Impact of Cadmium Contamination on Fertilizer Value and Associated Health Risks in Different Soil Types Following Anaerobic Digestate Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Plant Harvesting and Soil and Plant Analysis
2.3. Fertilizer Value
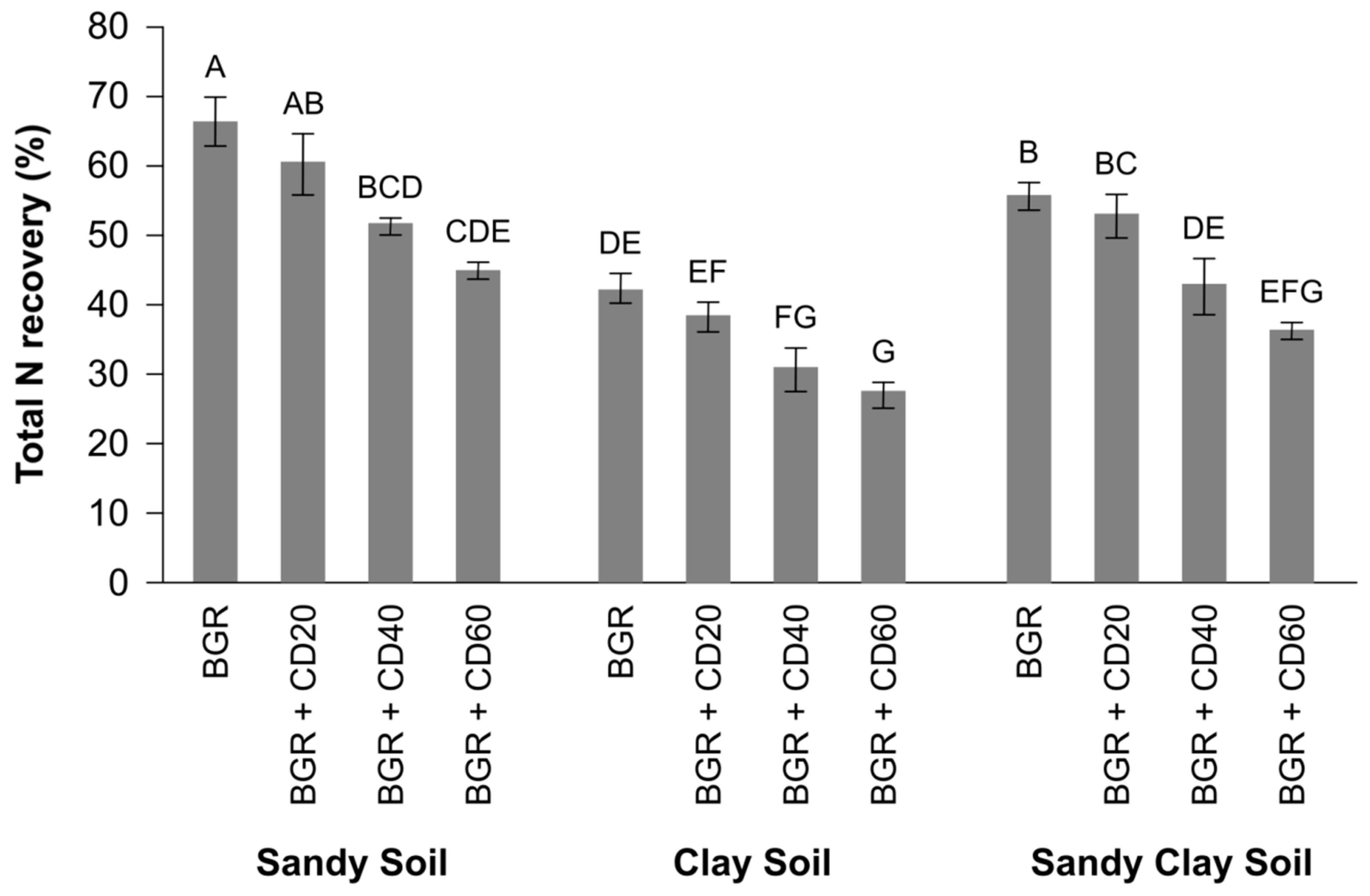
Nitrogen Recovery and Mineralization
2.4. Associated Health Risks Measurement
2.4.1. Daily Intake of Metals (DIM)
2.4.2. Health Risk Index (HRI)
2.5. Statistical Analysis
3. Result
3.1. Fertilizer Value
3.1.1. Total Dry Matter Yield of Spinach
3.1.2. Apparent N Recovery in Spinach Plant
3.1.3. N Mineralization
3.2. Human Health Risks
4. Discussion
4.1. Fertilizer Value (DM Yield, N Recovery and N-Mineralization)
4.2. Health Risk Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zheng, X.; He, X.; Lü, Q.; Qian, X.; Xiao, Q.; Lin, R. Effects of Pseudomonas TCd-1 on rice (Oryza sativa) cadmium uptake, rhizosphere soils enzyme activities and cadmium bioavailability under cadmium contamination. Ecotoxicol. Environ. Saf. 2021, 218, 112249. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. ATSDR’s Substance Priority List. 2022. Available online: http://www.atsdr.cdc.gov/SPL/index.html (accessed on 9 September 2023).
