The Degradation Process of Typical Neonicotinoid Insecticides in Tidal Streams in Subtropical Cities: A Case Study of the Wuchong Stream, South China
Abstract
1. Introduction
2. Materials and Methods
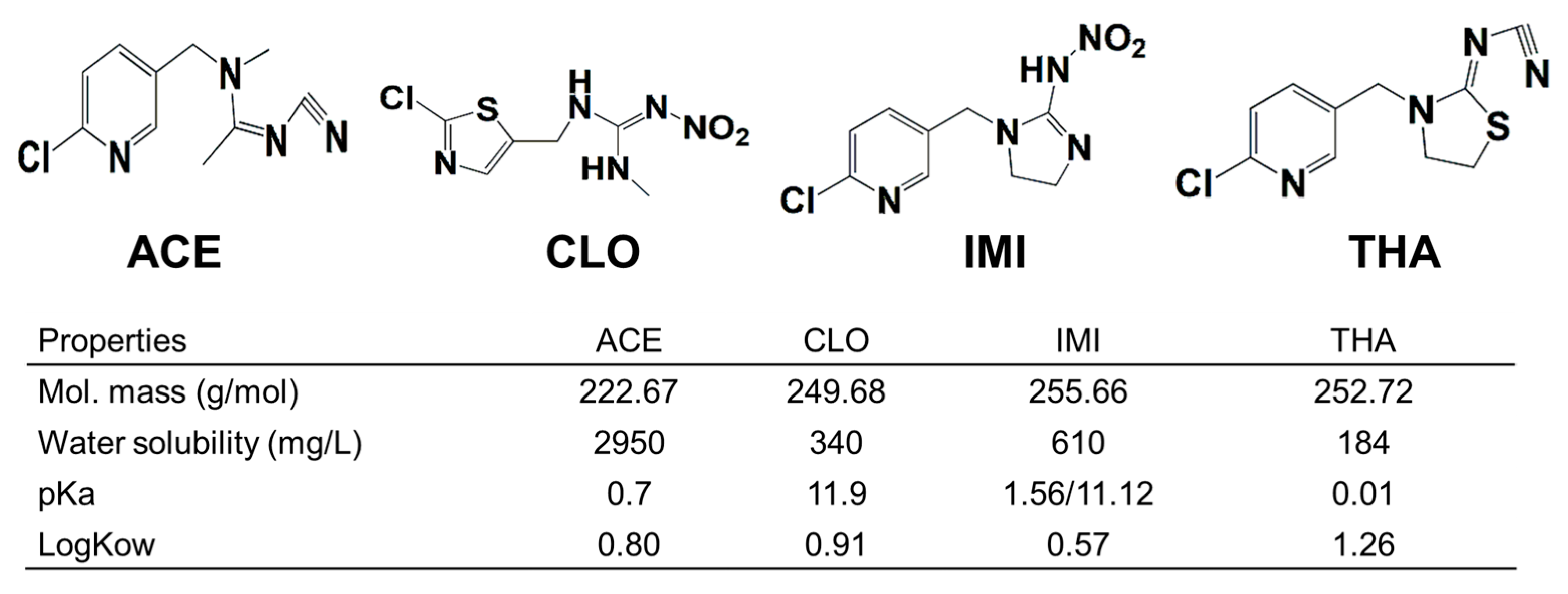
2.1. Chemicals and Reagents
2.2. Water Collection and Pretreatment
2.3. Central Composite Design of Three Degradation Experiments
2.4. Sample Preparation and Extraction
2.5. Instrumental Analysis and Quality Control
2.6. Data Analysis
3. Results and Discussion
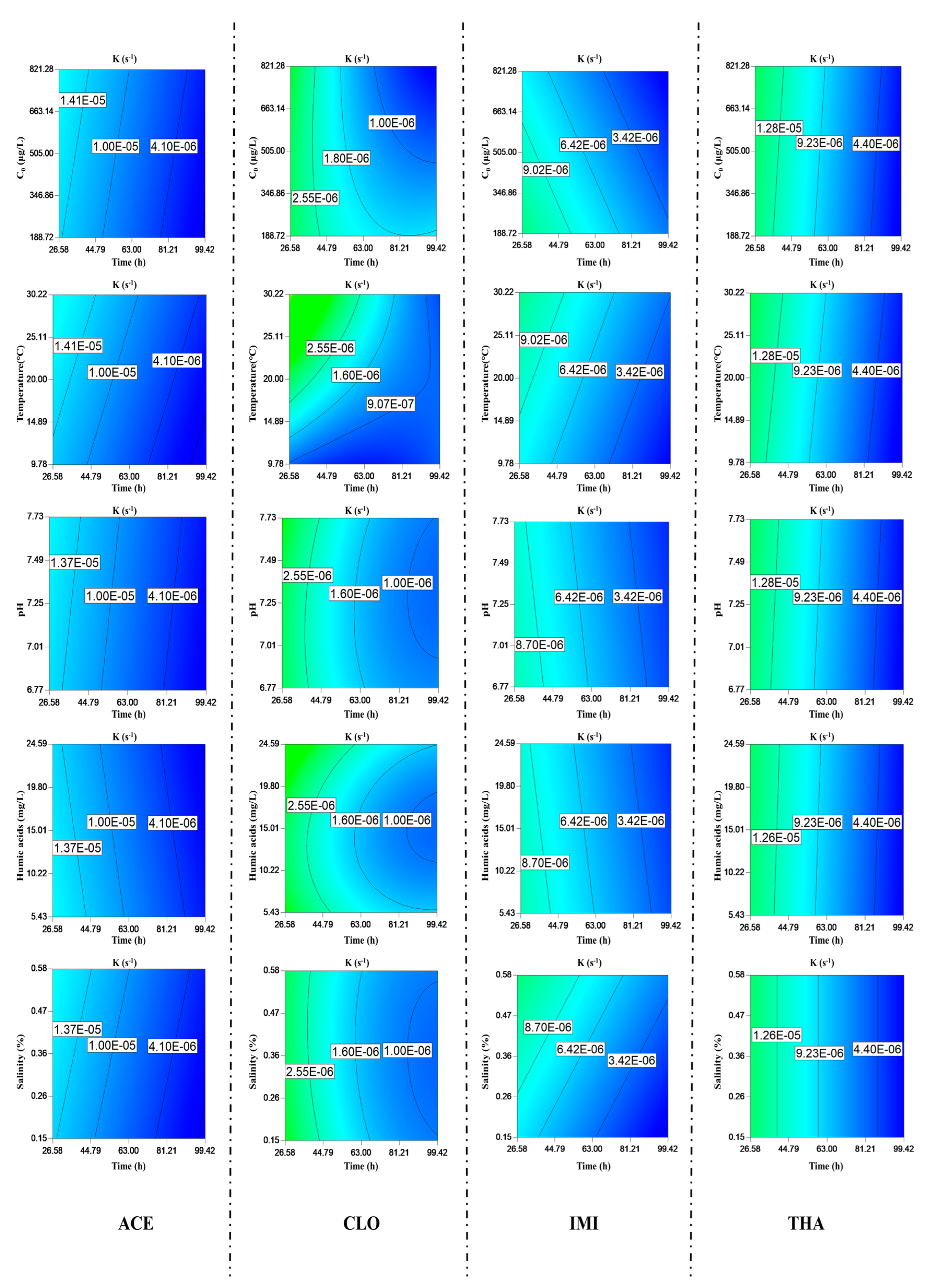
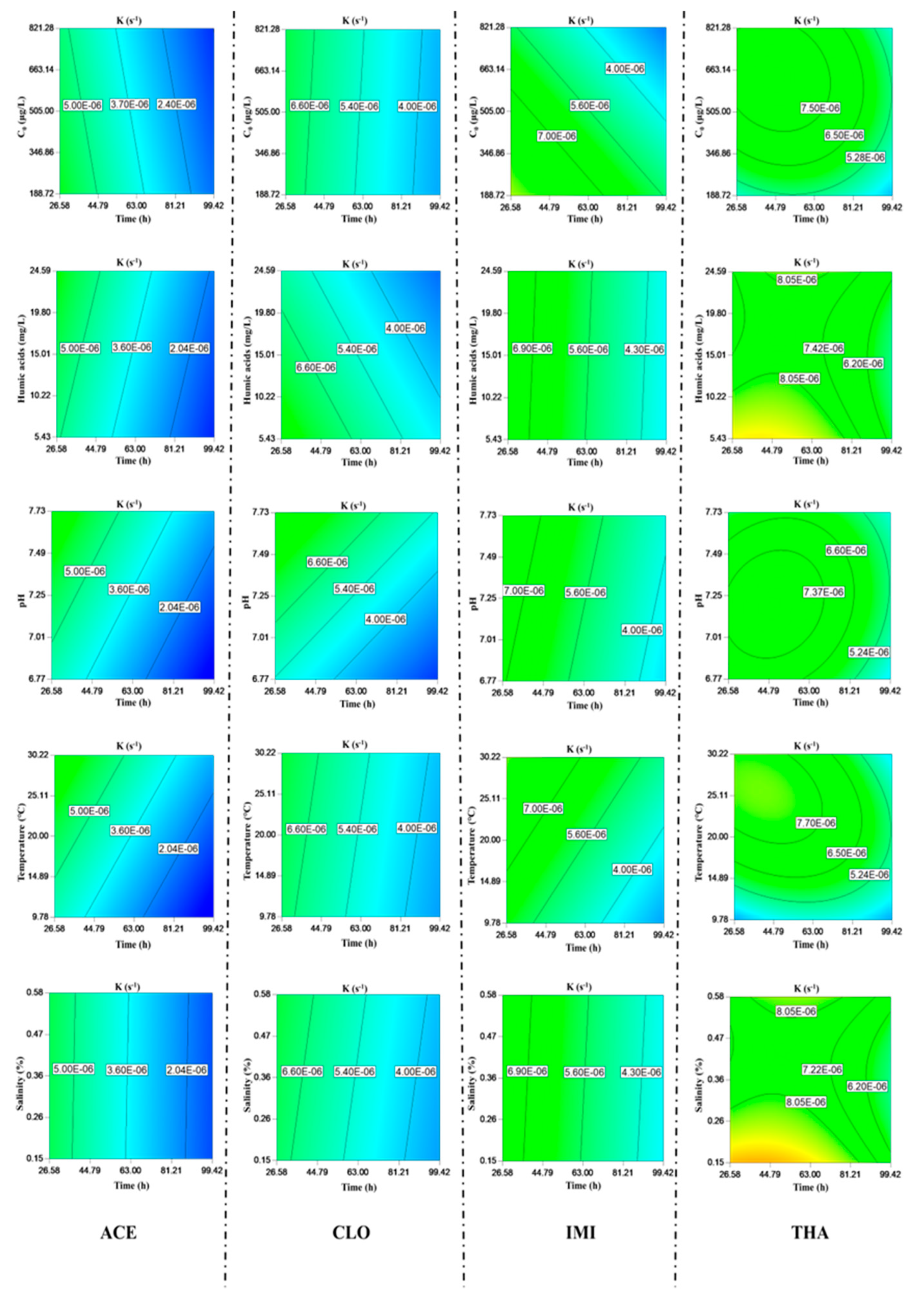
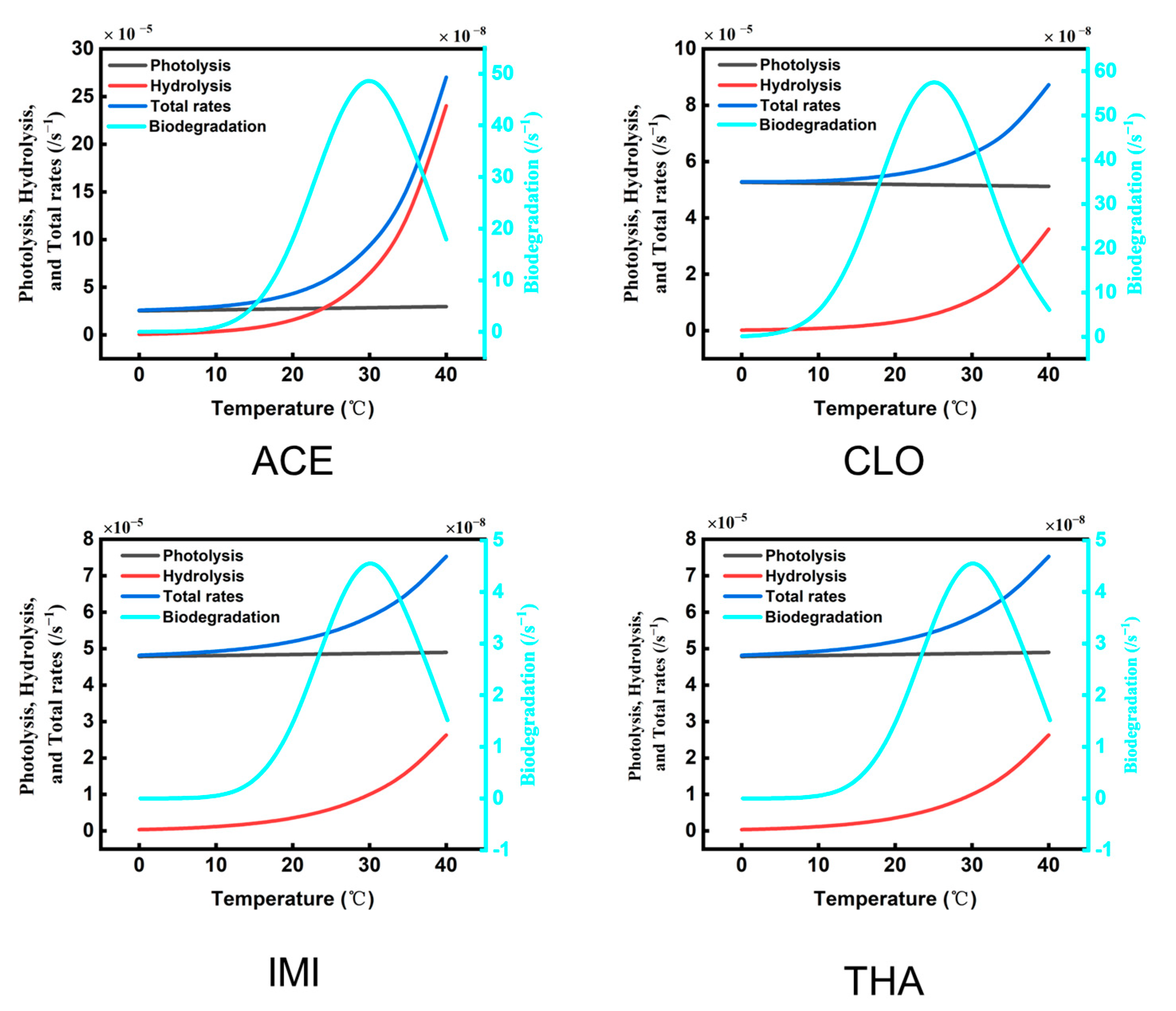
3.1. Hydrolysis
3.2. Photolysis
3.3. Biodegradation
3.4. The Total Degradation of Typical NEOs in the Wuchong Stream
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.; Whitehead, M.; Civil, W.; Goulson, D. Potential role of veterinary flea products in widespread pesticide contamination of English rivers. Sci. Total. Environ. 2021, 755, 143560. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, X.; Chen, C.; Tian, D.; Liu, H.; Xie, L.; Zhu, X.; Huang, M.; Ying, G.-G. Contamination of neonicotinoid insecticides in soil-water-sediment systems of the urban and rural areas in a rapidly developing region: Guangzhou, South China. Environ. Int. 2020, 139, 105719. [Google Scholar] [CrossRef]
- Batikian, C.M.; Lu, A.; Watanabe, K.; Pitt, J.; Gersberg, R.M. Temporal pattern in levels of the neonicotinoid insecticide, imidacloprid, in an urban stream. Chemosphere 2019, 223, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Granger, C.; Dong, H.; Mao, Y.; Duan, S.; Li, J.; Qiang, Z. Occurrences of 29 pesticides in the Huangpu River, China: Highest ecological risk identified in Shanghai metropolitan area. Chemosphere 2020, 251, 126411. [Google Scholar] [CrossRef]
