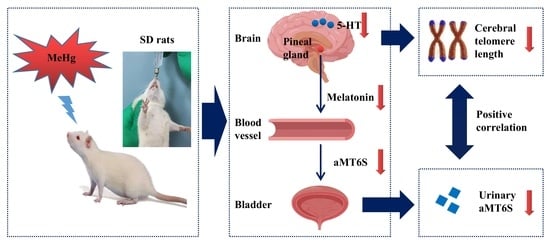
Subchronic Low-Dose Methylmercury Exposure Accelerated Cerebral Telomere Shortening in Relevant with Declined Urinary aMT6s Level in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Morris Water Maze
2.3. ELISA
2.4. Hematoxylin–Eosin (HE) Staining
2.5. Nissl Staining
2.6. Determine Blood-Hg Concentrations
2.7. Real-Time Quantitative PCR
2.8. Genomic DNA Isolation and Telomere Length Analysis
2.9. Statistical Analysis
3. Results
3.1. Influence of MeHg on Blood Mercury Concentration and Brain Somatic Index in Rats
3.2. The Effect of MeHg on Spatial Learning and Memory in Rats
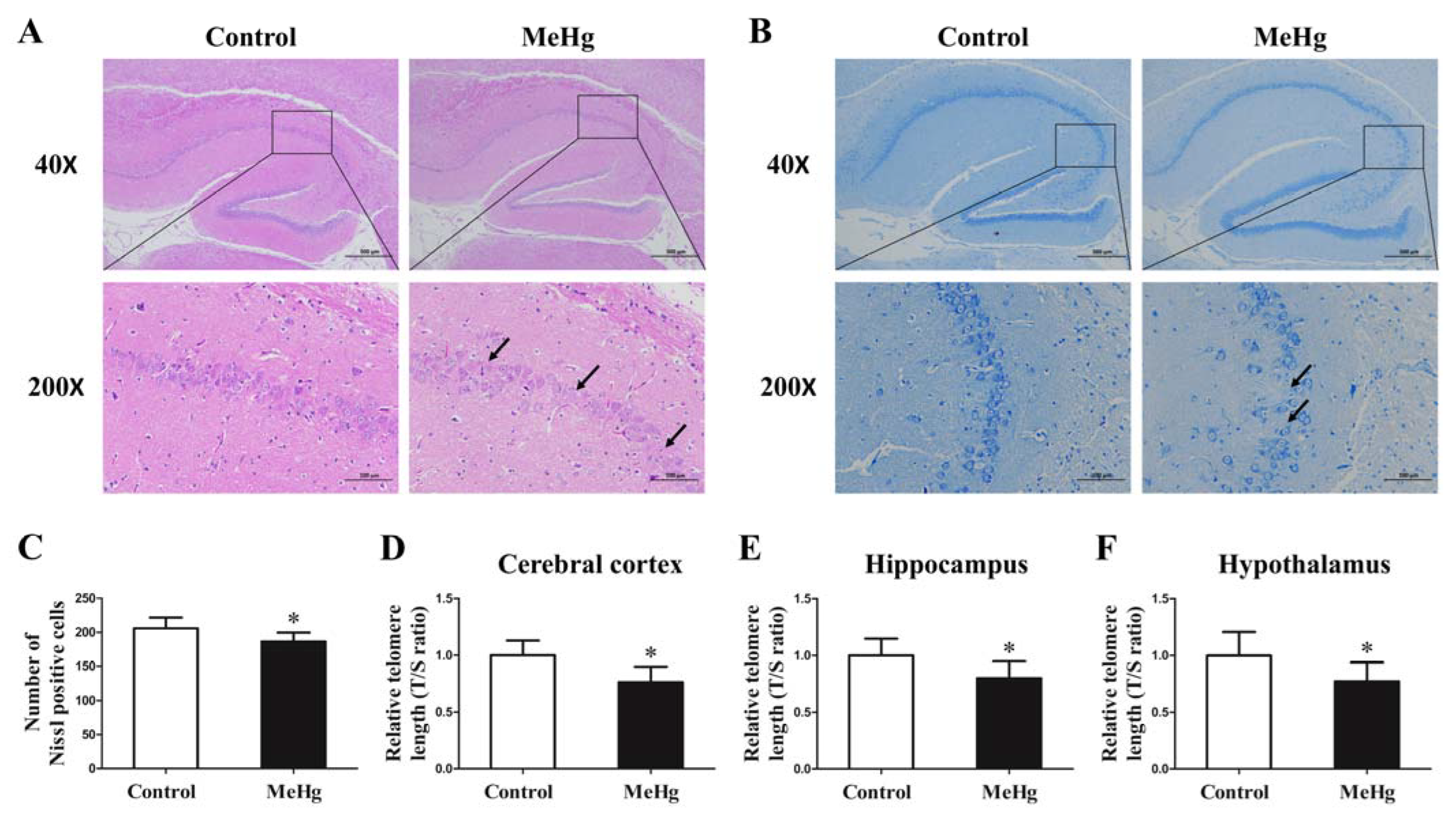
3.3. Impact of MeHg on Pathological Morphology and Telomere Length of Rat Brain Tissue
3.4. MeHg-Induced Alterations in the Levels of Serum Melatonin and Urinary aMT6s in Rats
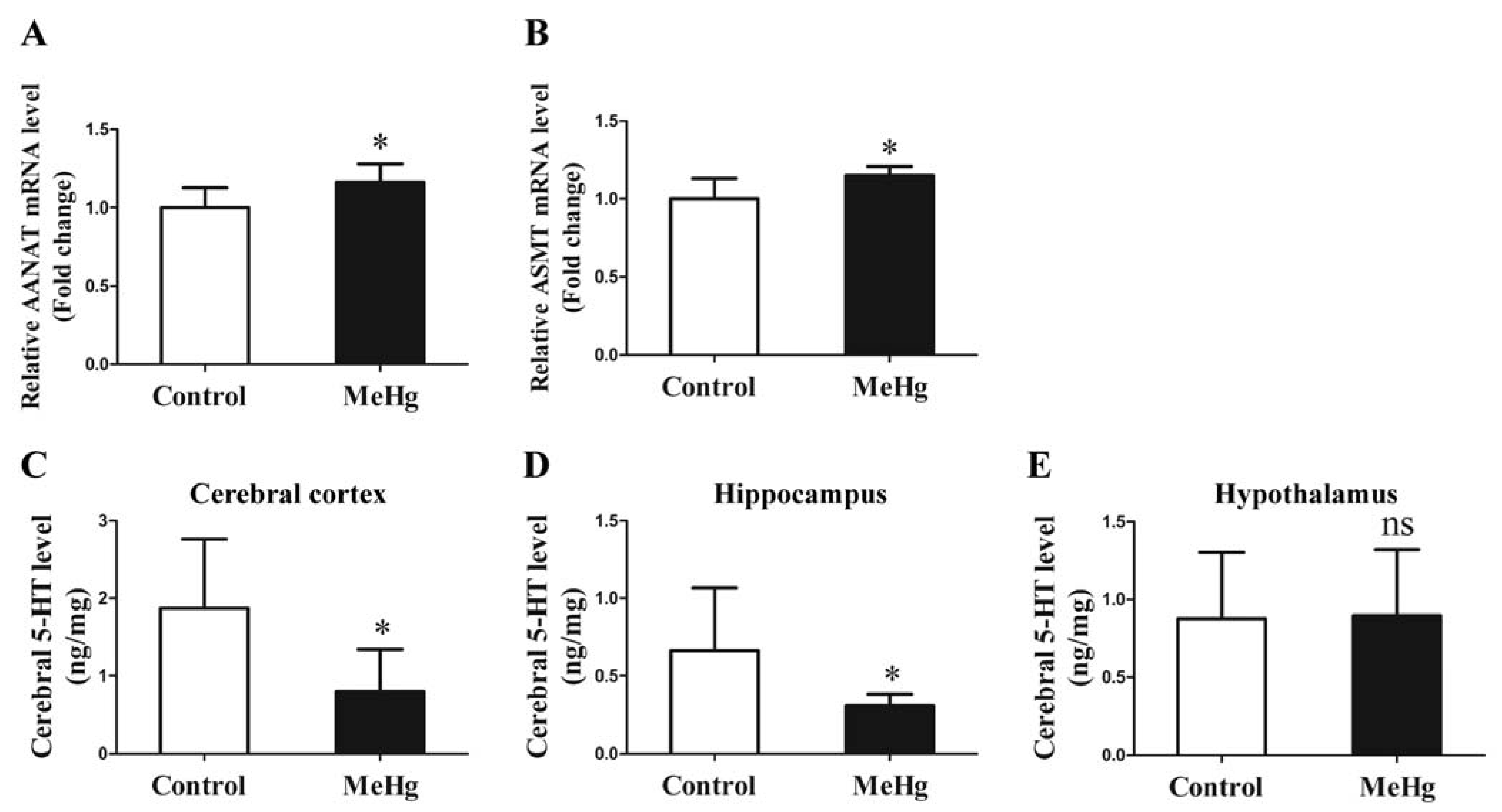
3.5. Influence of MeHg on the Expression of Melatonin Synthetase and 5-HT
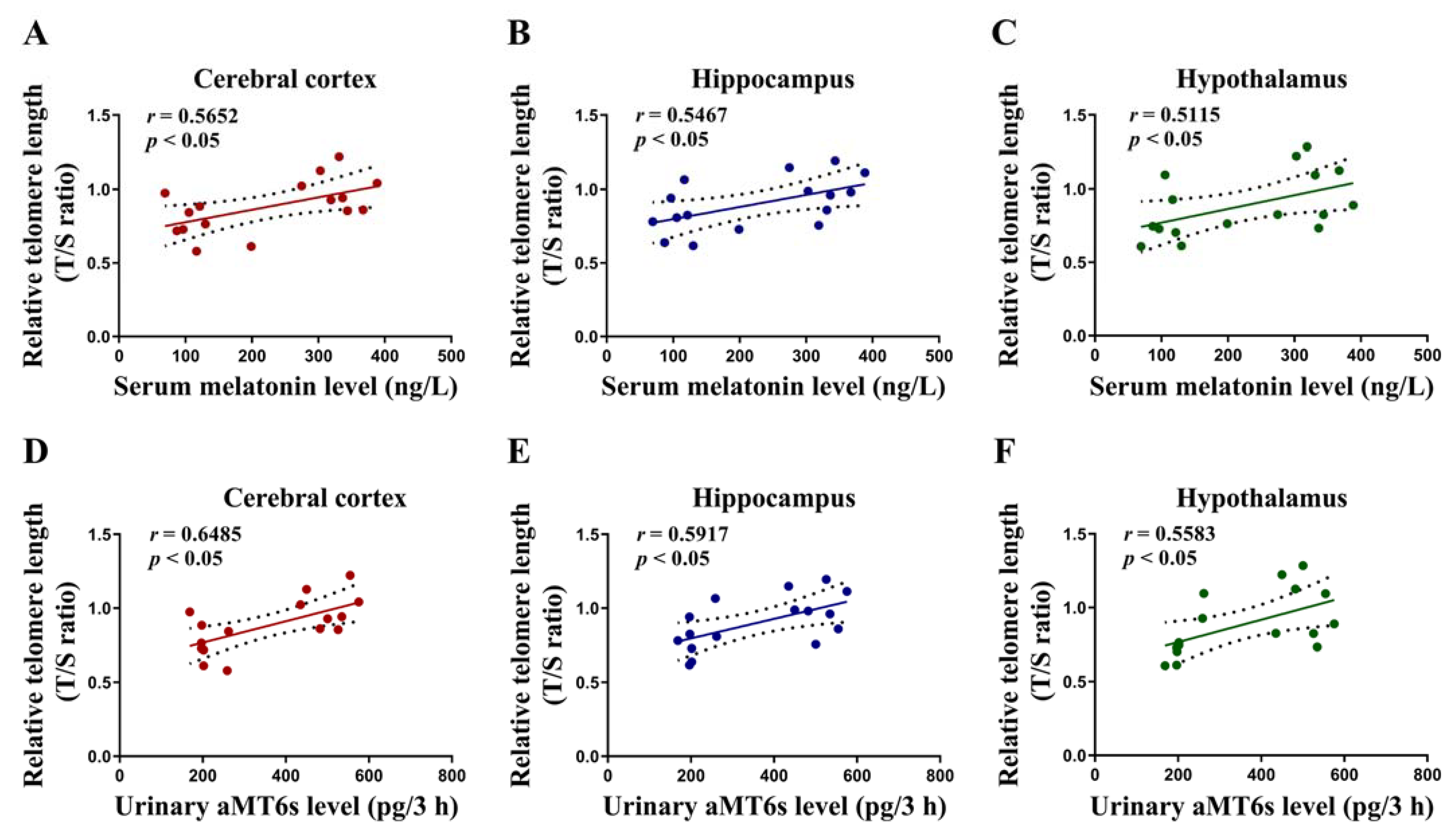
3.6. Correlations of the Level of Serum Melatonin and Urinary aMT6s with Cerebral Telomere Length in Rats
3.7. The Hypothesis of Adverse Impacts of MeHg on Cerebral Telomere Length and Causal Relationship with Reduced Melatonin Synthesis and Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, Y.; Lyu, Y.; Cao, Z.; Zhao, F.; Liu, Y.; Chen, C.; Li, C.; Gu, H.; Lu, F.; Zhou, J.; et al. Association of low cadmium and mercury exposure with chronic kidney disease among Chinese adults aged ≥80 years: A cross-sectional study. Chin. Med. J. 2022; in press. [Google Scholar] [CrossRef]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef]
