A Systematic Review of Nano- and Microplastic (NMP) Influence on the Bioaccumulation of Environmental Contaminants: Part I—Soil Organisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Literature Selection and Data Collection
3. Results
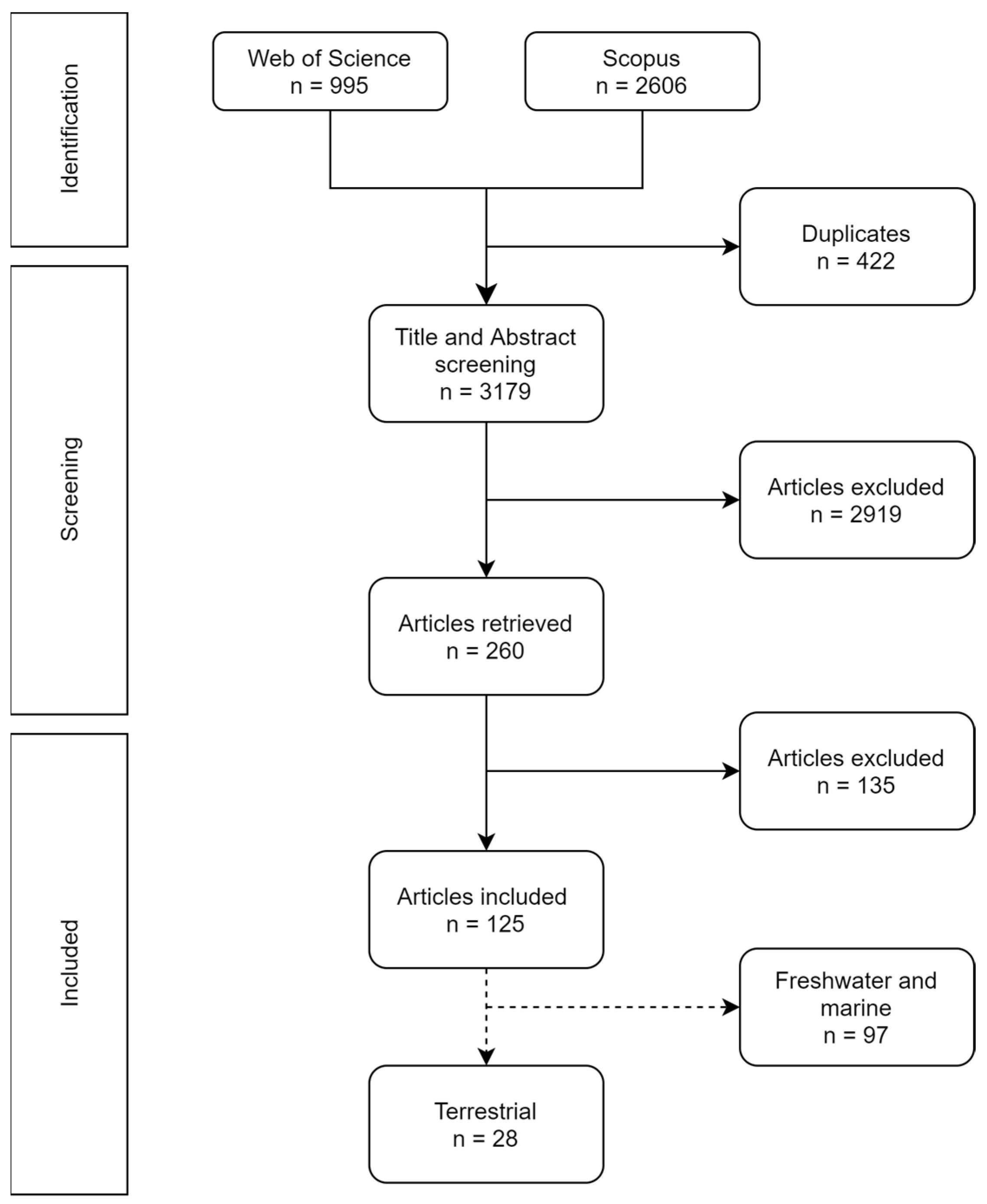
3.1. Literature Search and Selection
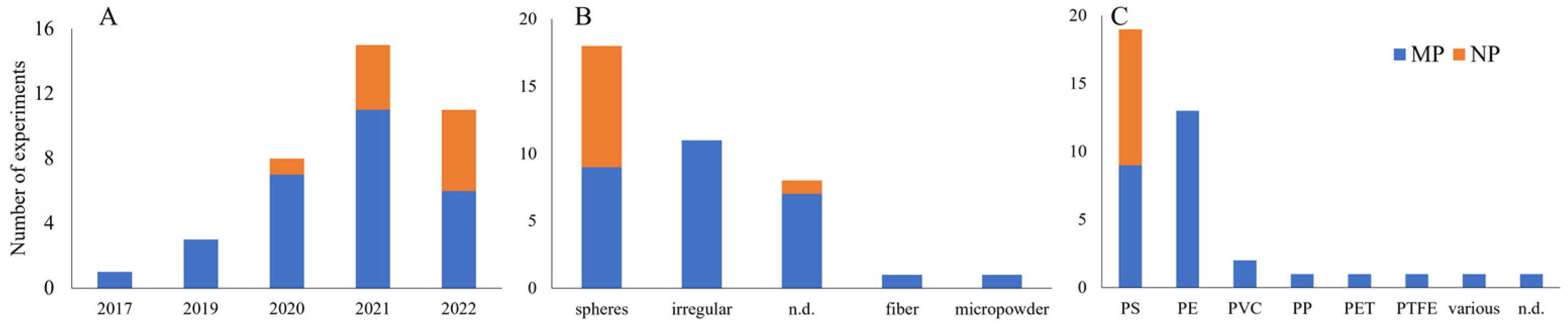
3.2. Characteristics of Selected Studies
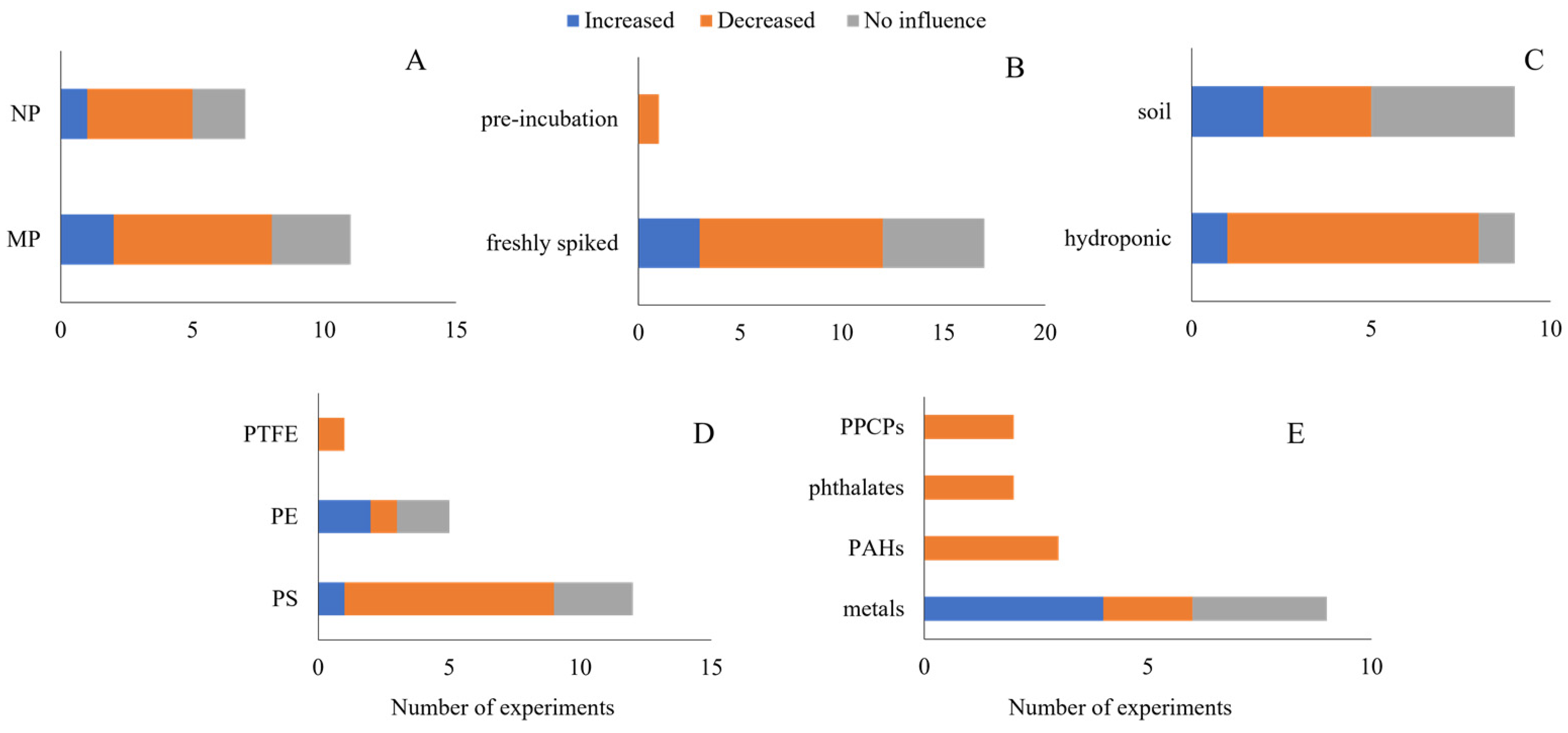
3.3. Studies with Earthworms and Potworms
3.4. Studies with Plants
4. Discussion
4.1. NMPs Are Most Likely to Affect Bioaccumulation
4.2. Knowledge Gaps and Limitations
5. Conclusions
- -
- Research is moving towards smaller particles, with an increasing number of publications using NP.
- -
- In plants, most studies observed a decrease in contaminants’ bioaccumulation. In earthworms, on the other hand, an increase in contaminant bioaccumulation was observed in most of the experiments.
- -
- In general, change in the bioavailable forms of the contaminants, either water or soil, was a major reason for the NMP to affect the contaminants’ bioaccumulation. This was valid for both plants and earthworms, even though the route of exposure differs between these two taxonomic groups.
- -
- Little consistency was found regarding NMP characteristics. This could be caused by the specific relation between NMP characteristics and the overall study design (from spiking methodology to contaminant used and its concentrations). Still, this topic should be better explored. For example, there is still inconsistency among studies on the NMP size that can enter the plant root.
- -
- Effects on bioaccumulation were more visible at high NMP concentrations, with few exceptions using realistic low concentrations. Future studies should focus on environmentally realistic concentrations.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Q.; Hu, X.; Yang, B.; Zhang, G.; Wang, J.; Ling, W. Distribution, abundance and risks of microplastics in the environment. Chemosphere 2020, 249, 126059. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Jeyavani, J.; Sibiya, A.; Shanthini, S.; Ravi, C.; Vijayakumar, S.; Rajan, D.K.; Vaseeharan, B. A Review on Aquatic Impacts of Microplastics and Its Bioremediation Aspects. Curr. Pollut. Rep. 2021, 7, 286–299. [Google Scholar] [CrossRef]
- Isobe, A.; Iwasaki, S.; Uchida, K.; Tokai, T. Abundance of non-conservative microplastics in the upper ocean from 1957 to 2066. Nat. Commun. 2019, 10, 4173. [Google Scholar] [CrossRef]
