Contamination Status, Environmental Factor and Risk Assessment of Polychlorinated Biphenyls and Hexachlorobutadiene in Greenhouse and Open-Field Agricultural Soils across China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Pretreatment
2.3. Chemical Analysis
2.4. Quality Assurance and Quality Control
2.5. Environmental Factors and Pollution Sources Analysis
2.6. Health Risk Assessment
2.7. Statistic Analysis
3. Results and Discussions
3.1. Levels and Spatial Distributions of Pollutants
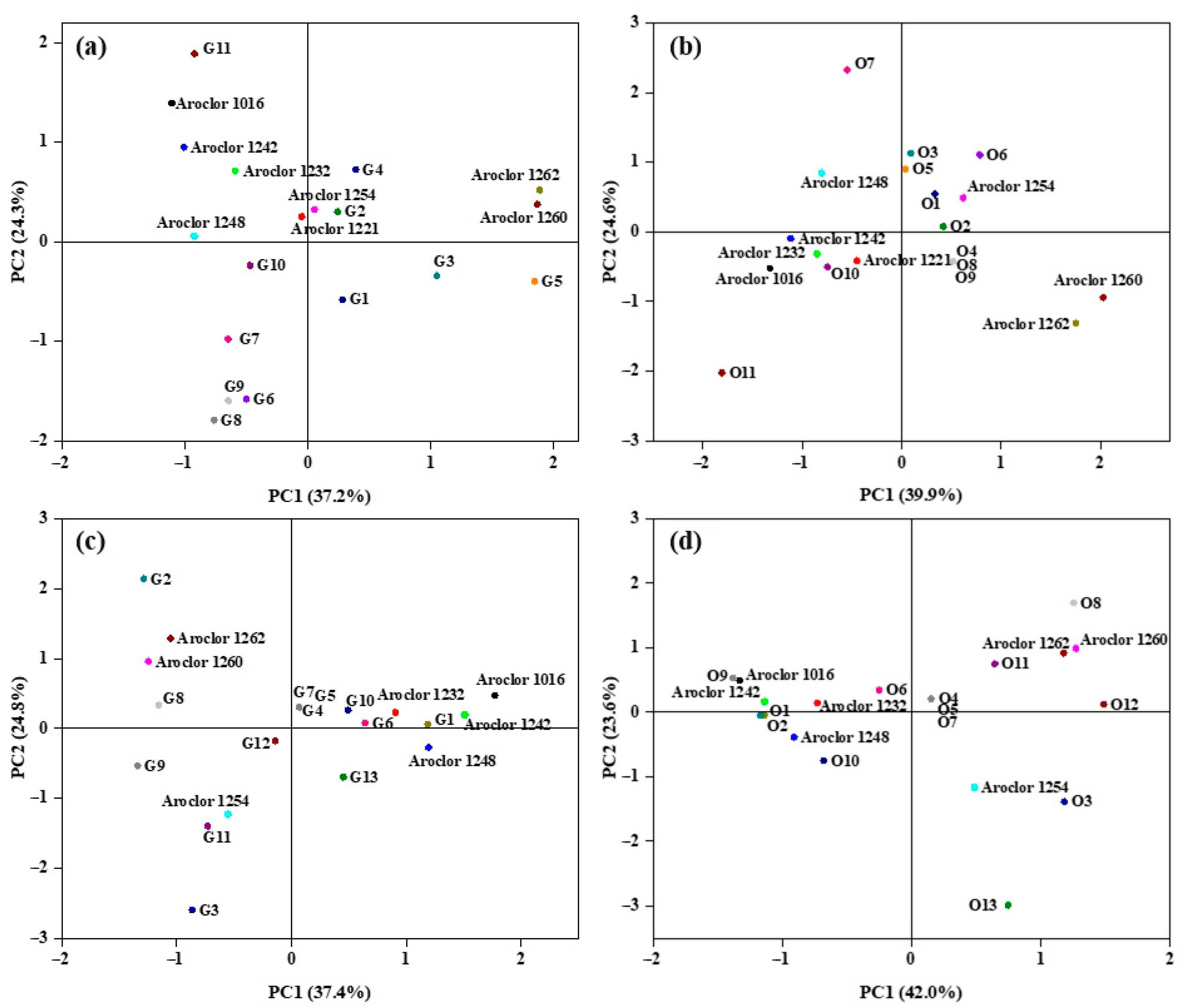
3.2. Regional Contamination and Source Analysis
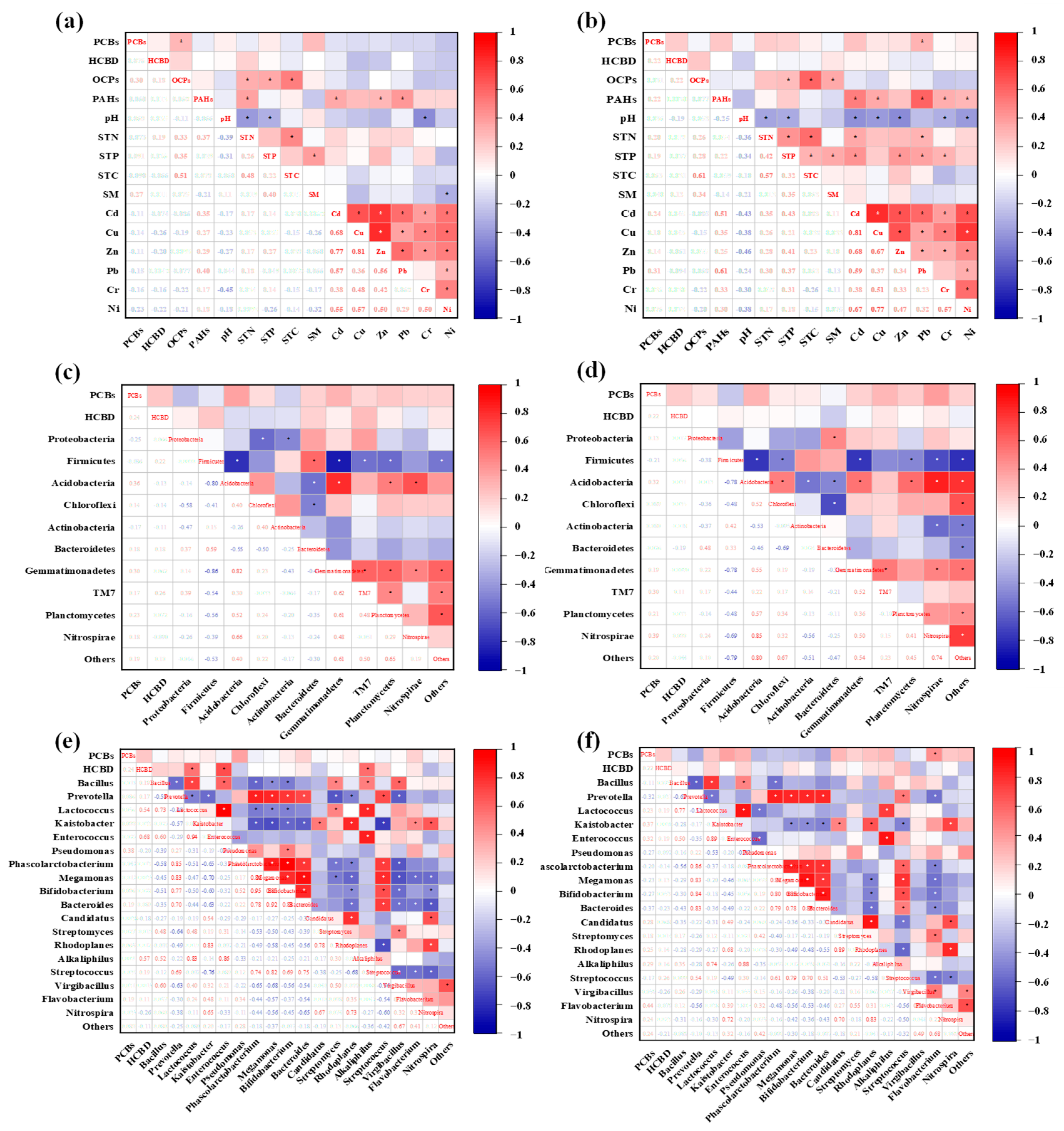
3.3. Correlation between Pollutants and Environmental Factors
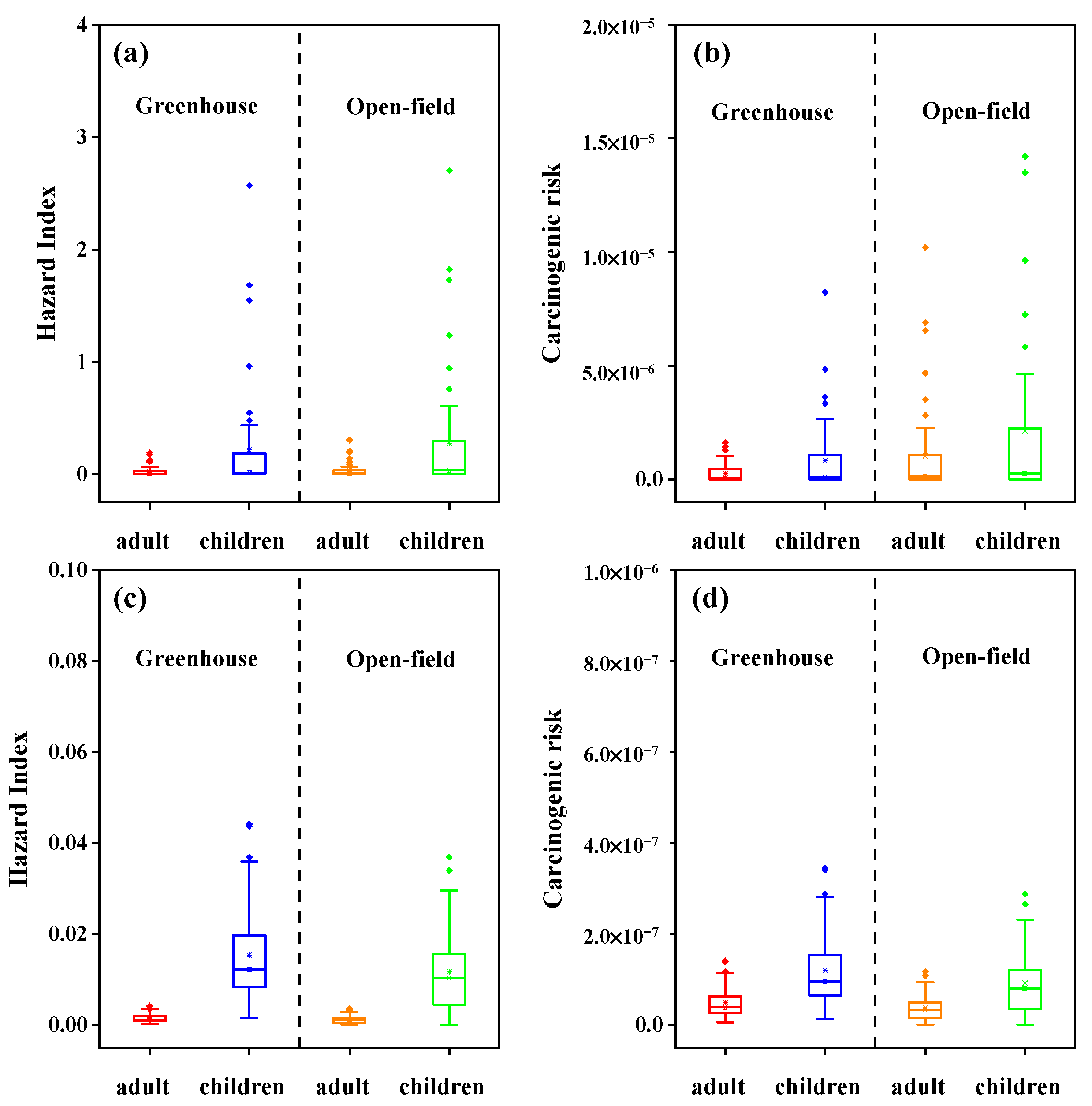
3.4. Risk Assessments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Tiwari, G.N.; Kumar, S.; Pandey, M. Role of greenhouse technology in agricultural engineering. Int. J. Agric. Res. 2006, 1, 364–372. [Google Scholar] [CrossRef]
- Zhang, M.H.; Yan, T.X.; Wang, W.; Jia, X.X.; Wang, J.; Klemes, J.J. Energy-saving design and control strategy towards modern sustainable greenhouse: A review. Renew. Sustain. Energy Rev. 2022, 164, 112602. [Google Scholar] [CrossRef]
- Hanan, J.J. Greenhouses: Advanced Technology for Protected Horticulture, 1st ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Wu, R.L.; He, W.; Li, Y.L.; Li, Y.Y.; Qin, Y.F.; Meng, F.Q. Residual concentrations and ecological risks of neonicotinoid insecticides in the soils of tomato and cucumber greenhouses in Shouguang, Shandong province, East China. Sci. Total Environ. 2020, 738, 140248. [Google Scholar] [CrossRef]
- Wang, X.B.; Wang, X.L.; Sheng, H.J.; Wang, X.Z.; Zhao, H.T.; Feng, K. Excessive nitrogen fertilizer application causes rapid degradation of greenhouse soil in China. Pol. J. Environ. Stud. 2022, 31, 1527–1534. [Google Scholar] [CrossRef]
- Badrudin, U.; Jazilah, S.; Prakoso, B. The effect of soil submersion and conditioner materials on residual organophosphate pesticides in soil and shallot bulbs. J. Agric. Sci. 2022, 44, 1–10. [Google Scholar] [CrossRef]
- Sun, J.T.; Pan, L.L.; Li, Z.H.; Zeng, Q.T.; Wang, L.W.; Zhu, L.Z. Comparison of greenhouse and open field cultivations across China: Soil characteristics, contamination and microbial diversity. Environ. Pollut. 2018, 243, 1509–1516. [Google Scholar] [CrossRef]
- Dou, R.N.; Sun, J.T.; Deng, F.C.; Wang, P.L.; Zhou, H.J.; Wei, Z.; Chen, M.Q.; He, Z.X.; Lai, M.L.; Ye, T.C.; et al. Contamination of pyrethroids and atrazine in greenhouse and open-field agricultural soils in China. Sci. Total Environ. 2020, 701, 134916. [Google Scholar] [CrossRef]