- Kim, S.; Song, S.H.; Lee, C.W.; Kwon, J.T.; Park, E.Y.; Oh, J.K.; Kim, H.J.; Park, E.; Kim, B. Low-Level Environmental Mercury Exposure and Thyroid Cancer Risk Among Residents Living Near National Industrial Complexes in South Korea: A Population-Based Cohort Study. Thyroid 2022, 32, 1118–1128. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res. 1998, 77, 68–72. [Google Scholar] [CrossRef]
- Gustin, K.; Tofail, F.; Mehrin, F.; Levi, M.; Vahter, M.; Kippler, M. Methylmercury exposure and cognitive abilities and behavior at 10 years of age. Environ. Int. 2017, 102, 97–105. [Google Scholar] [CrossRef]
- Weiss, B.; Clarkson, T.W.; Simon, W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ. Health Perspect. 2002, 110, 851–854. [Google Scholar] [CrossRef]
- Bittencourt, L.O.; Matta, P.P.M.; Nascimento, P.C.; Eiró-Quirino, L.; Aragão, W.A.B.; Dionizio, A.; Fernandes, L.M.P.; Silva, M.C.F.; Buzalaf, M.A.R.; Aschner, M.; et al. Deciphering the Global Proteomic Profile Involved in Methylmercury-Induced Cerebellar Neurodegeneration and Motor Dysfunction in Adult Rats. Toxics 2022, 10, 531. [Google Scholar] [CrossRef]
- Li, X.; Pan, J.; Wei, Y.; Ni, L.; Xu, B.; Deng, Y.; Yang, T.; Liu, W. Mechanisms of oxidative stress in methylmercury-induced neurodevelopmental toxicity. Neurotoxicology 2021, 85, 33–46. [Google Scholar] [CrossRef]
- Fujimura, M.; Usuki, F. Methylmercury-Mediated Oxidative Stress and Activation of the Cellular Protective System. Antioxidants 2020, 9, 1004. [Google Scholar] [CrossRef]
- O’Sullivan, R.J.; Karlseder, J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010, 11, 171–181. [Google Scholar] [CrossRef]
- Aviv, A.; Shay, J.W. Reflections on telomere dynamics and ageing-related diseases in humans. Philos. Trans. R. Soc. Lond B Biol. Sci. 2018, 373, 20160436. [Google Scholar] [CrossRef]