- Cai, Y.P.; Huang, G.H.; Yang, Z.F.; Lin, Q.G.; Tan, Q. Community-scale renewable energy systems planning under uncertainty—An interval chance-constrained programming approach. Renew. Sustain. Energy Rev. 2009, 13, 721–735. [Google Scholar] [CrossRef]
- Yi, X.; Zhang, C.; Liu, H.; Wu, R.; Tian, D.; Ruan, J.; Zhang, T.; Huang, M.; Ying, G.-G. Occurrence and distribution of neonicotinoid insecticides in surface water and sediment of the Guangzhou section of the Pearl River, South China. Environ. Pollut. 2019, 251, 892–900. [Google Scholar] [CrossRef]
- Sadaria, A.M.; Supowit, S.D.; Halden, R.U. Mass Balance Assessment for Six Neonicotinoid Insecticides During Conventional Wastewater and Wetland Treatment: Nationwide Reconnaissance in United States Wastewater. Environ. Sci. Technol. 2016, 50, 6199–6206. [Google Scholar] [CrossRef]
- Zhi, H.; Webb, D.T.; Schnoor, J.L.; Kolpin, D.W.; Klaper, R.D.; Iwanowicz, L.R.; LeFevre, G.H. Modeling risk dynamics of contaminants of emerging concern in a temperate-region wastewater effluent-dominated stream. Environ. Sci. Water Res. Technol. 2022, 8, 1408–1422. [Google Scholar] [CrossRef]
- Cai, Y.P.; Huang, G.H.; Yang, Z.F.; Tan, Q. Identification of optimal strategies for energy management systems planning under multiple uncertainties. Appl. Energy 2009, 86, 480–495. [Google Scholar] [CrossRef]
- Nicol, E.; Varga, Z.; Vujovic, S.; Bouchonnet, S. Laboratory scale UV–visible degradation of acetamiprid in aqueous marketed mixtures—Structural elucidation of photoproducts and toxicological consequences. Chemosphere 2020, 248, 126040. [Google Scholar] [CrossRef]
- Borsuah, J.F.; Messer, T.L.; Snow, D.D.; Comfort, S.D.; Mittelstet, A.R. Literature Review: Global Neonicotinoid Insecticide Occurrence in Aquatic Environments. Water 2020, 12, 3388. [Google Scholar] [CrossRef]