- Sana, S.S.; Dogiparthi, L.K.; Gangadhar, L.; Chakravorty, A.; Abhishek, N. Effects of microplastics and nanoplastics on marine environment and human health. Environ. Sci. Pollut. Res. 2020, 27, 44743–44756. [Google Scholar] [CrossRef]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard. Mater. 2020, 405, 123913. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Mar. Pollut. Bull. 2012, 64, 2782–2789. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Mouneyrac, C. Micro(Nano)Plastics: A threat to human health? Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. [Google Scholar] [CrossRef]
- Tourinho, P.S.P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hentschel, B.T.; Teh, S.J. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE 2014, 9, e85433. [Google Scholar] [CrossRef]
- Xie, M.; Huang, J.-L.; Lin, Z.; Chen, R.; Tan, Q.-G. Field to laboratory comparison of metal accumulation on aged microplastics in coastal waters. Sci. Total. Environ. 2021, 797, 149108. [Google Scholar] [CrossRef]
- Ding, T.; Wei, L.; Hou, Z.; Li, J.; Zhang, C.; Lin, D. Microplastics altered contaminant behavior and toxicity in natural waters. J. Hazard. Mater. 2021, 425, 127908. [Google Scholar] [CrossRef]
- Seidensticker, S.; Zarfl, C.; Cirpka, O.A.; Fellenberg, G.; Grathwohl, P. Shift in Mass Transfer of Wastewater Contaminants from Microplastics in the Presence of Dissolved Substances. Environ. Sci. Technol. 2017, 51, 12254–12263. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total. Environ. 2018, 657, 242–247. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total. Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Frias, J.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2018, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic Bag Derived-Microplastics as a Vector for Metal Exposure in Terrestrial Invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Loureiro, S.; Talluri, V.S.S.L.P.; Dolar, A.; Verweij, R.; Chvojka, J.; Michalcová, A.; Kočí, V.; van Gestel, C.A.M. Microplastic fibers influence Ag toxicity and bioaccumulation in Eisenia andrei but not in Enchytraeus crypticus. Ecotoxicology 2021, 30, 1216–1226. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. Microplastic particles increase arsenic toxicity to rice seedlings. Environ. Pollut. 2019, 259, 113892. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Xu, G.; Wang, Y.; Yu, Y. Impacts of polyethylene microplastics on bioavailability and toxicity of metals in soil. Sci. Total. Environ. 2020, 760, 144037. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Zhu, J.; Wang, J.; Wang, H.; Zhan, X. The joint toxicity of polyethylene microplastic and phenanthrene to wheat seedlings. Chemosphere 2021, 282, 130967. [Google Scholar] [CrossRef]
- Wang, J.; Coffin, S.; Sun, C.; Schlenk, D.; Gan, J. Negligible effects of microplastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environ. Pollut. 2019, 249, 776–784. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, G.; Yu, Y. Microplastics impact the accumulation of metals in earthworms by changing the gut bacterial communities. Sci. Total. Environ. 2022, 831, 154848. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, S.; Pei, L.; Sun, Y.; Wang, F. Ecotoxicological effects of polyethylene microplastics and ZnO nanoparticles on earthworm Eisenia fetida. Appl. Soil Ecol. 2022, 176, 104469. [Google Scholar] [CrossRef]
- Sobhani, Z.; Fang, C.; Naidu, R.; Megharaj, M. Microplastics as a vector of toxic chemicals in soil: Enhanced uptake of perfluorooctane sulfonate and perfluorooctanoic acid by earthworms through sorption and reproductive toxicity. Environ. Technol. Innov. 2021, 22, 101476. [Google Scholar] [CrossRef]
- Jia, H.; Wu, D.; Yu, Y.; Han, S.; Sun, L.; Li, M. Impact of microplastics on bioaccumulation of heavy metals in rape (Brassica napus L.). Chemosphere 2021, 288, 132576. [Google Scholar] [CrossRef]
- Sun, W.; Meng, Z.; Li, R.; Zhang, R.; Jia, M.; Yan, S.; Tian, S.; Zhou, Z.; Zhu, W. Joint effects of microplastic and dufulin on bioaccumulation, oxidative stress and metabolic profile of the earthworm (Eisenia fetida). Chemosphere 2020, 263, 128171. [Google Scholar] [CrossRef]
- Huang, C.; Ge, Y.; Yue, S.; Zhao, L.; Qiao, Y. Microplastics aggravate the joint toxicity to earthworm Eisenia fetida with cadmium by altering its availability. Sci. Total. Environ. 2020, 753, 142042. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total. Environ. 2021, 784, 147133. [Google Scholar] [CrossRef]
- Boughattas, I.; Zitouni, N.; Hattab, S.; Mkhinini, M.; Missawi, O.; Helaoui, S.; Mokni, M.; Bousserrhine, N.; Banni, M. Interactive effects of environmental microplastics and 2,4-dichlorophenoxyacetic acid (2,4-D) on the earthworm Eisenia andrei. J. Hazard. Mater. 2022, 424, 1275587. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, G.; Yu, Y. Effects of polystyrene microplastics on accumulation of pyrene by earthworms. Chemosphere 2022, 296, 134059. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J. Hazard. Mater. 2020, 392, 122273. [Google Scholar] [CrossRef]