- Li, Z.H.; Sun, J.T.; Zhu, L.Z. Organophosphorus pesticides in greenhouse and open-field soils across China: Distribution characteristic, polluted pathway and health risk. Sci. Total Environ. 2021, 765, 142757. [Google Scholar] [CrossRef]
- Krohn, C.; Zhang, P.; Wood, J.L.; Hayden, H.L.; Franks, A.E.; Jin, J.; Tang, C.X. Biochar reduced extractable dieldrin concentrations and promoted oligotrophic growth including microbial degraders of chlorinated pollutants. J. Hazard. Mater. 2020, 423, 127156. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.H.; Tao, M.L.; Zhou, D.D.; Zhang, Y.; Yao, J.; Kong, Q.N.; Guo, B.B. Occurrence and distribution characteristics of PCBs and PBDEs in farmland soils adjacent to electronic circuit board dismantling ruins. Front. Environ. Sci. 2023, 10, 1048345. [Google Scholar] [CrossRef]
- Breivik, K.; Sweetman, A.; Pacyna, J.M.; Jones, K.C. Towards a global historical emission inventory for selected PCB congeners—A mass balance approach-3. An update. Sci. Total Environ. 2007, 377, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Van der Gon, H.D.; van het Bolscher, M.; Visschedijk, A.; Zandveld, P. Emissions of persistent organic pollutants and eight candidate POPs from UNECE-Europe in 2000, 2010 and 2020 and the emission reduction resulting from the implementation of the UNECE POP protocol. Atmos. Environ. 2007, 41, 9245–9261. [Google Scholar] [CrossRef]
- Wang, L.; Bie, P.J.; Zhang, J.B. Estimates of unintentional production and emission of hexachlorobutadiene from 1992 to 2016 in China. Environ. Pollut. 2018, 238, 204–212. [Google Scholar] [CrossRef]
- Luo, C.L.; Hu, B.B.; Wang, S.R.; Wang, Y.; Zhao, Z.; Wang, Y.J.; Li, J.; Zhan, G. Distribution and chiral signatures of polychlorinated biphenyls (PCBs) in soils and vegetables around an e-waste recycling site. J. Agric. Food Chem. 2020, 68, 10542–10549. [Google Scholar] [CrossRef] [PubMed]
- Khuman, S.N.; Vinod, P.G.; Bharat, G.; Kumar, Y.S.M.; Chakraborty, P. Spatial distribution and compositional profiles of organochlorine pesticides in the surface soil from the agricultural, coastal and backwater transects along the south-west coast of India. Chemosphere 2020, 254, 126699. [Google Scholar] [CrossRef]
- Pena, A.; Delgado-Moreno, L.; Rodriguez-Liebana, J.A. A review of the impact of wastewater on the fate of pesticides in soils: Effect of some soil and solution properties. Sci. Total Environ. 2020, 718, 134468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.B.; Luo, Y.M.; Teng, Y.; Wan, H.F. PCB contamination in soils of the Pearl River Delta, South China: Levels, sources, and potential risks. Environ. Sci. Pollut. Res. 2013, 20, 5150–5159. [Google Scholar] [CrossRef]
- Mamontova, E.A.; Tarasova, E.N.; Mamontov, A.A.; Kuzmin, M.I.; McLachlan, M.S.; Khomutova, M.I. The influence of soil contamination on the concentration of PCBs in milk in Siberia. Chemosphere 2007, 67, 71–78. [Google Scholar] [CrossRef]