- Zhang, Q.; Wang, C. Natural and human factors affect the distribution of soil heavy metal pollution: A review. Water Air Soil Pollut. 2020, 231, 1–13. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Zhao, Y.; Deng, Q.; Lin, Q.; Zeng, C.; Zhong, C. Cadmium source identification in soils and high-risk regions predicted by geographical detector method. Environ. Pollut. 2020, 263, 114338. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Xie, Y.; Luo, Y.; Sheng, M.; Xu, F.; Xu, H. Ecological responses of soil microbial abundance and diversity to cadmium and soil properties in farmland around an enterprise-intensive region. J. Hazard. Mater. 2020, 392, 122478. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S.J.E. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Rashti, M.R.; Esfandbod, M.; Adhami, E.; Srivastava, P. Cadmium desorption behaviour in selected sub-tropical soils: Effects of soil properties. J. Geochem. Explor. 2014, 144, 230–236. [Google Scholar] [CrossRef]
- Jing, F.; Chen, X.; Wen, X.; Liu, W.; Hu, S.; Yang, Z.; Guo, B.; Luo, Y.; Yu, Q.; Xu, Y.J.S.U.; et al. Biochar effects on soil chemical properties and mobilization of cadmium (Cd) and lead (Pb) in paddy soil. Soil Use Manag. 2020, 36, 320–327. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Chen, G.P.J. Bioavailability of Cd in a soil–rice system in China: Soil type versus genotype effects. Plant Soil 2005, 271, 165–173. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Hu, Y.; Wang, X.; Ai, X.; Tang, L.; Matthew, C.; Cavanagh, J.; Qiu, J. Enrofloxacin at environmentally relevant concentrations enhances uptake and toxicity of cadmium in the earthworm Eisenia fetida in farm soils. J. Hazard. Mater. 2016, 308, 312–320. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Wang, M.; Chen, W.; Peng, C. Risk assessment of Cd polluted paddy soils in the industrial and township areas in Hunan, Southern China. Chemosphere 2016, 144, 346–351. [Google Scholar] [CrossRef]
- Knoell, D.L.; Wyatt, T.A. The adverse impact of cadmium on immune function and lung host defense. Semin. Cell Dev. Biol. 2021, 115, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Ashraf, M.N.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.; Yang, X. Organic soil additives for the remediation of cadmium contaminated soils and their impact on the soil-plant system: A review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Y.; Huang, B.; Teng, Y. Soil environmental quality in greenhouse vegetable production systems in eastern China: Current status and management strategies. Chemosphere 2017, 170, 183–195. [Google Scholar] [CrossRef]
- Shah, G.M.; Tufail, N.; Bakhat, H.F.; Imran, M.; Murtaza, B.; Farooq, A.B.U.; Saeed, F.; Waqar, A.; Rashid, M. Anaerobic degradation of municipal organic waste among others composting techniques improves N cycling through waste-soil-plant continuum. J. Soil Sci. Plant Nutr. 2017, 17, 529–542. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of soil, plant, and water analysis. A Man. West Asia N. Afr. Reg. 2013, 3, 65–119. [Google Scholar]
- Shah, G.M.; Tufail, N.; Bakhat, H.F.; Ahmad, I.; Shahid, M.; Hammad, H.M.; Nasim, W.; Waqar, A.; Rizwan, M.; Dong, R. Composting of municipal solid waste by different methods improved the growth of vegetables and reduced the health risks of cadmium and lead. Environ. Sci. Pollut. Res. 2019, 26, 5463–5474. [Google Scholar] [CrossRef]
- Cui, Y.-J.; Zhu, Y.-G.; Zhai, R.-H.; Chen, D.-Y.; Huang, Y.-Z.; Qiu, Y.; Liang, J.-Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ. Int. 2004, 30, 785–791. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund: Human Health Evaluation Manual (Part A): Interim Final; (EPA/540/1-89/002); U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- USEPA. Risk-Based Concentration Table; United States Environmental Protection Agency: Washington, DC, USA, 2011.