- Møller, P.; Wils, R.S.; Jensen, D.M.; Andersen, M.H.G.; Roursgaard, M. Telomere dynamics and cellular senescence: An emerging field in environmental and occupational toxicology. Crit. Rev. Toxicol. 2018, 48, 761–788. [Google Scholar] [CrossRef]
- Louzon, M.; Coeurdassier, M.; Gimbert, F.; Pauget, B.; de Vaufleury, A. Telomere dynamic in humans and animals: Review and perspectives in environmental toxicology. Environ. Int. 2019, 131, 105025. [Google Scholar] [CrossRef]
- Henle, E.S.; Han, Z.; Tang, N.; Rai, P.; Luo, Y.; Linn, S. Sequence-specific DNA cleavage by Fe2+-mediated fenton reactions has possible biological implications. J. Biol. Chem. 1999, 274, 962–971. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. Role and Therapeutic Potential of Melatonin in the Central Nervous System and Cancers. Cancers 2020, 12, 1567. [Google Scholar] [CrossRef]
- Pomianowski, K.; Gozdowska, M.; Burzyński, A.; Kalamarz-Kubiak, H.; Sokołowska, E.; Kijewska, A.; Kulczykowska, E. A study of aanat and asmt expression in the three-spined stickleback eye and skin: Not only “on the way to melatonin”. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 241, 110635. [Google Scholar] [CrossRef]
- He, R.; Cui, M.; Lin, H.; Zhao, L.; Wang, J.; Chen, S.; Shao, Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018, 199, 122–130. [Google Scholar] [CrossRef]
- Kim, C.Y.; Nakai, K.; Kameo, S.; Kurokawa, N.; Liu, Z.M.; Satoh, H. Protective effect of melatonin on methylmercury-Induced mortality in mice. Tohoku J. Exp. Med. 2000, 191, 241–246. [Google Scholar] [CrossRef]
- Said, E.S.; Ahmed, R.M.; Mohammed, R.A.; Morsi, E.M.; Elmahdi, M.H.; Elsayed, H.S.; Mahmoud, R.H.; Nadwa, E.H. Ameliorating effect of melatonin on mercuric chloride-induced neurotoxicity in rats. Heliyon 2021, 7, e07485. [Google Scholar] [CrossRef]