- Wang, C.F.; Gong, P.; Wang, X.P.; Yang, T.D. Distribution, environmental behavior, and health risks of polychlorinated biphenyls in the Tibetan agricultural soil and crops. Asian J. Ecotoxicol. 2016, 11, 339–346. [Google Scholar]
- Tang, Z.W.; Huang, Q.F.; Cheng, J.L.; Qu, D.; Yang, Y.F.; Guo, W. Distribution and accumulation of hexachlorobutadiene in soils and terrestrial organisms from an agricultural area, East China. Ecotoxicol. Environ. Saf. 2014, 108, 329–334. [Google Scholar] [CrossRef]
- Sun, J.T.; Pan, L.L.; Zhan, Y.; Zhu, L.Z. Spatial distributions of hexachloro-butadiene in agricultural soils from the Yangtze River Delta regions of China. Environ. Sci. Pollut. Res. Int. 2018, 25, 3378–3385. [Google Scholar] [CrossRef]
- Huang, S.Y.; Bao, J.P.; Shan, M.J.; Qin, H.; Wang, H.L.; Yu, X.J.; Chen, J.H.; Xu, Q.F. Dynamic changes of polychlorinated biphenyls (PCBs) degradation and adsorption to biochar as affected by soil organic carbon content. Chemosphere 2018, 211, 120–127. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Fang, G.D.; Herath, H.M.S.K.; Wang, Y.J.; Cang, L.; Xie, Z.B.; Zhou, D. Enhanced PCBs sorption on biochars as affected by environmental factors: Humic acid and metal cations. Environ. Pollut. 2013, 172, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.A.; Lin, L.S.; Just, C.L.; Hu, D.F.; Hornbuckle, K.C.; Schnoor, J.L.; Van Aken, B. The effects of individual PCB congeners on the soil bacterial community structure and the abundance of biphenyl dioxygenase genes. Environ. Int. 2010, 36, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.H.; Seeger, M. Bacterial metabolism of polychlorinated biphenyls. J. Mol. Microbiol. Biotechnol. 2008, 15, 121–138. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.D.; Fu, J.J.; Li, Y.M.; Wang, T.; Wang, Y.W.; Ren, D.W.; Ssebugere, P.; Zhang, Q.H.; Jiang, G.B. Temporal trends of PCBs, PCDD/Fs and PBDEs in soils from an E-waste dismantling area in East China. Environ. Sci. Process. Impacts 2013, 15, 1897–1903. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, Y.W.; Sun, C.; Yu, M.; Gao, Y.; Wang, T.; Liu, J.Y.; Jiang, G.B. Levels and distributions of hexachlorobutadiene and three chlorobenzenes in biosolids from wastewater treatment plants and in soils within and surrounding a chemical plant in China. Environ. Sci. Technol. 2014, 48, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.L.; Mao, S.D.; Zhou, J.Y.; Zhao, L.; Zhu, Y.Q.; Xu, C.; Sun, X.H.; Sun, J.Q.; Liu, W.P. Polychlorinated biphenyls (PCBs) in soils from typical paddy fields of China: Occurrence, influencing factors and human health risks. Environ. Pollut. 2022, 307, 119567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.F.; Miao, Q.F.; Pei, G.X.; Nan, Y.X.; Yu, S.Y.; Mei, X.L.; Feng, W.Y. Distribution, sources, and risk of polychlorinated biphenyls in the largest irrigation area in the Yellow River Basin. Water 2022, 14, 3472. [Google Scholar] [CrossRef]