- Shah, G.; Rashid, M.; Shah, G.; Groot, J.; Lantinga, E.A. Mineralization and herbage recovery of animal manure nitrogen after application to various soil types. Plant Soil 2013, 365, 69–79. [Google Scholar] [CrossRef]
- Möller, K.; Stinner, W.; Deuker, A.; Leithold, G. Effects of different manuring systems with and without biogas digestion on nitrogen cycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosyst. 2008, 82, 209–232. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.; Jensen, E.S.J.B. Mineralization of carbon and nitrogen from fresh and anaerobically stored sheep manure in soils of different texture. Biol. Fertil. Soils 1995, 19, 29–35. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Van Veen, J.; Kuikman, P.J.B. Soil structural aspects of decomposition of organic matter by micro-organisms. Biol. Fertil. Soils 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, Q.; Liu, Y.J.; Jia, F.A.; He, X.H. Responses of soil microbial activity to cadmium pollution and elevated CO2. Sci. Rep. 2014, 4, 4287. [Google Scholar] [CrossRef]
- Agrawal, S.B.; Mishra, S.J.E. Effects of supplemental ultraviolet-B and cadmium on growth, antioxidants and yield of Pisum sativum L. Ecotoxicol. Environ. Saf. 2009, 72, 610–618. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J.J.L. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Rieuwerts, J.; Thornton, I.; Farago, M.; Ashmore, M. Factors influencing metal bioavailability in soils: Preliminary investigations for the development of a critical loads approach for metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef]
- Andersson, A. Distribution of heavy metals as compared to some other elements between grain size fractions in soils. Swedish J. Agric. Res. 1979, 1979. 9, 7–13. [Google Scholar]
- Angelova, V.; Ivanova, R.; Pevicharova, G.; Ivanov, K. Effect of organic amendments on heavy metals uptake by potato plants. In Proceedings of the 19th World Congress of soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Reid, B.J.; Li, G.; Zhu, Y.-G. Application of biochar to soil reduces cancer risk via rice consumption: A case study in Miaoqian village, Longyan, China. Environ. Int. 2014, 68, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.; Zhang, X.; Zheng, J.; et al. Biochar soil amendment as a solution to prevent Cd-tainted rice from China: Results from a cross-site field experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Mohamed, I.; Zhang, G.-s.; Li, Z.-g.; Liu, Y.; Chen, F.; Dai, K. Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecol. Eng. 2015, 84, 67–76. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Bolan, N.; Pei, J.; Wang, H. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Sci. Total Environ. 2014, 468–469, 598–608. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. Chapter 2—A Review of Biochar and Its Use and Function in Soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Alia, N.; Sardar, K.; Said, M.; Salma, K.; Sadia, A.; Sadaf, S.; Toqeer, A.; Miklas, S.J.I.J.o.E.R.; Health, P. Toxicity and bioaccumulation of heavy metals in spinach (Spinacia oleracea) grown in a controlled environment. Int. J. Environ. Res. Public Health 2015, 12, 7400–7416. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.B. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Saleem, F.; Riaz, U.; Aziz, H.; Murtaza, G.; Saifullah; Naveed, M.; Shahid, M.; Murtaza, B. Health risk assessment of trace metals from spinach grown on compost-amended soil. Int. J. Phytoremediation 2018, 20, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Xing, M.; Yang, J.J.B.T. Speciation and transformation of heavy metals during vermicomposting of animal manure. Bioresour. Technol. 2016, 209, 397–401. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Burgos, P.; Madrid, F.; Camuña, I. Source separate collection of recyclables reduces chromium and nickel content in municipal solid waste compost. Air Water 2015, 43, 427–433. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
| Parameters | Units | BGR |
|---|---|---|
| Dry matter | % | 55.14 (±2.16) |
| Organic matter | % | 30.54 (±2.50) |
| Ntotal | % | 3.56 (±0.04) |
| Nmin | % | 0.98 (±0.06) |
| Total C | % | 46.54 (±3.92) |
| C:N ratio | - | 13.05 |
| Cd | mg kg−1 DM | 2.23 (±0.447) |
| pHH2O 1:10 | - | 7.45 (±0.18) |
| EC (1:5) | dS m−1 | 3.02 (±0.29) |
| Lignin | % DM | 28 (±1.60) |
| Cellulose | % DM | 26 (±0.97) |
| Hemicellulose | % DM | 20 (±1.23) |
| Parameters | Units | Sandy Soil | Clay Soil | Sandy Clay Soil |
|---|---|---|---|---|
| Dry matter | % | 95.96 (±9.82) | 42.54 (±3.30) | 55.14 (±2.16) |
| Organic matter | % | 16.92 (±1.33) | 27.60 (±5.72) | 30.54 (±2.50) |
| Ntotal | % | 01.23 (±0.10) | 3.75 (±0.02) | 3.56 (±0.04) |
| Total C | % | 19.80 (±0.12) | 51.36 (±5.61) | 46.54 (±3.92) |
| C:N ratio | - | 16.09 | 13.69 | 13.05 |
| Cd content | mg kg−1 DM | 2.4 (±0.447) | 2.23 (±0.447) | 2.5 (±0.447) |
| pHH2O 1:10 | - | 7.1 (±0.90) | 7.3 (±1.0) | 7.3 (±0.18) |
| EC (1:5) | dS m−1 | 243.4 (±0.07) | 306.7(±0.76) | 286.7 (±0.29) |
| Sand | % | 60 (±6.33) | 40 (±2.16) | 52 (±3.52) |
| Silt | % | 30 (±5.72) | 30 (±2.50) | 28 (±5.72) |
| Clay | % | 10 (±0.02) | 30 (±9.82) | 20 (±1.33) |
| Treatments | Total Dry Matter (g m−2) | N Uptake (g m−2) | N Use Efficiency | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sandy Soil | Clay Soil | Sandy Clay Soil | Sandy Soil | Clay Soil | Sandy Clay Soil | Sandy Soil | Clay Soil | Sandy Clay Soil | |
| Control | 611.43 c | 393.33 b | 500.48 c | 6.23 c | 4.00 d | 5.18 c | 98.2 | 98.33 | 96.56 |
| Cd20 | 595.24 c | 390.71 b | 494.29 c | 6.24 c | 3.99 d | 5.14 c | 95.39 | 98.01 | 96.24 |
| Cd40 | 585.71 c | 387.33 b | 486.67 c | 6.23 c | 4.02 d | 5.18 c | 94.02 | 96.37 | 93.98 |
| Cd60 | 578.57 c | 379.05 b | 484.95 c | 6.31 c | 3.90 d | 5.20 c | 91.73 | 97.2 | 93.34 |
| BGR | 1100 a | 723.81 a | 904.76 a | 12.85 a | 8.23 a | 10.74 a | 85.59 | 87.97 | 84.23 |
| Cd20 + BGR | 1038.1 ab | 704.76 a | 857.14 ab | 12.26 a | 7.80 ab | 10.42 a | 84.69 | 90.31 | 82.25 |
| Cd40 + BGR | 971.43 ab | 657.14 a | 819.05 ab | 11.36 ab | 7.09 bc | 9.44 ab | 85.51 | 99.47 | 86.79 |
| Cd60 + BGR | 904.76 b | 600.00 a | 780.95 b | 10.79 b | 6.60 c | 8.83 b | 83.89 | 106.7 | 88.47 |
| N Applied (g m−2) | N Uptake by Spinach (g m−2) | Residual Nmin (g m−2) | Net NOrganic Mineralized | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Organic | Mineral | Total | From BGR | Total | From BGR | A | B | |
| Sandy Soil | |||||||||
| Control | 0.00 | 0.00 | 0.00 | 6.23 | – | 0.67 | – | – | – |
