonized Approach Based on Existing HBM Data in the HBM4EU Initiative" Toxics 11, no. 3: 241. https://doi.org/10.3390/toxics11030241
APA StyleVogel, N., Lange, R., Schmidt, P., Rodriguez Martin, L., Remy, S., Springer, A., Puklová, V., Černá, M., Rudnai, P., Középesy, S., Janasik, B., Ligocka, D., Fábelová, L., Kolena, B., Petrovicova, I., Jajcaj, M., Eštóková, M., Esteban-Lopez, M., Castaño, A., ... Kolossa-Gehring, M. (2023). Exposure to Phthalates in European Children, Adolescents and Adults since 2005: A Harmonized Approach Based on Existing HBM Data in the HBM4EU Initiative. Toxics, 11(3), 241. https://doi.org/10.3390/toxics11030241