- Sun, J.T.; Pan, L.L.; Tsang, D.C.W.; Zhan, Y.; Liu, W.X.; Wang, X.L.; Zhu, L.Z.; Li, X.D. Polychlorinated biphenyls in agricultural soils from the Yangtze River Delta of China: Regional contamination characteristics, combined ecological effects and human health risks. Chemosphere 2016, 163, 422–428. [Google Scholar] [CrossRef]
- Wu, S.; Xia, X.H.; Yang, L.Y.; Liu, H. Distribution, source and risk assessment of polychlorinated biphenyls (PCBs) in urban soils of Beijing, China. Chemosphere 2011, 82, 732–738. [Google Scholar] [CrossRef]
- Li, Q.L.; Wang, Y.; Luo, C.L.; Li, J.; Zhang, G. Characterization and risk assessment of polychlorinated biphenyls in soils and rice tissues in a suburban paddy field of the Pearl River Delta, South China. Environ. Sci. Pollut. Res. 2015, 22, 11626–11633. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.; Ozturk, F.; Keles, M.; Yurdakul, S. PAHs and PCBs in an Eastern Mediterranean megacity, Istanbul: Their spatial and temporal distributions, air-soil exchange and toxicological effects. Environ. Pollut. 2017, 220, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Syed, J.H.; Mahmood, A.; Li, J.; Zhang, G.; Jones, K.C.; Malik, R.N. Influential role of black carbon in the soil-air partitioning of polychlorinated biphenyls (PCBs) in the Indus River Basin, Pakistan. Chemosphere 2015, 134, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Niu, L.L.; Zou, D.L.; Zhu, S.Y.; Liu, W.P. Congener-specific composition of polychlorinated biphenyls (PCBs) in soil-air partitioning and the associated health risks. Sci. Total Environ. 2019, 684, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.W.; Huang, Q.F.; Nie, Z.Q.; Yang, Y.F.; Yang, J.; Qu, D.; Cheng, J.L. Levels and distribution of organochlorine pesticides and hexachlorobutadiene in soils and terrestrial organisms from a former pesticide-producing area in Southwest China. Stoch. Environ. Res. Risk Assess. 2016, 30, 1249–1262. [Google Scholar] [CrossRef]
- Nieuwoudt, C.; Quinn, L.P.; Pieters, R.; Jordaan, I.; Visser, M.; Kylin, H.; Borgen, A.R.; Giesy, J.P.; Bouwman, H. Dioxin-like chemicals in soil and sediment from residential and industrial areas in central South Africa. Chemosphere 2009, 76, 774–783. [Google Scholar] [CrossRef]