- Pang, S.; Lin, Z.; Zhang, W.; Mishra, S.; Bhatt, P.; Chen, S. Insights Into the Microbial Degradation and Biochemical Mechanisms of Neonicotinoids. Front. Microbiol. 2020, 11, 868. [Google Scholar] [CrossRef]
- Mohammed, Y.M.M.; Badawy, M.E.I. Biodegradation of imidacloprid in liquid media by an isolated wastewater fungus Aspergillus terreus YESM3. J. Env. Sci. Health B 2017, 52, 752–761. [Google Scholar] [CrossRef]
- Chen, R.; Yin, H.; Zhang, C.; Luo, X.; Liang, G. Hydrolysis of a neonicotinoid: A theoretical study on the reaction mechanism of dinotefuran. Struct. Chem. 2017, 29, 315–325. [Google Scholar] [CrossRef]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef]
- Zhou, W.T.; Zhang, W.X.; Cai, Y.P. Laccase immobilization for water purification: A comprehensive review. Chem. Eng. J. 2021, 403, 126272. [Google Scholar] [CrossRef]
- Remucal, C.K. The role of indirect photochemical degradation in the environmental fate of pesticides: A review. Environ. Sci. Process. Impacts 2014, 16, 628–653. [Google Scholar] [CrossRef]
- Acero, J.L.; Real, F.J.; Benitez, F.J.; Matamoros, E. Degradation of neonicotinoids by UV irradiation: Kinetics and effect of real water constituents. Sep. Purif. Technol. 2019, 211, 218–226. [Google Scholar] [CrossRef]
- Hussain, S.; Hartley, C.J.; Shettigar, M.; Pandey, G. Bacterial biodegradation of neonicotinoid pesticides in soil and water systems. FEMS Microbiol. Lett. 2016, 363, fnw252. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Evans, A.; DeWinne, D.; White, P.; Mitchell, F. Modeling photodegradation kinetics of three systemic neonicotinoids-dinotefuran, imidacloprid, and thiamethoxam-in aqueous and soil environment. Environ. Toxicol. Chem. 2016, 35, 1718–1726. [Google Scholar] [CrossRef]