- Rao, M.V.; Purohit, A.; Patel, T. Melatonin protection on mercury-exerted brain toxicity in the rat. Drug Chem. Toxicol. 2010, 33, 209–216. [Google Scholar] [CrossRef]
- Minami, M.; Takahashi, H.; Inagaki, H.; Yamano, Y.; Onoue, S.; Matsumoto, S.; Sasaki, T.; Sakai, K. Novel tryptamine-related substances, 5-sulphatoxydiacetyltryptamine, 5-hydroxydiacetyltryptamine, and reduced melatonin in human urine and the determination of those compounds, 6-sulphatoxymelatonin, and melatonin with fluorometric HPLC. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 814–822. [Google Scholar] [CrossRef]
- Lv, X.D.; Liu, S.; Cao, Z.; Gong, L.L.; Feng, X.P.; Gao, Q.F.; Wang, J.; Hu, L.; Cheng, X.C.; Yu, C.H.; et al. Correlation between serum melatonin and aMT6S level for age-related macular degeneration patients. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4196–4201. [Google Scholar]
- Yang, T.H.; Chen, Y.C.; Ou, T.H.; Chien, Y.W. Dietary supplement of tomato can accelerate urinary aMT6s level and improve sleep quality in obese postmenopausal women. Clin. Nutr. 2020, 39, 291–297. [Google Scholar] [CrossRef]
- Passos, C.J.; Da Silva, D.S.; Lemire, M.; Fillion, M.; Guimarães, J.R.; Lucotte, M.; Mergler, D. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 76–87. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Jiang, P.; Chen, C.; Zhang, T. Levodopa improves learning and memory ability on global cerebral ischemia-reperfusion injured rats in the Morris water maze test. Neurosci. Lett. 2017, 636, 233–240. [Google Scholar] [CrossRef]
- Novo, J.P.; Martins, B.; Raposo, R.S.; Pereira, F.C.; Oriá, R.B.; Malva, J.O.; Fontes-Ribeiro, C. Cellular and Molecular Mechanisms Mediating Methylmercury Neurotoxicity and Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 3101. [Google Scholar] [CrossRef]