- Habibullah-Al-Mamun, M.; Ahmed, M.K.; Islam, M.S.; Tokumura, M.; Masunaga, S. Occurrence, distribution and possible sources of polychlorinated biphenyls (PCBs) in the surface water from the Bay of Bengal coast of Bangladesh. Ecotoxicol. Environ. Saf. 2019, 167, 450–458. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, H.; Liu, Y.; Li, S.Q. Residues Characters and Health Risk Assessment of PCBs in Agricultural Soils of Taiyuan City. J. Anhui Agric. Sci. 2017, 45, 96–101. [Google Scholar]
- Sun, L.X.; Mao, J.; Liu, T.F.; Yang, D.F. Analysis of polychlorinated biphenyls pollution status in farmland soils of south Jiangsu under different land-use types. J. Food Saf. Qual. 2019, 10, 5615–5620. [Google Scholar]
- Ding, J.F.; Zhang, J.L.; Dang, Z.; Lu, G.N.; Yi, X.J. Polychlorinated biphenyls contamination and its effect on microbial population in soil near E-waste dismantling area in Qingyuan, Guangdong, South China. Sci. Technol. Eng. 2015, 15, 48–53. [Google Scholar]
- Hu, T.P.; Mao, Y.; Ke, Y.P.; Liu, W.J.; Cheng, C.; Shi, M.M.; Zhang, Z.Q.; Zhang, J.Q.; Qi, S.H.; Xing, X.L. Spatial and seasonal variations of PAHs in soil, air, and atmospheric bulk deposition along the plain to mountain transect in Hubei province, central China: Air-soil exchange and long-range atmospheric transport. Environ. Pollut. 2021, 29, 118139. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Lin, T.; Li, Y.Y.; Guo, Z.G. Concentration and composition of polychlorinated biphenyls in the water of the East China Sea. Mar. Environ. Sci. 2019, 38, 589–593. [Google Scholar]
- Lee, R.G.M.; Coleman, P.; Jones, J.L.; Jones, K.C.; Lohmann, R. Emission factors and importance of PCDD/Fs, PCBs, PCNs, PAHs and PM10 from the domestic burning of coal and wood in the U.K. Environ. Sci. Technol. 2005, 39, 1436–1447. [Google Scholar] [CrossRef]
- Ba, T.; Zheng, M.H.; Zhang, B.; Liu, W.B.; Xiao, K.; Zhang, L.F. Estimation and characterization of PCDD/Fs and dioxin-like PCBs from secondary copper and aluminum metallurgies in China. Chemosphere 2009, 75, 73–1178. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.F.; Wang, X.T.; Wu, M.H.; Sheng, G.Y.; Fu, J.M. Characteristics and source of polychlorinated biphenyls in agricultural soil of Shanghai, China. J. Agro-Environ. Sci. 2010, 29, 899–903. [Google Scholar]
- Sun, J.T.; Pan, L.L.; Tsang, D.C.W.; Zhan, Y.; Zhu, L.Z.; Li, X.D. Organic contamination and remediation in the agricultural soils of China: A critical review. Sci. Total Environ. 2018, 615, 724–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wei, B.K.; Bao, J.S.; Wang, Y.; Hu, J.C.; Tang, Y.E.; Chen, T.; Jin, J. Polychlorinated biphenyls in the soil-crop-atmosphere system in e-waste dismantling areas in Taizhou: Concentrations, congener profiles, uptake, and translocation. Environ. Pollut. 2020, 257, 113622. [Google Scholar] [CrossRef]