- Lahive, E.; Cross, R.; Saarloos, A.I.; Horton, A.A.; Svendsen, C.; Hufenus, R.; Mitrano, D.M. Earthworms ingest microplastic fibres and nanoplastics with effects on egestion rate and long-term retention. Sci. Total. Environ. 2021, 807, 151022. [Google Scholar] [CrossRef]
- Xu, G.; Liu, Y.; Song, X.; Li, M.; Yu, Y. Size effects of microplastics on accumulation and elimination of phenanthrene in earthworms. J. Hazard. Mater. 2020, 403, 123966. [Google Scholar] [CrossRef]
- Huang, D.; Zhou, W.; Chen, S.; Tao, J.; Li, R.; Yin, L.; Wang, X.; Chen, H. Presence of polystyrene microplastics in Cd contaminated water promotes Cd removal by nano zero-valent iron and ryegrass (Lolium Perenne L.). Chemosphere 2022, 303, 134729. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Peijnenburg, W.J.; Li, R.; Yang, J.; Zhou, Q. Confocal measurement of microplastics uptake by plants. Methodsx 2019, 7, 100750. [Google Scholar] [CrossRef]
- Taylor, S.E.; Pearce, C.I.; Sanguinet, K.A.; Hu, D.; Chrisler, W.B.; Kim, Y.-M.; Wang, Z.; Flury, M. Polystyrene nano- and microplastic accumulation at Arabidopsis and wheat root cap cells, but no evidence for uptake into roots. Environ. Sci. Nano 2020, 7, 1942–1953. [Google Scholar] [CrossRef]
- Wang, H.-T.; Ding, J.; Xiong, C.; Zhu, D.; Li, G.; Jia, X.-Y.; Zhu, Y.-G.; Xue, X.-M. Exposure to microplastics lowers arsenic accumulation and alters gut bacterial communities of earthworm Metaphire californica. Environ. Pollut. 2019, 251, 110–116. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, Q.; Sun, Y. Microplastics as a Vector for HOC Bioaccumulation in Earthworm Eisenia fetida in Soil: Importance of Chemical Diffusion and Particle Size. Environ. Sci. Technol. 2020, 54, 12154–12163. [Google Scholar] [CrossRef]
- Ma, J.; Chen, F.; Zhu, Y.; Li, X.; Yu, H.; Sun, Y. Joint effects of microplastics and ciprofloxacin on their toxicity and fates in wheat: A hydroponic study. Chemosphere 2022, 303, 135023. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, J.; Zhu, J.; Zhang, L.; Jiang, L.; Yin, Y.; Guo, H. Effects of polystyrene microplastic on uptake and toxicity of copper and cadmium in hydroponic wheat seedlings (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2021, 217, 112217. [Google Scholar] [CrossRef]