- Tan, Q.; Huang, G.H.; Cai, Y.P. A Superiority-Inferiority-Based Inexact Fuzzy Stochastic Programming Approach for Solid Waste Management Under Uncertainty. Environ. Model. Assess. 2009, 15, 381–396. [Google Scholar] [CrossRef]
- Cai, Y.P.; Huang, G.H.; Tan, Q. An inexact optimization model for regional energy systems planning in the mixed stochastic and fuzzy environment. Int. J. Energy Res. 2009, 33, 443–468. [Google Scholar] [CrossRef]
- Carere, M.; Miniero, R.; Cicero, M.R. Potential effects of climate change on the chemical quality of aquatic biota. TrAC Trends Anal. Chem. 2011, 30, 1214–1221. [Google Scholar] [CrossRef]
- Delpla, I.; Jung, A.-V.; Baures, E.; Clement, M.; Thomas, O. Impacts of climate change on surface water quality in relation to drinking water production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef]
- Martins, I.; Soares, J.; Neuparth, T.; Barreiro, A.F.; Xavier, C.; Antunes, C.; Santos, M.M. Prioritizing the Effects of Emerging Contaminants on Estuarine Production under Global Warming Scenarios. Toxics 2022, 10, 46. [Google Scholar] [CrossRef]
- de Wit, C.A.; Vorkamp, K.; Muir, D. Influence of climate change on persistent organic pollutants and chemicals of emerging concern in the Arctic: State of knowledge and recommendations for future research. Environ. Sci. Process Impacts 2022, 1530–1543. [Google Scholar] [CrossRef]
- Emidio, E.S.; Calisto, V.; de Marchi, M.R.; Esteves, V.I. Photochemical transformation of zearalenone in aqueous solutions under simulated solar irradiation: Kinetics and influence of water constituents. Chemosphere 2017, 169, 146–154. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Sablas, M.M.; Hung, C.M.; Chen, C.W.; Garcia-Segura, S.; Dong, C.D. Modeling and optimization of imidacloprid degradation by catalytic percarbonate oxidation using artificial neural network and Box-Behnken experimental design. Chemosphere 2020, 251, 126254. [Google Scholar] [CrossRef]