- Meng, J.; Hong, S.; Wang, T.Y.; Li, Q.F.; Yoon, S.J.; Lu, Y.L.; Giesy, J.P.; Khim, J.S. Traditional and new POPs in environments along the Bohai and Yellow Seas: An overview of China and South Korea. Chemosphere 2017, 169, 503–515. [Google Scholar] [CrossRef]
- Diop, M.; Net, S.; Howsam, M.; Lencel, P.; Watier, D.; Grard, T.; Duflos, G.; Diouf, A.; Amara, R. Concentrations and potential human health risks of trace metals (Cd, Pb, Hg) and selected organic pollutants (PAHs, PCBs) in fish and seafood from the Senegalese Coast. Int. J. Environ. Res. 2017, 11, 349–358. [Google Scholar] [CrossRef]
- Dreyer, A.; Minkos, A. Polychlorinated biphenyls (PCB) and polychlorinated dibenzo-para-dioxins and dibenzofurans (PCDD/F) in ambient air and deposition in the German background*. Environ. Pollut. 2022, 316, 120511. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, X.P.; Gong, P.; Wang, C.F. Characterization of Tibetan soil as a source or sink of atmospheric persistent organic pollutants: Seasonal shift and impact of global warming. Environ. Sci. Technol. 2019, 53, 3589–3598. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, G.C.; Moodley, B. Kinetic and thermodynamic studies on partitioning of polychlorinated biphenyls (PCBs) between aqueous solution and modeled individual soil particle grain sizes. J. Environ. Sci. 2019, 76, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Terzaghi, E.; Vitale, C.M.; Salina, G.; Di Guardo, A. Plants radically change the mobility of PCBs in soil: Role of different species and soil conditions. J. Hazard. Mater. 2020, 388, 121786. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yu, L.; Xu, P.; Liang, Z.W.; Lu, Q.H.; Liang, D.W.; He, Z.L.; Wang, S.Q. Water content as a primary parameter determines microbial reductive dechlorination activities in soil. Chemosphere 2021, 267, 129152. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.F.; Zhao, X.J.; Zhao, S.Y.; Rogers, M.J.; He, J.Z. Salinity determines performance, functional populations, and microbial ecology in consortia attenuating organohalide pollutants. ISME J. 2023, 17, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Su, X.M.; Li, S.; Xie, M.Q.; Tao, L.Q.; Zhou, Y.; Xiao, Y.Y.; Lin, H.J.; Chen, J.R.; Sun, F.Q. Enhancement of polychlorinated biphenyl biodegradation by resuscitation promoting factor (Rpf) and Rpf-responsive bacterial community. Chemosphere 2021, 263, 128283. [Google Scholar] [CrossRef] [PubMed]
- Sadañoski, M.A.; Tatarin, A.S.; Velázquez, J.E.; Gonzalez, M.; Pegoraro, C.N.; Fonseca, M.I.; Villalba, L.L. PCB decomposition promoted by sugarcane bagasse organic waste. Rhizosphere 2023, 27, 100722. [Google Scholar] [CrossRef]