- Gao, M.; Xu, Y.; Liu, Y.; Wang, S.; Wang, C.; Dong, Y.; Song, Z. Effect of polystyrene on dibutyl phthalate (DBP) bioavailability and DBP-induced phytotoxicity in lettuce. Environ. Pollut. 2020, 268, 115870. [Google Scholar] [CrossRef]
- Xu, G.; Liu, Y.; Yu, Y. Effects of polystyrene microplastics on uptake and toxicity of phenanthrene in soybean. Sci. Total. Environ. 2021, 783, 147016. [Google Scholar] [CrossRef]
- Chen, L.; Han, L.; Feng, Y.; He, J.; Xing, B. Soil structures and immobilization of typical contaminants in soils in response to diverse microplastics. J. Hazard. Mater. 2022, 438, 129555. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Adams, C.A.; Sun, Y. Effects of Co-Contamination of Microplastics and Cd on Plant Growth and Cd Accumulation. Toxics 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Gao, P.; Zhang, M.; Wang, L.; Sun, H.; Liu, C. Adverse effects of microplastics on earthworms: A critical review. Sci. Total. Environ. 2022, 850, 158041. [Google Scholar] [CrossRef] [PubMed]
- Paul-Pont, I.; Lacroix, C.; Fernández, C.G.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Chua, E.M.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Clarke, B.O. Assimilation of Polybrominated Diphenyl Ethers from Microplastics by the Marine Amphipod. Allorchestes Compressa. Environ. Sci. Technol. 2014, 48, 8127–8134. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Liu, H.; Wang, H.; Lu, K.; Gao, S. Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. J. Hazard. Mater. 2020, 392, 122346. [Google Scholar] [CrossRef]
- Abbasi, S.; Moore, F.; Keshavarzi, B.; Hopke, P.K.; Naidu, R.; Rahman, M.M.; Oleszczuk, P.; Karimi, J. PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci. Total. Environ. 2020, 744, 140984. [Google Scholar] [CrossRef]
- Abbasi, S.; Moore, F.; Keshavarzi, B. PET-microplastics as a vector for polycyclic aromatic hydrocarbons in a simulated plant rhizosphere zone. Environ. Technol. Innov. 2021, 21, 101370. [Google Scholar] [CrossRef]
- van der Zande, M.; Kokalj, A.J.; Spurgeon, D.J.; Loureiro, S.; Silva, P.V.; Khodaparast, Z.; Drobne, D.; Clark, N.J.; Brink, N.W.V.D.; Baccaro, M.; et al. The gut barrier and the fate of engineered nanomaterials: A view from comparative physiology. Environ. Sci. Nano 2020, 7, 1874–1898. [Google Scholar] [CrossRef]