- Yuan, X.; Li, S.; Hu, J.; Yu, M.; Li, Y.; Wang, Z. Experiments and numerical simulation on the degradation processes of carbamazepine and triclosan in surface water: A case study for the Shahe Stream, South China. Sci. Total. Environ. 2019, 655, 1125–1138. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, J.; Li, S.; Yu, M. Occurrence, fate, and mass balance of selected pharmaceutical and personal care products (PPCPs) in an urbanized river. Environ. Pollut. 2020, 266, 115340. [Google Scholar] [CrossRef]
- Matamoros, V.; Duhec, A.; Albaigés, J.; Bayona, J.M. Photodegradation of Carbamazepine, Ibuprofen, Ketoprofen and 17α-Ethinylestradiol in Fresh and Seawater. Water Air Soil Pollut. 2008, 196, 161–168. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, D.; Yi, X.; Zhang, T.; Ruan, J.; Wu, R.; Chen, C.; Huang, M.; Ying, G. Occurrence, distribution and seasonal variation of five neonicotinoid insecticides in surface water and sediment of the Pearl Rivers, South China. Chemosphere 2019, 217, 437–446. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, X.; Fu, X.; Yu, B.; Wang, D.; Zhao, C.; Zhang, Q.; Tan, Y.; Wang, X. Development of a fast and sensitive method for measuring multiple neonicotinoid insecticide residues in soil and the application in parks and residential areas. Anal. Chim. Acta 2018, 1016, 19–28. [Google Scholar] [CrossRef]
- Todey, S.A.; Fallon, A.M.; Arnold, W.A. Neonicotinoid insecticide hydrolysis and photolysis: Rates and residual toxicity. Environ. Toxicol. Chem. 2018, 37, 2797–2809. [Google Scholar] [CrossRef]
- Daghrir, R.; Dimboukou-Mpira, A.; Seyhi, B.; Drogui, P. Photosonochemical degradation of butyl-paraben: Optimization, toxicity and kinetic studies. Sci. Total. Environ. 2014, 490, 223–234. [Google Scholar] [CrossRef]