- Maia, C.S.; Lucena, G.M.; Corrêa, P.B.; Serra, R.B.; Matos, R.W.; Menezes, F.C.; Santos, S.N.; Sousa, J.B.; Costa, E.T.; Ferreira, V.M. Interference of ethanol and methylmercury in the developing central nervous system. Neurotoxicology 2009, 30, 23–30. [Google Scholar] [CrossRef]
- Reardon, A.J.F.; Karathra, J.; Ribbenstedt, A.; Benskin, J.P.; MacDonald, A.M.; Kinniburgh, D.W.; Hamilton, T.J.; Fouad, K.; Martin, J.W. Neurodevelopmental and Metabolomic Responses from Prenatal Coexposure to Perfluorooctanesulfonate (PFOS) and Methylmercury (MeHg) in Sprague-Dawley Rats. Chem. Res. Toxicol. 2019, 32, 1656–1669. [Google Scholar] [CrossRef]
- Valentini, J.; Vicentini, J.; Grotto, D.; Tonello, R.; Garcia, S.C.; Barbosa Jr, F. Sub-Chronic Exposure to Methylmercury at Low Levels Decreases Butyrylcholinesterase Activity in Rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 95–99. [Google Scholar] [CrossRef]
- Grotto, D.; de Castro, M.M.; Barcelos, G.R.; Garcia, S.C.; Barbosa Jr, F. Low level and sub-chronic exposure to methylmercury induces hypertension in rats: Nitric oxide depletion and oxidative damage as possible mechanisms. Arch Toxicol. 2009, 83, 653–662. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging 2016, 8, 3–11. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Xia, Q.; Xia, Q.; Wang, B.; Yang, C.; Liang, J.; Liu, X. Potential roles of telomeres and telomerase in neurodegenerative diseases. Int. J. Biol. Macromol. 2020, 163, 1060–1078. [Google Scholar] [CrossRef]
- Liu, M.; Huo, Y.R.; Wang, J.; Wang, C.; Liu, S.; Liu, S.; Wang, J.; Ji, Y. Telomere Shortening in Alzheimer’s Disease Patients. Ann. Clin. Lab. Sci. 2016, 46, 260–265. [Google Scholar]