- Suenaga, H.; Fujihara, H.; Kimura, N.; Hirose, J.; Watanabe, T.; Futagami, T.; Goto, M.; Shimodaira, J.; Furukawa, K. Insights into the genomic plasticity of Pseudomonas putida KF715, a strain with unique biphenyl-utilizing activity and genome instability properties. Environ. Microbiol. Rep. 2017, 9, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Viktorova, J.; Jandova, Z.; Madlenakova, M.; Prouzova, P.; Bartunek, V.; Vrchotova, B.; Lovecka, P.; Musilova, L.; Macek, T. Native phytoremediation potential of Urtica dioica for removal of PCBs and heavy metals can be improved by genetic manipulations using constitutive CaMV 35S promoter. PLoS ONE 2017, 12, e0187053. [Google Scholar] [CrossRef]
- Du, J.J.; Hou, F.; Zhou, Q.X. Response of soil enzyme activity and soil bacterial community to PCB dissipation across different soils. Chemosphere 2021, 283, 131229. [Google Scholar] [CrossRef]
- Chang, Y.C.; Takada, K.; Choi, D.; Toyama, T.; Sawada, K.; Kikuchi, S. Isolation of biphenyl and polychlorinated biphenyl-degrading bacteria and their degradation pathway. Appl. Biochem. Biotechnol. 2013, 170, 381–398. [Google Scholar] [CrossRef]
- Niu, L.L.; Xu, C.; Yao, Y.J.; Liu, K.; Yang, F.X.; Tang, M.L.; Liu, W.P. Status, influences and risk assessment of hexachlorocyclohexanes in agricultural soils across China. Environ. Sci. Technol. 2013, 47, 12140–12147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Pei, G.X.; Zhang, Q.; Xu, M. Pollution characteristics and health risk of polychlorinated biphenyls in cultivated soil in the Hetao irrigation areas, Inner Mongolia, China. J. Agro-Environ. Sci. 2021, 40, 114–122. [Google Scholar]
- Rasmussen, P.E.; Subramanian, K.S.; Jessiman, B.J. A multi-element profile of housedust in relation to exterior dust and soils in the city of Ottawa, Canada. Sci. Total Environ. 2001, 267, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.X.; Tao, X.M.; Lv, K.L.; Zhang, N. Distribution characteristics and risk analysis of PAHs and PCBs in soils of Lanzhou. Adm. Technol. Environ. Monit. 2018, 30, 25–29. [Google Scholar]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.Q.; Wu, W.H.; Lin, D.H.; Yang, K. Microbial degradation of nondesorbable organic compounds on biochars by extracellular reactive oxygen species. J. Hazard. Mater. 2022, 439, 129625. [Google Scholar] [CrossRef]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]

| Compounds | Detection (%) | Mean (ng/g) | Median (ng/g) | Max (ng/g) | Min (ng/g) | |
|---|---|---|---|---|---|---|
| Greenhouse | PCBs | 84.31 | 62.33 | 52.22 | 241.22 | ND |
| HCBD | 100 | 8.19 | 6.64 | 24.18 | 0.85 | |
| Open-field | PCBs | 76.47 | 51.09 | 39.99 | 192.89 | ND |
| HCBD | 96.08 | 6.52 | 5.58 | 20.19 | ND |
| Different Region | PCBs | HCBD | ||
|---|---|---|---|---|
| Northeast China (n = 14) | Open-field soils | Max | 103.15 | 12.49 |