- Washington, J.W. Hydrolysis rates of dissolved volatile organic compounds: Principles, temperature effects and literature review. Groundwater 1995, 33, 415–424. [Google Scholar] [CrossRef]
- Anjos, C.S.; Lima, R.N.; Porto, A.L.M. An overview of neonicotinoids: Biotransformation and biodegradation by microbiological processes. Environ. Sci. Pollut. Res. 2021, 28, 37082–37109. [Google Scholar] [CrossRef]
- He, X.; Wubie, A.J.; Diao, Q.; Li, W.; Xue, F.; Guo, Z.; Zhou, T.; Xu, S. Biodegradation of neonicotinoid insecticide, imidacloprid by restriction enzyme mediated integration (REMI) generated Trichoderma mutants. Chemosphere 2014, 112, 526–530. [Google Scholar] [CrossRef]
- Sadaria, A.M.; Sutton, R.; Moran, K.D.; Teerlink, J.; Brown, J.V.; Halden, R.U. Passage of fiproles and imidacloprid from urban pest control uses through wastewater treatment plants in northern California, USA. Environ. Toxicol. Chem. 2017, 36, 1473–1482. [Google Scholar] [CrossRef]
- Wu, H.; Zhihong, Z.; Yu, Z. Analysis of climate change in Guangdong-Hong Kong-Macao Greater Bay Area from 1961 to 2018. Torrential. Rain Disasters 2019, 38, 303–310. (In Chinese) [Google Scholar]
- Cheng, J.; Bambrick, H.; Frentiu, F.D.; Devine, G.; Yakob, L.; Xu, Z.; Li, Z.; Yang, W.; Hu, W. Extreme weather events and dengue outbreaks in Guangzhou, China: A time-series quasi-binomial distributed lag non-linear model. Int. J. Biometeorol. 2021, 65, 1033–1042. [Google Scholar] [CrossRef]

| Environmental Factors | Units | −α c | −1 | 0 | 1 | α | Degradation Process |
|---|---|---|---|---|---|---|---|
| Initial concentration (C0) | ppb | 10 | 233 | 505 | 776 | 1000 | a |
| 200 | 525 | 1150 | 1675 | 2000 | b | ||