| Cd60 | 0.00 | 0.00 | 0.00 | 6.23 | – | 0.58 | |||
| BGR | 13.50 | 9.32 | 4.19 | 12.85 | 6.63 | 1.09 | 0.42 | 2.86 | 30.75 a |
| BGR + Cd60 | 13.50 | 9.32 | 4.19 | 10.79 | 4.48 | 1.16 | 0.89 | 0.93 | 10.04 c |
| Sandy Clay Soil | |||||||||
| Control | 0.00 | 0.00 | 0.00 | 5.18 | – | 0.45 | – | – | – |
| Cd60 | 0.00 | 0.00 | 0.00 | 5.01 | – | 0.42 | – | – | – |
| BGR | 13.50 | 9.32 | 4.19 | 10.74 | 5.56 | 0.91 | 0.46 | 1.82 | 19.74 b |
| BGR + Cd60 | 13.50 | 9.32 | 4.19 | 8.83 | 3.82 | 0.88 | 0.46 | 0.10 | 1.04 d |
| Clay Soil | |||||||||
| Control | 0.00 | 0.00 | 0.00 | 4.00 | 0.27 | – | – | – | |
| Cd60 | 0.00 | 0.00 | 0.00 | 3.90 | 0.30 | – | – | – | |
| BGR | 13.50 | 9.32 | 4.19 | 8.22 | 4.23 | 0.97 | 0.70 | 0.75 | 8.00 cd |
| BGR + Cd60 | 13.50 | 9.32 | 4.19 | 6.60 | 2.48 | 0.83 | 0.36 | 0.95 | –10.19 e |
| Treatments | C Metal Cd (mg kg−1) | DIM (mg day−1) | HRI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sandy Soil | Clay Soil | Sandy Clay Soil | Sandy Soil | Clay Soil | Sandy Clay Soil | Sandy Soil | Clay Soil | Sandy Clay Soil | |
| Control | 4.3 e | 6.3 e | 7.5 d | 0.0031 e | 0.0045 c | 0.0063 d | 0.19 c | 0.41 c | 0.75 e |
| Cd20 | 9.8 de | 11.8 bcd | 17.7 b | 0.0070 de | 0.0085 bc | 0.0150 b | 1.02 c | 1.66 bc | 3.97 bc |
| Cd40 | 18.7 bc | 17.2 b | 20.5 ab | 0.0134 bc | 0.0124 abc | 0.0174 ab | 3.6 c | 3.09 abc | 5.12 ab |
| Cd60 | 30.3 a | 24.4 a | 23.6 a | 0.0218 ab | 0.01766 a | 0.0200 a | 10.0 a | 6.38 a | 6.85 a |
| BGR | 7.1 e | 7.8 de | 7.3 d | 0.0051 e | 0.00567 bc | 0.0062 d | 0.51 c | 0.68 c | 0.69 e |
| Cd20 + BGR | 8.8 e | 9.7 cde | 12.2 c | 0.0063 e | 0.0070 bc | 0.0103 c | 0.81 c | 0.97 c | 1.83 de |
| Cd40 + BGR | 15.6 cd | 14.2 bc | 16.5 b | 0.0112 cd | 0.0102 abc | 0.0140 b | 2.61 bc | 2.10 bc | 3.35 cd |
| Cd60 + BGR | 25 ab | 24.8 a | 19.3 b | 0.0180 ab | 0.0208 ab | 0.0164 b | 6.49 ab | 4.9 ab | 4.53 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, G.M.; Farooq, U.; Shabbir, Z.; Guo, J.; Dong, R.; Bakhat, H.F.; Wakeel, M.; Siddique, A.; Shahid, N. Impact of Cadmium Contamination on Fertilizer Value and Associated Health Risks in Different Soil Types Following Anaerobic Digestate Application. Toxics 2023, 11, 1008. https://doi.org/10.3390/toxics11121008
Shah GM, Farooq U, Shabbir Z, Guo J, Dong R, Bakhat HF, Wakeel M, Siddique A, Shahid N. Impact of Cadmium Contamination on Fertilizer Value and Associated Health Risks in Different Soil Types Following Anaerobic Digestate Application. Toxics. 2023; 11(12):1008. https://doi.org/10.3390/toxics11121008
Chicago/Turabian StyleShah, Ghulam Mustafa, Umer Farooq, Zunaira Shabbir, Jianbin Guo, Renjie Dong, Hafiz Faiq Bakhat, Muhammad Wakeel, Ayesha Siddique, and Naeem Shahid. 2023. "Impact of Cadmium Contamination on Fertilizer Value and Associated Health Risks in Different Soil Types Following Anaerobic Digestate Application" Toxics 11, no. 12: 1008. https://doi.org/10.3390/toxics11121008
APA StyleShah, G. M., Farooq, U., Shabbir, Z., Guo, J., Dong, R., Bakhat, H. F., Wakeel, M., Siddique, A., & Shahid, N. (2023). Impact of Cadmium Contamination on Fertilizer Value and Associated Health Risks in Different Soil Types Following Anaerobic Digestate Application. Toxics, 11(12), 1008. https://doi.org/10.3390/toxics11121008