| Med | 32.95 | 5.05 | ||
| Mean | 30.21 | 5.27 | ||
| Greenhouse soils | Max | 123.86 | 10.89 | |
| Med | 23.05 | 3.93 | ||
| Mean | 38.51 | 5.26 | ||
| North China (n = 22) | Open-field soils | Max | 192.90 | 15.68 |
| Med | 77.35 | 5.84 | ||
| Mean | 82.16 | 6.75 | ||
| Greenhouse soils | Max | 241.21 | 23.91 | |
| Med | 97.54 | 8.24 | ||
| Mean | 111.05 | 10.39 | ||
| East China (n = 26) | Open-field soils | Max | 143.80 | 20.19 |
| Med | 43.50 | 4.25 | ||
| Mean | 48.59 | 5.37 | ||
| Greenhouse soils | Max | 171.21 | 20.19 | |
| Med | 34.75 | 6.48 | ||
| Mean | 51.64 | 8.89 | ||
| Central China (n = 4) | Open-field soils | Max | 66.55 | 7.23 |
| Med | 52.26 | 6.00 | ||
| Mean | 52.26 | 6.00 | ||
| Greenhouse soils | Max | 100.74 | 9.83 | |
| Med | 53.65 | 7.17 | ||
| Mean | 53.65 | 7.17 | ||
| South China (n = 4) | Open-field soils | Max | 104.98 | 15.33 |
| Med | 82.27 | 13.76 | ||
| Mean | 82.27 | 13.76 | ||
| Greenhouse soils | Max | 130.89 | 15.49 | |
| Med | 65.44 | 11.85 | ||
| Mean | 65.44 | 11.85 | ||
| Northwest China (n = 16) | Open-field soils | Max | 107.92 | 11.40 |
| Med | 57.27 | 5.31 | ||
| Mean | 51.12 | 5.38 | ||
| Greenhouse soils | Max | 85.52 | 16.87 | |
| Med | 8.29 | 6.51 | ||
| Mean | 28.68 | 7.46 | ||
| Southwest China (n = 16) | Open-field soils | Max | 132.09 | 18.60 |
| Med | 15.89 | 6.79 | ||
| Mean | 23.87 | 8.28 | ||
| Greenhouse soils | Max | 156.11 | 24.18 | |
| Med | 66.37 | 5.01 | ||
| Mean | 74.52 | 6.88 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hou, F.; Shi, R.; Li, X.; Lan, J.; Zhao, Z. Contamination Status, Environmental Factor and Risk Assessment of Polychlorinated Biphenyls and Hexachlorobutadiene in Greenhouse and Open-Field Agricultural Soils across China. Toxics 2023, 11, 941. https://doi.org/10.3390/toxics11110941
Li Y, Hou F, Shi R, Li X, Lan J, Zhao Z. Contamination Status, Environmental Factor and Risk Assessment of Polychlorinated Biphenyls and Hexachlorobutadiene in Greenhouse and Open-Field Agricultural Soils across China. Toxics. 2023; 11(11):941. https://doi.org/10.3390/toxics11110941
Chicago/Turabian StyleLi, Yaru, Fangwei Hou, Rongguang Shi, Xiaohua Li, Jing Lan, and Zongshan Zhao. 2023. "Contamination Status, Environmental Factor and Risk Assessment of Polychlorinated Biphenyls and Hexachlorobutadiene in Greenhouse and Open-Field Agricultural Soils across China" Toxics 11, no. 11: 941. https://doi.org/10.3390/toxics11110941
APA StyleLi, Y., Hou, F., Shi, R., Li, X., Lan, J., & Zhao, Z. (2023). Contamination Status, Environmental Factor and Risk Assessment of Polychlorinated Biphenyls and Hexachlorobutadiene in Greenhouse and Open-Field Agricultural Soils across China. Toxics, 11(11), 941. https://doi.org/10.3390/toxics11110941