| Time | h | 6 | 32 | 63 | 94 | 120 | a |
| min | 5 | 30 | 52.5 | 100 | 120 | b | |
| Temperature | °C | 4 | 12 | 20 | 29 | 36 | a |
| 10 | 15 | 25 | 34 | 40 | b | ||
| Solar radiation | W | 5 | 184 | 550 | 820 | 1000 | b |
| Humic acid content | mg/L | 0.02 | 5.79 | 15.01 | 24.23 | 30 | a/b |
| pH | 6.5 | 6.8 | 7.25 | 7.6 | 8 | a/b | |
| Salinity d | % | 0.05 | 0.18 | 0.425 | 0.66 | 0.8 | a/b |
| NEOs | MSE | Kp (min−1) | ||
|---|---|---|---|---|
| THA | 8.318 × 10−9 | 1.202 × 10−5 | 8.64 × 10−6 | 0.0032 |
| CLO | 1.322 × 10−8 | −4.26 × 10−5 | 3.58 × 10−6 | 0.091 |
| ACE | 1.901 × 10−9 | 1.04 × 10−4 | 1.56 × 10−6 | 0.012 |
| IMI | 1.55 × 10−9 | 1.4 × 10−4 | 1.60 × 10−6 | 0.0474 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Q.; Cai, Y.; Yuan, X.; Li, B.; Li, B. The Degradation Process of Typical Neonicotinoid Insecticides in Tidal Streams in Subtropical Cities: A Case Study of the Wuchong Stream, South China. Toxics 2023, 11, 203. https://doi.org/10.3390/toxics11030203
Jia Q, Cai Y, Yuan X, Li B, Li B. The Degradation Process of Typical Neonicotinoid Insecticides in Tidal Streams in Subtropical Cities: A Case Study of the Wuchong Stream, South China. Toxics. 2023; 11(3):203. https://doi.org/10.3390/toxics11030203
Chicago/Turabian StyleJia, Qunpo, Yanpeng Cai, Xiao Yuan, Bowen Li, and Bo Li. 2023. "The Degradation Process of Typical Neonicotinoid Insecticides in Tidal Streams in Subtropical Cities: A Case Study of the Wuchong Stream, South China" Toxics 11, no. 3: 203. https://doi.org/10.3390/toxics11030203
APA StyleJia, Q., Cai, Y., Yuan, X., Li, B., & Li, B. (2023). The Degradation Process of Typical Neonicotinoid Insecticides in Tidal Streams in Subtropical Cities: A Case Study of the Wuchong Stream, South China. Toxics, 11(3), 203. https://doi.org/10.3390/toxics11030203