- Baker, D.J.; Petersen, R.C. Cellular senescence in brain aging and neurodegenerative diseases: Evidence and perspectives. J. Clin. Investig. 2018, 128, 1208–1216. [Google Scholar] [CrossRef]
- Yeates, A.J.; Thurston, S.W.; Li, H.; Mulhern, M.S.; McSorley, E.M.; Watson, G.E.; Shamlaye, C.F.; Strain, J.J.; Myers, G.J.; Davidson, P.W.; et al. PUFA Status and Methylmercury Exposure Are Not Associated with Leukocyte Telomere Length in Mothers or Their Children in the Seychelles Child Development Study. J. Nutr. 2017, 147, 2018–2024. [Google Scholar] [CrossRef]
- Crespo-López, M.E.; Soares, E.S.; Macchi, B.M.; Santos-Sacramento, L.; Takeda, P.Y.; Lopes-Araújo, A.; Paraense, R.S.O.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Luz, D.A.; et al. Towards Therapeutic Alternatives for Mercury Neurotoxicity in the Amazon: Unraveling the Pre-Clinical Effects of the Superfruit Açaí (Euterpe oleracea, Mart.) as Juice for Human Consumption. Nutrients 2019, 11, 2585. [Google Scholar] [CrossRef]
- Price, M.R.; Kruse, J.A.; Galvez, M.E.; Lorincz, A.M.; Avigdor, M.; Heideman, P.D. Failure to respond to endogenous or exogenous melatonin may cause nonphotoresponsiveness in Harlan Sprague Dawley rats. J. Circadian Rhythms. 2005, 3, 12. [Google Scholar] [CrossRef]
- Yie, S.M.; Liu, G.Y.; Johansson, E.; Brown, C.; Brown, G.M. Age-associated changes and sex differences in urinary 6-sulphatoxymelatonin circadian rhythm in the rat. Life Sci. 1992, 50, 1235–1242. [Google Scholar] [CrossRef]
- do Amaral, F.G.; Cipolla-Neto, J.; Afeche, S.C. Melatonin Synthesis Enzymes Activity: Radiometric Assays for AANAT, ASMT, and TPH. Methods Mol. Biol. 2022, 2550, 33–43. [Google Scholar]