- Vaccari, F.; Forestieri, B.; Papa, G.; Bandini, F.; Huerta-Lwanga, E.; Boughattas, I.; Missawi, O.; Banni, M.; Negri, I.; Cocconcelli, P.S.; et al. Effects of micro and nanoplastics on soil fauna gut microbiome: An emerging ecological risk for soil health. Curr. Opin. Environ. Sci. Heallth 2022, 30, 100402. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ouyang, D.; Lei, J.; Tan, Q.; Xie, L.; Li, Z.; Liu, T.; Xiao, Y.; Farooq, T.H.; et al. Systematical review of inter-actions between microplastics and microorganisms in the soil environment. J. Hazard. Mater. 2021, 418, 126288. [Google Scholar] [CrossRef]
- Ardestani, M.M.; van Straalen, N.M.; van Gestel, C.A. Uptake and elimination kinetics of metals in soil invertebrates: A review. Environ. Pollut. 2014, 193, 277–295. [Google Scholar] [CrossRef]
- Golwala, H.; Zhang, X.; Iskander, S.M.; Smith, A.L. Solid waste: An overlooked source of microplastics to the environment. Sci. Total. Environ. 2021, 769, 144581. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is There any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef]
- van Gestel, C.A.M. Soil ecotoxicology: State of the art and future directions. Zookeys 2012, 176, 275–296. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tourinho, P.S.; Loureiro, S.; Pavlaki, M.D.; Mocová, K.A.; Ribeiro, F. A Systematic Review of Nano- and Microplastic (NMP) Influence on the Bioaccumulation of Environmental Contaminants: Part I—Soil Organisms. Toxics 2023, 11, 154. https://doi.org/10.3390/toxics11020154
Tourinho PS, Loureiro S, Pavlaki MD, Mocová KA, Ribeiro F. A Systematic Review of Nano- and Microplastic (NMP) Influence on the Bioaccumulation of Environmental Contaminants: Part I—Soil Organisms. Toxics. 2023; 11(2):154. https://doi.org/10.3390/toxics11020154
Chicago/Turabian StyleTourinho, Paula S., Susana Loureiro, Maria D. Pavlaki, Klará Anna Mocová, and Fabianne Ribeiro. 2023. "A Systematic Review of Nano- and Microplastic (NMP) Influence on the Bioaccumulation of Environmental Contaminants: Part I—Soil Organisms" Toxics 11, no. 2: 154. https://doi.org/10.3390/toxics11020154
APA StyleTourinho, P. S., Loureiro, S., Pavlaki, M. D., Mocová, K. A., & Ribeiro, F. (2023). A Systematic Review of Nano- and Microplastic (NMP) Influence on the Bioaccumulation of Environmental Contaminants: Part I—Soil Organisms. Toxics, 11(2), 154. https://doi.org/10.3390/toxics11020154