- Kamal, M.; Gbahou, F.; Guillaume, J.L.; Daulat, A.M.; Benleulmi-Chaachoua, A.; Luka, M.; Chen, P.; Kalbasi Anaraki, D.; Baroncini, M.; Mannoury la Cour, C.; et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J. Biol. Chem. 2015, 290, 11537–11546. [Google Scholar] [CrossRef]
- Ben Bacha, A.; Norah, A.O.; Al-Osaimi, M.; Harrath, A.H.; Mansour, L.; El-Ansary, A. The therapeutic and protective effects of bee pollen against prenatal methylmercury induced neurotoxicity in rat pups. Metab. Brain Dis. 2020, 35, 215–224. [Google Scholar] [CrossRef]
- Tsuzuki, Y. Effect of methylmercury exposure on different neurotransmitter systems in rat brain. Toxicol. Lett. 1982, 13, 159–162. [Google Scholar]
- De-Miguel, F.F.; Trueta, C. Synaptic and extrasynaptic secretion of serotonin. Cell Mol. Neurobiol. 2005, 25, 297–312. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Li, P.; Tao, J.; Chen, X.; Zhang, A. Subchronic Low-Dose Methylmercury Exposure Accelerated Cerebral Telomere Shortening in Relevant with Declined Urinary aMT6s Level in Rats. Toxics 2023, 11, 191. https://doi.org/10.3390/toxics11020191
Wu X, Li P, Tao J, Chen X, Zhang A. Subchronic Low-Dose Methylmercury Exposure Accelerated Cerebral Telomere Shortening in Relevant with Declined Urinary aMT6s Level in Rats. Toxics. 2023; 11(2):191. https://doi.org/10.3390/toxics11020191
Chicago/Turabian StyleWu, Xi, Ping Li, Junyan Tao, Xiong Chen, and Aihua Zhang. 2023. "Subchronic Low-Dose Methylmercury Exposure Accelerated Cerebral Telomere Shortening in Relevant with Declined Urinary aMT6s Level in Rats" Toxics 11, no. 2: 191. https://doi.org/10.3390/toxics11020191
APA StyleWu, X., Li, P., Tao, J., Chen, X., & Zhang, A. (2023). Subchronic Low-Dose Methylmercury Exposure Accelerated Cerebral Telomere Shortening in Relevant with Declined Urinary aMT6s Level in Rats. Toxics, 11(2), 191. https://doi.org/10.3390/toxics11020191